Abstract
Objective
To determine the disease characteristics and comorbidities predictive of vulvar cancer specific mortality and five year overall survival among older women, ages 65 and above.
Methods
A retrospective analysis was conducted of women diagnosed with vulvar cancer at a single regional cancer center from 1989 to 2003, with a follow up to 2009. Treatment records were extracted for: demographics and treatment information, Eastern Cooperative Oncology Group (ECOG) performance status and Charlson comorbidity index score. Probability of death from vulvar cancer was estimated using cumulative incidence, treating death by other known and unknown causes as competing risks. Predictors of overall survival were determined using multivariate Cox regression analyses.
Results
One hundred forty-six women were identified, with a median age at diagnosis of 79 years (range 65–95). Median follow up was 5.0 years (range 0.1–16.7 years). The cumulative incidence of vulvar cancer-specific mortality was 13% (95% CI: 0.08–0.19) at year one, 24% (95% CI: 0.17–0.31) at year three and 26% (95% CI: 0.19–0.33) at year five. Use of adjuvant therapy or surgical procedure performed did not differ by age at diagnosis (p=0.807 and 0.663) according to age group (65–74, 74–84 and 85+). Increasing age, Charlson comorbidity index score, lymph node involvement and type of surgery performed were associated with increased risk of death from any cause (all p<0.05).
Conclusion
Among women aged ≥65, vulvar cancer specific mortality was most significant in the first three years after diagnosis. Conversely other causes of mortality which can be attributed to comorbid conditions steadily increased with time.
Keywords: Vulvar cancer, Comorbidity, Treatment outcome
Introduction
Advancing age has been identified as a prognostic indicator for survival from vulvar cancer. Vulvar cancer is diagnosed at a median age of 69 years; the incidence of which begins to rise after age 50, then increases sharply after age 70 [1,2]. The life expectancy of women in the United States (US) as of 2006 has extended to the 8th decade of life and women at ages of 65, 75, and 85 years can expect to live, on average, another 20, 12, and 7 years respectively [3]. As the number of women in the US above the age of 65 increases, addressing unique aspects of cancer care among the elderly is of significance.
Analysis of US Federal Cancer Registry data from 1998 to 2003 found the 5-year relative survival rate from vulvar cancer in women less than 40 years of age to be significantly higher (96%) than for women above 65 years of age (72%) [1]. The high survival rate can, in part, be attributed to the large percentage of women who are diagnosed with early stage disease when the potential for cure is highest [4,5]. Women above the age 50 and in particular those greater than 75 years of age, experience a decreased vulvar cancer specific survival compared to a younger aged cohort [6-8].
The standard treatment for vulvar cancer involves radical vulvectomy with or without inguinal lymph node dissection. Adjuvant postoperative radiation therapy and chemotherapy have shown benefit in women with advanced vulvar cancer [9,10]. Although advances in the standard of care for treatment of vulvar cancer have improved survival, these treatments often invoke morbidity. The management of the elderly patient (7th and 8th decade of life) is therefore often individualized based on the patient’s performance status and comorbid conditions [8,11].
The trend of decreased survival in older women with invasive vulvar cancer has been attributed to increased comorbid conditions, poor performance status, and lack of appropriate referral. Beyond the clinically significant prognostic marker of lymph node involvement, the Eastern Cooperative Oncology Group (ECOG) performance status below that of fully active (0 score) has been associated with the use of nonstandard therapies [12]. The Surveillance, Epidemiology, and End Results (SEER) Program data from 1988 to 2005 shows that women less than 50 years of age were more likely to receive standard therapy including surgical resection while women older than 50 years tended to receive modified therapy with radiation resulting in differing survival rates of 87% and 52%, respectively [6].
In the current study we examined the treatment patterns of vulvar cancer in older women, ages 65 and above, from a single institution. The primary goal of this study is to determine disease and treatment characteristics that are predictive of mortality from vulvar cancer. We also explored predictors of overall survival, including all causes of death.
Patients and methods
We performed a retrospective analysis of vulvar cancer patients diagnosed from January 1989 to December 2003 at the Masonic Cancer Center (MCC) at the University of Minnesota using a tumor registry, with follow up to December 2009. Institutional Review Board approval was obtained prior to study initiation. Inclusion criteria included women 65 years or older diagnosed with pathologically confirmed invasive adenocarcinoma or squamous cell carcinoma of the vulva. Exclusion criteria included diagnosis of vulvar intraepithelial neoplasia (VIN) or lichen sclerosis. Clinical data was abstracted from outpatient and hospital charts.
Data extracted included: age, place of residence (nursing home or other), previous cancer diagnosis, and estimated distance to the MCC as determined by recording of zip code. Patients were grouped as residing within 30 mi or beyond from the MCC. ECOG performance status score, a 5 point scale that measures the ability to carry out normal daily activities (0) to being bed bound (4), was retrospectively assessed by two independent abstractors [13]. Charlson comorbidity index score was assigned to each patient based on review of documented medical diagnosis from the initial outpatient chart record or from the initial hospital admission. Charlson comorbidity index is tabulated from nineteen comorbid conditions which have assigned weights of 1, 2, 3, or 6, based on mortality risk for patients with the comorbid condition compared to mortality risk for those without [14]. All patients were staged according to the International Federation of Gynecology and Obstetrics (FIGO) system [15]. Treatment follow up data was obtained by review of outpatient charts and cancer registry data.
One-hundred forty six women diagnosed with vulvar cancer were included in this analysis. Demographics and disease characteristics for all patients were summarized. Age at diagnosis was collapsed into three groups: 65–74, 75–84 and over 85 years old for the purposes of this analysis as categorized by the U.S. Census Bureau. Disease stage, surgery performed and the use of adjuvant therapy were compared across age groups using chi-squared and Fisher’s exact tests. Patient deaths were divided into three categories: death from vulvar cancer, death from other known cause or death from unknown cause. Event times were calculated from the date of first diagnosis of vulvar cancer to the event or the date of last contact for patients still alive.
The probability of death from vulvar cancer was estimated using cumulative incidence, treating deaths by other known and unknown causes as competing risks. Differences in the incidence of death from vulvar cancer by age at diagnosis were analyzed using Gray’s test. Fine and Gray competing risks regression was used for multivariate analyses[16]. Predictors of overall survival (death from all causes) were determined using multivariate Cox regression [17]. Variables considered in the multivariate analyses included age at diagnosis (65–74/75–84/85+ years), FIGO stage (I/II/III/IV), previous cancer (yes/no), surgery (radical vulvectomy only/local excision only/unilateral or bilateral inguinal lymph node dissection (LND)/radical vulvectomy+inguinal LND), adjuvant therapy (none/chemotherapy only/radiation only/chemotherapy and radiation), lymph node involvement (yes/no), and Charlson comorbidity index score (0/1/2+). Final models were chosen using backward selection, removing variables with p-values >0.10. Performance status and place of residence were not included as possible covariates because of high correlation with other variables. Two patients with missing stage information and six with unknown lymph node involvement were excluded from the multivariate analyses.
All p values were two-sided and considered to be statistically significant if less than 0.05. All analyses were performed using SAS version 9.2 and R version 2.7.
Results
Patient demographics and clinical variables
One hundred and forty six women with vulvar cancer diagnosed over a fifteen year time period (1989–2003) were identified. The median age at diagnosis was 79 years (range 65–97). Thirty-three women were 85 years of age or older, of which 10 were 90 years or older. The women were primarily on Medicare (98%), lived within 30 mi of the clinic (56%), and were fully active (80%) with an ECOG performance status of 0 (Table 1). A Charlson comorbidity index score of 0 was recorded for 79 women (54%), score of 1 for 37 women (25%) and score of ≥2 for 30 women (21%). Thirteen women (9%) were nursing home residents and the majority of them (75%) had an ECOG performance status of 3 or 4. These women were more likely to have a Charlson comorbidity index score of ≥2. Common comorbid conditions were congestive heart failure, diabetes, peripheral vascular disease and chronic pulmonary disease. A history of previous cancer diagnosis was reported by 30 (21%) of the women.
Table 1.
Demographic and clinical characteristics (N = 146).
| Variable | N | % |
|---|---|---|
| Age at diagnosis | ||
| 65–74 | 43 | 29.45 |
| 75–84 | 70 | 47.95 |
| 85+ | 33 | 22.60 |
| Year of diagnosis | ||
| 1989–1994 | 47 | 32.19 |
| 1995–1999 | 66 | 45.21 |
| 2000–2003 | 33 | 22.60 |
| Insurance | ||
| Medicare | 143 | 97.95 |
| Other | 3 | 2.05 |
| Type of residence | ||
| Nursing home | 13 | 8.90 |
| Other | 133 | 91.10 |
| Distance of residence from university clinic | ||
| Within 30 mi | 82 | 56.16 |
| Greater than 30 mi | 62 | 42.47 |
| Unknown | 2 | 1.37 |
| ECOG performance status | ||
| 0 (Fully active) | 117 | 80.14 |
| 1 (Restricted in physically strenuous activity) | 13 | 8.90 |
| 2 (Ambulatory and capable of self-care) | 2 | 1.37 |
| 3 (Capable of only limited self-care) | 8 | 4.48 |
| 4 (Complete disabled) | 6 | 4.11 |
| Charlson score | ||
| 0 | 79 | 54.11 |
| 1 | 37 | 25.34 |
| 2+ | 30 | 20.55 |
| Histology | ||
| Squamous carcinoma | 143 | 97.95 |
| Adenocarcinoma | 3 | 2.05 |
| Disease stage (FIGO) | ||
| Stage I | 45 | 31.25 |
| Stage II | 48 | 33.33 |
| Stage III | 37 | 25.69 |
| Stage IV | 14 | 9.72 |
| Unstaged | 2 | |
| Lymph node involvement | ||
| No histological involvement | 103 | 73.57 |
| Involvement | 37 | 26.43 |
| Unknown | 6 | |
| Adjuvant therapy | ||
| None | 106 | 72.60 |
| Chemotherapy only | 1 | 0.68 |
| Radiation only | 15 | 10.27 |
| Chemotherapy and radiation | 24 | 16.44 |
| Surgeries performed | ||
| Radical vulvectomy only | 26 | 17.81 |
| Local excision only | 6 | 4.11 |
| Unilateral or bilateral inguinal LND | 3 | 2.05 |
| Radical vulvectomy + inguinal LND | 111 | 76.03 |
The predominant histologic subtype was squamous (143; 98%). The most common surgical procedure performed was radical vulvectomy with either unilateral or bilateral inguinal lymph node dissection (111; 76%), resulting with no histological metastasis to lymph nodes (103; 74%). FIGO stages I and II were the most common clinical stages (93; 65%) with the majority of patients receiving no adjuvant therapy (106; 73%).
The median follow up for the group was 5.0 years (range 0.1–16.7 years). Of the 146 patients, 44 (30.1%) were alive at last contact, 44 (30.1%) died from vulvar cancer, 49 (33.6%) died from other known causes and 9 (6.2%) died from an unknown cause. Other known causes of death include cardiovascular events, cerebrovascular disease and diabetes.
Vulvar cancer-specific mortality
The cumulative incidence of vulvar cancer-specific mortality was 13% (95% CI: 0.08–0.19) at year one, 24% (95% CI: 0.17–0.31) at year three and 26% (95% CI: 0.19–0.33) at year five (Fig. 1). Most of the deaths from vulvar cancer occurred in the first three years following diagnosis whereas the cumulative incidence of death from other causes increased steadily over time. Competing risks regression found age at diagnosis, disease stage, lymph node involvement, type of surgery performed and use of adjuvant therapy were all significantly associated with the risk of vulvar cancer-specific death in both univariate (data not shown) and multivariate models (Table 2). In particular, older age, higher disease stage, lymph node involvement, surgery removing lymph nodes only and receiving only chemotherapy or radiation therapy were associated with a greater risk of death (all p<0.01). These non-standard therapies were employed in individual cases at the discretion of the treating physician, but were equally distributed among the three age groups.
Fig. 1.
Cumulative incidence of death by cause: vulvar cancer or other known cause.
Table 2.
Predictors of vulvar cancer-specific mortality.
| Hazard ratio (95% CI) | p-value | |
|---|---|---|
| Age at diagnosis-category | 0.0046 | |
| 65–74 years olda | 1.0 | |
| 75–84 years old | 0.38 (0.18, 0.84) | |
| 85+ years old | 1.79 (0.78, 4.09) | |
| Stage | 0.0009 | |
| Ia | 1.0 | |
| II | 0.85 (0.31, 2.34) | |
| III | 0.62 (0.20, 1.94) | |
| IV | 3.17 (1.14, 8.80) | |
| Lymph node involvement | < 0.0001 | |
| Nonea | 1.0 | |
| Lymph node involvement | 7.47 (3.09, 18.04) | |
| Surgery performed | < 0.0001 | |
| Radical vulvectomy onlya | 1.0 | |
| Local excision only | 3.77 (0.75, 18.85) | |
| Unilateral or bilateral inguinal LND | 12.77 (2.52, 64.64) | |
| Radical vulvectomy + inguinal LND | 2.08 (0.49, 8.83) | |
| Adjuvant therapy | <0.0001 | |
| Nonea | 1.0 | |
| Chemo or radiation therapy only | 59.86 (19.21,186.54) | |
| Chemo and radiation therapy | 0.84 (0.36, 2.00) |
Reference group.
In our population, the data do not suggest that the use of adjuvant therapy or the type of surgical procedure performed differed by age at diagnosis (p=0.807 and 0.663 respectively; Table 3). Although limited by the small number of patients, FIGO stages III and IV present at a slightly higher proportion among the 65–74 year olds (44%), compared to 75–84 year olds (29%) and 85 year and older patients (36%) in this study population.
Table 3.
Treatment employed in patients by age at diagnosis (N = 146).
| 65–74 years old |
75–84 years old |
85+ years old |
p | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Disease stage (FIGO) | 0.639 | ||||||
| Unstaged | 1 | 2.33 | 1 | 1.43 | 0 | 0.00 | |
| I | 14 | 32.56 | 23 | 32.86 | 8 | 24.24 | |
| II | 9 | 20.93 | 26 | 37.14 | 13 | 39.39 | |
| III | 13 | 30.23 | 14 | 20.00 | 10 | 30.30 | |
| IV | 6 | 13.95 | 6 | 8.57 | 2 | 6.06 | |
| Adjuvant therapy | 0.807 | ||||||
| None | 29 | 67.44 | 53 | 75.71 | 24 | 72.73 | |
| Chemotherapy only | 0 | 0.00 | 1 | 1.43 | 0 | 0.00 | |
| Radiation only | 4 | 9.30 | 7 | 10.00 | 4 | 12.12 | |
| Chemo and radiation | 10 | 23.26 | 9 | 12.86 | 5 | 15.15 | |
| Surgery | 0.663 | ||||||
| Radical vulvectomy only | 7 | 16.28 | 10 | 14.29 | 9 | 27.27 | |
| Local excision only | 1 | 2.33 | 3 | 4.29 | 2 | 6.06 | |
| Unilateral or bilateral inguinal LND | 1 | 2.33 | 2 | 2.86 | 0 | 0.00 | |
| Radical vulvectomy + inguinal LND | 34 | 79.07 | 55 | 78.57 | 22 | 66.67 | |
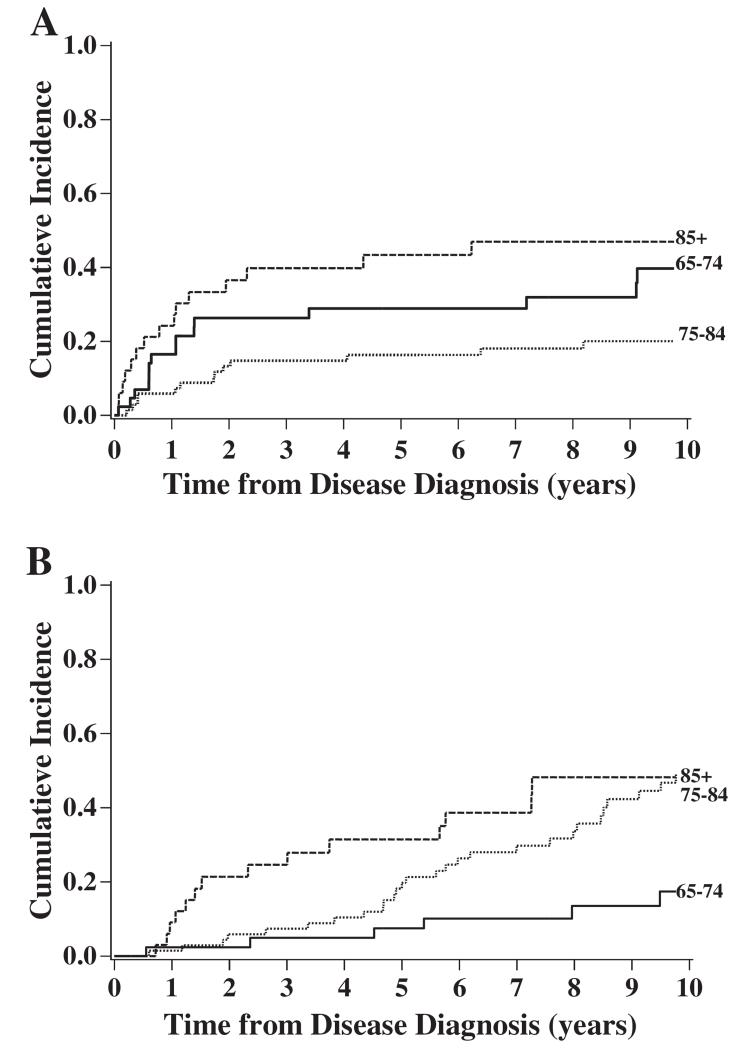
The cumulative incidence of death from vulvar cancer was most elevated among women 85 years and older and lowest among women 75–84 years old when comparing by age group (p=0.016; Fig. 2A). As expected, death from other causes increased with each increasing decade in age (p=0.008; Fig. 2B).
Fig. 2.
The cumulative incidence of death from vulvar cancer or other known causes by age at diagnosis. (A) Vulvar cancer specific mortality by age of diagnosis. (B) Other cause specific mortality by age of diagnosis.
Overall survival
The predictors of overall survival in the final Cox regression model included age at diagnosis, disease stage, lymph node involvement, Charlson score and the type of surgery performed (Table 4). As expected, increasing age, disease stage and lymph node involvement were associated with increased risk of death from any cause (all p<0.05). Patients with Charlson scores of 1 and 2+ had an increased risk of death of almost 2 and 3 times, respectively, compared to patients with a Charlson score of 0 (p=0.0006). The proportion of patients with Charlson scores of 0, 1 and 2+ surviving to/past 5 years from diagnosis were 0.66 (95% CI: 0.54, 0.75), 0.55 (95% CI: 0.37, 0.69) and 0.27 (95% CI: 0.12, 0.44), respectively.
Table 4.
Predictors of overall survival (all causes of death).
| Variable | Hazard ratio (95% CI) | P |
|---|---|---|
| Age category | <0.0001 | |
| 65–74 years olda | 1.0 | |
| 75–84 years old | 1.38 (0.81, 2.35) | |
| 85+ years old | 5.75 (3.06, 10.84) | |
| Stage | 0.048 | |
| Ia | 1.0 | |
| II | 0.98 (0.58, 1.66) | |
| III | 1.32 (0.58, 2.99) | |
| IV | 3.71 (1.26, 10.90) | |
| Lymph node involvement | 0.024 | |
| Nonea | 1.0 | |
| Lymph node involvement | 2.59 (1.13, 5.96) | |
| Charlson score | 0.0006 | |
| 0a | 1.0 | |
| 1 | 1.88 (1.12, 3.14) | |
| 2+ | 3.03 (0.70, 5.39) | |
| Surgery performed | 0.010 | |
| Radical vulvectomy onlya | 1.0 | |
| Local excision only | 1.93 (0.52, 7.16) | |
| Unilateral or bilateral inguinal LND | 7.17 (1.78, 28.90) | |
| Radical vulvectomy+inguinal LND | 0.85 (0.45, 1.59) |
Reference group.
Discussion
We examined the treatment patterns of women age 65 and above with vulvar cancer at a single regional cancer center. In this study population, vulvar cancer specific mortality was most significant in the first three years after diagnosis. Conversely other causes of mortality which can be attributed to comorbid conditions steadily increased with time. Age was a significant predictor of vulvar cancer specific mortality among women 85+ years, although women 65–74 and 75–84 had similar cancer mortality risk. Our data supports previous SEER database studies reporting increased cancer associated mortality and decreased overall survival with advancing age [6,7].
In this study, there was no difference in treatment patterns in women with vulvar cancer based on age. Women in the 85+ age group were able to undergo standard surgical treatment with radical vulvectomy and inguinal lymph node dissection with follow up adjuvant treatment. Age bias in the surgical and adjuvant treatment of the older 65+ population is well documented [18,19]. Previous studies on vulvar cancer outcome among older women are limited by high rates of non-standard surgical evaluation[8]. In the study by Stroup et al., less than 1% of Stage I and 55% of stage II underwent evaluation of regional lymph node status [7]. Lymph node involvement is a well documented significant and critical marker of survival and our data supports this consensus [4,20].
Competing causes of mortality must be acknowledged at the time of diagnosis and treatment of vulvar cancer among older patients. Within this study population, woman with Charlson comorbidity index score of 2+ experienced decreased overall survival. In a large study of 17,712 patients with prospectively documented concurrent conditions, the number of co-existing conditions and the severity of those conditions was documented to have significant impact on survival [21]. The Charlson comorbidity index score and the ECOG performance status are often utilized to provide an overall assessment of health [7,12]. Attempts to measure the impact of multiple medical conditions on the individual patience tolerance of cancer treatment and survival has been limited by the lack of validated comorbidity assessment tool that can be efficiently utilized in the oncology clinic setting.
Our study has some limitations. Due to inherent bias in a retrospective medical record review approach, women diagnosed with vulvar cancer and selectively not referred into the cancer center are not represented in this study. The data obtained concerning co-existing medical conditions while detailed is confined to clinic and hospital-based medical record information. The small sample size may underestimate potential differences in vulvar cancer specific mortality and overall survival between the three designated age groups.
In a disease such as vulvar cancer in which the median age of diagnosis is 69, cancer care must take into account age and comorbidity. Studies on aging are highlighting the ever increasing lifespan of the older woman. Although increasing age by decade is significant to vulvar cancer mortality, our data supports low rates of mortality for optimally early staged patients. As we expand our understanding of the impact of comorbid conditions on cancer treatment in older patients, further research should be supported in developing clinically relevant comorbidity assessment tools.
Acknowledgments
Role of the funding source
This work was supported by a Building Interdisciplinary Research Careers in Women’s Health to R. Ghebre, from the National Institutes of Child Health and Human Development (K12HD055887). This work was supported in part by NIH P30 CA77598 utilizing the Masonic Cancer Center, University of Minnesota Biostatistics and Bioinformatics Core.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
- [1].Saraiya M, Watson M, Wu X, King JB, Chen VW, Smith JS, et al. Incidence of in situ and invasive vulvar cancer in the US, 1998–2003. Cancer. 2008;113:2865–72. doi: 10.1002/cncr.23759. [DOI] [PubMed] [Google Scholar]
- [2].Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107:1018–22. doi: 10.1097/01.AOG.0000210268.57527.a1. [DOI] [PubMed] [Google Scholar]
- [3].Arias E. National vital statistics reports. 21. Vol. 58. National Center for Health Statistics; Hyattsville, MD: 2010. United States life tables, 2006. [PubMed] [Google Scholar]
- [4].Homesley HD, Bundy BN, Sedlis A, Yordan E, Berek JS, Jahshan A, et al. Assessment of current International Federation of Gynecology and Obstetrics staging of vulvar carcinoma relative to prognostic factors for survival (a Gynecologic Oncology Group study) Am J Obstet Gynecol. 1991;164:997–1003. doi: 10.1016/0002-9378(91)90573-a. [DOI] [PubMed] [Google Scholar]
- [5].Landrum LM, Lanneau GS, Skaggs VJ, Gould N, Walker JL, McMeekin DS, et al. Gynecologic Oncology Group risk groups for vulvar carcinoma: improvement in survival in the modern era. Gynecol Oncol. 2007;106:521–5. doi: 10.1016/j.ygyno.2007.04.029. [DOI] [PubMed] [Google Scholar]
- [6].Kumar S, Shah JP, Bryant CS, Imudia AN, Morris RT, Malone JM., Jr A comparison of younger vs older women with vulvar cancer in the United States. Am J Obstet Gynecol. 2009;200:e52–5. doi: 10.1016/j.ajog.2008.09.869. [DOI] [PubMed] [Google Scholar]
- [7].Stroup AM, Harlan LC, Trimble EL. Demographic, clinical, and treatment trends among women diagnosed with vulvar cancer in the United States. Gynecol Oncol. 2008;108:577–83. doi: 10.1016/j.ygyno.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vlastos AT, Usel M, Beffa V, Petignat P, Neyroud-Caspar I, Bouchardy C, et al. Treatments patterns of vulvar cancer in the elderly. Surg Oncol. 2004;13:187–91. doi: 10.1016/j.suronc.2004.10.002. [DOI] [PubMed] [Google Scholar]
- [9].Moore DH. Chemotherapy and radiation therapy in the treatment of squamous cell carcinoma of the vulva: are two therapies better than one? Gynecol Oncol. 2009;113:379–83. doi: 10.1016/j.ygyno.2009.01.004. [DOI] [PubMed] [Google Scholar]
- [10].Homesley HD, Bundey BN, Sedlis A, Adcock L. Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstet Gynecol. 1986;68:733–40. [PubMed] [Google Scholar]
- [11].Hurria A, Leung D, Trainor K, Borgen P, Norton L, Hudis C. Factors influencing treatment patterns of breast cancer patients age 75 and older. Crit Rev Oncol Hematol. 2003;46:121–6. doi: 10.1016/s1040-8428(02)00133-6. [DOI] [PubMed] [Google Scholar]
- [12].Hyde SE, Ansink AC, Burger MP, Schilthuis MS, van der Velden J. The impact of performance status on survival in patients of 80 years and older with vulvar cancer. Gynecol Oncol. 2002;84:388–93. doi: 10.1006/gyno.2001.6531. [DOI] [PubMed] [Google Scholar]
- [13].Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- [14].Charlson MEPP, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- [15].Pecorelli S, Benedet JL, Creasman WT, Shepherd JH. FIGO staging of gynecologic cancer. 1994-1997 FIGO Committee on Gynecologic Oncology. International Federation of Gynecology and Obstetrics. Int J Gynecol Obstet. 1999;65:243–9. doi: 10.1016/s0020-7292(99)00070-3. [DOI] [PubMed] [Google Scholar]
- [16].Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- [17].Cox DR. Regression models and life-tables. J R Stat Soc B Stat Methodol. 1972;34:187–220. [Google Scholar]
- [18].Yancik R, Wesley MN, Ries LAG, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–92. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- [19].Bouchardy C, Rapiti E, Blagojevic S, Vlastos A-Trs, Vlastos G. Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol. 2007;25:1858–69. doi: 10.1200/JCO.2006.10.4208. [DOI] [PubMed] [Google Scholar]
- [20].Maggino T, Landoni F, Sartori E, Zola P, Gadducci A, Alessi C, et al. Patterns of recurrence in patients with squamous cell carcinoma of the vulva. Cancer. 2000;89:116–22. doi: 10.1002/1097-0142(20000701)89:1<116::aid-cncr16>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- [21].Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–7. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]