Abstract
Marine sponges are found to be a rich source of bioactive compounds which show a wide range of biological activities including antiviral, antibacterial, and anti-inflammatory activities. This study aimed to investigate the possible anti-inflammatory, antioxidant and immunomodulator effects of the methanolic extract of the Red Sea marine sponge Xestospongia testudinaria. The chemical composition of the Xestospongia testudinaria methanolic extract was determined using Gas chromatography-mass spectroscopy (GC-MS) analysis. DPPH (2, 2-diphenyl-1-picryl-hydrazyl) was measured to assess the antioxidant activity of the sponge extract. Carrageenan-induced rat hind paw edema was adopted in this study. Six groups of rats were used: group1: Control, group 2: Carrageenan, group 3: indomethacin (10 mg/kg), group 4–6: Xestospongia testudinaria methanolic extract (25, 50, and 100 mg/kg). Evaluation of the anti-inflammatory activity was performed by both calculating the percentage increase in paw weight and hisopathologically. Assessment of the antioxidant and immunomodulatory activity was performed. GC-MS analysis revealed that there were 41 different compounds present in the methanolic extract. Sponge extract exhibited antioxidant activity against DPPH free radicals. Xestospongia testudinaria methanolic extract (100 mg/kg) significantly decreased % increase in paw weight measured at 1, 2, 3 and 4 h after carrageenan injection. Histopathologically, the extract caused a marked decrease in the capillary congestion and inflammatory cells infiltrate. The extract decreased paw malondialdehyde (MDA) and nitric oxide (NO) and increased the reduced glutathione (GSH), glutathione peroxidase (GPx), and catalase (CAT) activity. It also decreased the inflammatory cytokines, tumor necrosis factor-α (TNF-α), interleukin-1 β(IL-1β) and IL-6. The results of this study demonstrated the anti-inflammatory, antioxidant, and immunomodulatory effects of the methanolic extract of the Red Sea sponge Xestospongia testudinaria (100 mg/kg).
Introduction
Inflammatory reaction is a common physiologic response which protects the host from many harmful stimuli such as toxins, local injuries and pathogens [1]. Uncontrolled inflammation is considered as one of the pathophysiologic causes of most chronic diseases, including chronic inflammatory diseases, diabetes, cancer and cardiovascular diseases [2–3]. Over the years, reactive nitrogen species and reactive oxygen species (ROS) are found to play a key role in inflammation [4]. In addition, extensive scientific reports described the role played by various proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1 β(IL-1β) and IL-6 in inflammation [5]. Nowadays, non-steroidal anti-inflammatory drugs (NSAIDs) are the most commonly prescribed therapeutics for the treatment of many inflammatory diseases [6]. However, the long-term administration of these NSAIDs causes many harmful effects including gastrointestinal ulcers and bleeding and renal damage [7]. Therefore, the need for new anti-inflammatory drugs with fewer adverse effects is mandatory [8].
Since ancient times, marine environment has been documented to be a valuable source of bioactive metabolites including antioxidants, polyunsaturated fatty acids (PUFAs) and sterols [2]. These naturally occurring bioactive substances often have no matches on earth. Sponges are found to be a rich source of bioactive compounds with antiviral, antibacterial, and anti-inflammatory activities [2,3]. The most reported anti-inflammatory mechanisms of marine sponges metabolites were, inhibition of phospholipase A2 (PLA2) (a key enzyme in the arachidonic acid cascade); and inhibition of interleukine (IL)-1 mediated prostaglandin (PG)-E2 synthesis [9]. Clathriol B was a novel polyoxygenated steroids isolated from the marine sponge Clathria lissosclera, inhibited the production of superoxide from human peripheral blood neutrophils, which is known to be implicated in the pathogenesis of inflammatory disorders [10].
Sponges of the genus Xestospongia (class Desmospongia, order Haplosclerida, family Petrosiidae) are a large group among coral reef communities, known as barrel sponges, located all over the Caribbean Sea and Indo-Pacific Ocean at depths greater than 10 meters [11]. Members of the genus Xestospongia are considered a rich source of pharmacologically active compounds [10]. Recently, sterols have been isolated from the marine sponge Xestospongia testudinaria [11]. In addition, many recent studies show anti-inflammatory and antioxidant activity of many marine sponge-derived sterols [12]. Therefore, the aim of the present study is to investigate the possible anti-inflammatory, antioxidant and immunomodulator effects of the methanolic extract of the Red Sea sponge Xestospongia testudinaria, which is not studied before for drug discovery on murine model of paw edema.
Materials and Methods
Sponge material
The sponge was collected in May 2013 from Ghurab reef (North side) in in the Red Sea at Jazan, Saudi Arabia at depths of 13–25 m, after permission from Saudi General Directorate of Border Guard. The collection of marine sponge Xestospongia testudinaria was conducted as a part of the research work supported by the King Abdulaziz University. We confirm that the filed studies included in this paper did not involve any endangered or protected species.
The sponge Xestospongia testudinaria is volcano-shaped with sharp lengthwise ridges on the pink or pale red-brown outer side. The surface layer of sponge skeleton is a tangential irregular isotropic or alveolar reticulation of spicule bundles of 2–6 spicules in cross section of about 20–60 μm thick and individual loose spicules. The bundles contain some binding sponging, which is barely visible. Thin growth stages of the spicules are oxeas. The spicule length is quite variable and measuring about 100–400 × 7–10 μm. The sponge was identified by Prof. Rob van Soest at the Naturalis Biodiversity Center at Leiden, The Netherlands. A fragment is kept in the collections of the Naturalis Biodiversity Center in Leiden, The Netherlands, under registration number RMNH Por. 9176. Another voucher specimen was deposited in our Red Sea Marine Invertebrates Collection, Faculty of Pharmacy, King Abdulaziz University under the code No. DY-KSA-12.
Extraction and isolation
The freeze-dried sponge (180 g) was crushed and extracted by methanol (3 × 3 L). The crude extract was concentrated by evaporation under vacuum.
Derivatization
About 1 mg of sample was mixed with 100 μL of CH2Cl2, vortexed, and dried with nitrogen gas. The residue was mixed with 50 μL N-methyl-N-trimethylsilyl-trifluoroacetamide (MSTFA), heated at 80°C for 15 min, cooled and a volume of 1 μL was injected for gas chromatography–mass spectrometry (GC–MS) analysis.
Gas chromatography–mass spectrometry (GC–MS) analyses of the methanolic extract of Xestospongia testudinaria
The methanolic extract of Xestospongia testudinaria was analyzed by GC–MS. A Perkin Elmer Clarus 500 (Perkin Elmer, Shelton, CT, USA) was utilized throughout the experiments. The software controller/Integrator was TurboMass version 5.4.2.1617. An Elite-1 GC capillary column, Crossbond R at 100% dimethyl polysiloxane (30m×0.25mmid×0.25_mdf, Perkin Elmer) was used. The carrier gas was helium (purity 99.9999%) and the flow rate was 0.9 ml/min. Source (EI+): source temperature, 250°C. The GC line temperature was 200°C. Electron energy was 70 eV, and trap emission was 100 V. The oven was programmed as follows: initial temperature was 80°C (hold 5 min) to 250°C (rate 15°C/min, hold 5.0 min), followed by an increase to 280°C (rate 20°C/min, hold 2 min). The injector temperature was 260°C. The MS scan was from 45 to 350 m/z (500 scan/s). The injection volume was 1.0 μL, and the split ratio was 50:1. Samples were acquired by applying the positive total ion chromatogram (TIC). NIST2008 Program was used for matching characterized compounds.
In vitro antioxidant assay (DPPH test)
The DPPH method was commonly applied to measure the antioxidant properties of natural compounds [13]. Different concentrations (125,250,500,1000 and 2000 μg/ml) of the Xestospongia testudinaria extract in methanol were prepared. 3 mL of DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) solution was mixed to 2 mL of each concentration. The mixtures were allowed to stand for 10-minutes at room temperature [14]. The absorbance was measured at 517 nm using a Thermo Scientific GENESYS 10S UV-visible double beam spectrophotometer (USA). Methanol was used as a blank and Tocopherol (vitamin E) was used as the positive control [15]. Lower absorbance of the reaction mixture indicate higher free radical scavenging activity. The following equation [15] shows the radical scavenging activity of the sample, which is stated as the inhibition percentage: % inhibition = [(Ac −A sample)/Ac] × 100, where Ac is the absorbance of the control (DPPH in absence of extract) and A sample is the absorbance in the presence of the extract.
Animals and experimental design
Male Sprague Dawley rats (150–180 g) were used in this experiment. Animals were allowed free access to commercial pellet chow and water ad libitum. They were maintained at a temperature controlled room (22 ± 1°C) and humidity (55 ± 10%) with a 12 h light/dark cycle.
To minimize the suffering of laboratory animals, procedures including cervical dislocation were done under ether anaesthesia.
Thirty six rats were separated into six groups (n = 6). Xestospongia testudinaria methanolic extract (25, 50, and 100 mg/kg), indomethacin (Sigma, USA), the reference anti-inflammatory drug (10 mg/kg) [16], 0.9% saline, were injected intraperitoneal (i.p.) 2 h before carrageenan (Sigma, USA) injection. A noncarrageenan injected group was served as a control group. Animal study was implemented following the animal care and use Guideline for National Institutes of Health (NIH). The experimental protocol was approved by Unit of Biomedical Ethics Research Committee, Faculty of Medicine, King Abdulaziz University (Permit No:74–15).
Induction of paw edema in rat using carrageenan
The carrageenan-induced rat paw edema was performed according to the previously described technique [17]. Briefly, carrageenan (1.5% w/v, 0.1 ml/paw) was injected into right hind paw at the plantar side. Rats were observed for abnormal behavior and physical condition after carrageenan injection. The weight of the left (non-carrageenan injected) and right (carrageenan injected) paw was measured at 1, 2, 3, and 4 h of carrageenan injection [18]. For this purpose, rats were held firmly and each hind paw immersed in a beaker containing warm water (37°C) placed on a top-pan balance (Shinko Denshi Co.,Ltd.Japan) [18]. No deaths were reported throughout the experiment. Difference between the left and the right paw weight was obtained for assessment of edema at all the time points. The percentage increase in paw weight was calculated by dividing edema, weight after 1st, 2nd, 3rd and 4th hours by weight of left paw multiplied by 100.
Sample collection
Four h after carrageenan injection, 5 ml cardiac blood sample was taken into heparin from each rat under deep anesthesia, then the animal was killed by cervical dislocation. Hind paw specimens (left and right) were collected and Frozen at (-80°C) for estimation of lipid peroxides (measured as malondialdehyde, MDA), nitric oxide (NO), reduced glutathione (GSH) contents, glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase (CAT). After 3500 rpm centrifugation plasma aliquots were frozen at– 20°C for the analysis of TNFα, IL-1β and IL-6. Formalin fixed hind paw was used for the histopathologic examination.
Measurement of paw lipid peroxide (measured as malondialdehyde (MDA)
MDA was measured using Biodiagnostic kits, Egypt, according to the method of Uchiyama and Mihara [19]. The color of the adduct formed following the reaction of thiobarbituric acid with the tissue homogenate in a boiling waterbath was measured at 535 nm. Paw MDA concentration was expressed as nmol/g tissue.
Measurement of paw nitric oxide (NO)
The paw nitric oxide (NO) was measured using Biodiagnostic kits, Egypt, according to Tarpey et al. [20]. NO was determined in the liver homogenates using kits provided by Biodiagnostic, Egypt. Initially, nitrate was converted into nitrite by the enzyme nitrate reductase, followed by quantitation of nitrite using Griess reagent at the absorbance of 550 nm, as previously described [20]. NO was assayed by measuring total nitrate plus nitrite (NO3− +NO2−), the stable end products of NO metabolism. Paw NO concentration was expressed as μmol/g tissue.
Measurement of paw reduced glutathione (GSH)
GSH was quantified using Biodiagnostic kits, Egypt, according to the method of Ellman [21]. The principle of this procedure is based on the formation of 2-nitro-5- mercaptobenzoic acid from reduction of bis(3-carboxy-4- nitrophenyl) disulfide reagent by the SH group, which has a deep yellow color that was measured spectrophotometrically at 412 nm. Paw GSH concentration was expressed as U/g tissue.
Measurement of paw glutathione peroxidase enzyme activity (GPx)
Paw GPx activity was measured using Biodiagnostic kits, Egypt. GPx activity was determined in a coupled assay with glutathione reductase by measuring the rate of NADPH oxidation at 340 nm using H2O2 as the substrate [22]. GPx activity was expressed in U/g tissue.
Measurement of paw superoxide dismutase enzyme activity (SOD)
Paw SOD activity was measured using Biodiagnostic kits, Egypt, according to the method of Nishikimi et al. [23]. This assay depends on the ability of the SOD to inhibit the phenazine methosulphate-mediated reduction of nitroblue tetrazolium dye. SOD activity was expressed in U/mg tissue.
Measurement of paw catalase enzyme activity (CAT)
Paw CAT activity was measured using Biodiagnostic kits, Egypt, according to the method of Aebi [24]. H2O2 reacts with a known quantity of CAT. After adding catalase inhibitor, the reaction was stopped after exactly one minute. The remaining H2O2 reacts with 3,5-dichloro-2-hydroxybenzene sulfonic acid (DHBS) and 4- aminophenazone (AAP) to form a chromophore with color intensity inversely proportional to the amount of CAT in the original sample. The absorbance of samples was read at 510nm against a standard blank. CAT activity was expressed in U/g tissue.
Measurement of plasma TNFα, IL-1β and IL-6
TNFα, IL-1β and IL-6 levels were measured in an ELISA assay as part of an Assaypro TNFα, IL-1β and IL-6 kits (30 Triad South Drive St. Charles, MO 63304, USA) using monoclonal antibodies specific for TNFα, IL-1β and IL-6, respectively. Cytokine concentrations were calculated using a standard purified recombinant cytokines.
Histopathologic examination of the hind paws
Serial sections were taken from paraffin embedded paws and stained with hematoxylin and eosin (H&E), for the evaluation of histopathologic changes.
Statistical analysis
One-way analysis of variance test (ANOVA) was used for comparison between different groups followed by Dunnett t-test (two-sided) multiple comparisons to detect significant differences among individual mean values of all groups. Results are expressed as the mean ± SDM. The level of significance was set at P ≤ 0.05. Statistical analysis was generated using SPSS software for windows, version 14.0 SPSS Inc., Chicago.
Results
Chemical constituents of methanolic extracts of Xestospongia testudinaria
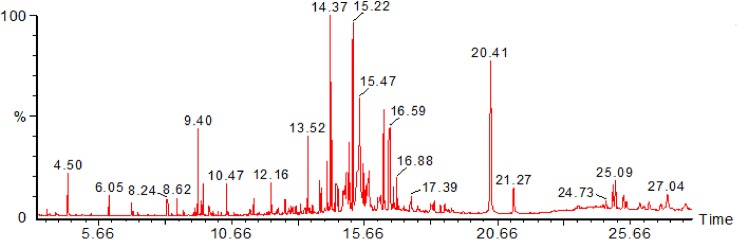
Several organic compounds were detected and indicated. They include 3α-Trimethylsiloxy) cholest-5-ene (2.46), l-alanine (1.13), propanoic acid (1.92), pyrimidine (0.65), glutamine (0.14), azelaic acid (0.34), hexadecanoic acid (12.75), Tetradecanoic acid (2.11), Oleic acid (1.91) (4.77), Octadecadiynoic acid (3.25) and Octanoic acid (2.11) (Table 1 and Fig 1).
Table 1. Chemical constituents of Xestospongia testudinaria methanolic extract, detected by GC-MS.
| Serial Number | Retention Time etention | Name of Compound | Peak Area (%) |
|---|---|---|---|
| 1 | 3.79 | Silane, trimethyl(3-phenoxypropoxy)- | 0.64 |
| 2 | 4.03 | N-Dimethylaminomethyl-tert.-butyl-isopropylphosphine | 0.42 |
| 3 | 4.78 | 3-Heptene, 2,2,4,6,6-pentamethyl- | 0.06 |
| 4 | 5.88 | I-Trimethylsiloxy-2-trimethylsilylaminoethane | 0.14 |
| 5 | 6.05 | Propanoic acid, 2-[(trimethylsilyl)oxy]-, trimethylsilyl ester | 1.92 |
| 6 | 6.89 | l-Alanine, N-(trimethylsilyl)-, trimethylsilyl ester | 1.13 |
| 7 | 7.12 | Glycine, N-(trimethylsilyl)-, trimethylsilyl ester | 0.35 |
| 8 | 8.62 | L-Valine, N-(trimethylsilyl)-, trimethylsilyl ester | 1.38 |
| 9 | 8.85 | Butanoic acid, 4-[bis(trimethylsilyl)amino]-, trimethylsilyl ester | 0.47 |
| 10 | 9.22 | Silanol, trimethyl-, phosphate (3:1) | 0.18 |
| 12 | 9.29 | N,O-Bis-(trimethylsilyl)leucine | 0.69 |
| 13 | 9.4 | Trimethylsilyl ether of glycerol | 6.22 |
| 14 | 9.49 | Pentenoic acid, 4-[(trimethylsilyl)oxy]-, trimethylsilyl ester | 0.89 |
| 15 | 9.54 | L-Isoleucine, N-(trimethylsilyl)-, trimethylsilyl ester | 1.93 |
| 16 | 9.82 | Pyrimidine, 2,4-bis[(trimethylsilyl)oxy]- | 0.65 |
| 17 | 9.97 | 2-Butenedioic acid (Z)-, bis(trimethylsilyl) ester | 0.06 |
| 18 | 10.24 | L-Serine, N,O-bis(trimethylsilyl)-, trimethylsilyl ester | 0.24 |
| 19 | 10.47 | Pentanedioic acid, bis(trimethylsilyl) ester | 2.26 |
| 20 | 10.53 | N,O,O-Tris(trimethylsilyl)-L-threonine | 0.22 |
| 21 | 10.75 | Octanoic acid, 7-oxo-, trimethylsilyl ester | 0.32 |
| 22 | 11.37 | Hexanedioic acid, bis(trimethylsilyl) ester | 0.52 |
| 23 | 11.4 | Pentonic acid, 2-deoxy-3,5-bis-O-(trimethylsilyl)-, γ-lactone | 1.32 |
| 24 | 11.63 | L-Aspartic acid, N-(trimethylsilyl)-, bis(trimethylsilyl) ester | 0.12 |
| 25 | 11.99 | L-Proline, 5-oxo-1-(trimethylsilyl)-, trimethylsilyl ester | 0.24 |
| 26 | 12.16 | Heptanedioic acid, bis(trimethylsilyl) ester | 2.22 |
| 27 | 12.39 | Glutamine, tris(trimethylsilyl)- | 0.14 |
| 28 | 12.7 | (±)-2-Hydroxyoctanoic acid, trimethylsilyl ester | 1.08 |
| 29 | 12.98 | D-Xylose, tetrakis(trimethylsilyl)- | 0.51 |
| 30 | 13.26 | Lyxose, tetra-(trimethylsilyl)-ether | 0.88 |
| 31 | 13.57 | Azelaic acid, bis(trimethylsilyl) ester | 0.34 |
| 32 | 13.96 | Tetradecanoic acid, trimethylsilyl ester | 2.11 |
| 33 | 14.03 | Oleic acid, trimethylsilyl ester | 1.91 |
| 34 | 14.37 | n-Pentadecanoic acid, trimethylsilyl ester | 12.14 |
| 35 | 14.43 | Undecanoic acid, 11-fluoro-, trimethylsilyl ester | 3.48 |
| 36 | 15.07 | Oleic acid, trimethylsilyl ester | 4.77 |
| 37 | 15.22 | Hexadecanoic acid, trimethylsilyl ester | 12.75 |
| 38 | 18.15 | 1-O-hexadecylglycerol—bis-trimethylsilyl ether derivative | 0.83 |
| 39 | 20.41 | 5,8,11-Eicosatriynoic acid, trimethylsilyl ester | 18.78 |
| 40 | 21.27 | 9,12-Octadecadiynoic acid, trimethylsilyl ester | 3.25 |
| 41 | 24.99 | 3α-(Trimethylsiloxy)cholest-5-ene | 2.46 |
Fig 1. Chromatogram obtained from GC-MS with the methanolic extracts of the Xestospongia testudinaria.
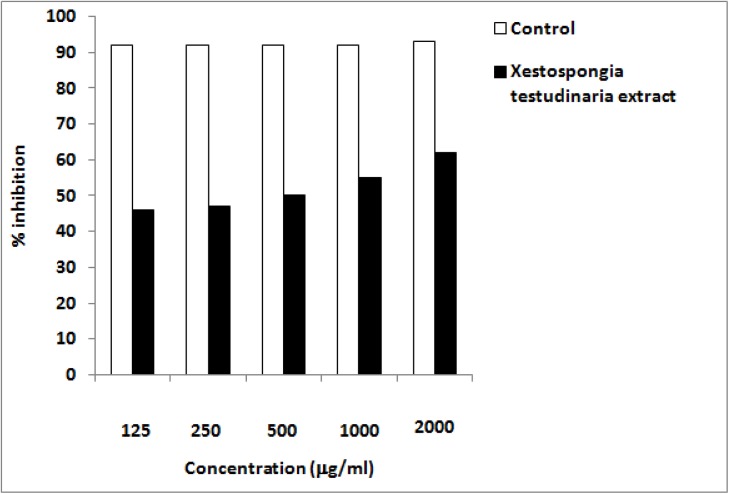
Effect of Xestospongia testudinaria sponge extract on In Vitro Antioxidant Activity (DPPH Test)
The scavenging effects of the Red sea sponge methanolic extract, Xestospongia testudinaria on DPPH radicals increased with increasing concentration. The results were stated in terms of percentage of inhibition (Fig 2). The maximum percentage of inhibition was 62% at concentration of 2 mg/ml.
Fig 2. Total antioxidant capacity of Xestospongia testudinaria methanolic extract at different concentrations in vitro.
Expressed as percent inhibition toward DPPH-induced oxidative stress.
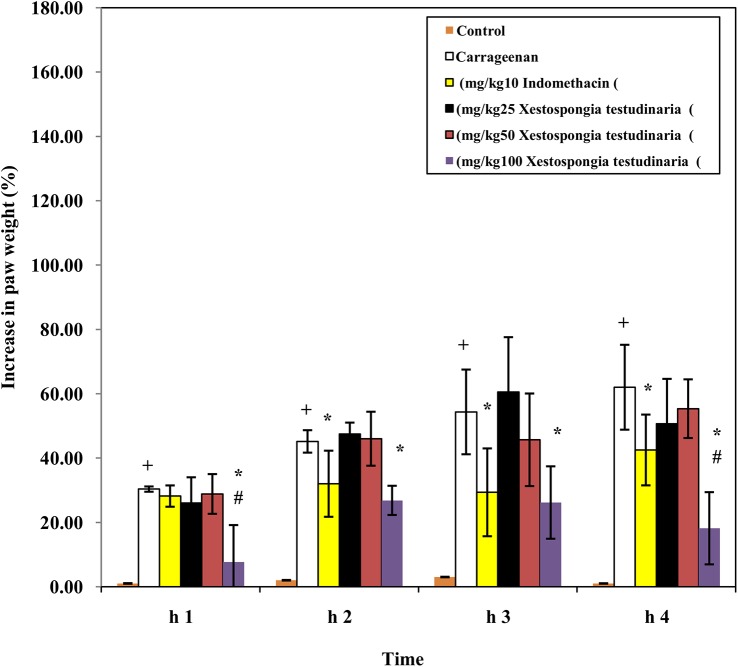
Effect of methanolic extract of Xestospongia testudinaria (25, 50, and 100 mg/kg) and indomethacin (10 mg/kg) on carrageenan-induced rat hind paw edema (Fig 3)
Fig 3. Effect of Xestospongia testudinaria methanolic extract (25, 50, and 100 mg/kg) and indomethacin (10 mg/kg) on carrageenan-induced rat hind paw edema.
Results were expressed as a percentage increase in paw weight (mean ± SDM) of six rats. * Significant difference compared to carrageenan group (P ≤ 0.05). # Significant difference compared to indomethacin group (P ≤ 0.05).
Injection of rats with carrageenan caused a significant increase in the paw weight % measured after 1, 2, 3 and 4 h compared to the control values (p = 0.000). Pretreatment of carrageenan injected rats with indomethacin (10 mg/kg) significantly decreased paw weight % measured at 2, 3 and 4 h (29%, 46% and 31%, respectively) (p = 0.014, 0.009 and 0.019 respectively) compared to carrageenan injected rats. Pretreatment of carrageenan injected rats with Xestospongia testudinaria (25 and 50 mg/kg) did not decrease paw weight % measured at 1, 2, 3 and 4 h compared to carrageenan injected rats. On the other hand, pretreatment of carrageenan injected rats with Xestospongia testudinaria (100 mg/kg) significantly decreased paw weight % measured at 1, 2, 3 and 4 h (75%, 41%, 52% and 71% respectively) (p = 0.001, 0.000, 0.003 and 0.000 respectively) compared to carrageenan injected rats. In addition, Xestospongia testudinaria (100 mg/kg) significantly decreased paw weight % measured after 1 and 4 h of carrageenan injection (73% and 57%, respectively) (p = 0.002 and 0.004 respectively) compared to indomethacin injected rats.
Effect of methanolic extract of Xestospongia testudinaria (100 mg/kg) and indomethacin (10 mg/kg) on paw lipid peroxides (MDA), nitric oxide (NO) and reduced glutathione (GSH) concentrations measured in carrageenan-induced rat hind paw edema
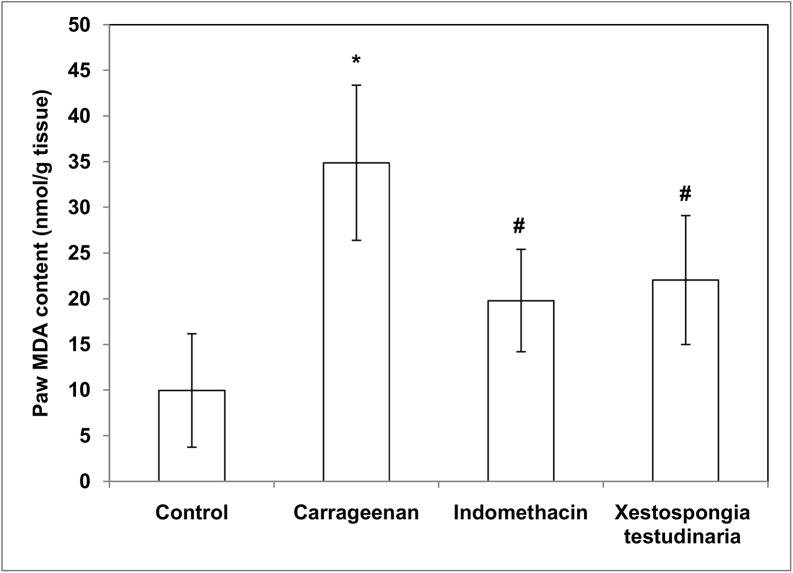
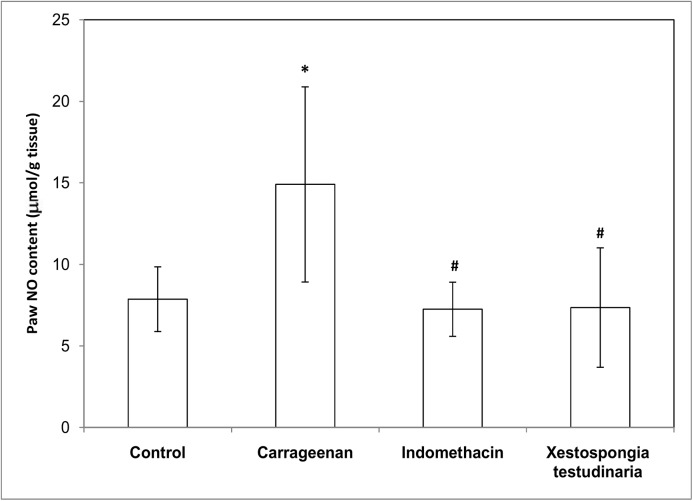
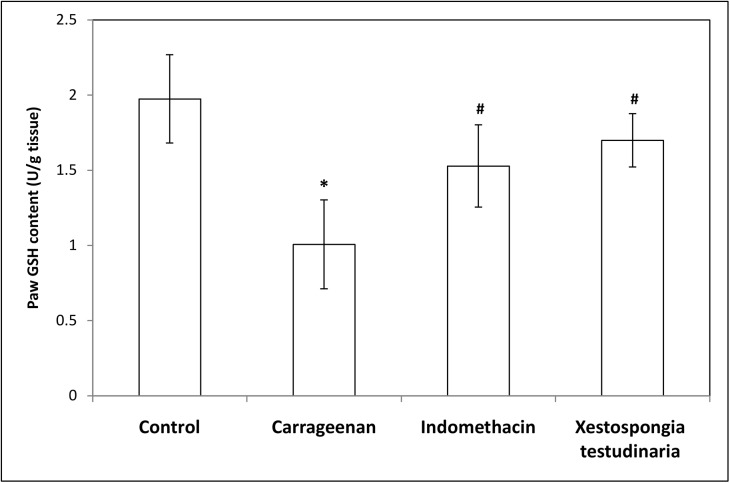
Treatments of rats with carrageenan caused a significant increase in both paw MDA and NO contents (~ 3.5 folds and 2 folds, respectively) compared to the control contents (p = 0.000 and 0.021, respectively) (Figs 4 and 5). On the other hand, treatments of rats with carrageenan caused a significant decrease (49%) in paw GSH contents compared to the control rats (p = 0.000) (Fig 6). Pretreatment of carrageenan injected rats with both Xestospongia testudinaria (100 mg/kg) and indomethacin (10 mg/kg) significantly decreased both paw MDA (37% and 43%, respectively) (p = 0.000) and NO contents (51% both) (p = 0.025 and 0.013 respectively) (Figs 4 and 5). On the other hand, pretreatment of carrageenan injected rats with both Xestospongia testudinaria (100 mg/kg) and indomethacin (10 mg/kg) significantly increased paw GSH contents (69% and 52%, respectively) (p = 0.001 and 0.010, respectively) compared to the carrageenan treated rats (Fig 6).
Fig 4. Effect of Xestospongia testudinaria methanolic extract (100 mg/kg) and indomethacin (10 mg/kg) on paw lipid peroxides (MDA) content (nmol/g tissue) measured in carrageenan-induced rat hind paw edema.
Results were expressedas mean ± SDM of six rats.*Significant versus control (P ≤ 0.05). #Significant versus carrageenan (P ≤ 0.05).
Fig 5. Effect of Xestospongia testudinaria methanolic extract (100 mg/kg) and indomethacin (10 mg/kg) on paw NO content (μmol/g tissue) measured in carrageenan-induced rat hind paw edema.
Results were expressed as mean ± SDM of six rats. *Significant versus control (P ≤ 0.05).#Significant versus carrageenan (P ≤ 0.05).
Fig 6. Effect of Xestospongia testudinaria methanolic extract (100 mg/kg) and indomethacin (10 mg/kg) on paw GSH content (U/g tissue) measured in carrageenan-induced rat hind paw edema.
Results were expressed as mean ± SDM of six rats. *Significant versus control (P ≤ 0.05). #Significant versus carrageenan (P ≤ 0.05).
Effect of methanolic extract of Xestospongia testudinaria (100 mg/kg) and indomethacin (10 mg/kg) on paw glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase (CAT) activities measured in carrageenan-induced rat hind paw edema
Briefly, the activities of SOD were not changed in all treatment regimens. Treatments of rats with carrageenan caused a significant decrease in both paw GPx (43%) and CAT activities (63%) compared to the control rats (p = 0.017 and 0.025, respectively). Pretreatment of carrageenan injected rats with both Xestospongia testudinaria (100 mg/kg) and indomethacin (10 mg/kg) significantly increased paw GPx (55% and 62%, respectively) (p = 0.021 and 0.046, respectively) and SOD enzymes activity (129% and 106%, respectively) (p = 0.000) compared to the carrageenan treated rats (Table 2).
Table 2. Effect of Xestospongia testudinaria methanolic extract (100 mg/kg) and indomethacin (10 mg/kg) on paw GPX, SOD and CAT enzymes activity measured in carrageenan-induced rat hind paw edema.
| Treatment regimen | GPX(U/g tissue) | SOD(U/mg tissue) | CAT(U/g tissue) |
|---|---|---|---|
| Control | 302 ± 94 | 0.22 ± 0.01 | 0.51 ± 0.29 |
| Carrageenan | 172 ± 58 a | 0.13 ± 0.01 | 0.19 ± 0.03 a |
| Indomethacin | 279 ± 91 b | 0.14 ± 0.04 | 0.39 ± 0.09 b |
| Xestospongia testudinaria extract | 265 ± 61 b | 0.13 ± 0.04 | 0.43 ± 0.08 b |
Data are mean ± SD (n = 6).
a Significant versus control (P ≤ 0.05).
b Significant versus carrageenan (P ≤ 0.05).
Effect of methanolic extract of the sponge Xestospongia testudinaria (100 mg/kg) and indomethacin (10 mg/kg) on plasma TNF-α, IL-1β and IL-6 measured in carrageenan-induced rat hind paw edema
Treatments of rats with carrageenan caused a significant increase in plasma TNF-α, IL-1β and IL-6 (30%, 12 folds and 106%, respectively) (p = 0.000) compared to the control rats. Pretreatment of carrageenan injected rats with both Xestospongia testudinaria (100 mg/kg) and indomethacin (10 mg/kg) caused a significant decrease in plasma TNF-α (18% and 17%, respectively) (p = 0.000 and 0.003, respectively), IL-1β (30% and 34%, respectively) (p = 0.000) and IL-6 (53% and 51%, respectively) (p = 0.000) compared to the carrageenan treated rats (Table 3).
Table 3. Effect of Xestospongia testudinaria methanolic extract (100 mg/kg) and indomethacin (10 mg/kg) on plasma TNF-α, IL-1β and IL-6 measured in carrageenan-induced rat hind paw edema.
| Treatment regimen | TNF-α(pg/ml) | IL-1β (pg/ml) | IL-6 (pg/ml) |
|---|---|---|---|
| Control | 309 ± 20 | 127 ± 36 | 129 ± 7 |
| Carrageenan | 402 ± 26 a | 1529 ± 159 a | 266 ± 21 a |
| Indomethacin | 333 ± 33 b | 1011 ± 106 b | 131 ± 5 b |
| Xestospongia testudinaria extract | 330 ± 17 b | 1076 ± 61 b | 124 ± 2 b |
Data are mean ± SD (n = 6).
a Significant versus control (P ≤ 0.05).
b Significant versus carrageenan (P ≤ 0.05).
Histopathological study
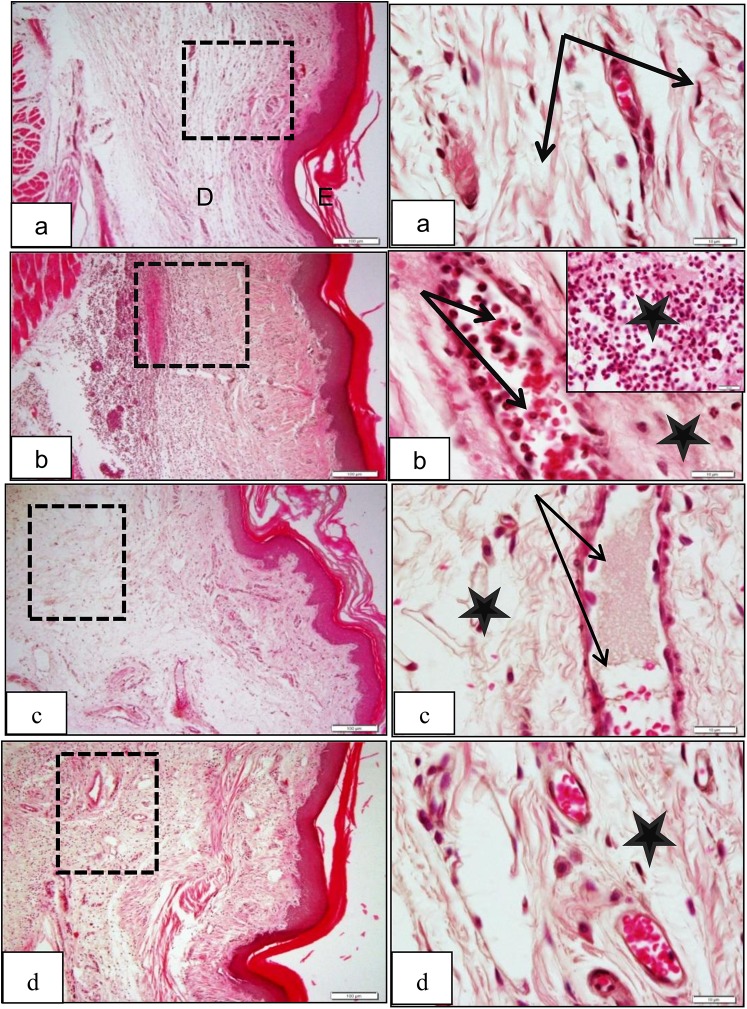
Carrageenan injections into rat paw induced a good model of acute inflammatory reaction that characterized by blood capillary dilation and congestion with marked inflammatory exudate consists mainly of polymorphnuclear leucocytes and occasionally mast cells (Fig 7B). Injection of Xestospongia testudinaria (100 mg/kg) 2 h before carrageenan injection exerted marked anti-inflammatory effect represented by marked decrease in the capillary congestion and inflammatory cells infiltrate (Fig 7C) which is more or less similar to the action of the reference anti-inflammatory drug, indomethacin (10 mg/kg) (Fig 7D).
Fig 7. Low and magnified power of rat paw sections stained by H&E, (a) Control group, E: epidermis, D: dermis with no signs of vascular congestion or inflammatory cells (dotted square) with normal capillaries (black arrows) and connective tissue dermis (stars). (b) Carrageenan group, showing marked inflammatory infiltration in the deep dermis (D), capillary dilation and congestion with neutrophils margination prior to escape into the surrounding tissue (arrows;) (c) Xestospongia testudinaria methanolic extract group, showing significant decrease of the inflammatory cells in the dermis and within blood vessels. (d) Indomethacin group, showing a decrease in both inflammatory cells infiltration and vascular congestion.
Discussion
Assessment of anti-inflammatory activity of marine natural products from sponges using carrageenan-induced inflammation in the rat paw is the most common assay [25,26]. In the current study, Xestospongia testudinaria methanolic extract revealed significant anti-inflammatory activity at a dose of 100mg/ kg even than the indomethacin treated group. Several compounds possess varied chemical structures and potent anti-inflammatory activity has been isolated from marine sponges [9]. In the present study, GC-MS analysis revealed five compounds exhibit antiinflamatory activity, Propanoic acid [27], Pyrimidine [28], Glutamine [29]. Also GC-MS revealed the presence of several fatty acids as (Tetradecanoic acid, Oleic acid, Octadecadiynoic acid, Octanoic acid) with sterol derivative 3α-(Trimethylsiloxy) cholest-5-ene. Pervious study on sea star found that, maximum antiinflammatory activity was obtained after combination of steroid and fatty acid [30]. Hexadecanoic acid possesses anti-inflammatory and antioxidant activity [31,32].
Recently, five sterols (cholesterol, 24-hydroperoxy-24-vinylcholesterol, saringosterol, methylcholest-5-ene-3β, 25-diol, and 29-hydroperoxystigmasta-5,24(28)-dien-3β-ol) were isolated from the marine sponge Xestospongia testudinaria [11]. Recently, we reported that the Red Sea sponges Scalarispongia aqabaensis and Callyspongia siphonella produced two new sterols, which exhibit anti-inflammatory activity using paw induced edema in rat [33]. Xestospongia testudinaria methanolic extract also had antioxidant activity as revealed by DPPH assay. GC-MS showed the presence of Azalic acid and l-Alanine which could be responsible for the antioxidant activity of Xestospongia testudinaria sponge extract [34,35]. ROS such as superoxide anion, hydroxyl radicals and hydrogen peroxide play major roles in terms of producing cellular damage in the inflammatory processes [36]. NO and MDA, the end product of lipid peroxidation [37], are considered important markers of oxidative stress [37–39]. In the present study, paw MDA and NO were increased while GSH, GPx, CAT and SOD were reduced in the carrageenan inflamed group. The results of the current work revealed that both Xestospongia testudinaria methanolic extract and indomethacin reduced paw MDA and NO and increased GSH formation. Moreover, both elevated the decreased activities of the antioxidant enzymes, GPx and CAT. GPx, in the existence of GSH are considered the second line defense against hydroperoxides where it accelerates hydrogen peroxide reduction or other organic hydroperoxides [40]. Phytochemicals present in sponges can act as antioxidants and prevent disorders due to oxidative damage [41]. In our recent published work, the extract of the Red Sea sponge Suberea mollis exhibited a significant antioxidant activity against CCl4-induced oxidative stress in rat liver [42]. Furthermore, sterols isolated from the sponge Fascaplysinopsis sp. are found to display antioxidant activity [43].
Inflammatory cytokines TNF-α, IL-1β and IL-6 play an important role in the inflammatory process and are responsible for production of acute phase proteins [44]. Treatment strategies are based on the presence of these inflammatory cytokines [45]. TNF-α, IL-1β and IL-6, are released mainly from monocytes and macrophages at the inflammatory sites [46]. In the present study, pretreatment of carrageenan-treated rats with Xestospongia testudinaria methanolic extract or indomethacin result in reduction in elevated TNF-α, IL-1β and IL-6. Previous studies found that proactive metabolites isolated from marine sponge-derived fungus Penicillium species, inhibit TNF-α, IL-1β and IL-6 in murine macrophage cells [47]. Similarly, marine sponge metabolite hymenialdisine was found to be a potent inhibitor of IL-2 and TNF-α production [48].
Conclusion
The results of this study demonstrated that pretreatment with the methanolic extract of the Red Sea marine sponge Xestospongia testudinaria prevents carrageenan-induced acute local inflammation in rats. The protective effect is based on the augmentation of antioxidant enzyme system which in turn causes a reduction in the oxidative stress measures. Xestospongia testudinaria methanolic extract may also prevent acute local inflammation through inhibition of proinflammatory cytokines formation.
Recommendation
Future study is warneted with orally consumed extract instead of injected intraperitoneal. In addition, bioassay guided fractionation of the crude Xestospongia testudinaria methanolic extract is important.
Acknowledgments
We would like to thank the Saudi General Directorate of Border Guard for the permission to collecting the sponge materials.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Chang CW, Chang WT, Liao JC, Chiu YJ, Hsieh MT, Peng WH, et al. Analgesic and anti-inflammatory activities of methanol extract of Cissus repens in mice. Evid Based Complement Alternat Med. 2012; 2012: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Senthilkumar K, Kim SK. Marine invertebrates natural products for anti-inflammatory and chronic diseases. Evid Based Complement Alternat Med. 2013; 2013:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Azevedo LG, Peraza GG, Lerner C, Soares A, Murcia N, Muccillo-Baisch AL. Investigation of the anti-inflammatory and analgesic effects from an extract of Aplysina caissara, a marine sponge. Fundam Clin Pharmacol. 2008; 22: 549–556. 10.1111/j.1472-8206.2008.00624.x [DOI] [PubMed] [Google Scholar]
- 4. Gualillo O, Eiras S, Lago F, Dieguez C, Casanueva FF. Evaluated serum leptin concentrations induced by experimental acute inflammation. J. Ethnopharmacol. 2001; 75: 213–218. [DOI] [PubMed] [Google Scholar]
- 5. Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002; 10:787–95. [DOI] [PubMed] [Google Scholar]
- 6. Inotai A, Hankó B, Mészáros A. Trends in the nonsteroidal anti-inflammatory drug market in six central-Eastern European countries based on retail information. Pharmacoepidemiol Drug Saf. 2010; 19: 183–190. 10.1002/pds.1893 [DOI] [PubMed] [Google Scholar]
- 7. Qin HY, Wu JC, Tong XD, Sung JJ, Xu HX. Bian ZX. Systematic review of animal models of post-infectious/post-inflammatory irritable bowel syndrome. J Gastroenterol. 2011; 46: 164–174. 10.1007/s00535-010-0321-6 [DOI] [PubMed] [Google Scholar]
- 8. Cai C, Chen Y, Zhong S, Ji B, Wang J, Bai X, et al. Anti-Inflammatory Activity of N-Butanol Extract from Ipomoea stolonifera In Vivo and In Vitro. PLoS One. 2014; 2: e95931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keyzers RA, Davies-Coleman MT. Anti-inflammatory metabolites from marine sponges. Chem. Soc. Rev. 2005; 34: 355–365. [DOI] [PubMed] [Google Scholar]
- 10. D'Orazio N, Gammone MA, Gemello E, De Girolamo M, Cusenza S, Riccioni G. Marine bioactives: pharmacological properties and potential applications against inflammatory diseases. Mar Drugs. 2012; 10: 812–33. 10.3390/md10040812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou X, Lu Y, Lin X, Yang B, Yang X, Liu Y. Brominated aliphatic hydrocarbons and sterols from the sponge Xestospongia testudinaria with their bioactivities. Chem Phys Lipids. 2011; 164:703–6. 10.1016/j.chemphyslip.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 12. Liolios CC, Sotiroudis GT, Chinou I. Fatty acids, sterols, phenols and antioxidant activity of Phoenix theophrasti fruits growing in Crete, Greece. Plant Foods Hum Nutr. 2009; 64: 52–61. 10.1007/s11130-008-0100-1 [DOI] [PubMed] [Google Scholar]
- 13. Bendary E, Francis RR, Ali HMG, Sarwat MI, El Hady S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Annals of Agricultural Sciences. 2013; 58: 173–181. [Google Scholar]
- 14. Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity, LWT—Food Science and Technology. 1995; 28: 25–30. [Google Scholar]
- 15. Marxen K, Vanselow KH, Lippemeier S, Hintze R, Ruser A, Hansen UP. Determination of DPPH radical oxidation caused by methanolic extracts of some microalgal species by linear regression analysis of spectrophotometric measurements. Sensors. 2007; 7: 2080–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garbacki N, Tits M, Angenot L, Damas J. Inhibitory effects of proanthocyanidins from Ribes nigrum leaves on carrageenan acute inflammatory reactions induced in rats. BMC Pharmacology. 2007; 4: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Winter CA, Risley EA, Nuss GW. Carragenin-induced oedema in hind paw on the rat as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962; 111: 544–547. [DOI] [PubMed] [Google Scholar]
- 18. Handy RL, Moore PK. A comparison of the effects of L-NAME, 7-NI and L-NIL on carrageenan-induced hindpaw oedema and NOS activity. Br. J. Pharmacol. 1998; 123: 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. 1978; Anal Biochem. 86: 271–278. [DOI] [PubMed] [Google Scholar]
- 20. Tarpey MM, Wink DA, Grisham MB. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am J Physiol Regul Integr Comp Physiol. 2004; 286: R431–R444. [DOI] [PubMed] [Google Scholar]
- 21. Ellman GL. Tissue sulfhydryl groups, Arch Biochem Biophys. 1959; 82: 70–77. [DOI] [PubMed] [Google Scholar]
- 22. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70:158–169. [PubMed] [Google Scholar]
- 23. Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972; 46: 849–854. [DOI] [PubMed] [Google Scholar]
- 24. Aebi H. Catalase in vitro. Methods in Enzymology. 1984, 105: 121–126. [DOI] [PubMed] [Google Scholar]
- 25. Robert AK, Michael T. Davies-Coleman. Anti-inflammatory metabolites from marine sponges. Chem Soc Rev. 2005; 34:355–365. [DOI] [PubMed] [Google Scholar]
- 26. Glaser KB, De Carvalho MS, Jacobs RS, Kernan MR, Faulkner DJ. Manoalide: Structure-activity studies and definition of the pharmacophore for phospholipase A2 inactivation. Mol Phys. 1989; 36:782–788. [PubMed] [Google Scholar]
- 27. Van Esch A, Van Steensel-Moll HA, Steyerberg EW, Offringa M, Habbema JD, Derksen-Lubsen G. Antipyretic efficacy of ibuprofen and acetaminophen in children with febrile seizures. Archives of Pediatrics & Adolescent Medicine. 1995; 149: 632–7. [DOI] [PubMed] [Google Scholar]
- 28. Amir M, Javed SA, Kumar H. Pyrimidine as anti-inflammatory agent: a review. Indian Journal of Pharmaceutical Sciences. 2007; 68: 337. [Google Scholar]
- 29. Jain P, Khanna NK. Evaluation of anti-inflammatory and analgesic properties of L-glutamine. Agents Actions. 1981; 11: 243–9. [DOI] [PubMed] [Google Scholar]
- 30. Pereira DM, Correia-da-Silva G, Valentão P, Teixeira N, Andrade PB. Anti-inflammatory effect of unsaturated fatty acids and Ergosta-7,22-dien-3-ol from Marthasterias glacialis: prevention of CHOP-mediated ER-stress and NF-κB activation. PLoS One. 2014; 13: e88341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harada H, Yamashita U, Kurihara H, Fukushi E, Kawabata J, Kamei Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002;22: 2587–2590. [PubMed] [Google Scholar]
- 32. Gobalakrishnan R, Manikandan P, Bhuvaneswari R. Antimicrobial potential and bioactive constituents from aerial parts of Vitis setosa wall. J. Med. Plant Res. 2014; 8: 454–460. [Google Scholar]
- 33. Youssef DT, Ibrahim AK, Khalifa SI, Mesbah MK, Mayer AM, van Soest RW. New anti-inflammatory sterols from the Red Sea sponges Scalarispongia aqabaensis and Callyspongia siphonella. Nat Prod Commun. 2010; 1:27–31. [PubMed] [Google Scholar]
- 34. Passi S, Picardo M, De Luca C, Breathnach AS, Nazzaro-Porro M. Scavenging activity of azelaic acid on hydroxyl radicals “in vitro.” Free Radic Res Commun. 1991;11:329–338. [DOI] [PubMed] [Google Scholar]
- 35. Grosser N, Oberle S, Berndt G, Erdmann K, Hemmerle A, Schröder H. Antioxidant action of L-alanine: heme oxygenase-1 and ferritin as possible mediators. Biochem Biophys Res Commun. 2004; 314:351–5. [DOI] [PubMed] [Google Scholar]
- 36. Kumar PP, Kuttan G. Vernonia cinerea L. scavenges free radicals and regulates nitric oxide and proinflammatory cytokines profile in carrageenan induced paw edema model, Immunopharmacol Immunotoxicol. 2009; 31: 94–102. 10.1080/08923970802438391 [DOI] [PubMed] [Google Scholar]
- 37. Wang R, Liu YQ, Ha W, Shi YP, Hwang TL, Huang GJ, et al. In vitro anti-inflammatory effects of diterpenoids and sesquiterpenoids from traditional Chinese medicine Siegesbeckia pubescens. Bioorg Med Chem Lett. 2014; 15:3944–7. [DOI] [PubMed] [Google Scholar]
- 38. Wu XL, Li CW, Chen HM, Su ZQ, Zhao XN, Chen JN,et al. Anti-Inflammatory Effect of Supercritical-Carbon Dioxide Fluid Extract from Flowers and Buds of Chrysanthemum indicum Linnén. Evid Based Complement Alternat Med. 2013; 2013:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999; 222: 236–45. [DOI] [PubMed] [Google Scholar]
- 40. Kumaran A, Karunakaran RJ. Activity-guided isolation and identification of free radical-scavenging components from an aqueous extract of Coleus aromaticus. Food Chemistry. 2007; 100: 356–361. [Google Scholar]
- 41. Mannix D, Langridge S, Lander GH, Rebizant J, Longfield MJ, Stirling WG, et al. Experiments on transuranium compoundswith x-ray resonant exchange scattering. Physica B: Physica Condensed Matter. 2007; 262: 125–40. [Google Scholar]
- 42. Abbas AT, El-Shitany NA, Shaala LA, Ali SS, Azhar EI, Abdel-Dayem UA, et al. Red Sea Suberea mollis Sponge Extract Protects against CCl4-Induced Acute Liver Injury in Rats via an Antioxidant Mechanism. Evid Based Complement Alternat Med. 2014; 2014: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aknin M1, Gros E, Vacelet J, Kashman Y, Gauvin-Bialecki A. Sterols from the Madagascar sponge Fascaplysinopsis sp. Mar Drugs. 2010; 17:2961–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kushner I. Regulation of the acute phase response by cytokines. Perspect Biol Med. 1993; 36: 611–622. [DOI] [PubMed] [Google Scholar]
- 45. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011; 1813:878–88. 10.1016/j.bbamcr.2011.01.034 [DOI] [PubMed] [Google Scholar]
- 46. Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res. 2006; Ther. 8 Suppl 2:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li JL, Zhang P, Lee YM, Hong J, Yoo ES, Bae KS, et al. Oxygenated hexylitaconates from a marine sponge-derived fungus Penicillium sp. Chem Pharm Bull (Tokyo). 2011; 59:120–3. [DOI] [PubMed] [Google Scholar]
- 48. Sharma V, Lansdell TA, Jin G, Tepe JJ. Inhibition of Cytokine Production by Hymenialdisine Derivatives. J. Med. Chem. 2004; 47: 3700–3703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.