Abstract
Background: Women with pregnancy complications benefit from closer monitoring postpartum and beyond. Increased postpartum emergency room (ER) use may indicate unmet need for outpatient obstetrics and primary care. The purpose of this study was to evaluate whether women with pregnancy complications (gestational diabetes [GDM], gestational hypertension, and preeclampsia) have increased ER use in the first 6 months postpartum, compared with women without these complications.
Methods: We conducted a retrospective population-based cohort study using a 2003–2010 Maryland Medicaid managed care claims data set, linked with U.S. Census data. Data included claims for outpatient and ER visits for women aged 12–45 years who were continuously enrolled in Medicaid for at least 100 days of pregnancy and 90 days postpartum. We used logistic regression to calculate the association between pregnancy complications and having ≥1 ER visit in the 6 months postpartum.
Results: We identified 26,074 pregnancies, of which 20% were complicated by GDM, gestational hypertension, or preeclampsia. Of these complicated pregnancies, 42.1% had GDM, 35.4% had gestational hypertension, and 42.5% had preeclampsia (diagnoses were not mutually exclusive). In the 6 months postpartum, 25% of women had ≥1 ER visits. Of the complicated pregnancy group, 27.7% had ≥1 ER visit, versus 23.6% of the comparison group (p<0.0001). In adjusted analyses, women with a pregnancy complication were more likely to have ≥1 ER visit compared with women without these complications (odds ratio [OR]1.14, 95% confidence interval [CI] 1.05–1.23). The strength of association was highest in women under age 25 (OR 1.20, 95% CI 1.09–1.33). Preconception medical comorbidities (type 2 diabetes, chronic hypertension, obesity, asthma, mental health, and substance abuse diagnoses) were also strongly associated with postpartum ER use (OR 1.61, 95% CI 1.51–1.73).
Conclusions: Pregnancy complications increased ER utilization during the 6 months postpartum, especially among women under age 25 years. Interventions that improve discharge planning and early postpartum care may decrease ER use.
Introduction
Of the 6,578,000 annual pregnancies1,2,3 in 2008 in the United States, 5%–17% are complicated by gestational diabetes,4–7 and 6%–8% by one or more hypertensive disorders of pregnancy.8–10 The prevalence of gestational diabetes,4 gestational hypertension, and preeclampsia4,10–12 have increased, particularly in younger women.4,11–12 They are among the leading causes of perinatal maternal and fetal morbidity and mortality in the United States and worldwide.4,8,9,13–15 Preeclampsia and gestational diabetes are additionally associated with future risk of maternal metabolic4,16 and premature cardiovascular diseases.17–19
Frequent interactions with the health care system during pregnancy and the postpartum period present opportunities for health promotion,20–22 preventative health screenings,4,9,20 and engagement in long-term behavioral changes to reduce future risk.20,22–23 Yet even women with pregnancy complications often miss their postpartum visits.22,24–28 Low-income women—who have disproportionate prevalence of gestational diabetes and hypertensive disorders in pregnancy4,29–30—have multiple barriers to outpatient follow-up in the postpartum period.31 These barriers include lack of childcare, schedule demands, difficulty accessing care, and lack of understanding about the long-term health risks associated with pregnancy complications31 and may increase women's reliance on emergency room (ER) settings.32–33 However, ER visits are often more costly, less efficient, and less equipped for longitudinal care of chronic medical diagnoses.34–36 Understanding the characteristics of women who are more likely to use the ER in the postpartum period will inform strategies to reduce unnecessary visits, through improved discharge planning and provision of outpatient care.
Our objectives were to use a Medicaid claims dataset to (1) describe postpartum ER utilization in the 6 months after delivery among women with and without pregnancy complications (gestational diabetes, gestational hypertension, preeclampsia), and (2) examine the association between pregnancy complications and ER utilization after adjustment for maternal sociodemographic characteristics, insurance coverage duration, and other health care utilization. We hypothesized that women with complicated pregnancies,37–38 preconception medical comorbidities,37,39–40 and cesarean delivery41 would be more likely to use the ER in the 6-month postpartum period compared with women without these conditions, and that primary care visit attendance in the 6 months after delivery would attenuate these associations.
Materials and Methods
Study design and data set description
We conducted a retrospective cohort study between 2003 and 2010 using a claims database from one of the seven Medicaid managed care programs in Maryland.42 Patients eligible for Medicaid managed care in Maryland, which offers the same benefits as fee for service plans,42–43 can choose from the available plan in their county and will be randomly assigned to a plan if they do not make a designation. The dataset included claims for every delivery, as well as outpatient and ER visit claims during pregnancy, 6 months preconception, and 12 months after delivery. To describe socioeconomic variables not available in our claims data we used zip code at the time of delivery to link with zip code–level neighborhood characteristics in the 2000 U.S. Census Bureau's American Community Survey using methods from the Dartmouth Atlas of Healthcare.24,44
This data set met the definition of a limited data set under the Health Insurance Portability and Accountability Act, and was approved by the Johns Hopkins University School of Medicine Institutional Review Board.
Sample selection
We selected all pregnant women aged 12–45 years and required 100 days or more of continuous Medicaid coverage during pregnancy and 90 days or more of continuous postpartum Medicaid coverage (to include those women who qualified for Medicaid based on pregnancy and therefore lost coverage after 90 days postpartum). Seventy-one percent of our sample was comprised of women who became eligible for Medicaid based on pregnancy. The benefit structure is the same for women who had preconception Medicaid and those who qualified because of pregnancy. We identified pregnancies and deliveries using an International Classification of Diseases, ninth revision (ICD-9) and current procedural terminology code–based definition from the National Center for Quality Assurance's Healthcare Effectiveness Data and Information Set measures and additionally added codes for stillborn and multiple gestation births.24,45,46 We required at least one insurance claim in the 2 weeks prior and subsequent to the delivery date to ensure an accurate delivery date.
Definitions of complicated and comparison pregnancy groups
We evaluated the role of individual pregnancy complications: gestational diabetes, gestational hypertension, and preeclampsia using ICD-9 codes (see Supplementary Table S1 for definitions; Supplementary Data are available online at www.liebertpub.com/jwh).46 We created a composite variable for “any of these complications.” Our rationale for focusing on these three conditions was to distinguish ER use associated with the pregnancy-related diagnoses (which are usually not chronic but do confer higher risk of future disease), separate from ER use related to preconception or chronic medical disorders, such as type 2 diabetes or chronic hypertension. For women with one or more complicated pregnancies in the dataset, we selected the first complicated pregnancy as the index pregnancy. Complications associated with the index pregnancy were classified under each of the individual complications. Women in the comparison group did not have any diagnoses of gestational diabetes, gestationals hypertension or preeclampsia. For women in the comparison group, we selected the first pregnancy in the dataset for analysis.
Outcome definitions
The primary outcome was one or more ER visits in the 6 months postpartum. We chose 6 months postpartum in order to capture both delivery-related as well as subsequent medical complications that may occur later after delivery.37 We additionally described two time periods after the index delivery (within 8 weeks and 9–24 weeks) to better understand temporal variations in the predictors of ER use.
Covariates
Age was categorized into five categories: ≤18, 19–24, 25–29, 30–34, and ≥35 years. Race/ethnicity was self-reported at the time of Medicaid enrollment. Other covariates included cesarean delivery, and preconception medical comorbidities (type 2 diabetes, chronic hypertension, obesity, asthma, mental health, substance abuse). See Supplementary Table S1 for the ICD-9 code–based definitions.46
Health care utilization covariates included whether the patient had received early prenatal care, defined as prenatal care received in the first 104 days of pregnancy, if they attended a postpartum obstetrics appointment (defined as an outpatient obstetrics visit with any provider, within 56 days of delivery), and/or attended one or more primary care visits. We included the proportion living below the federal poverty line, categorized into tertiles, from neighborhood variables from the U.S. Census in our analysis.
Statistical analyses
We used descriptive statistics to describe ER utilization in our cohort for the first 8 weeks, 8–12 weeks, and the full 24 weeks postpartum, among women with and without pregnancy complications (gestational diabetes, gestational hypertension, preeclampsia), preconception medical comorbidities and cesarean delivery. We used bivariate analyses to evaluate the relationships between age, pregnancy complications, and ER use.
Our primary analysis was a logistic regression model to assess the association of pregnancy complications with utilization of one or more ER visits. Our secondary analysis was a multinomial logistic regression to assess for differences in the association between pregnancy complications and use of ER in the postpartum period by outcomes of ER visit frequency: 1 ER visit, 2–3 ER visits, and 4–10 ER visits. For the multinomial modeling, we excluded women with more than 10 ER visits (n=23; 0.06% of sample) because it represented an extreme outlier. All analyses were adjusted for age, race, receipt of early prenatal care, attendance at at least one postpartum primary care visit and obstetrics visit within 56 days postpartum, any preconception insurance coverage, duration of postpartum insurance coverage, and the neighborhood proportion of people living below the federal poverty level. We also adjusted for delivery year to control for changes in Maryland Medicaid policy and other secular trends. Because medical comorbidities and cesarean delivery were identified as confounders they were included in the final model.47
Additional analysis
Based on our exploratory analysis, we hypothesized that the relationship between pregnancy complication and ER use might vary based on age.5,11,39,48 We assessed effect modification by age by testing an age*pregnancy complication interaction term based on a dichotomized age variable derived from the exploratory analysis, and by describing a stratified analysis by age ≥25 versus age <25 years.
Results
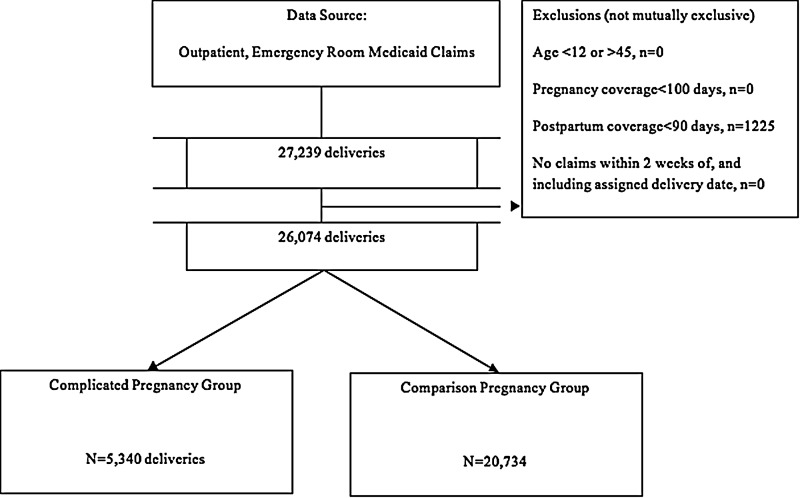
We identified 27,329 pregnancies between 2003 and 2010, of which 1,255 were excluded because they did not meet the required 90 days of continuous postpartum insurance coverage, resulting in 26,074 deliveries in our final sample (Fig. 1). Twenty percent of the sample were complicated pregnancies. In the complicated pregnancy group, 42.1% had gestational diabetes, 35.4% had gestational hypertension, and 42.5% had preeclampsia. These diagnoses were not mutually exclusive; the overlap between gestational diabetes and preeclampsia was 5.8%, and between gestational diabetes and gestational hypertension was 5.2%.
FIG. 1.
Selection of the sample of women with complicated and comparison pregnancies, 2003–2010.
Characteristics of the sample of pregnant women are in Table 1. Women with complicated pregnancies were older (p<0.0001), more likely to be African American (p<0.0001), have cesarean delivery (p<0.0001), and have preconception medical comorbidities (p<0.0001), when compared with women without these complications. In contrast to the women in the comparison pregnancy group, women in the complicated pregnancy group were more likely to attend one or more postpartum outpatient primary care visits in the 6 months after delivery (p<0.0001), receive early prenatal care (p<0.0001), and attend the 6-week postpartum obstetrics visit (p<0.0001). Women with pregnancy complications were also more likely to continue Medicaid insurance coverage beyond 3 months postpartum (p=0.001).
Table 1.
Characteristics of Women Insured by Medicaid, with Complicated and Comparison Pregnancies, 2003–2010
| Covariates | Overall N=26,074Number (%) | Pregnancy complications N=5,340Number (%) | Comparison pregnancies N=20,734Number (%) | p-Value |
|---|---|---|---|---|
| Maternal age at delivery | <0.0001 | |||
| <18 | 2,058 (7.8) | 337 (6.3) | 1,721 (8.3) | |
| 18–24 | 13,324 (51.1) | 2,441 (45.7) | 10,883 (52.5) | |
| 25–29 | 5,832 (22.4) | 1,232 (23.1) | 4,600 (22.2) | |
| 30–34 | 3,071 (11.9) | 794 (14.9) | 2,277 (11.0) | |
| ≥35 | 1,789 (6.8) | 536 (10.0) | 1,253 (6.0) | |
| Maternal race/ethnicity | <0.0001 | |||
| African American | 11,739 (45.0) | 2,538 (47.5) | 9,201 (44.4) | |
| Caucasian | 8,668 (33.2) | 1,708 (32.0) | 6,960 (33.6) | |
| Hispanic | 1,252 (4.8) | 255 (4.8) | 977 (4.8) | |
| Other race | 429 (1.7) | 101 (1.9) | 328 (1.6) | |
| Unknown race | 3,986 (15.3) | 738 (13.8) | 3,248 (15.6) | |
| Neighborhood characteristicsa | ||||
| Proportion below the FPLb | 0.34 | |||
| First tertile | 8,819 (33.8) | 1,824 (34.2) | 6,995 (33.7) | |
| Second tertile | 8,583 (32.9) | 1,785 (33.4) | 6,798 (32.8) | |
| Third tertile | 8,672 (33.3) | 1,731 (32.4) | 6,941 (33.5) | |
| Medical and delivery complications | ||||
| Preeclampsia | 2,588 (9.9) | 2,588 (48.5) | N/A | |
| Eclampsia | 133 (0.5) | 133 (2.5) | N/A | |
| Gestational hypertension | 1,892 (7.3) | 1,892 (35.4) | N/A | |
| Gestational diabetes mellitus | 2,249 (8.6) | 2,249 (42.1) | N/A | |
| Cesarean delivery | 7,054 (27.1) | 1,996 (37.4) | 5,058 (24.4) | <0.0001 |
| Preconception medical comorbidities | ||||
| Any medical comorbidity | 9,712 (37.3) | 2,812 (52.67) | 7,184 (34.7) | <0.0001 |
| Any mental illnessc | 4,371 (16.8) | 887 (16.6) | 3,484 (16.8) | 0.740 |
| Drug and alcohol abuse | 1,293 (5.0) | 267 (0.1) | 1,026 (5.0) | 0.880 |
| Obesity | 2,617 (10.0) | 944 (17.67) | 1,673 (8.1) | <0.0001 |
| Chronic hypertension | 1,675 (6.4) | 815 (15.3) | 860 (4.2) | <0.0001 |
| Asthma | 3,121 (12.0) | 735 (13.8) | 2,386 (11.5) | <0.0001 |
| Pregestational diabetesd | 331 (1.3) | 74 (1.4) | 257 (1.2) | 0.40 |
| Healthcare utilization | ||||
| ER visits after delivery | <0.0001 | |||
| 0 Visits | 19,688 (75.5) | 3857 (72.2) | 15831 (76.4) | |
| 1 Visit | 3,971 (15.2) | 895 (16.8) | 3076 (14.8) | |
| 2–3 Visit | 1,928 (7.34) | 453 (8.4) | 1475 (7.1) | |
| 4–10 Visitse | 464 (1.8) | 128 (2.4) | 336 (1.6) | |
| PCP visit in 6 months postpartum,f ≥1 | 8,675 (33.3) | 1,911 (35.8) | 6,764 (32.6) | <0.0001 |
| Early prenatal care, ≥1 | 6,982 (26.8) | 1,503 (28.2) | 5,479 (26.4) | 0.01 |
| Postpartum 6-week OB Visit | 14,553 (55.8) | 3,157 (59.1) | 11,396 (55.0) | <0.0001 |
| Insurance coverage | ||||
| Preconception coverage | 0.7 | |||
| None | 17,333 (66.45) | 3,526 (66.0) | 13,807 (66.6) | |
| Any | 8,741 (33.5) | 1,814 (34.0) | 6,927 (33.4) | |
| Postpartum coverage | 0.001 | |||
| ≤<=95 days | 10,714 (41.1) | 2,071 (38.8) | 8,643 (41.7) | |
| 96–185 days | 1,766 (6.8) | 385 (7.2) | 1,381 (6.7) | |
| >185 days | 13,594 (52.1) | 2,884 (54.0) | 10,710 (51.7) | |
Neighborhood characteristics denominator 25,688 total; 5,263 complicated and 20,425 comparison pregnancies due to missing zip codes.
Definitions according to 2000 U.S. Census, reported by zip code.
Any mental illness: Defined as any preconception or pregnancy-related diagnoses of mental illness. See Supplementary Table S1 for definitions.
Any diabetes mellitus diagnosis in the 3 months prior to conception. See Supplementary Table S for diabetes definition.
Only 30 women (0.11%) had more than 10 ER visits in the 6 months postpartum.
Postpartum primary care physician attendance related to low rate of postpartum insurance retention. 39% of those women with complicated pregnancies, and 42% of those in the comparison pregnancy group lost insurance after 3 months, and an additional 7% of each group lost insurance between 3 and 6 months postpartum.
ER, emergency room; FPL, federal poverty limit; OB, obstetrician.
In the 6 months postpartum, 25% of women had one or more ER visits, for a total of 11,138 ER visits, ranging from 0–40 total visits per woman. Of the women who went to the ER during the 6 months after delivery, 3,971 (62.2%) made only one visit, 1,928 (30.2%) made 2–3 visits, and 464 (7.3%) made 4–10 visits. Women with pregnancy complications had higher rates of ER utilization in the 6 months postpartum compared with women in the comparison pregnancy group (27.7% vs. 23.6%, p<0.0001). In the complicated pregnancy group, 16.8% had 1 ER visit, 8.5% had 2–3 visits, 2.3% had 4–10 visits, and 0.13% had more than 10 visits in the 6 months postpartum. In the comparison group, 14.8% had 1 visit, 7.1% had 2–3 visits, 1.6% had 4–10 visits, and 0.08% had greater than 10 visits (p<0.0001 for trend).
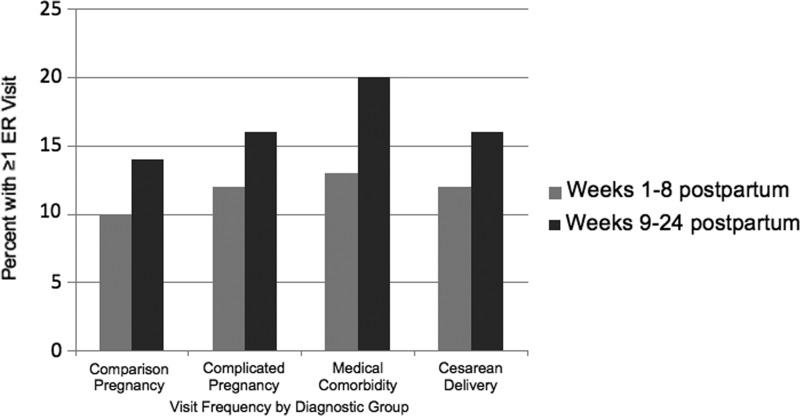
Figure 2 demonstrates temporal differences in the ER visit rates between the pregnancy complication and comparison groups of women within 8 and 24 weeks after delivery. It also demonstrates the utilization between women who had other characteristics that potentially affect ER use: cesarean delivery and preconception medical comorbidity. Overall, a greater proportion of women with pregnancy complications attended one or more ER visits (27.8%) than those in the comparison group (23.6%). The difference in their ER visit rates was stable over the two time periods (12% in the initial 8 weeks and 16% in weeks 9–24 after delivery). ER use among women with a cesarean delivery also remained fairly stable between the early to late postpartum periods (12% and 16% respectively). For women with preconception medical comorbidities, however, only 13% used the ER in the first 8 weeks postpartum, while usage increased to 20% in the later weeks 9–24.
FIG. 2.
Percentage of women attending one or more ER visits at 1–8 weeks and 9–24 weeks postpartum by pregnancy complication, medical comorbidity, and delivery method status.
Table 2 presents results of the adjusted multivariate logistic regression and multinomial logistic regression models to evaluate the association between pregnancy complications and ER use. In the adjusted multivariate logistic regression analysis, women with a pregnancy complication were more likely to attend one or more ER visits in the 6 months after delivery (odds ratio [OR] 1.14–95% confidence interval [CI] 1.06–1.24). Cesarean delivery (OR 1.24, CI 1.16–1.33) and preconception medical comorbidities (OR 1.63, CI 1.52–1.75) were also associated with higher odds of ER use in the 6 months postpartum.
Table 2.
Multinomial Regression Analysis Evaluating the Relationship Between Pregnancy Complications and ER Frequency Categories
| Logistic regression | Multinomial logistic regression | |||
|---|---|---|---|---|
| Covariates | ≥1 ER visitOR (CI) | 1 ER visitOR (CI) | 2–3 ER visitsOR (CI) | 4–10 ER visitsOR (CI) |
| Pregnancy complicationa | 1.14 (1.06–1.24) | 1.13 (1.03–1.24) | 1.13 (1.00–1.29) | 1.27 (1.00–1.61) |
| Gestational diabetesb | 1.12 (1.00–1.26) | 1.12 (0.98–1.29) | 1.12 (0.93–1.03) | 1.08 (0.75–1.56) |
| Preeclampsiac | 1.11 (1.00–1.24) | 1.11 (0.97–1.26) | 1.02 (0.85–1.22) | 1.08 (0.78–1.85) |
| Gestational hypertensiond | 1.17 (1.04–1.32) | 1.05 (0.90–1.22) | 1.30 (1.07–1.58) | 1.27 (0.87–1.85) |
| Maternal age, years (REF, <18) | ||||
| 18–24 | 1.03 (0.91–1.16) | 0.95 (0.83–1.09) | 1.16 (0.96–1.41) | 1.19 (0.82–1.72) |
| 25–29 | 0.82 (0.72–0.93) | 0.78 (0.67–0.91) | 0.89 (0.72–1.11) | 0.76 (0.50–1.15) |
| 30–34 | 0.72 (0.62–0.84) | 0.69 (0.58–0.82) | 0.81 (0.63–1.03) | 0.47 (0.41–1.07) |
| ≥35 | 0.66 (0.55–0.78) | 0.65 (0.53–0.79) | 0.70 (0.53–1.05) | 0.47 (0.26–0.84) |
| Maternal race (REF, black) | ||||
| Caucasian | 1.07 (0.98–1.15) | 1.01 (0.92–1.11) | 1.06 (0.94–1.20) | 1.69 (1.33–2.14) |
| Hispanic | 0.88 (0.74–1.04) | 0.83 (0.68–1.02) | 0.84 (0.62–1.02) | 1.64 (0.96–2.81) |
| Other/unknown | 0.94 (0.82–1.01) | 0.92 (0.81–1.05) | 0.80 (0.72–1.06) | 1.32 (0.87–2.00) |
| Cesarean section | 1.24 (1.16–1.33) | 1.23 (1.13–1.34) | 1.26 (1.12–1.41) | 1.31 (1.05–1.63) |
| Medical comorbiditye | 1.63 (1.52–1.75) | 1.40 (1.29–1.52) | 1.91 (1.71–2.13) | 3.10 (2.50–3.85) |
| Obesity | 1.34 (1.22–1.47) | 1.25 (1.12–1.40) | 1.47 (1.28–1.70) | 1.20 (0.91–1.59) |
| Preconception diabetesf | 1.30 (1.03–1.65) | 1.19 (0.89–1.60) | 1.49 (1.06–2.10) | 1.73 (0.95–3.14) |
| Chronic hypertension | 1.11 (0.99–1.13) | 1.12 (0.98–1.29) | 1.12 (0.93–1.34) | 1.23 (0.87–1.73) |
| Asthma | 1.43 (1.32–1.56) | 1.26 (1.14–1.40) | 1.55 (1.36–1.76) | 1.20 (1.76–2.72) |
| Substance abuse | 1.29 (1.14–1.47) | 1.22 (1.50–1.43) | 1.37 (1.13–1.66) | 1.58 (1.14–2.17) |
| Psychiatric diagnosisg | 1.46 (1.35–1.58) | 1.30 (1.18–1.43) | 1.63 (1.44–1.84) | 2.35 (1.90–2.92) |
| Received early prenatal care | 1.12 (1.04–1.21) | 1.12 (1.03–1.22) | 1.18 (1.05–1.33) | 0.90 (0.72–1.14) |
| Attended postpartum obstetrics visit | 1.06 (0.99–1.25) | 1.05 (0.97–1.13) | 1.07 (0.96–1.20) | 1.09 (0.88–1.35) |
| Attended postpartum PCP visit | 1.40 (1.31–1.50) | 1.28 (1.18–1.38) | 1.51 (1.36–1.68) | 2.30 (1.86–2.83) |
| Any preconception insurance coverage | 1.19 (1.10–1.28) | 1.14 (0.97–1.25) | 1.21 (1.07–1.36) | 1.42 (1.13–1.79) |
| Postpartum insurance coverage (REF, ≤90 days) | ||||
| 91–180 days | 1.74 (1.52–1.98) | 152 (1.30–1.78) | 2.22 (1.76–2.80) | 3.80 (2.25–6.41) |
| 180–365 days | 2.42 (2.23–2.61) | 1.98 (1.80–2.17) | 3.29 (2.84–3.81) | 6.76 (4.69–9.72) |
| Neighborhood proportion living below FPL,h (REF, tertile 1) | ||||
| Tertile 2 | 1.00 (0.92–1.09) | 0.99 (0.90–1.09) | 1.02 (0.89–1.17) | 1.03 (0.80–1.34) |
| Tertile 3 | 1.07 (0.99–1.17) | 1.09 (0.99–1.20) | 1.07 (0.93–1.23) | 0.98 (0.74–1.28) |
| Delivery year | 1.02 (1.00–1.04) | 1.02 (1.00–1.03) | 1.02 (0.99–1.05) | 1.06 (1.01–1.11) |
Boldface indicates statistically significant results at or below the level of p<0.05.
OR for pregnancy complication (gestational diabetes, gestational hypertension or preeclampsia): model adjusted for all covariates.
OR for gestational diabetes (gestational hypertension, preeclampsia not included): model adjusted for all covariates.
OR for preeclampsia (gestational diabetesand gestational hypertension not included): model adjusted for all covariates.
OR for preeclampsia (gestational diabetesand gestational hypertension not included): model adjusted for all covariates.
Model run with any medical comorbidity, and separately without any medical comorbidity to obtain OR for individual comorbidities.
Preconception diabetes diagnoses: Defined as any preconception diagnoses of diabetes.
Defined as any preconception or pregnancy-related diagnoses of mental illness. See Supplementary TableS1 for definitions.
Definitions according to 2000 U.S. Census, reported by zip code.
CI, 95% confidence interval; OR, odds ratio; PCP, primary care physician; REF, reference group; SD, standard deviation.
We evaluated the association between ER use and individual medical comorbidities and outpatient care utilization. Prior mental illness (OR 1.46, CI 1.35–1.58), asthma (OR 1.43, CI 1.32–1.56), obesity (OR 1.34, CI 1.22–1.47), preconception diabetes (1.30, CI 1.03–1.65), and substance abuse (OR 1.29, CI 1.14–1.47) increased the odds or ER use in the 6 months postpartum, while chronic hypertension did not. Occurrence of an outpatient primary care visit in the 12 months after delivery was associated with increased ER utilization (OR 1.40, CI 1.31–1.50), but attendance of the postpartum obstetric visit did not change the association with ER use (OR 1.06, CI 0.99–1.25) (See Supplementary Table S2 for model development).
The results of the adjusted multinomial logistic regression confirmed the findings from the multivariate logistic regression, showing increased odds of ER use among women with pregnancy complications. Results also suggested an even stronger association of pregnancy complications with 4–10 ER visits, those these results were not statistically significant (OR 1.27, CI 1.00–1.61). Consistent with the primary analysis, both cesarean delivery and preconception medical comorbidities were also associated with increased odds of ER use. The relationship between preconception medical comorbidities and ER use was also strengthened with increasing ER visit frequency, with an OR of 3.01 (CI 2.50–3.85) for the outcome of 4–10 ER visits (Table 2).
Stratified analysis to assess effect modification by age
In our exploratory analysis, results of bivariate analyses by age and complication demonstrated statistically significant increase in the odds of a complication in all age groups as compared with women under age 18, with the greatest odds in the ≥35 age group (OR 2.18–95% CI 1.87–2.54). In contrast, multivariate regression evaluating the odds of an ER visit in the complicated group was lower in all age categories when compared with those in the <18 group (OR 0.86, CI 0.76–0.93). In a stratified analysis by age, women younger than 25 years of age had increased odds of ER use (OR 1.20, CI 1.09–1.33), while women 25 or older did not (OR 1.05. CI 0.94–1.19).
Comments
In a large sample of 26,074 postpartum women in a Medicaid managed care plan in Maryland, we found a modest association between having a pregnancy complication (gestational diabetes, gestational hypertension, and preeclampsia) and ER use in the first 6 months postpartum (OR 1.14). This positive association was stronger in women under the age of 25 (OR 1.20), and similar in women making 2–3 ER visits. Women who had a cesarean delivery were at higher odds of ER use, and the relationship was stronger still for those with preconception medical comorbidities, especially among those making 4–10 ER visits. We observed that the increased ER use associated with medical complications in pregnancy began even before women attended their 6-week postpartum visit, and then utilization continued at similar rates throughout the 6 months postpartum. ER utilization may represent reduced access to outpatient care services and thus missed opportunities for follow-up primary and outpatient care. Our results demonstrating the relationship between medical complications and increased ER utilization could inform health services and community-based strategies to improve the delivery of and access to postpartum health care services for this high-risk population.
In addition to our study, two other studies have examined the role of medical complications of pregnancy and preconception medical comorbidities on postpartum ER use.37,38 In a secondary data analysis of 171 low income women, Hamilton and colleagues also reported high rates of urgent care, ER visits and re-hospitalizations in the year postpartum among women with gestational diabetes and chronic hypertension.37 Of those women who attended an acute care visit (urgent care or ER), 38.5% occurred during the first 8 weeks after delivery, while 39% occurred in the period between 24 and 36 weeks after delivery.37 In a retrospective cohort study using claims data from 222,084 deliveries at a large health care delivery system in the United States, Clark and colleagues also found increased odds of ER use in the 6 weeks immediately postpartum, as well as increased ER use in women with a hypertension or preeclampsia related visit within 100 days postpartum.38 Our study confirms the findings from these two studies in a large cohort of women with Medicaid, with a longer duration of follow-up through 6 months after delivery. In addition, our analysis has enabled us to establish a comparison group of women without pregnancy complications for evaluation of utilization rates between women with complicated and uncomplicated pregnancies. Our analyses also controlled for key sociodemographic confounders, insurance coverage, and comorbid medical diagnoses and enabled us able to examine other potential predictors of ER use, thereby informing future hypotheses on strategies to reduce ER use.
Contrary to our hypothesis, attendance at outpatient primary care and obstetrics visits during pregnancy,48 or in the 6 months following delivery, did not attenuate the association between pregnancy complication and ER use. In fact, women with one or more primary care physician visits in the postpartum period were even more likely to have an ER visit. Similarly, those who received early prenatal care had higher odds of ER use. Finally, attending the 6-week postpartum obstetrics visit had no impact on the association of pregnancy complication and postpartum ER use. We identified several possible explanations for these counterintuitive findings. Women may have been sent to the ER from the primary care provider's office and outpatient visits may have represented ER followup care, prompting the direct association between receipt of outpatient care and using the ER. We also found that women with more prenatal care visits had disproportionately experiened higher pregnancy complication rates (17.4% of those making 16 or more prenatal care vists had pregnancy complications vs. 9.6% of those making fewer than 16 visits, p<0.0001, data not shown). These women may be a sicker population, requiring appropriately higher use of intensive outpatient and ER services.39–40 Finally, the postpartum visit may have had little impact on postpartum ER use due to the timing of the visit. The majority (60%, data not shown) of the ER visits among those with pregnancy complications were made prior to the 6-week postpartum visit, and thus this visit may be too late in the postpartum course to have a large impact on ER use.
Our findings have several important implications for current clinical care and health services delivery for high-risk populations of women. First, because we found high ER use immediately following discharge, interventions focused on enhancing discharge planning in obstetrics populations may reduce ER visits and hospital readmissions, as seen in nonobstetrics populations.49–52 Studies of “intensive discharge planning” using the Perceived Readiness for Discharge after Birth scale have shown that the scale predicts risk of post discharge problems.53 Use of this scale could aid in targeting those at high risk post discharge, allowing for intensive intervention to potentially decrease postpartum ER utilization.53 Second, women we identified as high risk because of pregnancy complications may be eligible for postpartum home visits aimed at improving newborn care and outcomes, but which could aid their successful transition into outpatient care and prevent ER use. In fact, studies in socially or medically complicated women have demonstrated that a benefit of postpartum home visits is a reduction in maternal acute care visits, postpartum hospital stays, costs,54 repeat pregnancies,55–56 and months on Medicaid.57 The Affordable Care Act's Maternal, Infant, and Early Childhood Home Visiting Program was recently expanded, presenting an opportunity to link maternal and child care to improve outcomes for both.58 Third, our study and others38 have observed increases in ER use even prior to the 6-week postpartum visit. Further study is needed to identify the optimal timing and content of the postpartum visit to enhance women's transitions into community-based and primary care settings to reduce ER use. Lastly, through Medicaid expansion and use of the insurance health care insurance exchanges for low income women, the Affordable Care Act will likely improve women's access to insurance coverage after delivery.59–61 Whether this insurance coverage access results in greater primary care and preventive services utilization and decreased reliance on the ER is not yet clear. While some studies demonstrated that frequent utilizers of the ER tend to be uninsured or lack access to outpatient care,48 other studies examining ER use in the general population suggest that the situation is more complicated and may be related to higher levels of chronic illness.39–40
The major strength of this analysis is the large sample size on which the analysis was performed. The large sample size allowed sufficient power to evaluate pregnancy complications while controlling for multiple covariates, including individual, insurance, and neighborhood characteristics. This enabled assessment of significant predictors of ER use, which has practical implications for developing and targeting interventions. Our ethnically diverse sample may also increase the generalizability of these results to other low income populations in the United States.
This study has several limitations. First, in regard to our sample selection, our study duration was 8 years. The 2008 Maryland Medicaid expansion62 overlapped with our study from July 2008 through December 2010, resulting in an additional 60,000 newly insured adults in Maryland during this period. It is possible that post expansion enrollees differed in important ways from those enrolled prior to expansion. Additionally there may have been changes in secular trends during this period. To control for these we adjusted our analyses for delivery year. Second, we only evaluated women who had Medicaid managed by Johns Hopkins Healthcare, one of seven potential Medicaid managed care plans in Maryland. To assess the external generalizability of our Medicaid sample population to the Medicaid population of Maryland, we evaluated ER frequency rates by year and age group and compared these results with available Medicaid statistics for the state of Maryland. The rates of ER use in our sample (25%) were comparable to statewide data of 27.9% in 2008, 31.9% in 2009, and 30% in 2010. For age-adjusted rates during the same period, our rate of 25% was lower than adults aged 19–39, which were 31.8% in 2008, 36.9% in 2009, and 36.9% in 2010.43 Third, 67% of our sample had a temporary form of Medicaid granted to pregnant women, which is discontinued at 8 weeks postpartum. There is no way to know how many of these women qualified for regular Medicaid postpartum; however, there was no statistically significant difference between the percentage of women with pregnancy-related Medicaid in the complicated and comparison pregnancy groups, suggesting a nondifferential bias. Fourth, with respect to our data set, diagnoses included within claims data are prone to errors (under- and over-coding). However, for assessing utilization of health care services, claims are reliable data sources. Fifth, our analyses were limited to women who had Medicaid insurance for the 6 months postpartum. We do not have data on women who changed to a different insurance plan, who lost Medicaid and became uninsured postpartum, or who moved out of the state of Maryland. We are unable to assess if the women who left our dataset would be more or less likely to use the ER, and thus we are unable to make a determination of the direction of bias for this limitation. Sixth, we limited our analysis to those women with pregnancies resulting in deliveries, thus missing potentially important information on health care utilization in women whose pregnancies resulted in early miscarriage or abortion. Women who miscarried may be more likely to have had a pregnancy complication, suggesting that inclusion of these women may have strengthened the associations. Seventh, our claims data did not include sociodemographic data. We addressed this by linking our data to the U.S. Census data to obtain neighborhood level sociodemographic characteristics. Lastly, we were unable to obtain meaningful coding data due to the way in which our dataset was constructed. Due to the unreliability of the codes, we were concerned about making any inferences from the data to the nature of the visit. This limits our ability to connect postpartum visits with specific diagnoses relating to pregnancy or the medical complications of pregnancy included in our analysis.
Conclusions
We found that young women with medical complications of pregnancy had increased odds of ER utilization during the 6 months postpartum. The increasing prevalence of medical complications of pregnancy among young, low-income women supports efforts to target postpartum interventions to improve their health care utilization decrease ER use and improve health outcomes.
Supplementary Material
Acknowledgments
Ashley Harris is supported by Hopkins Health Resources and Services Administration National Research Service Award, Primary Care Health Services Fellowship Training Program T32HP10025BO-20, as well as Johns Hopkins Behavioral Research in Heart and Vascular Disease Fellowship Training Program T32HL007180-39, and by a National Institute of Health Loan Repayment Award, Health Disparities Loan Repayment Program, National Institute on Minority Health and Health Disparities. This funding was used to design the study; analyze and interpret the data; and prepare, review and publish the manuscript. Dr. Wendy Bennett and the development of this dataset are supported by a career development award from the National Heart, Lung, and Blood Institute, 5K23HL098476-02. This funding was also used to prepare, review and publish the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ventura SJ, Mosher WD, Curtin SC, Abma JC, Henshaw S. Trends in pregnancy rates for the United States, 1976–97: An update. Natl Vital Stat Rep. 2001;49:1–9 [PubMed] [Google Scholar]
- 2.Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990–2008. Natl Vital Stat Rep 2012;60:1–21 [PubMed] [Google Scholar]
- 3.Martin JA, Hamilton BE, Osteman MJK, et al. Births: Final data for 2012. Natl Vital Stat Rep 2013;62:1–68 [PubMed] [Google Scholar]
- 4.Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the fifth international workshop-conference on gestational diabetes mellitus. Diabetes Care 2007;30:S251–60 [DOI] [PubMed] [Google Scholar]
- 5.Desisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev Chronic Dis 2014;11:130415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Association of Diabetes in Pregnancy Study Group Consensus Panel, Metzger BE, Gabbe SG, Persson B, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara A. Increasing prevalence of gestational diabetes: A public health perspective. Diabetes Care 2007;30:S141–146 [DOI] [PubMed] [Google Scholar]
- 8.ACOG Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin. diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol 2002;99:159–167 [DOI] [PubMed] [Google Scholar]
- 9.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000;183:S1–S22 [PubMed] [Google Scholar]
- 10.Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol 2003;102:181–192 [DOI] [PubMed] [Google Scholar]
- 11.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens 2008;21:521–526 [DOI] [PubMed] [Google Scholar]
- 12.Zhang T, Zeisler J, Hatch MC, Berkowitz G. Epidemiology of pregnancy-induced hypertension. Epidemiol Rev 1997;19:218–232 [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy 2003;22:203–212 [DOI] [PubMed] [Google Scholar]
- 14.Chang J, Elam-Evans LD, Berg CJ, et al. Pregnancy-related mortality surveillance—United States, 1991–1999. MMWR Surveill Summ 2003;52:1–8 [PubMed] [Google Scholar]
- 15.Persson B, Hanson U. Neonatal morbidities in gestational diabetes mellitus. Diabetes Care 1998;21:B79–84 [PubMed] [Google Scholar]
- 16.Di Cianni G, Lencioni C, Volpe L, et al. C-reactive protein and metabolic syndrome in women with previous gestational diabetes. Diabetes Metab Res Rev 2007;23:135–140 [DOI] [PubMed] [Google Scholar]
- 17.Kessous R, Shoham-Vardi I, Pariente G, Sherf M, Sheiner E. An association between gestational diabetes mellitus and long-term maternal cardiovascular morbidity. Heart 2013;99:1118–1121 [DOI] [PubMed] [Google Scholar]
- 18.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: A systematic review and meta-analyses. Am Heart J 2008;156:918–930 [DOI] [PubMed] [Google Scholar]
- 20.Ratner RE, Christophi CA, Metzger BE, et al. Prevention of diabetes in women with a history of gestational diabetes: Effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93:4774–4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002;25:1862–1868 [DOI] [PubMed] [Google Scholar]
- 22.Ferrara A, Ehrlich SF. Strategies for diabetes prevention before and after pregnancy in women with GDM. Curr Diabetes Rev 2011;7:75–83 [DOI] [PubMed] [Google Scholar]
- 23.Azen SP, Peters RK, Berkowitz K, Kjos S, Xiang A, Buchanan TA. TRIPOD (TRoglitazone in the prevention of diabetes): A randomized, placebo-controlled trial of troglitazone in women with prior gestational diabetes mellitus. Control Clin Trials 1998;19:217–231 [DOI] [PubMed] [Google Scholar]
- 24.Bennett WL, Chang HY, Levine DM, et al. Utilization of primary and obstetric care after medically complicated pregnancies: An analysis of medical claims data. J Gen Intern Med 2014;29:636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim C, Tabaei BP, Burke R, et al. Missed opportunities for type 2 diabetes mellitus screening among women with a history of gestational diabetes mellitus. Am J Public Health 2006;96:1643–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell MA, Phipps MG, Olson CL, Welch HG, Carpenter MW. Rates of postpartum glucose testing after gestational diabetes mellitus. Obstet Gynecol 2006;108:1456–1462 [DOI] [PubMed] [Google Scholar]
- 27.Bryant AS, Haas JS, McElrath TF, McCormick MC. Predictors of compliance with the postpartum visit among women living in Healthy Start Project areas. Matern Child Health J 2006;10:511–516 [DOI] [PubMed] [Google Scholar]
- 28.Chu SY, Callaghan WM, Shapiro-Medndoza CD, et al. Postpartum care visits: 11 states and New York City, 2004. MMWR 2007;756:1312–1316 [PubMed] [Google Scholar]
- 29.Dooley SL, Metzger BE, Cho NH. Gestational diabetes mellitus. Influence of race on disease prevalence and perinatal outcome in a U.S. population. Diabetes 1991;40:25–29 [DOI] [PubMed] [Google Scholar]
- 30.Miranda ML, Swamy GK, Edwards S, Maxson P, Gelfand A, James S. Disparities in maternal hypertension and pregnancy outcomes: Evidence from North Carolina, 1994–2003. Public Health Rep 2010;125:579–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett WL, Ennen CS, Carrese JA, et al. Barriers to and facilitators of postpartum follow-up care in women with recent gestational diabetes mellitus: A qualitative study. J Womens Health (Larchmt) 2011;20:239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidson RA, Giancola A, Gast A, et al. Evaluation of access, a primary care program for indigent patients: inpatient and emergency room utilization. J Community Health 2003;28:59–64 [DOI] [PubMed] [Google Scholar]
- 33.Rust G, Ye J, Baltrus P, et al. Practical barriers to timely primary care access: Impact on adult use of emergency department services. Arch Intern Med 2008;168:1705–1710 [DOI] [PubMed] [Google Scholar]
- 34.Hansagi H, Olsson M, Sjoberg S, Tomson Y, Goransson S. Frequent use of the hospital emergency department is indicative of high use of other health care services. Ann Emerg Med 2001;37:561–567 [DOI] [PubMed] [Google Scholar]
- 35.Brook RH, Stevenson RL., Jr. Effectiveness of patient care in an emergency room. N Engl J Med 1970;283:904–907 [DOI] [PubMed] [Google Scholar]
- 36.Brook RH, Berg MH, Schechter PA. Effectiveness of nonemergency care via an emergency room. A study of 116 patients with gastrointestinal symptoms. Ann Intern Med 1973;78:333–339 [DOI] [PubMed] [Google Scholar]
- 37.Hamilton MS, Brooten D, Youngblut JM. High-risk pregnancy: Postpartum rehospitalization. J Perinatol 2002;22:566–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark SL, Belfort MA, Dildy GA, et al. Emergency department use during the postpartum period: Implications for current management of the puerperium. Am J Obstet Gynecol 2010;203:38.e1–e6 [DOI] [PubMed] [Google Scholar]
- 39.Vinton DT, Capp R, Rooks SP, Abbott JT, Ginde AA. Frequent users of US emergency departments: Characteristics and opportunities for intervention. Emerg Med J 2014. DOI: 10.1136/emermed-2013-202407 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.LaCalle E, Rabin E. Frequent users of emergency deparments: The myths, the data and the policy implications. Ann Emerg Med 201;56:42–48 [DOI] [PubMed] [Google Scholar]
- 41.Lydon-Rochelle M, Holt VL, Martin DP, Easterling TR. Association between method of delivery and maternal rehospitalization. JAMA 2000;283:2411–2416 [DOI] [PubMed] [Google Scholar]
- 42.https://mmcp.dhmh.maryland.gov/healthchoice/SitePages/Home.aspx Accessed January18, 2015
- 43.Maryland Department of Health and Mental Hygiene. HealthChoice. Available at: https://mmcp.dhmh.maryland.gov/healthchoice/Documents/2014%20Health%20Choice%20Evaluation.pdf Accessed January18,2015
- 44.The Dartmouth Atlas of Healthcare. Available at: www.dartmouthatlas.org/tools/downloads.aspx?tab=37 Accessed November12, 2013
- 45.National Committee for Quality Assurance (NCQA). Prenatal and postpartum care (PPC) definitions, 1994–2009. Available at: www.ncqa.org/portals/0/Prenatal%20Postpartum%20Care.pdf Accessed July30, 2013
- 46.Center for Medicare and Medicaid Services. ICD-9 code lists. Available at: www.cms.gov/medicare-coverage-database/staticpages/icd-9-code-lookup.aspx Accessed July30, 2013
- 47.Bliss R, Weinberg J, Vieira V, Webster T. Detecting confounding and evaluating the 10% rule. 2011. Joint Statistical Meetings. Brigham and Women's Hospital and Boston University School of Public Health; Available at: www.amstat.org/meetings/jsm/2011/onlineprogram/AbstractDetails.cfm?abstractid=302094 Accessed May22, 2014 [Google Scholar]
- 48.Hunt KA, Weber EJ, Showstack JA. Characteristics of frequent users of emergency departments. Ann Emerg Med 2006;48:1–8 [DOI] [PubMed] [Google Scholar]
- 49.Friedman B, Basu J. The rate and cost of hospital readmissions for preventable conditions. Med Care Res Rev 2004;61:225–240 [DOI] [PubMed] [Google Scholar]
- 50.Mistiaen P, Francke AL, Poot E. Interventions aimed at reducing problems in adult patients discharged from hospital to home: A systematic meta-review. BMC Health Serv Res 2007;7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas JW, Holloway JJ. Investigating early readmission as an indicator for quality of care studies. Med Care 1991;29:377–394 [DOI] [PubMed] [Google Scholar]
- 52.Kossovsky MP, Sarasin FP, Perneger TV, Chopard P, Sigaud P, Gaspoz J. Unplanned readmissions of patients with congestive heart failure: Do they reflect in-hospital quality of care or patient characteristics? Am J Med 2000;109:386–390 [DOI] [PubMed] [Google Scholar]
- 53.Weiss ME, Ryan P, Lokken L. Validity and reliability of the perceived readiness for discharge after birth scale. J Obstet Gynecol Neonatal Nurs 2006;35:34–45 [DOI] [PubMed] [Google Scholar]
- 54.Brooten D, Youngblut JM, Brown L, Finkler SA, Neff DF, Madigan E. A randomized trial of nurse specialist home care for women with high-risk pregnancies: Outcomes and costs. Am J Manag Care 2001;7:793–803 [PMC free article] [PubMed] [Google Scholar]
- 55.Olds DL, Kitzman H, Cole R, et al. Effects of nurse home-visiting on maternal life course and child development: Age 6 follow-up results of a randomized trial. Pediatrics 2004;114:1550–1559 [DOI] [PubMed] [Google Scholar]
- 56.O'Sullivan AL, Jacobsen BS. A randomized trial of a health care program for first-time adolescent mothers and their infants. Nurs Res 1992;41:210–215 [PubMed] [Google Scholar]
- 57.Olds DL, Henderson CR, Jr, Chamberlin R, Tatelbaum R. Preventing child abuse and neglect: A randomized trial of nurse home visitation. Pediatrics 1986;78:65–78 [PubMed] [Google Scholar]
- 58.U.S. Department of Health and Human Services. Health Resources and Services Administration: Maternal and Child Health Segment. Maternal, infant and early childhood home visiting program. Available at http://mchb.hrsa.gov/programs/homevisiting Accessed June10, 2014
- 59.Committee on Health Care for Underserved Women. American College of Obstetricians and Gynecologists. Benefits to women of Medicaid expansion through the Affordable Care Act. Committee Opinion No. 552. Obstet Gynecol 2013;121:223–225 [DOI] [PubMed] [Google Scholar]
- 60.Center on Budget and Policy Priorities. Expanding Medicaid will benefit both low income women and their babies. April 17, 2013. Available at: www.cbpp.org/files/Fact-Sheet-Impact-on-Women.pdf Accessed August28, 2014
- 61.Salganicoff A, Ranji U, Beamesderfer A, Kurani N. Women and health care in the early years of the Affordable Care Act: Key findings from the 2013 Kaiser Women's Health Survey. Available at: http://kff.org/womens-health-policy/report/women-and-health-care-in-the-early-years-of-the-aca-key-findings-from-the-2013-kaiser-womens-health-survey Accessed November11, 2014
- 62.Families USA. Description of Medicaid expansions by state. Available at: www.familiesusa.org/resources/state-information/expansions/maryland-expansion.html Accessed August28, 2013
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.