Abstract
Perinatal generalized anxiety disorder (GAD) has a high prevalence of 8.5%–10.5% during pregnancy and 4.4%–10.8% postpartum. Despite its attendant dysfunction in the patient, this potentially debilitating mental health condition is often underdiagnosed. This overview will provide guidance for clinicians in making timely diagnosis and managing symptoms appropriately. A significant barrier to the diagnosis of GAD in the perinatal population is difficulty in distinguishing normal versus pathological worry. Because a perinatal-specific screening tool for GAD is nonexistent, early identification, diagnosis and treatment is often compromised. The resultant maternal dysfunction can potentially impact mother–infant bonding and influence neurodevelopmental outcomes in the children. Comorbid occurrence of GAD and major depressive disorder changes the illness course and its treatment outcome. Psychoeducation is a key component in overcoming denial/stigma and facilitating successful intervention. Treatment strategies are contingent upon illness severity. Cognitive behavior therapy (CBT), relaxation, and mindfulness therapy are indicated for mild GAD. Moderate/severe illness requires pharmacotherapy and CBT, individually or in combination. No psychotropic medications are approved by the FDA or Health Canada in pregnancy or the postpartum; off-label pharmacological treatment is instituted only if the benefit of therapy outweighs its risk. SSRIs/SNRIs are the first-line treatment for anxiety disorders due to data supporting their efficacy and overall favorable side effect profile. Benzodiazepines are an option for short-term treatment. While research on atypical antipsychotics is evolving, some can be considered for severe manifestations where the response to antidepressants or benzodiazepines has been insufficient. A case example will illustrate the onset, clinical course, and treatment strategies of GAD through pregnancy and the postpartum.
Introduction
The nomenclature of generalized anxiety disorder (GAD) has evolved since Freud first described occurrence of chronic, free-floating anxiety1 over a century ago. With the introduction of the Diagnostic and Statistical Manual of Mental Disorders, third edition (DSM-III) in 1980, GAD became a clear psychiatric condition. Changes to the classification of GAD continued with updated empirical evidence in DSM-IV-TR,2 which focused on the characteristics of excessive and uncontrollable worry. In the DSM-5,3 the diagnostic criteria for GAD remained largely unchanged with only minimal re-organization of some criteria. While awareness of GAD in general population has increased among clinicians, the illness has received little attention in the perinatal population.
Prevalence of GAD
Prevalence of GAD during pregnancy varies between 8.5% and 10.5%,4–6 which is as high as or higher than in the general population (1.2% to 6.4%).7 However, in the postpartum period there appears to be a greater variance in the reported prevalence of GAD, between 4.4% and 10.8%.8–11 This difference in rates is contingent on recruitment strategies, methodology, and presence of a control group. Studies show subjects selected from a hospital outpatient population display a higher rate of GAD versus those from wide scale epidemiological surveys.
Although specific risk factors for development of perinatal GAD have not been established, in clinical practice the disease appears to manifests in women who are excessive worriers, have a positive family history of anxiety disorders, and suffer from other anxiety or mood disorders.
Clinical Presentation in Perinatal Period
GAD is a clinical entity distinct from feelings of anxiety associated with postpartum depression; GAD is characterized by excessive, uncontrollable worry that can cause functional impairment. Despite the frequent manifestation of GAD during the perinatal period, the DSM specifier “with peripartum onset” is presently designated only for mood disorders, not for anxiety disorders.3 Applying the DSM-5 criteria for the general population to pregnant/postpartum women potentially excludes those consumed with excessive worries for less than 6 months. Some researchers therefore define perinatal GAD if the illness meets other DSM criteria for a minimum duration of one month.5,10
Problematically for diagnosis, physical symptoms of GAD such as fatigue, irritability, tension, concentration difficulties, and insomnia may be mistaken as normal in pregnancy and the postpartum. Distinguishing normal pregnancy-related concerns from pathological worry, particularly in first trimester primiparous women, can also make diagnosing perinatal GAD difficult.12 These worries become disabling if they are recurrent, time-consuming, intrusive, and acquire a quality of irrationality. The general themes of perinatal worries revolve around: (1) fears of fetal wellbeing, (2) maternal wellness, (3) illness in the partner, and (4) parental mortality. If these worry symptoms persist for an extended period of time they can cause functional impairment in every area of the mother's life.
Consequences of GAD in Mother and Infant
GAD specific studies in the perinatal population remain sparse; however, several studies have addressed the impact of anxiety symptoms not diagnosed as GAD.13–16
Stein and colleagues17 found that recurrent negative thinking in mothers with GAD resulted in their being less responsive and engaged in interactions with their infants. In addition, these infants appeared to be withdrawn and were more likely to display lowered emotional tone. In a study by Arteche et al.,18 women suffering from postpartum depression or GAD were less likely to identify happy infant faces accurately due to excessive maternal worry.
Women diagnosed with GAD at 10 weeks after childbirth were found to report sexual fear, avoidance, and body image self-consciousness compared with women without the illness. This group of women was sensitive to experiencing distress but not dysfunction in their sexual relationships.19
Few studies have investigated the impact of maternal GAD on short- and long-term child development outcome; the available data suggests that maternal GAD negatively impacts neurodevelopment and growth of the infant. Uguz and colleagues20 found that maternal GAD during pregnancy led to significantly lower levels of fetal brain-derived neurotrophic factor potentially negatively impacting the neurodevelopment of the fetus.
A recent meta-analysis and systematic review suggested that there was a modest but statistically significant association between maternal anxiety during pregnancy and an increased risk of preterm birth and low birth weight.21 The occurrence of low birth weight and preterm birth has also been found to be a risk factor in itself for the development of GAD in later years.22
Several studies have shown a link between stress in utero and adverse neurobehavioral outcomes in the neonatal period. This correlation has been proposed to be a result of dysregulation of the hypothalamic–pituitary axis of the mother and the offspring.23 Although not specific to GAD, the relationship between maternal anxiety/stress and its negative impact on child development has been extensively studied. Consequences include: negative infant behavioral reactivity at 4 months,13 difficult infant temperament at 4/6 months,14 lower mental development at age 2 years,15 negative affectivity at 2 years,16 lower inhibitory control in girls, and lower visuospatial working memory in boys and girls aged 6–9 years.24
Comorbidity with GAD During Pregnancy and Postpartum
While coexistence of perinatal GAD with depression has been well studied, its association with other anxiety disorders and substance use disorders has limited data.
Grigoriadis and colleagues25 found a high comorbid depression rate of 49.5% in perinatal women with GAD. Comorbidity with other anxiety disorders included: OCD (4.4%), agoraphobia (8.8%), panic disorder (9.9%), and phobia (19.8%). In a study by Wenzel et al.26 75% of postpartum women with GAD also met criteria for a depression.
A longitudinal study by Reck et al.27 supported the observation that women with anxiety disorders often display accompanying symptoms of depression, and vice-versa. Comorbidity between anxiety and depressive disorders result in a more severe and protracted course of illness.28 In the general population, anxiety disorders are considered significant risk factors for the onset of depressive disorders.29–31 Coelho et al.32 found that anxiety disorders in pregnancy independently predicted a threefold increase in the development of postpartum depression up to two years postpartum. The sequential occurrence of GAD and depression throughout the perinatal period follows an interesting pattern. While antenatal GAD has been recognized to be a risk factor for development of postpartum depression, this relationship appears to be bidirectional;33 midtrimester depression has also been found to be related to anxiety in late pregnancy.34 Collectively, these studies support the observation that women with anxiety disorders often display accompanying symptoms of depression, and vice-versa.
Arch35 found that pregnancy anxiety was the strongest predictor of alcohol consumption in the antenatal period. Specific domains of pregnancy anxiety that accounted for elevated drinking risk were fears of bearing a handicapped child and fears of pregnancy-related impact on one's appearance.
Patient Engagement in Treatment Planning
Explaining to patients and their families that symptoms of worry might represent an illness is not easy, as the patient may experience disbelief, embarrassment, or shame related to their emotional turmoil, while dysfunction resulting from all-consuming worry means their insight into their suffering is diminished. Untreated excessive worry over time can lead the patient to be apprehensive of situations that produce normal anxiety.36 One way in which patients cope is by intensely focusing on controlling the external environment, including the suggested treatment.
Managing GAD patients agreeing to treatment is often complex due to the limited availability of accessible and affordable psychotherapy resources. The use of medication in pregnancy is at best controversial. The National Institute for Health and Care Excellence has published an overview of anxiety disorders in perinatal women and clinical management and service guidelines for antenatal and postnatal mental health, both including sections on GAD.37,38 Generally, the management plan is determined by consensual agreement to treatment between the patient and the clinician.
Case Example of Perinatal GAD
Thirty-four year old Tiffany, a married teacher, was referred by her family doctor to the Reproductive Mental Health Program at 18 weeks gestation with excessive, irrational worry that occupied 80%–90% of her time almost every day, with varying intensity. Her worries focused on: (1) the baby's development, (2) birth-related fear, (3) her ability to mother. Additional symptoms included increased irritability, restlessness, and fatigue; she met the DSM-5 criteria for GAD.
During her first pregnancy with Jack, her 18-month-old toddler, Tiffany received four sessions of cognitive behavioral therapy (CBT) for illogical worries with regard to Jack's welfare. After birth, a trial of selective serotonin reuptake inhibitor (SSRI) resulted in remission of GAD. She discontinued medication after conceiving unexpectedly; her worries gradually resurfaced.
Follow-Up of the Case Study
Tiffany was assessed by a psychiatrist and administered the GAD-7 and confirmed the diagnosis of GAD in second pregnancy. Tiffany struggled with the idea of worries being diagnosed as an illness. At a follow-up appointment with her husband Mark 2 weeks later, the challenges and struggles of Tiffany became clearer. High irritability, short fuse, and lack of patience resulted in frequent arguments between the couple; her interactions with Jack became a concern.
After discussing treatment options with Mark and Tiffany, the couple opted to seek CBT from a Psychologist rather than restart medication during pregnancy because of worries about potential adverse effects on the growing fetus. The CBT helped her cope fairly well through the pregnancy. The birth was uneventful with delivery of a healthy, 8-pound newborn.
In the postpartum period, one month later, symptoms of excessive worry returned accompanied by depressive mood. At three months postpartum, Tiffany could no longer nurse; the symptoms continued to escalate. She began an antidepressant trial combined with the CBT sessions, which led to full remission of mood symptoms, though she continued to experience manageable levels of anxiety.
Diagnosing and Screening for GAD During Pregnancy and Postpartum
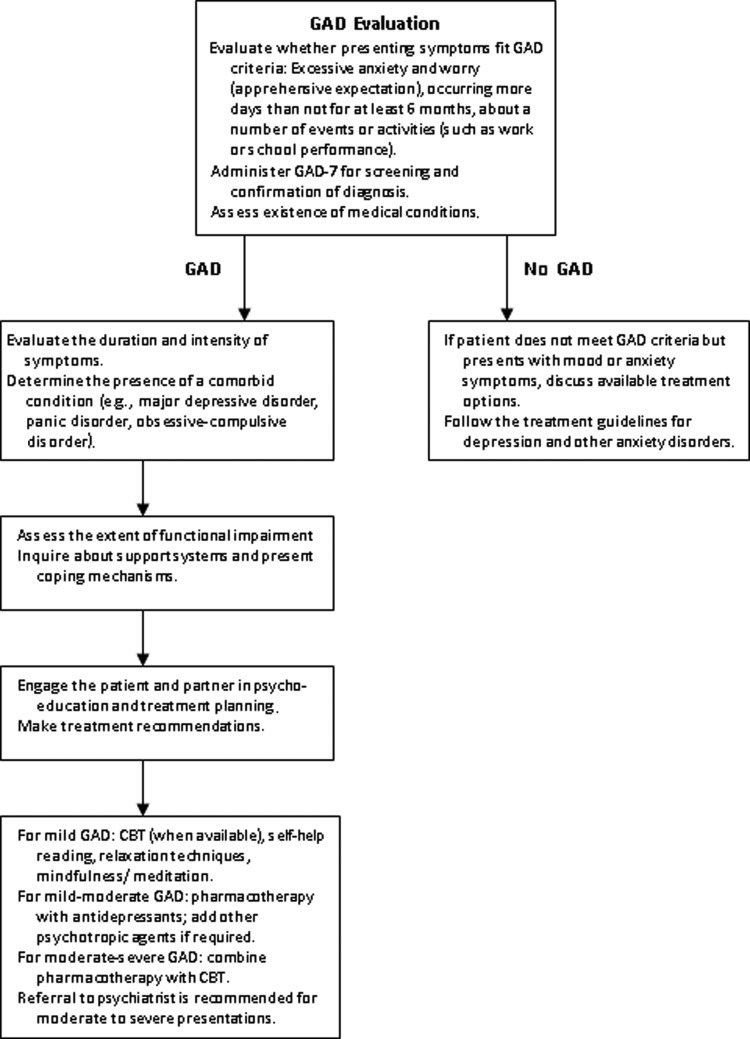
Specific screening tools to diagnose perinatal GAD do not appear to exist.39 The most commonly validated subjective measures in perinatal population are the General Health Questionnaire,40 State-Trait Anxiety Inventory,41 and the Hospital Anxiety and Depression Scales42 and GAD-7.43 Other screening measures for non-GAD anxiety include the Postpartum Worry Scale,10 the Pregnancy Anxiety Scale,44 and the Perinatal Anxiety Screening Scale.45 In clinical practice, healthcare providers rely on a diagnostic interview and available anxiety tools for confirming the diagnosis. Although the GAD-743 is not validated for perinatal population, this screening tool appears to capture the symptomatology and severity of the illness in pregnant and postpartum mothers. We recommend antenatal GAD screening around 28–30 weeks, when depression screening is often done routinely. A repeat screening with GAD-7 can be undertaken at 6-8 weeks postpartum, at a standard postpartum visit. An algorithm for evaluating and treating GAD is presented in Figure 1.
FIG. 1.
Clinical algorithm for treatment of generalized anxiety disorder (GAD). CBT, cognitive behavioral therapy.
Pharmacological Treatment of Perinatal Generalized Anxiety Disorder
Cognitive-behavioral therapies are the first-line choice for the treatment of mild to moderate perinatal GAD. However, in moderate to severe cases, pharmacological treatment should be considered. While medication treatments have potential risks, they must be weighed against the risks of untreated maternal anxiety and its consequences for the developing infant.
Possible risks associated with pharmacotherapy in the perinatal period include spontaneous abortion, major fetal malformations, acute neonatal toxicity, long-term neurobehavioral effects, and drug transmission to the infant via breast milk.46 For these reasons, health care practitioners must take particular care to prescribe those medications believed to have an appropriate safety profile for use in pregnant and lactating women. Pharmacotherapy in the perinatal period should favor medications with the least transmission across the placenta and with minimal active metabolites.47 Doses should begin as low as possible and should be titrated upward as necessary. Monotherapy is preferred if possible. Sudden discontinuation should be avoided.
Antidepressants
This class of medication has not been rigorously tested in the peripartum population with controlled studies, for ethical and practical reasons; extant data are primarily based on retrospective case studies, or other observational or population-based research. SSRIs are the most frequently used class of antidepressant medications in general and during pregnancy.48 Their use in nursing mothers is more frequent compared to other antidepressants. Tricyclic antidepressants and monoamine oxidase inhibitors are not generally prescribed, primarily because of their less favorable side-effect profile.49
Research in the perinatal population suggests that SSRIs and serotonin–norepinephrine reuptake inhibitors (SNRIs) cross the placenta, with 70%–86% of the maternal dosage being transferred to the infant.50 Several reviews and meta-analyses have not found compelling evidence that SSRI/SNRIs as a class are generally associated with birth defects.51–43 The exception is first-trimester exposure to paroxetine, which has been correlated with cardiac malformations,54 although a direct causal relationship has not been established. Some researchers suggest that risk increases only at high dosage,55 while others have found no relationship between paroxetine use during pregnancy and congenital malformation.56 In light of conflicting data, our recommendation is to avoid paroxetine exposure in women planning pregnancies. There are also accounts of other SSRI-related malformations such as hypertrophic stenosis, omphalocele, and neural tube defects, although evidence is limited.57,58 There have been small but significant associations between the use of SSRI/SNRIs and spontaneous abortions, as well as shorter gestational age, preterm delivery, and lower APGAR scores in neonates.59–61 Additionally, an increased risk of persistent pulmonary hypertension of the newborn has been associated with third trimester SSRI/SNRI exposure;62 the absolute risk, however, is small—3 to 4 cases per thousand.63 Third trimester exposure has been related to neonatal withdrawal symptoms, such as jitteriness, irritability, tremulousness, difficulty feeding, difficulty sleeping, hypertonia, and other adverse symptoms such as seizures. These withdrawal effects are common, appearing in 30% of infants exposed to SSRIs, but they are generally mild, transient, and self-limiting.64 They appear to be particularly associated with the use of paroxetine.65
It is worth noting that in many cases, these correlations between SSRI/SNRI usage and adverse effects are confounded by underlying maternal depression, thereby precluding any firm causal conclusions from being drawn. For example, SSRI/SNRI use is associated with an increased rate of preterm birth; however, there is evidence that preterm birth rates are equivalently elevated in women with untreated depression, suggesting that underlying depression, and not SSRI/SNRI exposure, may ultimately be responsible for the observed association.66
The current data do not, on the whole, suggest that SSRI/SNRIs are related to negative long-term neurobehavioral effects; children exposed to antidepressants have not consistently been found to differ on cognitive, motor, or behavioral outcomes relative to nonexposed controls.67–69 Recently, some investigations have found an association between autism and prenatal antidepressant exposure,70,71 while other large-scale studies have failed to detect such a connection.72,73 Further research into this correlation is needed.
The use of SSRI/SNRIs during breastfeeding also requires careful consideration. Infant exposure to SSRIs via breast milk has generally been associated with low or negligible levels of compounds in infant serum;74 mild transient symptoms after breastfeeding have been reported, but only in isolated case studies.75 Of the SSRI/SNRIs, sertraline is preferred for breast-feeding women, as it has a low milk/plasma ratio and relative infant dose.76 Similarly, paroxetine has a very low milk/plasma ratio; preliminary data also suggest that escitalopram has a low relative infant dose and is largely undetectable in the infant after feeding.77
Overall, treatment of perinatal GAD with SSRI/SNRIs should be determined on a case-by-case basis. Patients with prior history of SSRI/SNRI intake should continue on the drug they have been taking in order to reduce the probability of relapse, with the exception perhaps of paroxetine, due to the potential cardiac risks to the fetus. For those women beginning pharmacological treatment for the first time, an SSRI/SNRI with evidence for efficacy in GAD should be chosen. In addition, preference among these may be based on which possess a short half-life, a low accumulation in breast milk, a track record of safety in pregnancy, and a low ratio of cord to serum drug concentration.
Benzodiazepines
The class of benzodiazepines is among the most commonly used prescription drug among pregnant women.77 They appear effective at rapidly treating short-term anxiety, particularly its somatic symptoms.78 Because antidepressants require several weeks before the onset of their action, benzodiazepines can be used for brief, pro re nata treatment in order to produce an immediate anxiolytic response. Despite their usefulness, there is serious concern about cognitive impairment with long-term use of benzodiazepines.79 Extended use of this class of drug is associated with a substantial risk of dependence, withdrawal, and abuse.80,81
In pregnant women, with regard to teratogenicity, earlier studies of benzodiazepines tend to report an association with oral clefts;82 however, later research generally failed to find this association.83 Their use in pregnancy can elevate the risk of neonatal withdrawal symptoms such as hypertonia, hyperreflexia, tremors, bradycardia, and sleep disturbance.84,85 Third trimester exposure has also been associated with the “floppy infant syndrome,” characterized by low APGAR scores, hypothermia, muscular hypotonia, and sluggish response to cold temperatures.37,85 Because of these risks, benzodiazepines should be particularly avoided in the third trimester. Lorazepam may be favored over other benzodiazepines due to its low accumulation in fetal tissue.47 Excretion of benzodiazepines in breast milk appears to be low in general;37 however, one should consider avoiding diazepam due to its active metabolites and long-lived presence in breast milk.86,87
Atypical antipsychotics
This class of medication is generally not used as a primary modality of treatment for GAD. In nonperinatal population, these medications are prescribed to augment other pharmacologic agents for severe forms of the illness when response to other types of pharmacotherapy has been insufficient. While meta-analyses do not support augmentation for GAD with olanzapine, risperidone, and ziprasidone,81 in preliminary studies, newer atypicals such as quetiapine and aripiprazole seem to show promise.88,89 Side effects related to atypical antipsychotics include extrapyramidal symptoms, weight gain, diabetes, hyperlipidemia, and a prolonged QTc interval.90
Research into the perinatal use of newer antipsychotics is so far confined to case studies. Atypical antipsychotics have been associated with an increased probability of therapeutic abortions as well as low neonatal birth weight.91 In one study, atypical antipsychotic usage has been linked to an increased risk of congenital malformation, gestational diabetes, and caesarean section delivery.92 No long-term studies track the effects of atypical antipsychotics on infant neurodevelopment.93
Nonpharmacological Treatment Options
Psychotherapeutic approaches to GAD are vital in targeting cognitive processes underlying the excessive generalized worrying that debilitate the sufferer. The goal of psychotherapy in perinatal women with GAD is to reduce the overall level of autonomic arousal, lessen the mother's concern over her worries, and provide support in reducing her worries to a more reasonable level.
Limitations to accessing appropriate psychotherapy are generally due to high cost of therapy. In addition, it is difficult to find a trained therapist who specializes in perinatal mental health. In general, pregnant women tend to prefer treatment with psychotherapy over pharmacotherapy, as pharmacological agents usually add another layer of worry for the mother.94,95 In one study, preference for psychotherapy alone was stronger among pregnant (74%) than nonpregnant (47%) women; both groups of women rated exposure-based CBT more favorably than pharmacotherapy.94
Cognitive behavioral therapy
CBT is a form of psychotherapy focused on changing maladaptive patterns of thinking and behavior. The effectiveness of CBT in the treatment of anxiety disorders, and especially GAD, has been well established in the general population,96–98 with improvement rates estimated between 34% and 68%.99 A study showed that a group CBT intervention for antenatal mothers with mild to moderate anxiety symptoms resulted in significantly decreased levels of anxiety; the effect was sustained into the postpartum period.100 However, researchers have cautioned that CBT's overall effects are still clinically modest; patients receiving CBT could still require further treatment in order to reach remission.99 Despite evidence that CBT is promising, this treatment can be difficult to implement due to the lack of easy accessibility.
Mindfulness training
Based on meditation techniques, mindfulness training is the practice of awareness and attention exercises focused on accepting one's present state of emotions, thoughts, and physical sensations. During the perinatal period this has been shown to significantly reduce negative affect, state anxiety, pregnancy-specific anxiety, and stress in nonclinical populations.101–103 An evaluation of group mindfulness-based cognitive therapy for perinatal anxiety showed statistically and clinically significant improvements in anxiety, worry, and depression, as well as a significant increase in self-compassion and mindfulness.104 Additionally, in this study 94% of women with GAD no longer met diagnostic criteria posttreatment.
Relaxation techniques
Activites such as progressive muscle relaxation and diaphragmatic breathing are an integral part of many CBT-oriented treatments for GAD.105 In a randomized controlled trial (RCT), Bastani and colleagues106 showed that applied relaxation training significantly reduced anxiety and perceived stress among pregnant women compared to those who received routine prenatal care. In another RCT, guided imagery and progressive muscle relaxation was found to be most effective in enhancing levels of relaxation and decreasing heart rate.107 Hoyer et al.108 compared worry exposure with applied relaxation in the treatment of GAD and results showed that the treatments were equally effective. However, a recent meta-analysis indicated that CBT may be more effective in treatment of GAD than applied relaxation.97
Psychoeducation
Psychoeducation provides information about both the disorder and treatment rationale. This is often the most important initial intervention and frequently improves adherence to treatment. It is inexpensive, easy to implement, and can be applied immediately by any clinician.109 However, because there is no objective measure of anxiety in pregnancy, clinicians often find it challenging to engage a pregnant patient in psychoeducation, as women who have worried their entire lives may not recognize their experience as pathological. Furthermore, GAD can be hard to separate from personality traits. Individuals who are perfectionistic and highly persistent are more likely to have anxiety disorders than mood disorders.110 These individuals are high functioning, organized, and prefer to feel in control of their surroundings.
Conclusion and Future Perspectives
Marcé in 1858111 first described psychological symptoms in perinatal women in “Treatise on Insanity in Pregnant, Postpartum, and Lactating Women.” Prior to the inclusion of the postpartum onset specifier in DSM-IV, clinicians struggled to advocate for treatment and research related to perinatal women with mood disorders. Similarly, until peripartum GAD is formally classified in the DSM, its status will remain questionable. Without a defined classification of this condition, researchers are unable to investigate this illness in a methodologically consistent way, and it may continue to be under-recognized in clinical practice. This review attempts to increase the awareness and knowledge of perinatal GAD to the readership and to familiarize them to the unique aspects of this challenging clinical disorder.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Freud S. Inhibitions, symptoms and anxiety. In: Strachey J, eds. The standard edition of the complete psychological works of Sigmund Freud, volume 2 London: Hogarth Press, 1959:77–174 [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. (DSM-IV-TR). Arlington, VA: American Psychiatric Association, 2000 [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th ed. (DSM-5). Arlington, VA: American Psychiatric Association, 2013 [Google Scholar]
- 4.Adewuya AO, Ola BA, Aloba OO, Mapayi BM. Anxiety disorders among Nigerian women in late pregnancy: A controlled study. Arch Womens Ment Health 2006;9:325–328 [DOI] [PubMed] [Google Scholar]
- 5.Buist A, Gotman N. Generalized anxiety disorder: Course and risk factors in pregnancy. J Affect Disord 2011;131:277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutter-Dallay AL, Giaconne-Marcesche V, Glatigny-Dallay E, Verdoux H. Women with anxiety disorders during pregnancy are at increased risk of intense postnatal depressive symptoms: A prospective survey of the MATQUID cohort. Eur Psychiatry 2004;19:459–463 [DOI] [PubMed] [Google Scholar]
- 7.Hoge EA, Oppenheimer JE, Simon NM. Generalized anxiety disorder. FOCUS 2004;2:346–359 [Google Scholar]
- 8.Phillips J, Sharpe L, Matthey S. Rates of depressive and anxiety disorders in a residential mother-infant unit for unsettled infants. Aust N Z J Psychiatry 2007;41:836–842 [DOI] [PubMed] [Google Scholar]
- 9.Navarro P, García-Esteve L, Ascaso C, Aguado J, Gelabert E, Martín-Santos R. Non-psychotic psychiatric disorders after childbirth: Prevalence and comorbidity in a community sample. J Affect Disord 2008;109:171–176 [DOI] [PubMed] [Google Scholar]
- 10.Wenzel A, Haugen EN, Jackson LC, Robinson K. Prevalence of generalized anxiety at eight weeks postpartum. Arch Womens Ment Health 2003;6:43–49 [DOI] [PubMed] [Google Scholar]
- 11.Rowe HJ, Fisher JRW, Loh WM. The Edinburgh Postnatal Depression Scale detects but does not distinguish anxiety disorders from depression in mothers of infants. Arch Womens Ment Health 2008;11:103–108 [DOI] [PubMed] [Google Scholar]
- 12.Weisberg RB, Paquette JA. Screening and treatment of anxiety disorders in pregnant and lactating women. Womens Health Issues 2002;12:32–36 [DOI] [PubMed] [Google Scholar]
- 13.Davis EP, Snidman N, Wadhwa PD, Glynn LM, Schetter CD, Sandman CA. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy 2004;6:319–331 [Google Scholar]
- 14.Austin MP, Hadzi-Pavlovic D, Leader L, Saint K, Parker G. Maternal trait anxiety, depression and life event stress in pregnancy: Relationships with infant temperament. Early Hum Dev 2005;81:183–190 [DOI] [PubMed] [Google Scholar]
- 15.Brouwers EPM, Baar AL. Van, Pop VJM. Maternal anxiety during pregnancy and subsequent infant development. Infant Behav Dev 2001;24:95–106 [Google Scholar]
- 16.Blair MM, Glynn LM, Sandman CA, Davis EP. Prenatal maternal anxiety and early childhood temperament. Stress 2011;14:644–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein A, Craske MG, Lehtonen A, et al. Maternal cognitions and mother-infant interaction in postnatal depression and generalized anxiety disorder. J Abnorm Psychol 2012;121:795–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arteche A, Joormann J, Harvey A, et al. The effects of postnatal maternal depression and anxiety on the processing of infant faces. J Affect Disord 2011;133:197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenzel A, Haugen EN, Goyette M. Sexual adjustment in postpartum women with generalized anxiety disorder. J Reprod Infant Psychol 2005;23:365–366 [Google Scholar]
- 20.Uguz F, Sonmez EO, Sahingoz M, et al. Maternal generalized anxiety disorder during pregnancy and fetal brain development: A comparative study on cord blood brain-derived neurotrophic factor levels. J Psychosom Res 2013;75:346–350 [DOI] [PubMed] [Google Scholar]
- 21.Ding X-X, Wu Y-L, Xu S-J, et al. Maternal anxiety during pregnancy and adverse birth outcomes: A systematic review and meta-analysis of prospective cohort studies. J Affect Disord 2014;159:103–110 [DOI] [PubMed] [Google Scholar]
- 22.Vasiliadis H-M, Buka SL, Martin LT, Gilman SE. Fetal growth and the lifetime risk of generalized anxiety disorder. Depress Anxiety 2010;27:1066–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mina TH, Reynolds RM. Mechanisms linking in utero stress to altered offspring behaviour. Curr Top Behav Neurosci 2014;18:93–122 [DOI] [PubMed] [Google Scholar]
- 24.Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A 2012;109:E1312–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grigoriadis S, de Camps Meschino D, Barrons E, et al. Mood and anxiety disorders in a sample of Canadian perinatal women referred for psychiatric care. Arch Womens Ment Health 2011;14:325–333 [DOI] [PubMed] [Google Scholar]
- 26.Wenzel A, Haugen EN, Jackson LC, Brendle JR. Anxiety symptoms and disorders at eight weeks postpartum. Anxiety Disord 2005;19:295–311 [DOI] [PubMed] [Google Scholar]
- 27.Reck C, Struben K, Backenstrass M, et al. Prevalence, onset and comorbidity of postpartum anxiety and depressive disorders. Acta Psychiatr Scand 2008;118:459–468 [DOI] [PubMed] [Google Scholar]
- 28.Van Balkom AJLM, van Boeijen CA, Boeke AJP, van Oppen P, Kempe PT, van Dyck R. Comorbid depression, but not comorbid anxiety disorders, predicts poor outcome in anxiety disorders. Depress Anxiety 2008;25:408–415 [DOI] [PubMed] [Google Scholar]
- 29.Bittner A, Goodwin RD, Wittchen H-U, Beesdo K, Höfler M, Lieb R. What characteristics of primary anxiety disorders predict subsequent major depressive disorder? J Clin Psychiatry 2004;65:618–626, quiz 730 [DOI] [PubMed] [Google Scholar]
- 30.Wittchen H-U, Beesdo K, Bittner A, Goodwin RD. Depressive episodes–evidence for a causal role of primary anxiety disorders? Eur Psychiatry 2003;18:384–393 [DOI] [PubMed] [Google Scholar]
- 31.Hofmeijer-Sevink MK, Batelaan NM, van Megen HJGM, et al. Clinical relevance of comorbidity in anxiety disorders: A report from the Netherlands Study of Depression and Anxiety (NESDA). J Affect Disord 2012;137:106–112 [DOI] [PubMed] [Google Scholar]
- 32.Coelho HF, Murray L, Royal-Lawson M, Cooper PJ. Antenatal anxiety disorder as a predictor of postnatal depression: A longitudinal study. J Affect Disord 2011;129:348–353 [DOI] [PubMed] [Google Scholar]
- 33.Prenoveau J, Craske M, Counsell N, et al. Postpartum GAD is a risk factor for postpartum MDD: The course and longitudinal relationships of postpartum GAD and MDD. Depress Anxiety 2013;30:506–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skouteris H, Wertheim EH, Rallis S, Milgrom J, Paxton SJ. Depression and anxiety through pregnancy and the early postpartum: An examination of prospective relationships. J Affect Disord 2009;113:303–308 [DOI] [PubMed] [Google Scholar]
- 35.Arch JJ. Pregnancy-specific anxiety: Which women are highest and what are the alcohol-related risks? Compr Psychiatry 2013;54:217–228 [DOI] [PubMed] [Google Scholar]
- 36.Andrews G, Creamer M, Crino R, Hunt C, Lampe L, Page A. treatment of anxiety disorders: Clinician guides and patient manuals, 2nd ed. Cambridge: Cambridge University Press, 2003 [Google Scholar]
- 37.National Collaborating Centre for Mental Health (NCCMH). Antenatal and postnatal mental health: The NICE guideline on clinical management and service guidance. Leicester and London: The British Psychological Society and the Royal College of Physicians, 2007. [PubMed] [Google Scholar]
- 38.National Institute for Health and Care Excellence (NICE). Antenatal and postnatal health overview. Manchester: NICE, 2014. [Google Scholar]
- 39.Argyropoulos SV, Ploubidis GB, Wright TS, et al. Development and validation of the Generalized Anxiety Disorder Inventory (GADI). J Psychopharmacol 2007;21:145–152 [DOI] [PubMed] [Google Scholar]
- 40.Goldberg DP, Hillier VF. A scaled version of the General Health Questionnaire. Psychol Med 1979;9:139–145 [DOI] [PubMed] [Google Scholar]
- 41.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, 1970. [Google Scholar]
- 42.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361–370 [DOI] [PubMed] [Google Scholar]
- 43.Spitzer R, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine 2006;166:1092–1097 [DOI] [PubMed] [Google Scholar]
- 44.Levin JS. The factor structure of the Pregnancy Anxiety Scale. J Heal Soc Behav 1991;32:368–381 [PubMed] [Google Scholar]
- 45.Somerville S, Dedman K, Hagan R, et al. The Perinatal Anxiety Screening Scale: Development and preliminary validation. Arch Womens Mental Health 2014;17:443–454 [DOI] [PubMed] [Google Scholar]
- 46.Cohen L, Altshuler L. Pharmacologic management of psychiatric illness during pregnancy and the postpartum period. In: Dunner D, Rosenbaum JF, eds. The psychiatric clinics of North America annal of drug therapy. Philadelphia: WB Saunders Company, 1997:21–60 [Google Scholar]
- 47.Avni-Barron O, Wiegartz PS. Issues in treating anxiety disorders in pregnancy. Psychiatr Times 2011;28 [Google Scholar]
- 48.Alwan S, Reefhuis J, Rasmussen S a, Olney RS, Friedman JM. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med 2007;356:2684–2692 [DOI] [PubMed] [Google Scholar]
- 49.Peretti S, Judge R, Hindmarch I. Safety and tolerability considerations: Tricyclic antidepressants vs. selective serotonin reuptake inhibitors. Acta Psychiatr Scand 2000;101(s403):17–25 [DOI] [PubMed] [Google Scholar]
- 50.Einarson A. Studying the safety of drugs in pregnancy: And the gold standard is? J Clin Pharmacol Pharmacoepidemiol 2008;1:3–8 [Google Scholar]
- 51.Byatt N, Deligiannidis KM, Freeman MP. Antidepressant use in pregnancy: A critical review focused on risks and controversies. Acta Psychiatr Scand 2013;127:94–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gentile S. Selective serotonin reuptake inhibitor exposure during early pregnancy and the risk of birth defects. Acta Psychiatr Scand 2011;123:266–275 [DOI] [PubMed] [Google Scholar]
- 53.Grigoriadis S, Vonderporten EH, Mamisashvili L, et al. Antidepressant exposure during pregnancy and congenital malformations: Is there an association? A systematic review and meta-analysis of the best evidence. J Clin Psychiatry 2013;74:e293–e308 [DOI] [PubMed] [Google Scholar]
- 54.GlaxoSmithKline. Use of paroxetine in first trimester of pregnancy may have a small increased risk of birth defects, compared to other antidepressants. Missisauga, ON: GlaxoSmithKline, 2005. [Google Scholar]
- 55.Bérard A, Ramos E, Rey E, Blais L, St-André M, Oraichi D. First trimester exposure to paroxetine and risk of cardiac malformations in infants: The importance of dosage. Birth Defects Res B Dev Reprod Toxicol 2007;80:18–27 [DOI] [PubMed] [Google Scholar]
- 56.Einarson A, Pistelli A, DeSantis M, et al. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry 2008;165:749–752 [DOI] [PubMed] [Google Scholar]
- 57.Bakker MK, De Walle HEK, Wilffert B, de Jong-Van den Berg LTW. Fluoxetine and infantile hypertrophic pylorus stenosis: A signal from a birth defects-drug exposure surveillance study. Pharmacoepidemiol Drug Saf 2010;19:808–813 [DOI] [PubMed] [Google Scholar]
- 58.Malm H, Artama M, Gissler M, Ritvanen A. Selective serotonin reuptake inhibitors and risk for major congenital anomalies. Obstet Gynecol 2011;118:111–120 [DOI] [PubMed] [Google Scholar]
- 59.Einarson A, Choi J, Einarson TR, Koren G. Rates of spontaneous and therapeutic abortions following use of antidepressants in pregnancy: Results from a large prospective database. J Obstet Gynaecol Can 2009;31:452–456 [DOI] [PubMed] [Google Scholar]
- 60.Hemels MEH, Einarson A, Koren G, Lanctôt KL, Einarson TR. Antidepressant use during pregnancy and the rates of spontaneous abortions: A meta-analysis. Ann Pharmacother 2005;39:803–809 [DOI] [PubMed] [Google Scholar]
- 61.Ross LE, Grigoriadis S, Mamisashvili L, et al. Selected pregnancy and delivery outcomes after exposure to antidepressant medication:A a systematic review and meta-analysis. J Am Med Assoc Psychiatry 2013;70:436–443 [DOI] [PubMed] [Google Scholar]
- 62.Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 2006;354:579–587 [DOI] [PubMed] [Google Scholar]
- 63.Grigoriadis S, Vonderporten EH, Mamisashvili L, et al. Prenatal exposure to antidepressants and persistent pulmonary hypertension of the newborn: Systematic review and meta-analysis. BMJ 2014;348:f6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oberlander TF, Misri S, Fitzgerald CE, Kostaras X, Rurak DW, Riggs W. Pharmacologic Factors Associated With Transient Neonatal Symptoms Following Prenatal Psychotropic Medication Exposure. J Clin Psychiatry 2004;65:230–237 [DOI] [PubMed] [Google Scholar]
- 65.Sanz EJ, De-las-Cuevas C, Kiuru A, Bate A, Edwards R. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: A database analysis. Lancet 2005;365:482–487 [DOI] [PubMed] [Google Scholar]
- 66.Wisner KL, Sit DKY, Hanusa BH, et al. Major depression and antidepressant treatment: Impact on pregnancy and neonatal outcomes. Am J Psychiatry 2009;166:557–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Misri S, Reebye P, Kendrick K, et al. Internalizing behaviors in 4-year-old children exposed in utero to psychotropic medications. Am J Psychiatry 2006;163:1026–1032 [DOI] [PubMed] [Google Scholar]
- 68.Oberlander TF, Reebye P, Misri S, Papsdorf M, Kim J, Grunau RE. Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med 2007;161:22–29 [DOI] [PubMed] [Google Scholar]
- 69.Reebye PN, Morison SJ, Panikkar H, Misri S, Grunau RE. Affect expression in prenatally psychotropic exposed and nonexposed mother-infant dyads. Infant Ment Health J 2002;23:403–416 [Google Scholar]
- 70.Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011;68:1104–1112 [DOI] [PubMed] [Google Scholar]
- 71.Harrington RA, Lee L-C, Crum RM, Zimmerman AW, Hertz-Picciotto I. Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay. Pediatrics 2014;133:e1241–-e1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hviid A, Melbye M, Pasternak B. Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. N Engl J Med 2013;369:2406–2415 [DOI] [PubMed] [Google Scholar]
- 73.Sørensen MJ, Grønborg TK, Christensen J, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol 2013;5:449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gentile S. The safety of newer antidepressants in pregnancy and breastfeeding. Drug Saf 2005;28:137–152 [DOI] [PubMed] [Google Scholar]
- 75.Hale TW, Shum S, Grossberg M. Fluoxetine toxicity in a breastfed infant. Clin Pediatr 2001;40:681–684 [DOI] [PubMed] [Google Scholar]
- 76.Hale TW. Medications and mothers' milk, 15th ed. Amarillo, TX: Hale Publishing, 2012 [Google Scholar]
- 77.Daw JR, Mintzes B, Law MR, Hanley GE, Morgan SG. Prescription drug use in pregnancy: A retrospective, population-based study in British Columbia, Canada (2001–2006). Clin Ther 2012;34:239–249.e2 [DOI] [PubMed] [Google Scholar]
- 78.Hoffman EJ, Mathew SJ. Anxiety disorders: A comprehensive review of pharmacotherapies. Mt Sinai J Med 2008;75:248–262 [DOI] [PubMed] [Google Scholar]
- 79.Barker MJ, Greenwood KM, Jackson M, Crowe SF. Cognitive effects of long-term benzodiazepine use. CNS Drugs 2004;18:37–48 [DOI] [PubMed] [Google Scholar]
- 80.Baldwin DS, Waldman S, Allgulander C. Evidence-based pharmacological treatment of generalized anxiety disorder. Int J Neuropsychopharmacol 2011;14:697–710 [DOI] [PubMed] [Google Scholar]
- 81.NCCMH. Generalised anxiety disorder in adults: The NICE guidelines on clinical management and service guidance. Leicester and London: The British Psychological Society and the Royal College of Physicians, 2011. [Google Scholar]
- 82.Dolovich LR, Addis A, Vaillancourt JMR, Power JDB, Koren G, Einarson TR. Benzodiazepine use in pregnancy and major malformations or oral cleft: Meta-analysis of cohort and case-control studies. BMJ 1998;317:839–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wikner BN, Stiller C-O, Bergman U, Asker C, Källén B. Use of benzodiazepines and benzodiazepine receptor agonists during pregnancy: Neonatal outcome and congenital malformations. Pharmacoepidemiol Drug Saf 2007;16:1203–1210 [DOI] [PubMed] [Google Scholar]
- 84.Gentile S. Anxiety and sleep disorders, psychopharmacology, and pregnancy. In: Galbally M, Snellen M, Lewis A, eds. Psychopharmacology and pregnancy. Heidelberg: Springer; 2014:87–102 [Google Scholar]
- 85.Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatr Serv 2002;53:39–49 [DOI] [PubMed] [Google Scholar]
- 86.Committee on Drugs. Use of psychoactive medication during pregnancy and possible effects on the fetus and newborn. Pediatrics 2000;105:880–887 [DOI] [PubMed] [Google Scholar]
- 87.Levine RE, Oandasan AP, Primeau L a, Berenson AB. Anxiety disorders during pregnancy and postpartum. Am J Perinatol 2003;20:239–248 [DOI] [PubMed] [Google Scholar]
- 88.Katzman MA. Aripiprazole: A clinical review of its use for the treatment of anxiety disorders and anxiety as a comorbidity in mental illness. J Affect Disord 2011;128:S11–S20 [DOI] [PubMed] [Google Scholar]
- 89.Stein DJ, Bandelow B, Merideth C, Olausson B, Szamosi J, Eriksson H. Efficacy and tolerability of extended release quetiapine fumarate (quetiapine XR) monotherapy in patients with generalised anxiety disorder: An analysis of pooled data from three 8-week placebo-controlled studies. Hum Psychopharmacol 2011;26:614–628 [DOI] [PubMed] [Google Scholar]
- 90.Üçok A, Gaebel W. Side effects of atypical antipsychotics: A brief overview. World Psychiatry 2008;7:58–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McKenna K, Koren G, Tetelbaum M, et al. Pregnancy outcome of women using atypical antipsychotic drugs: A prospective comparative study. J Clin Psychiatry 2005;66:444–449 [DOI] [PubMed] [Google Scholar]
- 92.Reis M, Källén B. Maternal use of antipsychotics in early pregnancy and delivery outcome. J Clin Psychopharmacol 2008;28:279–288 [DOI] [PubMed] [Google Scholar]
- 93.ACOG Committee on Practice Bulletins; Stowe ZN, Ragan K. ACOG Practice Bulletin No. 92: Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol 2008;111:1001–1020 [DOI] [PubMed] [Google Scholar]
- 94.Arch JJ. Cognitive behavioral therapy and pharmacotherapy for anxiety: Treatment preferences and credibility among pregnant and non-pregnant women. Behav Res Ther 2014;52:53–60 [DOI] [PubMed] [Google Scholar]
- 95.Goodman J. Women's attitudes, preferences, and perceived barriers to treatment for perinatal depression. Birth 2009;36:60–69 [DOI] [PubMed] [Google Scholar]
- 96.Covin R, Ouimet AJ, Seeds PM, Dozois DJA. A meta-analysis of CBT for pathological worry among clients with GAD. J Anxiety Disord 2008;22:108–116 [DOI] [PubMed] [Google Scholar]
- 97.Cuijpers P, Sijbrandij M, Koole S, Huibers M, Berking M, Andersson G. Psychological treatment of generalized anxiety disorder: A meta-analysis. Clin Psychol Rev 2014;34:130–140 [DOI] [PubMed] [Google Scholar]
- 98.Leichsenring F, Salzer S, Jaeger U, et al. Cognitive-behavioral therapy in generalized anxiety disorder: A randomized, controlled trial. Am J Psychiatry 2009;166:875–881 [DOI] [PubMed] [Google Scholar]
- 99.Huppert JD, Rynn M. Generalized anxiety disorder. In: Stein DJ, ed. Clinical manual of anxiety disorders. Arlington, VA: American Psychiatric Publishing; 2004:147–171 [Google Scholar]
- 100.Austin M-P, Frilingos M, Lumley J, et al. Brief antenatal cognitive behaviour therapy group intervention for the prevention of postnatal depression and anxiety: A randomised controlled trial. J Affect Disord 2008;105:35–44 [DOI] [PubMed] [Google Scholar]
- 101.Duncan LG, Bardacke N. Mindfulness-based childbirth and parenting education: Promoting family mindfulness during the perinatal period. J Child Fam Stud 2010;19:190–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dunn C, Hanieh E, Roberts R, Powrie R. Mindful pregnancy and childbirth: Effects of a mindfulness-based intervention on women's psychological distress and well-being in the perinatal period. Arch Womens Ment Health 2012;15:139–143 [DOI] [PubMed] [Google Scholar]
- 103.Vieten C, Astin J. Effects of a mindfulness-based intervention during pregnancy on prenatal stress and mood: Results of a pilot study. Arch Womens Ment Health 2008;11:67–74 [DOI] [PubMed] [Google Scholar]
- 104.Goodman JH, Guarino A, Chenausky K, et al. CALM Pregnancy: Results of a pilot study of mindfulness-based cognitive therapy for perinatal anxiety. Arch Womens Ment Health 2014;17:373–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Borkovec BTD, Newman MG, Castonguay LG. Cognitive-behavioral therapy for generalized anxiety disorder with integrations from interpersonal and experiential therapies 2003;8:382–389 [DOI] [PubMed] [Google Scholar]
- 106.Bastani F, Hidarnia A, Kazemnejad A, Vafaei M, Kashanian M. A randomized controlled trial of the effects of applied relaxation training on reducing anxiety and perceived stress in pregnant women. J Midwifery Womens Health 2005;50:e36–e40 [DOI] [PubMed] [Google Scholar]
- 107.Urech C, Fink NS, Hoesli I, Wilhelm FH, Bitzer J, Alder J. Effects of relaxation on psychobiological wellbeing during pregnancy: A randomized controlled trial. Psychoneuroendocrinology 2010;35:1348–1355 [DOI] [PubMed] [Google Scholar]
- 108.Hoyer J, Beesdo K, Gloster AT, Runge J, Höfler M, Becker ES. Worry exposure versus applied relaxation in the treatment of generalized anxiety disorder. Psychother Psychosom 2009;78:106–115 [DOI] [PubMed] [Google Scholar]
- 109.Donker T, Griffiths KM, Cuijpers P, Christensen H. Psychoeducation for depression, anxiety and psychological distress: A meta-analysis. BMC Med 2009;7:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cloninger CR, Zohar AH, Hirschmann S, Dahan D. The psychological costs and benefits of being highly persistent: Personality profiles distinguish mood disorders from anxiety disorders. J Affect Disord 2012;136:758–766 [DOI] [PubMed] [Google Scholar]
- 111.Marcé LV. Traité de la folie des femmes enceintes des nouvelles accouchées et des nourrices et considérations médico-légales qui se rattachent à ce sujet. Paris: Baillière, 1858. [Google Scholar]