Abstract
Pathogen density and genetic diversity fluctuate in the outside-host environment during and between epidemics, affecting disease emergence and the severity and probability of infections. Although the importance of these factors for pathogen virulence and infection probability has been acknowledged, their interactive effects are not well understood. We studied how an infective dose in an environmentally transmitted opportunistic fish pathogen, Flavobacterium columnare, affects its virulence both in rainbow trout, which are frequently infected at fish farms, and in zebra fish, a host that is not naturally infected by F. columnare. We used previously isolated strains of confirmed high and low virulence in a single infection and in a co-infection. Infection success (measured as host morbidity) correlated positively with dose when the hosts were exposed to the high-virulence strain, but no response for the dose increase was found when the hosts were exposed to the low-virulence strain. Interestingly, the co-infection resulted in poorer infection success than the single infection with the high-virulence strain. The rainbow trout were more susceptible to the infection than the zebra fish but, in both species, the effects of the doses and the strains were qualitatively similar. We suggest that as an increase in dose can lead to increased host morbidity, both the interstrain interactions and differences in infectivity in different hosts may influence the severity and consequently the evolution of disease. Our results also confirm that the zebra fish is a good laboratory model to study F. columnare infection.
Introduction
Virulence (the harm caused to the host by a pathogen) is influenced by several ecological and evolutionary processes that often involve a trade-off between host exploitation and pathogen reproduction [1, 2]. The key factors driving the virulence of a pathogen (e.g. host susceptibility, pathogen growth rate and host-specificity) coevolve in an arms race between the pathogen and its host [1]. Opportunistic pathogens are often host-generalists and can have the ability to survive and replicate outside the host, thus not being restricted by the transmission-virulence trade-off [3]. Despite the opportunists having a great impact on general health, their infection dynamics and infectivity in different host species are poorly characterized, and not covered by the traditional theories of virulence.
In nature, populations of hosts and their pathogens are diverse, and the hosts are often infected by several pathogen genotypes or species, which often leads to increased virulence [4, 5, 6, 7]. Interactions of co-infecting pathogens can have a significant role in virulence evolution, either due to strain competition or by facilitating infection with cooperative interactions [4, 8, 9, 10]. Co-infections may be especially important for generalist pathogens that have a wide host range and, thus, a higher likelihood of coming across potential hosts than host-specialists [3, 11]. However, pathogen virulence may face trade-offs as a result of the ecological and evolutionary costs of generalism [12], which could lead to higher pathogen doses needed for the initiation of an infection. As a consequence, the virulence of a generalist pathogen in different hosts may not always be easy to predict [11].
Infective dose (the number of cells needed to infect a host) varies greatly among pathogen species [13]. The infective dose is generally recognized to influence disease dynamics and severity [14, 15], as virulence typically increases with the dose [8, 14]. Although dose effects in multiply infected hosts can have important evolutionary consequences [16], the strain interactions in the context of dose effect are still poorly understood.
Previous studies on co-infections with eukaryotic parasites have demonstrated the virulence increase in the fish pathogen Flavobacterium columnare [17, 18]. However, it has remained unknown how the interstrain interactions of different F. columnare strains affect the virulence during co-infection. Using two host species we investigate how an increasing pathogen dose and co-infection (with two bacterial strains differing in their virulence), affect the virulence of this host-generalist pathogen in two phylogenetically distant host species. Our aim is to shed light on how the infective doses and co-infections in opportunistic pathogens shape the disease outcome in different host species, and thus increase the present understanding of disease evolution and how disease epidemics emerge in differing conditions. As an infection model we use the opportunistic fish pathogen F. columnare, and as hosts the rainbow trout (Oncorhynchus mykiss), a Salmonid host frequently infected in fish farms, and the zebra fish (Danio rerio), a Cyprinid host.
Materials and Methods
Pathogen
Flavobacterium columnare is a globally important fish pathogen in freshwater aquaculture [19, 20] and known to affect several fish species in fish farming and in the wild as a causative agent of columnaris disease [21, 22, 23]. The common clinical signs of the disease include gill necrosis, fin erosion and skin lesions such as the typical saddleback symptom around the dorsal fin [20, 24]. The disease is transmitted from infected fish via water and biofilms [25, 26]. In Europe, F. columnare is an especially difficult pathogen in salmonid fish farming, where it can cause severe fish mortality within the rearing units [20, 25]. The disease outbreaks occur in the summer when the water temperature naturally rises above 20°C [27]. Two previously isolated F. columnare strains were used in this study: a high-virulence strain B185 isolated during a columnaris disease outbreak at a salmonid fish farm in Central Finland (farm L, see details on the strain isolation and virulence in our previous studies [28, 29]) and a low-virulence strain B398 isolated from the inlet water of another salmonid fish farm in the same area (farm V, see [25]). Pure cultures were stored frozen at -80°C in a stock containing 10% glycerol and 10% fetal calf serum. For the experiments, the bacterial strains were grown in modified Shieh medium [30] at 26°C with constant agitation (150 rpm).
Host species
The rainbow trout is a cold-adapted fish species, occurring naturally in the Pacific Ocean and cold streams in the North American continent from Alaska to Mexico [31]. After introduction into Finland around 1900, the rainbow trout has become the most important commercially farmed fish species in the country [32], and since the 1990’s has been severely affected by columnaris disease during warm water periods [20]. As F. columnare is prevalent at salmonid farms and their inlet waters in Finland, we used rainbow trout as a model species representing a natural host of F. columnare. For the study, apparently healthy fingerling rainbow trout with no known history with F. columnare were obtained from a stock of a fish farm (farm V) in Central Finland. The fish were obtained from the farm during a cold water season (when no outbreaks occur), brought to our fish rearing facilities where F. columnare-free well water is used, and maintained for two months at 15.0–16.0°C before conducting the experiments. The average weight of the fish was 1.25 g.
The zebra fish is a well-established laboratory animal that shares the temperature optimum of the pathogen (for zebra fish, see [33]; for F. columnare, see [24]). It is a tropical species indigenous to South Asia, and thus it does not have a recent co-evolutionary history with the bacterial strains used in this study. The adult, unsexed, disease-free zebra fish (average weight 0.21 g) were obtained from Core Facilities (COFA) and Research Services of Tampere (University of Tampere, Finland).
Both rainbow trout and zebra fish have been previously used as experimental hosts for F. columnare [21, 25, 34, 35], but how the bacterial strain and the dose affect the onset of columnaris disease has not yet been thoroughly studied. If the zebra fish are found to respond to the experimental columnaris infection in a similar way to the rainbow trout, they could be used as a reliable model in further columnaris disease experiments.
Infection treatments
To examine the interactions between virulence, infection dose, and host species, the fish were infected with the two F. columnare strains, and with a 1:1 mixture of these strains, by bath challenge [29]. The fish were individually challenged in 50 ml of aerated ground water with 5.×105, 1.0×106, 3.0×106, 6.0×106, 9.0×106, 1.2×107, 1.6×107, 2.0×107 and 3.0×107 CFU (colony forming units) ml-1 of overnight-grown bacteria for 2 hours at 25°C in two fish per dose. The dose was treated as a continuous variable, totaling 18 replicate fish per species per each treatment group (high-virulence strain, low-virulence strain and co-infection). Per species, 5 replicates of negative control fish (sham-exposed to sterile Shieh medium) were used. After being challenged, the fish were transferred individually into 1 liter aquaria with 0.5 liter of ground water, and monitored for clinical signs of disease and morbidity for 5 days, the first 48 hours at 2-hour intervals. The water temperature was maintained at 25.0–26.3°C throughout the experiment. The fatally moribund fish were euthanized by decapitation. Also the surviving and the control fish were euthanized in the end of the experiment. To verify the columnaris infection, cultivations from gills were spread on Shieh agar supplemented with tobramycin [36]. The yellow colonies with the rhizoid morphology typical to F. columnare were considered as an indicator of columnaris infection. The experiment was conducted under permission ESAVI-2010-05569/Ym-23, granted by the National Animal Experiment Board at the Regional State Administrative Agency for Southern Finland.
Statistical analysis
The data were analyzed using a generalized linear model (GLM) for binomial distribution. Two factors (‘host species’ and ‘treatment’ (high-virulence strain, low-virulence strain or co-infection)), a continuous covariate (‘dose’), and all their possible interactions were included as variables to explain the fate of the fish (dead or surviving) within the time from the beginning of the experiment (see [37]). All the infected rainbow trout ‘died’ during the experiment. The last moribund rainbow trout were euthanized 42 hours before the end of the experiment. As the gill cultures taken from the infected and moribund fish were positive for F. columnare and cultures from the surviving fish were negative, it can rather safely be assumed that zebra fish surviving up to 141 hours (i.e. until the end-point of the experiment) were able to resist, or tolerate and survive, the infection. Thus, we did not consider the surviving individuals as censored cases. The model selection was based on Akaike information criteria (AIC; Table 1) and the analysis was conducted with the software R 2.15.2 and the package Lme4. When interpreting the effects of the terms included in the model, a significance level of 0.05 or less was used.
Table 1. Model selection based on Akaike information criteria (AIC).
| Model | AIC | df | P |
|---|---|---|---|
| host*treatment*dose | 234.23 | ||
| host+treatment+dose+host:treatment+host:dose+dose:treatment | 231.22 | 2 | 0.611 |
| host+treatment+dose+host:treatment+dose:treatment | 230.72 | 2 | 0.173 |
| host+treatment+dose+host:treatment | 232.93 | 2 | 0.104 |
| host+treatment+dose+dose:treatment | 236.78 | 1 | 0.009 |
The model with smallest AIC value estimating the morbidity risk of the host (rainbow trout or zebra fish) within time is underlined.
The degrees of freedom (df) and significance levels (P) are given for the goodness of fit compared to the next higher level model.
Single- and co-infections are included in the term ‘treatment’.
Results
The risk of fatal infection of the host was significantly influenced by the dose, the treatment (high-virulence strain, low-virulence strain or co-infection) and the host species (Fig 1, Tables 2 and 3). We found that 1) the increase in the dose correlates positively with the host morbidity risk when the hosts are exposed to the high-virulence strain or the mixture; 2) the infection success in the co-infected hosts is approximately an average of that of hosts infected with the high-virulence and the low-virulence strains (Fig 1), indicating that only the high-virulence strain is responsible for the host morbidity; and 3) the rainbow trout is more susceptible to the columnaris infection than the zebra fish (Fig 1), but both hosts respond to the bacterial doses and strains qualitatively similarly. All the moribund hosts were found positive for F. columnare in bacterial culture taken from fish gills, whereas the unexposed hosts and hosts surviving the infection were found negative.
Fig 1. Estimated mortality risk per hour of A) zebra fish (Danio rerio), and B) rainbow trout (Oncorhynchus mykiss) infected with a high-virulence (continuous line) and a low-virulence (dotted line) strain of F. columnare, and their mixture, i.e. co-infection (dashed line).
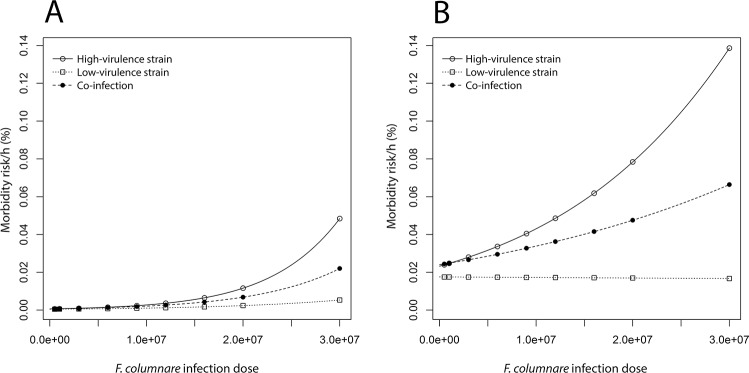
Table 2. The significance and test values of the bacterial dose, the treatment and the host species on the morbidity risk of the hosts.
| Source | Df | Deviance | Residual deviance | P |
|---|---|---|---|---|
| Host | 1,106 | 84.350 | 116.663 | <0.001 |
| Dose | 1,105 | 9.606 | 107.057 | 0.002 |
| Treatment | 2,103 | 14.654 | 92.403 | <0.001 |
| Host:Dose | 1,102 | 10.205 | 82.198 | 0.002 |
| Dose:Treatment | 2,100 | 4.522 | 77.677 | 0.104 |
Significant P values are denoted in bold.
Table 3. The effect of the bacterial dose, the treatment and the host species on the host morbidity risk.
| Source | Estimate | SE |
|---|---|---|
| (Intercept) a | -3.738 | 0.390 |
| Host(Zebra fish) | -3.633 | 0.571 |
| Dose | 6.372−8 | 2.805−8 |
| Treatment(Co-infection) | 3.337−2 | 0.505 |
| Treatment(Single infection, low) | -2.861−1 | 0.496 |
| Host(Zebra fish):Dose | 8.272−8 | 3.197−8 |
| Dose:Treatment(Co-infection) | -2.832−8 | 3.353−8 |
| Dose:Treatment(Single infection, low) | -6.542−8 | 3.186−8 |
a Intercept includes the effects of the host (rainbow trout) and the treatment (single infection, high).
Discussion
Opportunistic pathogens are often host-generalists and may survive and replicate outside the host [3], thus having different environmental dynamics than obligate pathogens. Pathogens that are durable in the outside-host environment may not have high fitness costs related to virulence [38], which has also been observed for F. columnare [39, 40]. The ability to survive and replicate outside the host can contribute significantly to the infective bacterial populations in the environment, and therefore information on the relationship between the number of free-living bacteria (the infection dose) and disease virulence is needed. Although the influence of dose on disease dynamics has been widely acknowledged, its effect on parasite virulence and reproduction is sometimes unclear or even contradictory [14]. Additionally, the experimental evidence from the infective doses of opportunistic pathogens is scarce, especially in different host species. We addressed these issues by infecting two host species with increasing doses of a high-virulence and a low-virulence bacterial strain in a single and in a co-infection.
We found a strong positive relationship between the dose and the host morbidity risk in the treatments in which the high-virulence strain was involved (Fig 1). As the host morbidity risk in this study is a measure of pathogen virulence (as demonstrated in e.g. [2, 41, 42, 43]), our result suggests that the virulence of F. columnare is strongly dose-dependent. This finding is in agreement with the experimental evidence from obligatory pathogens [14, 44]. Interestingly, in contradiction with numerous previous studies [5, 7, 45, 46, 47], we did not observe any additive effects of co-infection on the host morbidity risk. This indicates that the outcome of the infection in this study is affected by the interplay between the bacterial strain and the dose. Previous studies (e.g. [48, 49]) have shown that the more virulent strains have a competitive advantage in mixed infections, whereas in some systems, like Schistosoma mansoni, co-infections may favor the less virulent strains [50]. Our result suggests that the presence of a low-virulence strain may significantly alter the co-infection outcome, most likely by diluting the infection dose. Indeed, if the low-virulence strain lacks the ability to produce essential virulence factors needed for a successful infection, its presence may reduce the total severity of the disease outbreak. This is an important finding as the interactions between high-virulence and low-virulence strains are generally poorly understood. Yet, pathogen strains with variable levels of virulence often co-occur in the environment [20, 22], thus influencing the onset of disease outbreaks or host immune response.
Also interference competition via antimicrobial compounds like colicins (i.e. inhibitory compounds targeted to hamper the growth of other conspecific strains) may have trade-offs with virulence [51]. F. columnare has been reported to produce bacteriocins that are equivalent to colicins, as demonstrated in [52]. However, in order to find out if the mechanism leading to reduced virulence in our system builds upon the competitive interactions between the bacterial strains, more studies are needed in the context of virulence evolution.
Although maintaining the ability to infect multiple host species can be an efficient survival strategy, it may result in a trade-off, leading to lower pathogen virulence [11, 12, 53]. We found the two host species to respond qualitatively similarly to the increase in the infection dose, but the rainbow trout was more sensitive to the increase than the zebra fish. Similar associations between F. columnare strains and the host species have also been found in salmonids in general and in channel catfish [35, 54, 55]. However, more extensive studies on a variety of host species would be needed to find out if our results are due to adaptation of the strains to the rainbow trout, or if host generalism has trade-offs with virulence of F. columnare. Nevertheless, our result has important implications because F. columnare populations encounter a wide range of host species both in the wild and at fish farms [21, 22, 23, 56].
The sensitivity of the rainbow trout in this study can also be partly caused by the experimental conditions. The water temperature during the experiment was not optimal for the cold-adapted rainbow trout, although it still was within the temperature range naturally occurring in fish farming conditions. Indeed, columnaris disease outbreaks at fish farms are typically prevalent during the warm water season [20, 57]. Yet, our findings confirm that zebra fish is a suitable model species for experimental studies of F. columnare infections. Zebra fish has been successfully used as an infection model for columnaris disease already in prior studies [34, 35], but the infection dynamics of F. columnare in zebra fish compared to rainbow trout has remained unclear. Information about the comparability of the dose responses in these two species is therefore intensely needed to be able to replace the stress-sensitive rainbow trout in the demanding laboratory experiments. Unlike the rainbow trout, the zebra fish is well suited to laboratory conditions; it is a small-sized species that thrives in warm temperatures (as does the pathogen) and does not require constant water flow [33]. Additionally, zebra fish are available year-round.
Our results suggest that an increase in dose can lead to more severe disease and poorer host survival in host-generalist opportunistic pathogens, but the host survival may be dependent on the original ability of each bacterial strain to cause disease in a strain-specific manner. For the same reason, different pathogen strains may not necessarily have additive effects on disease virulence. Based on our results, it seems that the interactions between the dose and the pathogen strains are important drivers of infection in different host species, and warrant for more studies for evolution of virulence and pathogen host range. Furthermore, from an applied perspective, using zebra fish as an infection model can provide valuable information on the virulence of F. columnare, as the zebra fish shares the temperature optimum of the pathogen and tolerates the experimental conditions well.
Acknowledgments
We would like to thank Dr. Heidi Kunttu and Dr. Elina Laanto for bacterial strains, MSc Reetta Penttinen and Mr. Petri Papponen for help in laboratory, Dr. Bibiana Rojas, Dr. Emily Burdfield-Steel, Dr. Lauri Mikonranta and Prof. Jaana Bamford for valuable comments. This work was supported by the Finnish Centre of Excellence Program of the Academy of Finland; the CoE in Biological Interactions 2012–2017 (#252411), and by the Academy of Finland grant #272995 (L.-R.S.).
Data Availability
All data are contained in the paper.
Funding Statement
This work was supported by the Centre of Excellence in Biological Interactions, grant #252411 (https://www.jyu.fi/bioenv/en/divisions/coe-interactions), and the Academy of Finland, grant #272995 (http://www.aka.fi/en). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ebert D & Herre EA. The evolution of parasitic diseases. Parasitol Today 1996; 12: 96–101. 10.1016/0169-4758(96)80668-5 [DOI] [PubMed] [Google Scholar]
- 2. Frank SA. Models of parasite virulence. Q Rev Biol 1996; 71: 37–78. 10.1086/419267 [DOI] [PubMed] [Google Scholar]
- 3. Brown SP, Cornforth DM, Mideo N. Evolution of virulence in opportunistic pathogens: generalism, plasticity, and control. Trends Microbiol 2012; 20: 336–342. 10.1016/j.tim.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Read AF, Taylor LH. The ecology of genetically diverse infections. Science 2001; 292: 1099–1102. 10.1126/science.1059410 [DOI] [PubMed] [Google Scholar]
- 5. Karvonen A, Rellstab C, Louhi K-R, Jokela J. Synchronous attack is advantageous: mixed genotype infections lead to higher infection success in trematode parasites. Proc R Soc B 2012; 279: 171–176. 10.1098/rspb.2011.0879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alizon S, de Roode JC, Michalakis Y. Multiple infections and the evolution of virulence. Ecol Lett 2013; 16: 556–567. 10.1111/ele.12076 [DOI] [PubMed] [Google Scholar]
- 7. Susi H, Barrés B, Vale PF, Laine A-L. Co-infection alters population dynamics of infectious disease. Nat Commun 2014; 6: 5975 10.1038/ncomms6975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Read AF, Aaby P, Antia R, Ebert D, Ewald PW, Gupta S, et al. What can evolutionary biology contribute to understanding virulence? In: Stearns SC, Koella JC, editors. Evolution in Health and Disease. Oxford University Press; 1999. pp. 205–215. [Google Scholar]
- 9. Jokela J, Schmid-Hempel P, Rigby MC. Dr. Pangloss restrained by the Red Queen–steps towards a unified defence theory. Oikos 2000; 89: 267–274. 10.1034/j.1600-0706.2000.890207.x [DOI] [Google Scholar]
- 10. Leggett HC, Benmayor R, Hodgson DJ, Buckling A. Experimental evolution of adaptive phenotypic plasticity in a parasite. Curr Biol 2013; 23: 139–142. 10.1016/j.cub.2012.11.045 [DOI] [PubMed] [Google Scholar]
- 11. Leggett HC, Buckling A, Long GH, Boots M. Generalism and the evolution of parasite virulence. Trends Ecol Evol 2013; 28: 592–596. 10.1016/j.tree.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 12. Benmayor R, Hodgson DJ, Perron GG, Buckling A. Host mixing and disease emergence. Curr Biology 2009; 19: 764–767. 10.1016/j.cub.2009.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leggett HC, Cornwallis CK, West SA. Mechanisms of pathogenesis, infective dose and virulence in human parasites. PLoS Pathog 2012; 8: e1002512 10.1371/journal.ppat.1002512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Regoes RR, Ebert D, Bonhoeffer S. Dose-dependent infection rates of parasites produce the Allee effect in epidemiology. Proc Biol Sci 2002; 269: 271–279. 10.1098/rspb.2001.1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Y, Handel A. Modeling inoculum dose dependent patterns of acute virus infections. J Theor Biol 2014; 347: 63–73. 10.1016/j.jtbi.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 16. Ebert D, Zschokke-Rohringer CD, Carius HJ. Dose effects and density-dependent regulation of two microparasites of Daphnia magna . Oecologia 2000; 122: 200–209. 10.1086/303404 [DOI] [PubMed] [Google Scholar]
- 17. Bandilla M, Valtonen ET, Suomalainen L-R, Aphalo PJ, Hakalahti T. A link between ectoparasite infection and susceptibility to bacterial disease in rainbow trout. Int J Parasitol 2006; 36: 987–991. 10.1016/j.ijpara.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 18. Xu D-H, Shoemaker CA, LaFrentz BR. Enhanced susceptibility of hybrid tilapia to Flavobacterium columnare after parasitism by Ichthyophthirius multifiliis . Aquaculture 2014; 430: 44–49. 10.1016/j.aquaculture.2014.03.041 [DOI] [Google Scholar]
- 19. Wagner BA, Wise DJ, Khoo LH, Terhune JS. The epidemiology of bacterial diseases in food-size channel catfish. J Aquat Anim Health 2002; 14: 263–272. [DOI] [PubMed] [Google Scholar]
- 20. Pulkkinen K, Suomalainen L-R, Read AF, Ebert D, Rintamäki P, Valtonen ET. Intensive fish farming and the evolution of pathogen virulence: the case of columnaris disease in Finland. Proc R Soc B 2010; 277: 593–600. 10.1098/rspb.2009.1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suomalainen L-R, Kunttu H, Valtonen ET, Hirvelä-Koski V, Tiirola M. Molecular diversity and growth features of Flavobacterium columnare strains isolated in Finland. Dis Aquat Org 2006; 70: 55–61. 10.3354/dao070055 [DOI] [PubMed] [Google Scholar]
- 22. Olivares-Fuster A, Baker JL, Terhune JS, Shoemaker CA, Klesius PH, Arias CA. Host-specific association between Flavobacterium columnare genomovars and fish species. Syst Appl Microbiol 2007; 30: 624–633. 10.1016/j.syapm.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 23. Scott SJ, Bollinger TK. Flavobacterium columnare: an important contributing factor to fish die-offs in southern lakes of Saskatchewan, Canada. J Adv Lab Res Biol 2014; 26: 832–836. 10.1177/1040638714553591 [DOI] [PubMed] [Google Scholar]
- 24. Declercq AM, Haesebrouck F, Van den Broeck W, Bossier P, Decostere A. Columnaris disease in fish: a review with emphasis on bacterium-host interactions. Vet Res 2013; 44:27 10.1186/1297-9716-44-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kunttu HMT, Suomalainen L-R, Pulkkinen K, Valtonen ET. Environment may be the source of Flavobacterium columnare outbreaks at fish farms. Environ Microbiol Rep 2012; 4: 398–402. 10.1111/j.1758-2229.2012.00342.x [DOI] [PubMed] [Google Scholar]
- 26. Cai W, De La Fuente L, Arias CR. Biofilm formation by the fish pathogen Flavobacterium columnare: Development and parameters affecting surface attachment. Appl Environ Microbiol 2013; 79: 5633–5642. 10.1128/AEM.01192-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suomalainen L-R, Tiirola M, Valtonen ET. Influence of rearing conditions on Flavobacterium columnare infection of rainbow trout. J Fish Dis 2005; 28: 271–277. 10.1111/j.1365-2761.2005.00631.x [DOI] [PubMed] [Google Scholar]
- 28. Laanto E, Sundberg L-R, Bamford JKH. Phage specificity of the freshwater fish pathogen Flavobacterium columnare . Appl Environ Microbiol 2011; 77: 7868–7872. 10.1128/AEM.05574-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laanto E, Bamford JKH, Laakso J, Sundberg L-R. Phage-driven loss of virulence in a fish pathogenic bacterium. PLOS ONE 2012; 7: e53157 10.1371/journal.pone.0053157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Song YL, Fryer JL, Rohovec JS. Comparison of six media for the cultivation of Flexibacter columnaris . Fish Pathol 1988; 23: 91–94. doi: 10.3147/jsfp.23.91. [DOI] [Google Scholar]
- 31.United States Department of Agriculture. Rainbow trout (Oncorhynchus mykiss). Fish and Wildlife Habitat Management Leaflet 13. 2000. Available: http://www.fws.gov/northeast/wssnfh/pdfs/rainbow1.pdf.
- 32. Finnish Game and Fisheries Research Institute. Rainbow trout (Oncorhynchus mykiss) Natural Resources Institute Finland; 2009. Available: http://www.rktl.fi/english/fish/fish_atlas/rainbow_trout/rainbow_trout.html. [Google Scholar]
- 33. Lawrence C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture 2007; 269: 1–20. 10.1016/j.aquaculture.2007.04.077 [DOI] [Google Scholar]
- 34. Moyer TR, Hunnicutt DW. Susceptibility of zebra fish Danio rerio to infection by Flavobacterium columnare and F. johnsoniae . Dis Aquat Org 2007; 76: 39–44. 10.3354/dao076039 [DOI] [PubMed] [Google Scholar]
- 35. Olivares-Fuster O, Bullard SA, McElwain A, Llosa MJ, Arias CR. Adhesion dynamics of Flavobacterium columnare to channel catfish Ictalurus punctatus and zebrafish Danio rerio after immersion challenge. Dis Aquat Org 2011; 96: 221–227. 10.3354/dao02371 [DOI] [PubMed] [Google Scholar]
- 36. Decostere A, Haesebrouck F, Devriese LA. Shieh medium supplemented with tobramycin for selective isolation of Flavobacterium columnare (Flexibacter columnaris) from diseased fish. J Clin Microbiol 1997; 35: 322–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. Mixed effects models and extensions in ecology with R, 1st ed. New York: Springer; 2009. [Google Scholar]
- 38. Walther BA, Ewald PW. Pathogen survival in the external environment and the evolution of virulence. Biol Rev 2004; 79: 849–869. 10.1017/S1464793104006475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kunttu HMT, Valtonen ET, Jokinen EI, Suomalainen L-R. Saprophytism of a fish pathogen as a transmission strategy. Epidemics 2009; 1: 96–100. 10.1016/j.epidem.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 40. Sundberg L-R, Kunttu HMT, Valtonen ET. Starvation can diversify the population structure and virulence strategies of an environmentally transmitting fish pathogen. BMC Microbiol 2014; 14: 1–6. 10.1186/1471-2180-14-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bull JJ. Perspective: Virulence. Evolution 1994; 48: 1423–1437. 10.2307/2410237 [DOI] [PubMed] [Google Scholar]
- 42. Read AF. The evolution of virulence. Trends Microbiol 1994; 2: 73–76. 10.1016/0966-842X(94)90537-1 [DOI] [PubMed] [Google Scholar]
- 43. Levin BR. The evolution and maintenance of virulence in microparasites. Emerg Infect Dis 1996; 2: 93–102. 10.3201/eid0202.960203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fellous S, Koella JC. Cost of co-infection controlled by infectious dose combinations and food availability. Oecologia 2010; 162: 935–940. 10.1007/s00442-009-1535-2 [DOI] [PubMed] [Google Scholar]
- 45. Taylor LH, Walliker D, Read AF. Mixed-genotype infections of the rodent malaria Plasmodium chabaudi are more infectious to mosquitoes than single-genotype infections. Parasitology 1997; 115: 121–132. doi: 10.1017/S0031182097001145. [DOI] [PubMed] [Google Scholar]
- 46. Griffiths EC, Pedersen AB, Fenton A, Petchey OL. The nature and consequences of coinfection in humans. J Infection 2011; 63: 200–206. 10.1016/j.jinf.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lass S, Hudson PJ, Thakar J, Saric J, Harvill E, Albert R, et al. Generating super-shedders: co-infection increases bacterial load and egg production of a gastrointestinal helminth. J R Soc Interface 2012; 10: 20120588 10.1098/rsif.2012.0588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. De Roode JC, Pansini R, Cheesman SJ, Helinski MEH, Huijben S, Wargo AR, et al. Virulence and competitive ability in genetically diverse malaria infections. Proc Natl Acad Sci USA 2005; 102: 7624–7628. 10.1073/pnas.0500078102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ben-Ami F, Mouton L, Ebert D. The effects of multiple infections on the expression and evolution of virulence in a Daphnia-endoparasite system. Evolution 2008; 62:1700–1711. 10.1111/j.1558-5646.2008.00391.x [DOI] [PubMed] [Google Scholar]
- 50. Gower CM, Webster JP. Intraspecific competition and the evolution of virulence in a parasitic trematode. Evolution 2005; 59: 544–553. 10.1111/j.0014-3820.2005.tb01014.x [DOI] [PubMed] [Google Scholar]
- 51. Kerr B, Riley MA, Feldman MW, Bohannan BJ. Local dispersal promotes biodiversity in a real-life game of rock-paper-scissors. Nature 2002; 418: 171–174. 10.1038/nature00823 [DOI] [PubMed] [Google Scholar]
- 52. Anacker RL, Ordal EJ. Studies on the myxobacterium Chondrococcus columnaris. II. Bacteriocins. J Bacteriol 1959; 781: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rigaud T, Perrot-Minnot M-J, Brown MJF. Parasite and host assemblages: embracing the reality will improve our knowledge of parasite transmission and virulence. Proc R Soc B 2010; 277: 3693–3702. 10.1098/rspb.2010.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. LaFrentz BR, LaPatra SE, Shoemaker CA, Klesius PH. Reproducible challenge model to investigate the virulence of Flavobacterium columnare genomovars in rainbow trout Oncorhynchus mykiss . Dis Aquat Org 2012; 101: 115–122. 10.3354/dao02522 [DOI] [PubMed] [Google Scholar]
- 55. Shoemaker CA, Olivares-Fuster O, Arias CR, Klesius PH. Flavobacterium columnare genomovar influences mortality in channel catfish (Ictalurus punctatus). Vet Microbiol 2008; 127: 353−359. 10.1016/j.vetmic.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 56. Decostere A, Haesebrouck F, Devriese LA. Characterization of four Flavobacterium columnare (Flexibacter columnaris) strains isolated from tropical fish. Vet Microbiol 1998; 62: 35–45. 10.1016/S0378-1135(98)00196-5 [DOI] [PubMed] [Google Scholar]
- 57. Karvonen A, Rintamäki P, Jokela J, Valtonen ET. Increasing water temperature and disease risks in aquatic systems: climate change increases the risk of some, but not all, diseases. Int J Parasitol 2010; 40: 1483–1488. 10.1016/j.ijpara.2010.04.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained in the paper.


