Abstract
Background
MRSA infections are becoming more prevalent throughout the HIV community. MRSA infections are a challenge to both physicians and patients due to limited choice of therapeutic options and increased cost of care.
Objectives
This study was aimed to determine the prevalence of colonization and co-resistance patterns of MRSA species among HIV positive pediatric patients in the Amhara National Regional State, Northwest Ethiopia.
Methods
Culture swabs were collected from the anterior nares, the skin and the perineum of 400 participants. In vitro antimicrobial susceptibility testing was done on Muller Hinton Agar by the Kirby-Bauer disk diffusion method, using 30 μg cefoxitin (OXOID, ENGLAND) according to the recommendations of the Clinical and Laboratory Standards Institute. Methicillin sensitivity/resistance was tested using cefoxitin. Data was analyzed by descriptive statistics and logistic regression model using Epi Info 7.
Results
S. aureus was detected in 206 participants (51.5%). The prevalence of MRSA colonization in this study was 16.8%. Colonization by S. aureus was associated with male gender (OR = 0.5869; 95% CI: 0.3812–0.9036; p-value = 0.0155), history of antibiotic use over the previous 3 months (OR = 2.3126; 95% CI: 1.0707–4.9948; p-value = 0.0329) and having CD4 T-cell counts of more than 350 x 106 cells / L (OR = 0.5739; 95% CI = 0.3343–0.9851; p-value = 0.0440). Colonization by MRSA was not associated with any one of the variables. Concomitant resistance of the MRSA to clindamycin, chloramphenicol, co-trimoxazole, ceftriaxone, erythromycin and tetracycline was 7.6%, 6%, 5.25%, 20.9%, 23.9% and 72.1%, respectively.
Conclusion
High rates of colonization by pathogenic MRSA strains is observed among HIV positive pediatric patients in the Amhara National Regional state.
Introduction
HIV-infected patients have increased Staphylococcus aureus colonization [1,2]. As colonization by Methicillin Resistant S. aureus(MRSA) is associated with increased risk of infection by MRSA [1,3,4], individuals at risk for both the colonization and infection by MRSA may serve as sources of outbreaks in both hospital and community settings.
Generally, MRSA colonization is said to occur in individuals who have frequent exposure to healthcare settings and in those with frequent antibiotic usage as well as immune suppression [1, 5–7]. Other factors listed in the literature as risk for health facility associated MRSA infection are age, duration and place of hospitalization, underlying disease, invasive procedures or devices, previous hospitalization, intensity of care, proximity to a MRSA-colonized patient, underlying dermatological diseases [8–10]. Male-to-male sexual intercourse and housing conditions are also cited as potential risk factors for community acquired MRSA (CA-MRSA) [11,12].
MRSA infections are a challenge for physicians in developing countries to treat because of the limited choice of therapeutic options available [13] and due to the possibility of concomitant drug resistance of the MRSA to other antimicrobials. MRSA are also a challenge to patients in developing settings due to increased cost of care [14, 15]. The financial burden of MRSA care in regions of limited resource, such as Ethiopia, is not expected to be easy because there are other priorities such as TB, HIV and malaria already [16].
Infection prevention and control measures have been the primary means to attempt to limit the spread of MRSA. Management of MRSA infections among HIV infected children in the study area generally tends to be empirical based on reports from literature. To our knowledge, there are no reports on colonization rates and antibiotic susceptibility patterns of MRSA among HIV infected patients in the study area and Ethiopia in general. This study is aimed to provide an overview of the epidemiology and microbiology of MRSA in the Amhara National Regional State (ANRS) with special focus on HIV positive pediatric patients.
Patients and Methods
Study Design
A total of 400 study participants were recruited from the Pediatric HIV clinics of FelegeHiwot Referral Hospital, Dessie Referral hospital and Debre-Tabor Referral Hospital, in the Amhara National Regional State. Eligible participants were HIV-infected, under 15 years of age, receiving medical care at the aforementioned health facilities. Patients who were on antibiotic treatment for any bacterial infection during the time of data collection were excluded from the study. Any voluntary participants who visited the clinics from December 2013 through April 2014 were invited to participate in the study.
Variables
A structured questionnaire was used to collect microbiologic data (such as colonization by S.aureus and antibiotic suceptibility profiles) as well as pertinent data from patient records (such as demographic characteristics, immune status, type of ARV drugs used for therapy, history of antimicrobial drug use within the past 3 months, living condition, and history of skin infections).
Laboratory Procedures
From each participant, specimens for S. aureus culture were collected from the anterior nares, the skin of the back and the perineum using sterile broth moistened swabs.
The swabs were aseptically inoculated onto Sheep Blood Agar (SBA) and Mannitol Salt Agar (MSA) (Becton Dickinson)media. The culture plates were examined after 24–48 hours of incubation at 35°C (under anaerobic conditions for SBA and aerobic conditions for MSA plates). Sub culturing was done on both SBA and MSA.
Isolates were confirmed using Gram stain. Colonies with Gram positive cocci isolates were further tested for catalase and coagulase activity. In vitro antimicrobial susceptibility testing was done on Muller Hinton Agar by the Kirby-Bauer disk diffusion method using chloramphenicol (30ug), ceftriaxone (30 ug), ciprofloxacin (5 ug), clindamycin (10ug), erythromycin (15ug), tetracycline (30 ug) and trimethoprim-sulfamethoxazole (25ug).
Clinical and Laboratory Standards Institute (CLSI) guidelines were used for interpretation of zones of inhibition. Methicillin sensitivity/resistance was tested using 30 μg cefoxitin (OXOID, ENGLAND). The methicillin-susceptible strain of S. aureus ATCC 29213 and the MRSA isolate BMB9393 were used as controls.
Definitions
At each study visit, participants were classified as MRSA colonized if MRSA was detected from any one of the specimen collection sites. Participants were classified as colonized with methicillin-susceptible S. aureus (MSSA) if MSSA was detected and MRSA was not detected. Participants colonized with both MSSA and MRSA were classified as MRSA colonized.
Statistical Methods
The primary analysis compared participants in whom a MRSA colonization had occurred with those without MRSA colonization. All analyses were performed using Epi Info 7.
By using a binary logistic regression model, adjusted Odds ratios (AOR) and 95% Confidence Intervals were calculated to identify variables associated independently with the development of MRSA colonization. All variables with the p value less than zero point two (p<0.2) in univariate analysis were included in a multivariate model. Statistical significance was indicated by a p value <0.05.
Ethics Statement
Ethical clearance was obtained from the Institutional Review Boards (IRB) of the Biotechnology Research Institute (BRI) of Bahir Dar University. Verbal consent was obtained from the participants' guardians in order to avoid cultural issues that would have been raised by signing documents amongst the highly stigmatized HIV patients in the study area. Additionally, an affirmation of a desire to give specimen was secured from older participants (7–15 years of age). A script of the oral consent written in Amharic language, which was approved by the IRBs, was used as a guide to obtaining the verbal consent. A log was prepared to document responses to the invitations to participate in the study. Permission to conduct the study was obtained from the Amhara National Regional Health Bureau and the medical directors’ offices of the respective hospitals included in the study.
Results
From the total 400 HIV-infected pediatric patients, 1200 specimens were collected. Most participants (77%) were urban dwellers, with the majority having a follow up at the Felege Hiwot Referral Hospital in Bahir Dar city (47%), followed by Dessie Referral Hospital in Dessie city (37.5%) and the rest from Debre-tabor Referral Hospita in Debre-tabor city (15.5%).
Males accounted for 56% of the cases (Male: Female ratio = 1.3) with median age of 10 (IQR = 4). The majority of participants (37%) had a base line WHO stage III HIV diagnosis. The median most recent CD4 cell count was 597 cells/μL. Antiretroviral therapy had been taken by 79.8% of the patients, with the majority (36%) on Zidovudine based standard regimen. Nearly 80% of the patients were on Co-trimoxazole prophylaxis therapy (CPT). About 2.5% of the patients had history of treatment failure while 3.5% had history of hospital admission within the past 1 year. The demographic and clinical parameters of the participants is summarized on Table 1.
Table 1. Baseline demographic data in HIV-infected children who gave skin, nasal and perineal samples, Amhara National Regional State, 2014.
| Variable | Patients, n (%) |
|---|---|
| Female | 176 (44) |
| Type of residence, n (%) | |
| Urban | 397 (99.2) |
| Rural | 3 (0.8) |
| Median age (IQ range) | 10 (4) |
| WHO Stage, n(%) | |
| I | 93 (23.2) |
| II | 139 (34.8) |
| III | 149 (37.2) |
| IV | 19 (4.8) |
| CD4 count, Median (IQR) | |
| Baseline | 322 (392) |
| Most recent | 597 (493) |
| Hospitalization within the past 12 months, n (%) | |
| Yes | 14 (3.5) |
| No | 386 (96.5) |
| History of antibiotic use in the past 3 months, n(%) | |
| Yes | 33 (8.2) |
| No | 367 (91.8) |
| History of treatment with HAART, n (%) | |
| Taking HAART | 318 (79.5) |
| Not on ARVs | 82 (20.5) |
| Months on HAART, Mean | 36.24 |
| History of ARV treatment failure, n (%) | |
| Yes | 10 (2.5) |
| No | 390 (97.5) |
| Co-trimoxazole Preventive Therapy, n (%) | |
| Yes | 318 (79.5) |
| No | 82 (20.5) |
| Months on CPT, Mean | 34.91 |
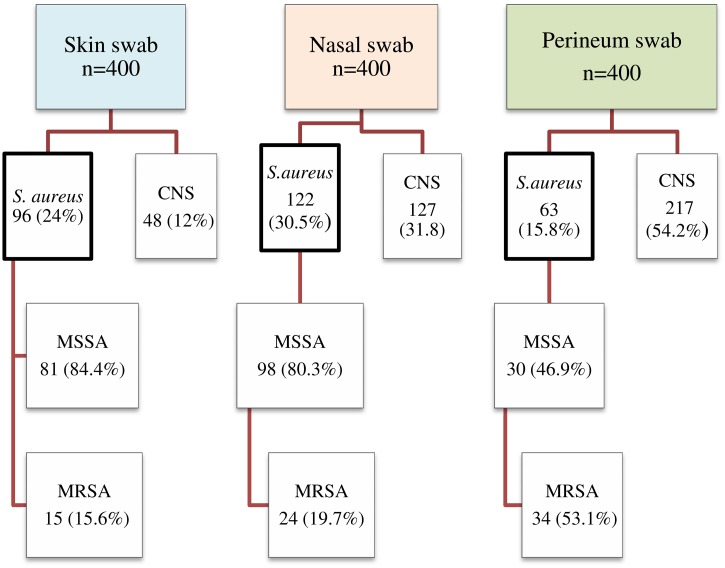
Overall, 281 of the specimens (23.4%) contained S. aureus, of which 73 (26%) were identified to be MRSA isolates. These isolates were identified from any of the three specimen collection sites of 206 participants (51.5%). Out of these 206 participants with S. aureus colonization, 67 of them (32.5%) were colonized by MRSA. Thus, the prevalence of MRSA colonization in this study was calculated to be 16.8% (i.e., 67 out of 400 total participants).
The majority of the MRSA species (47.5%) were isolated from the perineum specimens, followed by nasal (32.8%) and skin specimens (19.7%) (Fig 1). In addition, MRSA colonization at two distinct specimen collection sites was detected in 1.25% of the participants. However, no patient had reports of MRSA colonization at all three sites simultaneously.
Fig 1. Numbers of S. aureus clinical isolates by type of specimen among 400 HIV-infected children, Amhara National Regional State, 2014.
Additionally, MSSA was detected in 209 (17.4%) patient samples, while coagulase negative staphylococci (CNS) were identified from 392 (32.7%) of the patient samples.
In logistic regression analysis (Tables 2 and 3), there were no significant associations between MRSA colonization and the independent variables. However, colonization by S. aureus was observed to have a statistically significant association with each of male gender (OR = 0.5869; 95% CI: 0.3812–0.9036; p-value = 0.0155), history of antibiotic use over the previous 3 months (OR = 2.3126; 95% CI: 1.0707–4.9948; p-value = 0.0329) and having CD4 T-cell counts of more than 350 x 106 cells / L (OR = 0.5739; 95% CI = 0.3343–0.9851; p-value = 0.0440).
Table 2. Correlation between Variables and Colonization by S. aureus among 400 HIV-Infected Pediatric Patients, Amhara National Regional State, 2014.
| Covariate | Odds ratio | p-value | 95% CI |
|---|---|---|---|
| Gender (Male/Female) | 0.5869 | 0.0155 | 0.3812–0.9036 |
| Age Groups | 0.9364 | 0.858 | 0.4557–1.9242 |
| History of hospitalization in the past 3 months | 0.7334 | 0.6398 | 0.2002–2.6871 |
| History of antibiotic therapy in the previous 3 months (yes/No) | 2.3126 | 0.0329 | 1.0707–4.9948 |
| Initiation of ART | 0.818 | 0.5594 | 0.4167–1.6059 |
| Type of HAART regimen | 0.9062 | 0.0928 | 0.8078–1.0165 |
| Duration on HAART | 1.2616 | 0.069 | 0.9821–1.6208 |
| Initiation of CPT | 1.3286 | 0.4248 | 0.6612–2.6695 |
| Duration on CPT | 1.3377 | 0.167 | 0.8854–2.0210 |
| History of ART failure | 0.6613 | 0.5425 | 0.1748–2.5024 |
| Baseline WHO stage | 1.0798 | 0.5545 | 0.8370–1.3931 |
| Most recent WHO stage | 0.9988 | 0.9845 | 0.8855–1.1266 |
| Baseline CD4Category* | 0.7745 | 0.2294 | 0.5106–1.1749 |
| Most Recent CD4Category* | 0.5739 | 0.044 | 0.3343–0.9851 |
*CD4T-cell count of 350 x 106 cells / L or more per ml versus CD4T-cell counts under 350 x 106 cells / L
Table 3. Correlation between Variables and Colonization by MRSA among 400 HIV-Infected Pediatric Patients, Amhara National Regional State, 2014.
| Covariate | Odds ratio | p-value | 95% CI |
|---|---|---|---|
| Gender (Male/Female) | 0.9772 | 0.9324 | 0.5733–1.6657 |
| Age Groups | 1.0104 | 0.9615 | 0.6633–1.5392 |
| History of hospitalization in the past 3 months | 1.0297 | 0.9704 | 0.2193–4.8352 |
| History of antibiotic therapy in the previous 3 months (yes/No) | 2.608 | 0.027 | 0.1010–2.5420 |
| Initiation of ART | 1.0167 | 0.9696 | 0.4346–2.3786 |
| Type of HAART regimen | 0.9679 | 0.6562 | 0.8383–1.1175 |
| Duration on HAART | 0.9472 | 0.7921 | 0.6329–1.4176 |
| Initiation of CPT | 0.9714 | 0.93 | 0.5090–1.8541 |
| Duration on CPT | 1.0226 | 0.9367 | 0.5886–1.7767 |
| History of ART failure | 0.394 | 0.2076 | 0.0926–1.6771 |
| Baseline WHO stage | 0.863 | 0.4037 | 0.6107–1.2195 |
| Most recent WHO stage | 1.0877 | 0.305 | 0.9263–1.2771 |
| Baseline CD4 Category* | 0.8436 | 0.5508 | 0.4825–1.4751 |
| Most Recent CD4Category* | 0.6809 | 0.2547 | 0.3514–1.3193 |
*CD4T-cell count of 350 x 106 cells / L or more per ml versus CD4T-cell counts under 350 x 106 cells / L
In toto, as much as 20.9% of the MRSA isolates were resistant to ceftriaxone.Resistance to each of ciprofloxacin or erythromycin was noticed in 23.9% of the isolates.
While the figure for tetracycline resistance was highest (72.1%), clindamycin, chloramphenicol and co-trimoxazole had resistance rates of 7.6%, 6% and 5.25% respectively. Table 4 shows the antibiotic susceptibility pattern of the MRSA isolates to common antimicrobial agents in the study area.
Table 4. Antibiotic co-resistance pattern of MRSA isolated from HIV positive pediatric patients, Amhara National Regional state, 2014.
| Antibiotic | Per cent | 95% CI |
|---|---|---|
| Chloramphenicol | 6 | 0.32–2.72 |
| Ceftriaxone | 20.9 | 2.00–5.94 |
| Ciprofloxacin | 23.9 | 2.38–6.55 |
| Clindamycin | 7.6 | 0.46–3.06 |
| Co-trimoxazole | 5.25 | 3.36–8.04 |
| Erythromycin | 23.9 | 2.38–6.55 |
| Tetracycline | 72.1 | 9.28–15.96 |
Discussion
This study is the first of its kind to assess rates of S. aureus colonization of HIV infected pediatric patients in the study area particularly and Ethiopia at large. The findings underscore the existence of alarming rates of MRSA colonizers that are simultaneously resistant to some commonly prescribed antimicrobial agents.
Overall, the rate of S. aureus colonization reported from this study (51.5%) was higher than that of a study involving HIV positive children in Durban, South Africa (26.5%) [17].
More S. aureus were identified from the nasal specimen than the perineal swabs, at rates of approximately half of the values for nasal carriage. Although intestinal carriage rates of S. aureus among HIV infected children have not been widely investigated, studies involving different sets of adult population generally indicate comparatively higher figures of nasopharyngeal carriage than those of perineal carriage [18–20].
Our finding on the prevalence rate of nasopharyngeal colonization by S. aureus from this study was consistent with specific figures from West Bengal (24%) [21] and Cambodia (30.4%) [22]. However, higher rates were observed among children with HIV-1 infection in a hospital-based cross-sectional study from Brazil (45.16% vs. 19%) [23]. While these differences could be attributed to differing use of antimicrobials in the corresponding study areas, all these figures are generally indicative of requiring high attention.
The proportion of MRSA identified in this study was compared with reports from two different studies conducted in Soweto (South Africa) (39% and 60%) [24,25]. A small retrospective case-control study undertaken among patients followed at the Texas Children's Hospital Retrovirology Clinic indicated even higher rates of MRSA (82%) [26].
In our study, colonization by S. aureus had a significant statistical association with some variables. Nonetheless, we observed differing levels of consistency with findings of previous studies. For example, history of antibiotic use over the previous 3 months increased the likelihood of colonization by Staphylococci (OR = 2.3126; 95% CI: 1.0707–4.9948; p-value = 0.0329), while high CD4 T-cell counts were found to have got a protective effect. In contrary, Bhattacharya et al reported no association with immune status or recent antibiotic use [21]. Also, male gender had lower odds for colonization by S. aureus (OR = 0.5869; 95% CI: 0.3812–0.9036; p-value = 0.0155), while Bogaert et al indicated the opposite amongst healthy children (OR 1.46, 1.25–1.70) [27]. Other reports had found associations between age and S. aureus carriage in both healthy as well as HIV infected children, though there are inconsistencies with the specific age groups for the peak incidence for the colonization by S. aureus [27, 28]. No statistical relationship was observed with age in this study.
The assessment of the antibiotic susceptibility profiles of the MRSA isolates generally indicated high rates of co-resistance of MRSA to commonly prescribed antibiotics such as ceftriaxone, erythromycin and tetracycline. Groom et al [24] had similarly reported high figures of antibiotic resistance of MRSA to tetracyclines and erythromycin, while their findings on TMP—SMX and clindamycin co-resistance were much higher than our observation (94% and 65% respectively). Clindamycin resistance was observed in 19% of the MRSA isolates [29] in a different study.
The observed low rates of resistance to clindamycin, chloramphenicol and co-trimoxazole might suggest that most of these colonizations were caused by CA-MRSA rather than HA-MRSA strains [30–33]. Strain typing of the MRSA clinical isolates by molecular tools is recommended. Nevertheless, the high rates of concomitant drug resistance to the commonly available reserve intravenous antibiotics for use among the pediatric population needs to be given a special attention. This is because S. aureus is among the most common pathogens encountered in pediatric practice [34]. Additionally, reports of epidemics due to MRSA are not uncommon [34]. As all of the participants of this study were ambulatory, those that are colonized by the MRSA could easily transmit the pathogenic bacteria to other members of the community.
Staphylococcal infections tend to be serious necessitating aggressive treatment with specific and effective antimicrobial agents. Therefore, colonization by multidrug resistant MRSA of immunocompromised patients warrants intervention.
The authors of this study know, from anecdotal information, that intravenous ceftriaxone is one of the antibiotics recommended for the treatment of Staph infections in the study area. However, in the era of growing resistance to cephalosporins, cheaper alternatives need to be available. The cost of vancomycin and intravenous clindamycin is well above the affordability range of poor HIV patients and families, who need to meet the cost of other demands such as that of nutrition and treatment for opportunistic infections.
Based on the findings of this study, we recommend the use of chloramphenicol, co-trimoxazole or clindamycin for the management of MRSA infections among HIV infected patients in the ANRS. Additionally, decolonization and prompt institution of MRSA surveillance need to be considered by policy makers as these individuals might act as sources of outbreak.
One limitation of this study is its design in that it is a cross sectional study that does not allow comparison with the MRSA colonization rate of HIV negative children. Additionally, it was difficult to determine whether the colonization by S. aureus was persistent or intermittent. Lack of incorporation of viral load results was another limitation which would have made checking statistical associations between level of immune suppression of participants and colonization by MRSA more objective.
Conclusion
High rates of colonization by pathogenic MRSA strains was observed among HIV positive pediatric patients in the Amhara National Regional state. The MRSA isolates from colonizers of skin, nose or perineum of HIV-infected children were found to be concomitantly antibiotic resistant to cephalosporin, tetracycline and erythromycin.
Recommendation
We recommend antibiotics appropriate for multidrug resistant MRSA infections be introduced to the ANRS. There is an urgent need to further document the spectrum and antibiotic resistance profiles of HIV-infected children in under-resourced communities. Molecular analysis of the methicillin resistant S. aureus bacterial isolates for strain typing is planned as a continuation of this study.
Acknowledgments
We are deeply indebted to the Biotechnology Research Institute of Bahir Dar University for funding this study and the Research and Community Service for approving the study. We are grateful to Dr KefyalewAlemayehu for his kind guidance during times of challenge and to his wise advices. We also thank staff at the Pediatric HIV clinics and laboratories of the study sites. Last, we would like to appreciate our participants without whom this study would not have been possible.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was granted by the Research and Community Service Office of Bahir Dar University (http://www.bdu.edu.et/) under the watchful eye of the "Biotechnology Research Institute" of Bahir Dar University. MTL and YZC received the funding and submitted progress reports to the Biotechnology Institute as per the agreement.
References
- 1. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. ClinMicrobiol Rev. 1997; 10:505–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shet A, Mathema B, Mediavilla JR, Kishii K, Mehandru S, Jeane-Pierre P, et al. Colonization and subsequent skin and soft tissue infection due to methicillin-resistant Staphylococcus aureus in a cohort of otherwise healthy adults infected with HIV type 1. J Infect Dis. 2009; 200:88–93. 10.1086/599315 [DOI] [PubMed] [Google Scholar]
- 3. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus . Am J Med. 2008. April; 121(4):310–5. 10.1016/j.amjmed.2007.07.034 [DOI] [PubMed] [Google Scholar]
- 4. Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005; 5(12):751–762. [DOI] [PubMed] [Google Scholar]
- 5. Nguyen MH, Kauffman CA, Goodman RP, Squier C, Arbeit RD, Singh N, et al. Nasal carriage of and infection with Staphylococcus aureus in HIV-infected patients. Ann Intern Med. 1999; 130:221–5. [DOI] [PubMed] [Google Scholar]
- 6. Drapeau CM, Angeletti C, Festa A, Petrosillo N. Role of previous hospitalization in clinically-significant MRSA infection among HIV-infected inpatients: results of a case—control study. BMC Infect Dis. 2007, 7:36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klevens RM, Morrison MA, Fridkin SK, Reingold A, Petit S, Gershman K, et al. Community-associated methicillin-resistant Staphylococcus aureus and healthcare risk factors. Emerg Infect Dis. 2006, 12(12):1991–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Onorato M, Borucki MJ, Baillargeon G, Paar DP, Freeman DH, Cole CP, et al. Risk factors for colonization or infection due to methicillin-resistant Staphylococcus aureus in HIV-positive patients: a retrospective case-control study. Infect Control HospEpidemiol. 1999. January;20(1): 26–30. [DOI] [PubMed] [Google Scholar]
- 9. Warshawsky B, Hussain Z, Gregson DB, Alder R, Austin M, Bruckschwaiger D, et al. Hospital- and community-based surveillance of methicillin-resistant Staphylococcus aureus: Previous hospitalization is the major risk factor. Infect Control HospEpidemiol 2000. November; 21(11): 724–7. [DOI] [PubMed] [Google Scholar]
- 10. Santoro-Lopes G, de Gouvea EF, Monteiro RC, Branco RC, Rocco JR, Halpern M, et al. Colonization with methicillin-resistant Staphylococcus aureus after liver transplantation. Liver Transpl 2005. February; 11(2): 203–9. [DOI] [PubMed] [Google Scholar]
- 11. Diep BA, Chambers HF, Graber CJ, Szumowski JD, Miller LG, Han LL, et al. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med 2008; 148:249–57. [DOI] [PubMed] [Google Scholar]
- 12. Popovich KJ, Weinstein RA, Aroutcheva A, Rice T, Hota B. Community associatedmethicillin-resistant Staphylococcus aureus and HIV: intersecting epidemics. Clin Infect Dis. 2010; 50:979–87. 10.1086/651076 [DOI] [PubMed] [Google Scholar]
- 13. Herwaldt LA. Control of methicillin-resistant Staphylococcus aureus in the hospital setting. Am J Med 1999. May; 106 (5A): 11S–18S. [DOI] [PubMed] [Google Scholar]
- 14. Abramson MA, Sexton DJ. Nosocomial methicillin-resistant and methicillin-susceptible Staphylococcus aureus primary bacteremia: at what costs? Infect Control HospEpidemiol 1999. June; 20(6): 408–11. [DOI] [PubMed] [Google Scholar]
- 15. Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006; 42(Suppl 2):S82–S89. [DOI] [PubMed] [Google Scholar]
- 16.Federal Ministry of Health of Ethiopia; Ethiopia’s Fourth National Health Accounts, 2007/08; accessed in August 2012 on http://www.who.int/nha/country/eth/ethiopia_nha_4.pdf
- 17. McNally LM, Jeena PM, Gajee K, Sturm AW, Tomkins AM, Coovadia HM, et al. Lack of association between the nasopharyngeal carriage of Streptococcus pneumonia and Staphylococcus aureus in HIV-1-infected South African children. J Infect Dis.2006; 194, 385–390. [DOI] [PubMed] [Google Scholar]
- 18. Acton DS, Plat-Sinnige MJ, van Wamel W, de Groot N, van Belkum A. Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its clinical impact? Eur J ClinMicrobiol Infect Dis. 2009. February;28(2):115–27. [DOI] [PubMed] [Google Scholar]
- 19. Szumowski JD, Wener KM, Gold HS, Wong M, Venkataraman L, Runde CA, et al. Methicillin-resistant Staphylococcus aureus colonization, behavioral risk factors, and skin and soft-tissue infection at an ambulatory clinic serving a large population of HIV-infected men who have sex with men. Clin Infect Dis. 2009. July 1;49(1):118–21. 10.1086/599608 [DOI] [PubMed] [Google Scholar]
- 20. Joore IK, van Rooijen MS, Schim van der Loeff MF, de Neeling AJ, van Dam A, de Vries HJ. Low prevalence of methicillin-resistant Staphylococcus aureus among men who have sex with men attending an STI clinic in Amsterdam: a cross-sectional study. BMJ Open. 2013. March 5;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhattacharya SD, Niyogi SK, Bhattacharyya S, Arya BK, Chauhan N, Mandal S. Associations between potential bacterial pathogens in the nasopharynx of HIV infected children. Indian J Pediatr. 2012;79(11):1447–53. 10.1007/s12098-012-0762-4 [DOI] [PubMed] [Google Scholar]
- 22. Krcmery V, Sokolova J, Kulkova N, Liskova A, Shahum A, Benca G. Nasopharyngeal bacterial colonisation in HIV-positive children in Cambodia. Trop Med Int Health. October 2013; 18 (10):1267–1268 10.1111/tmi.12178 [DOI] [PubMed] [Google Scholar]
- 23. D'Avila NE, Zhang L, Miller RG, D'Avila AC, Conceição AP, Boffo MS. High prevalence of nasopharyngeal colonization by Staphylococcus aureus among children with HIV-1 infectionin extreme southern Brazil. J Trop Pediatr. 2008;54(6):410–2. 10.1093/tropej/fmn051 [DOI] [PubMed] [Google Scholar]
- 24. Groome MJ, Albrich WC, Wadula J, Khoosal M, Madhi SA. Community-onset Staphylococcus aureusbacteraemia in hospitalised African children: high incidence in HIV-infected children and high prevalence of multidrug resistance. PaediatrInt Child Health. 2012;32(3):140–146 [DOI] [PubMed] [Google Scholar]
- 25. Madhi SA, Petersen K, Madhi A, Khoosal M, Klugman KP. Increased disease burden and antibiotic resistance of bacteria causing severe community-acquired lower respiratory tract infections in human immunodeficiency virus type 1-infected children.Clin Infect Dis. 2000;31:170–6. [DOI] [PubMed] [Google Scholar]
- 26. McNeil JC, Hulten KG, Kaplan SL, Schwarzwald HL, Mason EO. Staphylococcus aureus infections in HIV-positive children and adolescents. Pediatr Infect Dis J. 2012;31(3):284–6. 10.1097/INF.0b013e318239c1fe [DOI] [PubMed] [Google Scholar]
- 27. Bogaert D, van Belkum A, Sluijter M, Luijendijk A, de Groot R, Rümke HC, et al. Colonization by Streptococcus pneumonia and Staphylococcus aureus in healthy children. Lancet. 2004b; 363, 1871–1872 [DOI] [PubMed] [Google Scholar]
- 28. Kinabo GD, van der Ven A, Msuya LJ, Shayo AM, Schimana W, Ndaro A, et al. Dynamics of nasopharyngeal bacterial colonization in HIV exposed young infants in Tanzania. Trop Med Int Health. 2013; 18(3):286–95. 10.1111/tmi.12057 [DOI] [PubMed] [Google Scholar]
- 29. Srinivasan A, Seifried S, Zhu L, Bitar W, Srivastava DK, Shenep JL, et al. Short communication: methicillin-resistantStaphylococcus aureus infections in children and young adults infected with HIV. AIDS Res Hum Retroviruses. 2009;25(12):1219–24. 10.1089/aid.2009.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen AE, Goldstein M, Carroll K, Song X, Perl TM, Siberry GK. “Evolving epidemiology of pediatric Staphylococcus aureuscutaneous infections in a Baltimore hospital.”PediatrEmerg Care. 2006; 22(10): 717–723. [DOI] [PubMed] [Google Scholar]
- 31. Dietrich WD, Auld BD, and Mermel AL. Community-acquired methicillin-resistantStaphylococcus aureus in southern New England children. Pediatr. 2004; 113(4): e347–e352. [DOI] [PubMed] [Google Scholar]
- 32. Fergie EJ, Purcell K. Community-acquired methicillin-resistant Staphylococcus aureusinfections in South Texas children. Pediatr Infect Dis J. 2001; 20(9):860–863. [DOI] [PubMed] [Google Scholar]
- 33. Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, et al. EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006; 355(7):666–74. [DOI] [PubMed] [Google Scholar]
- 34. Parras F, Rodríguez M, Bouza E, Muñoz P, Cercenado E, Guerrero C, et al. Epidemic outbreak of methicillin-resistant Staphylococcus aureus in a general hospital. Preliminary report. EnfermInfeccMicrobiolClin. 1991. April;9(4):200–7. Spanish. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.