Abstract
BACKGROUND
Timing of solid food introduction in infancy has been associated with several chronic diseases. To explore potential mechanisms, we investigated the relationship between timing of solid food introduction and F2-isoprostanes – a marker of oxidative stress.
METHODS
Urinary F2-isoprostanes were assessed in 336 healthy children <age 11.5 years with 1,266 clinic visits (mean=3.8 visits per child) in the Diabetes Autoimmunity Study in the Young. We analyzed the association between F2-isoprostane concentrations and infant diet exposures using linear mixed models adjusted for age, age2, HLA-DR3/4, DQB1*0302 genotype, first-degree relative with type 1 diabetes, maternal age, maternal education, sex, and exposure to in utero cigarette smoke.
RESULTS
Later solid food introduction was associated with lower F2-isoprostane concentrations in childhood (on average, 0.10 ng/mg per month of age at introduction) (estimate: −0.10, 95% CI: −0.18, −0.02, P-value=0.02). Moreover, childhood F2-isoprostane concentrations were, on average, 0.24 ng/mg lower in individuals breastfed at solid food introduction (estimate: −0.24, 95% CI: −0.47, −0.01, P-value=0.04) compared with those who were not. Associations remained significant after limiting analyses to F2-isoprostanes after age 2 years.
CONCLUSION
Our results suggest a long-term protective effect of later solid food introduction and breastfeeding at solid food introduction against increased F2-isoprostane concentrations throughout childhood.
INTRODUCTION
Timing of complementary food introduction has been implicated in the development of a number of chronic diseases, such as obesity(1,2), celiac disease(3,4), type 1 diabetes (T1D)(5,6), and atopic disorders(7,8). Previous guidelines established by the American Academy of Pediatrics recommended introduction of solid foods at 4 to 6 months of age, exclusive breastfeeding for the first 4 to 6 months of age, continued breastfeeding to the first birthday and beyond if possible, and the use of infant formula for the first year of life for those infants who are not breastfed(9). In 2012, the American Academy of Pediatrics updated their recommendations to include exclusive breastfeeding for about 6 months, followed by continued breastfeeding as complementary foods are introduced, with continuation of breastfeeding for 1 year or longer as mutually desired by mother and infant(10). The European Society of Pediatric Gastroenterology, Hepatology and Nutrition recommends not introducing solid foods before 4 to 6 months of age(11). However, a study by Clayton et al. (2013) recently showed 40.4% of US mothers introduced solid foods before age 4 months(12). It is important to understand the short- and long-term effects of infant feeding in order to better educate mothers and inform recommendations.
One possible long-term effect of early introduction of solid foods is increased oxidative stress, which results from an imbalance between the production of free radicals and reactive oxygen species, and the natural antioxidant capacity of the body that blocks and scavenges these radicals(13). Early life exposures have been shown to increase oxidative stress levels, such as exposure to maternal smoke in utero(14,15) and infant formula feeding compared to breastfeeding(16,17). Oxidative stress can damage lipids, proteins, and nucleic acids, and has been implicated in the pathogenesis of many diseases, including pediatric diseases, such as asthma, cystic fibrosis, and juvenile rheumatoid arthritis(13,18,19). A well-studied biomarker of in vivo oxidative stress is F2-isoprostanes, which are formed during the non-enzymatic oxidation of arachidonic acid by free radicals, including reactive oxygen species. Measurement of urinary F2-isoprostanes is an index of systemic oxidative stress in humans.
We previously investigated factors associated with urinary F2-isoprostanes in a cohort of healthy children at increased genetic risk for developing T1D(20). Being female, having the HLA-DR3/4 genotype, a higher plasma γ-tocopherol:total lipids ratio, and a lower α-carotene:total lipids ratio were associated with higher F2-isoprostane concentrations(20). In the current analysis, we explore whether timing of infant diet exposures is associated with increased urinary F2-isoprostanes.
RESULTS
Table 1 describes the 328 children in the subcohort with F2-isoprostane measurements and the individual associations between these characteristics and creatinine adjusted urinary F2-isprostane concentrations. Increasing age of the child, greater than 12 years of maternal education, and increasing maternal age at the birth of the child were associated with decreased urinary F2-isoprostane concentrations. Having the high T1D-risk HLA-DR3/4, DQB1*0302 genotype, being female, and being exposed to maternal cigarette smoke in utero were associated with increased F2-isprostane concentrations throughout childhood.
Table 1.
Association between population characteristics and creatinine-adjusted urinary F2-isoprostane concentrations in the 328 children in the subcohort of the Diabetes Autoimmunity Study in the Young
| Characteristic | n | Unadjusted Estimate (95% CI) |
|---|---|---|
| Child’s age, mean (SD), years | 4.2 (2.4) | −0.24 (−0.28, −0.20)* |
| HLA-DR3/4, DQB1*0302 | 95 (28.3%) | 0.29 (0.04, 0.54)* |
| First-degree relative with T1D | 110 (32.7%) | 0.19 (−0.08, 0.46) |
| Female | 162 (48.2%) | 0.30 (0.07, 0.53)* |
| Race/ethnicity, non-Hispanic white | 248 (73.8%) | −0.04 (−0.31, 0.22) |
| Maternal education, > 12 years | 259 (77.5%) | −0.38 (−0.66, −0.10)* |
| Maternal age, mean (SD), years | 29.7 (5.5) | −0.02 (−0.04, −0.003)* |
| Exposure to maternal cigarette smoke in utero | 41 (12.4%) | 0.39 (0.03, 0.75)* |
| Exposure to environmental tobacco smoke | 362 (29.1%) | 0.10 (−0.14, 0.34) |
P value < 0.05.
Table 2 displays the associations between infant diet predictors and F2-isoprostane concentrations. Adjusting for age, age2, HLA-DR3/4, DQB1*0302, first-degree relative with T1D, sex, maternal education, maternal age, and exposure to maternal cigarette smoke in utero, the later a child was introduced to solid foods, the lower their F2-isoprostane concentrations in childhood (on average, 0.10 ng/mg per month of age at introduction) (estimate: −0.10 (95% CI: −0.18, −0.02), P-value: 0.02). The full final model for the association between age at first exposure to solid foods and F2-isoprostane concentrations is displayed in Supplemental Table 1 (online) where in addition to age at first exposure to solid foods, age, age2, and sex remained significant. We then examined individual components of the solid foods variable and found that lower childhood F2-isoprostane concentrations were associated with later introduction to any cereal (wheat/barley/oats/rice) (on average, 0.10 ng/mg per month of age at introduction to any cereal, estimate: −0.10 (95% CI: −0.17, −0.02), P-value: 0.02) and meat (0.04 ng/mg per month of age at introduction to meat, estimate: −0.04 (95% CI: −0.07, −0.005), P-value: 0.03).
Table 2.
Infant diet predictors of creatinine-adjusted urinary F2-isoprostane concentrations in healthy DAISY children (1,246 observations, 328 subjects)
| Infant Diet Characteristic | Mean Duration or Age at First Exposure (SD) |
Adjusted Estimate At All Agesa (95% CI) |
Adjusted Estimate After Age 2a (95% CI) |
|---|---|---|---|
| Exclusive breastfeeding duration (months) | 1.4 (1.8) | −0.02 (−0.09, 0.04) | −0.04 (−0.11, 0.03) |
| Breastfeeding duration (months) | 6.5 (7.0) | −0.01 (−0.02, 0.01) | −0.01 (−0.03, 0.005) |
| Breast-milk months | 4.2 (3.4) | −0.02 (−0.05, 0.02) | −0.03 (−0.06, 0.01) |
| Age at first exposure to cow’s milk (months) | 4.0 (3.6) | −0.005 (−0.04, 0.03) | −0.01 (−0.04, 0.02) |
| Age at first exposure to any solid food (months) | 4.5 (1.4)b | −0.10 (−0.18, −0.02)* | −0.11 (−0.19, −0.03)* |
| Age at first exposure to any cereal (wheat/barley/oats/rice) (months) | 4.6 (1.4) | −0.10 (−0.17, −0.02)* | −0.10 (−0.18, −0.03)* |
| Age at first exposure to foods containing wheat/barley (months) | 6.9 (1.9) | −0.04 (−0.10, 0.02) | −0.04 (−0.10, 0.01) |
| Age at first exposure to foods containing rice/oat (months) | 4.7 (1.5) | −0.07 (−0.14, 0.01) | −0.08 (−0.15, −0.005)* |
| Age at first exposure to fruit, excluding fruit juice (months) | 5.8 (1.5) | −0.06 (−0.13, 0.01) | −0.06 (−0.13, 0.02) |
| Age at first exposure to vegetables (months) | 6.0 (1.4) | −0.02 (−0.09, 0.06) | −0.03 (−0.10, 0.05) |
| Age at first exposure to meat (months) | 9.3 (3.1) | −0.04 (−0.07, −0.005)* | −0.04 (−0.07, −0.003)* |
| Breastfeeding at Food Introduction Variables | n (%) Breastfed at Food Introduction | ||
| Breastfeeding at introduction of solid foods | 188 (56.5%) | −0.24 (−0.47 −0.01)* | −0.26 (−0.50, −0.03)* |
| Breastfeeding at introduction of cereal (wheat/barley/oats/rice) | 186 (55.9%) | −0.22 (−0.45, 0.01) | −0.24 (−0.47, −0.01)* |
| Breastfeeding at introduction of wheat/barley | 147 (44.1%) | −0.12 (−0.35, 0.10) | −0.13 (−0.36, 0.09) |
Adjusted for age, age2, HLA-DR3/4, DQB1*0302, first-degree relative with T1D, sex, maternal education, maternal
age, and exposure to maternal cigarette smoke in utero.
The mean age at first exposure to any solid food is less than the means of its components because 13 children were introduced to cheese before any cereal.
P value < 0.05.
The associations between breastfeeding at complementary food introduction and F2-isoprostane concentrations throughout childhood are also described in Table 2. Adjusting for age, age2, HLA-DR3/4, DQB1*0302, first-degree relative with T1D, sex, maternal education, maternal age, and exposure to maternal cigarette smoke in utero, childhood F2-isoprostane concentrations were, on average, 0.24 ng/mg lower in those who were breastfed at introduction of solid foods (estimate: −0.24 (95% CI: −0.47, −0.01), P-value: 0.04) compared with those who were not breastfed at introduction of solid foods. The full final model for breastfeeding at introduction of solid foods is displayed in Supplemental Table 1 (online) where in addition to breastfeeding at introduction of solid foods, age, age2, and sex remained significant.
To further investigate the relationship between F2-isoprostane concentrations, timing of introduction of solid foods, and breastfeeding at the introduction of solid foods, a categorical variable with six levels was created. It represented whether the child was introduced to solid foods early (< 4 months of age), according to previous recommendations by the American Academy of Pediatrics (4–5 months of age), or late (≥ 6 months of age) and whether or not the child was breastfed when introduced to solid foods. We chose being introduced to solid foods between 4–5 months of age and breastfed at introduction of solid foods as the reference group. Being introduced to solid foods before the 4-month birthday and not being breastfed at the time of introduction was significantly associated with increased F2-isoprostane concentrations compared to being introduced to solid foods between 4–5 months of age and breastfed at introduction of solid foods (Figure 1).
Figure 1.
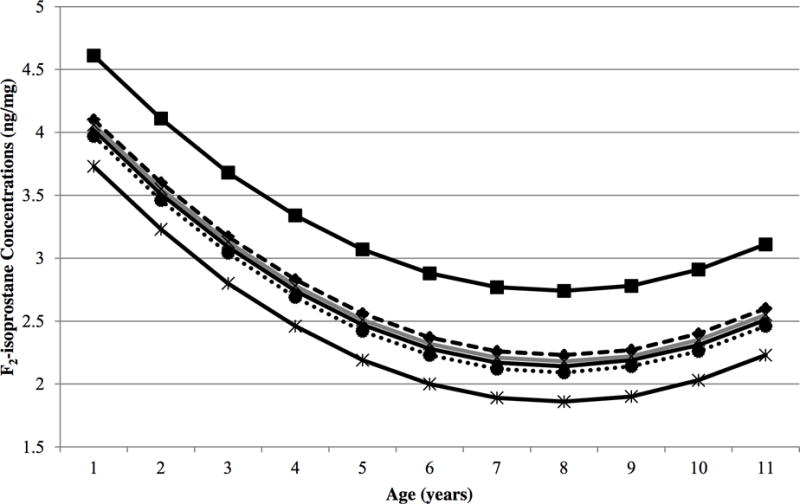
Urinary F2-isoprostane concentrations are related to age at first exposure to any solid food and being breastfed at introduction of solid foods in DAISY children. DAISY children introduced to solid foods before the 4-month birthday and not breastfed at introduction of solid foods (solid black line with square symbols) had, on average, significantly higher F2-isoprostane concentrations compared to children introduced to solid foods between 4–5 months of age and breastfed at introduction of solid foods (solid black line with triangle symbols) (P = 0.0004) after adjusting for age, age2, HLA-DR3/4, DQB1*0302, first-degree relative with T1D, sex, maternal education, maternal age, and exposure to maternal cigarette smoke in utero. The lowest F2-isoprostane concentrations were seen in children introduced to solid foods on or after the 6-month birthday and breastfed at introduction of solid foods (solid black line with asterisk symbols). The additional groups are represented by the following lines: introduced to solid foods before the 4-month birthday and breastfed at introduction of solid foods (dashed black line with diamond symbols), introduced to solid foods between 4–5 months of age and not breastfed at introduction of solid foods (solid gray line with x symbols), introduced to solid foods on or after the 6-month birthday and not breastfed at introduction of solid foods (dotted black line with circle symbols).
To see if age at solid food introduction had an impact on F2-isoprostane concentrations long after the initial exposure, we ran the same analyses as described in the preceding paragraph, but limited F2-isoprostane measurements to those collected after 2 years of age (Table 2). Later introduction to solid foods, including any cereal (wheat/barley/oats/rice) and meat, as well as being breastfed at introduction of solid foods were still associated with decreased F2-isoprostane concentrations in childhood after limiting F2-isoprostane measurements to those after 2 years of age. Additionally, breastfeeding at introduction of cereal (wheat/barley/oats/rice) and later introduction to rice/oat were associated with decreased F2-isoprostane concentrations in childhood after limiting F2-isoprostane measurements to those after 2 years of age. The final full models for first exposure to solid foods and breastfeeding at introduction of solid foods limited to F2-isoprostane measurements after 2 years are displayed in Supplemental Table 1 (online).
DISCUSSION
This longitudinal analysis in a prospective cohort demonstrates a novel association between timing of solid food introduction and F2-isoprostane concentrations, a measure of oxidative stress. Oxidative stress plays an influential role in the development of many chronic diseases. Understanding how early life exposures affect oxidative stress throughout childhood may help to prevent the long-term effects oxidative stress appears to have on development of chronic disease both in childhood and later in life.
One of the strengths of this study is its prospective design. Infant diet data were collected prospectively at 3 month intervals for the first 15 months of life, increasing accuracy of the data. Another strength of this study is that F2-isoprostane measurements collected at multiple time points throughout childhood allowed us to estimate the effect of timing of infant diet exposures on F2-isoprostane concentrations throughout early childhood. To our knowledge, this is the first study to examine the effect of timing of infant diet exposures on F2-isoprostane concentrations.
Previous studies examining oxidative stress in relation to infant feeding type have found higher F2-isoprostane values in children who were formula fed compared to children who were breastfed(16,17). Human colostrum has been shown to exhibit antioxidant and anti-inflammatory properties(21). Additionally, scavengers of free radicals, including α-tocopherol, cysteine, and ascorbate are higher in breast-milk than cow’s milk(22). We did not find an association between breastfeeding duration and F2-isoprostane concentrations. However, our results suggest that breastfeeding at solid food introduction is associated with decreased F2-isoprostane concentrations and average F2-isoprostane concentrations were highest in children who were introduced to solid foods before the 4-month birthday and not breastfed at introduction of solid foods.
Breastfeeding at introduction of solid foods has been explored as a protective factor for inflammatory diseases, such as celiac disease, T1D, and food allergies. A number of studies have observed a protective association between breastfeeding while introducing gluten and celiac disease risk(4,23–25). Previously the Diabetes Autoimmunity Study in the Young (DAISY) showed reduced islet autoimmunity (IA) risk if cereals were introduced while the child was still breastfeeding(26), and more recently a protective association between breastfeeding at introduction of solid foods and T1D risk was shown(5). A nested case-control study exploring introduction of complementary foods and its relationship to developing food allergies found that infants diagnosed with food allergies before 2 years of age were less likely to be breastfed when cow’s milk protein was first introduced(8). The results of our current analyses provide another reason for breastfeeding while introducing solid foods – reducing urinary F2-isoprostane concentrations in childhood.
Regarding timing of introduction to solid foods, much of the literature supports introducing solid foods after the 4-month birthday. Before 4 months of age, the child has a relatively immature gut immune system that may have an abnormal response to solid food antigens. Grimshaw et al. (2013) found infants diagnosed with a food allergy before 2 years of age were introduced to solid foods earlier (≤ 16 weeks of age)(8). Early introduction to gluten-containing foods has been associated with celiac disease autoimmunity(3). Early introduction to solid foods(5), gluten-containing foods(6), and dairy products(27) have been associated with increased T1D risk and early introduction to cereals(26,28), gluten-containing foods(6,29), fruits and berries(30), root vegetables(28), and egg(28) have all been associated with increased risk of IA. Early introduction to solid foods has also been associated with childhood obesity(1,2).
The results of this study suggest an increase in urinary F2-isoprostane concentrations may be a potential mechanism through which timing of solid food introduction increases risk of childhood diseases, such as T1D, celiac disease, and allergies. The gut microbiota may play a role in this pathway with infant diet leading to increased oxidative stress, changing the gut microbiota and eventually leading to chronic disease as shown in a murine model(31). This murine model showed a long-term high-fat diet induced oxidative stress that then influenced gut microbiota based on different strains of intestinal bacteria having varying degrees of growth sensitivity to oxidative stress, believed to eventually lead to metabolic syndrome(31). A study examining the influence of milk-feeding type and HLA-genotype on intestinal microbiota in infants with a family history of celiac disease found that while both milk-feeding type and HLA-genotype influenced intestinal microbiota, breastfeeding reduced the differences in microbiota composition attributed to HLA-genotype(32). This is encouraging for those with the increased risk HLA genotypes, as the effect these genotypes have on the microbiota associated with increased risk of celiac disease, can be reduced through a modifiable exposure, early infant diet.
In conclusion, our results suggest a long-term protective effect of later solid food introduction and breastfeeding at solid food introduction against increased urinary F2-isoprostane concentrations throughout childhood. Future research that investigates pathways between infant diet, gut microbiota, oxidative stress, and chronic disease in human populations may inform new strategies for chronic disease prevention starting in infancy.
METHODS
Study Population
DAISY recruited two groups of children between 1993 and 2004 who are at increased risk for T1D, and followed them prospectively for IA and T1D development. One group is composed of first-degree relatives of patients with T1D, identified through the Barbara Davis Center for Childhood Diabetes and recruited mainly between birth and age 2 years. The second group is composed of infants born at St. Joseph’s Hospital in Denver, CO, whose umbilical cord blood was screened for T1D-susceptibility HLA-DR, DQ genotypes and were recruited if they had these genotypes(33). Details of the newborn screening, sibling and offspring recruitment, and follow-up of both cohorts have been published previously(34). All study protocols were approved by the Colorado Multiple Institutional Review Board, and informed consent was given by parents of all participating children.
The DAISY cohort is composed of 2,547 children at increased genetic risk for developing T1D. F2-isoprotanes were investigated in a representative sample of 380 children (i.e. subcohort) selected from the DAISY cohort via stratified sampling based on HLA-DR genotype and family history of T1D. F2-isoprostane measurements were obtained on 336 children in the subcohort between the ages of 6 months and 11.5 years from August 1997 – December 2005. Of the 336 with F2-isoprostane measurements, 1 child was missing maternal education, 3 children were missing exposure to maternal smoke in utero, 3 children were missing first exposure to solid food, and 1 child was missing both maternal education and exposure to maternal smoke in utero, which results in 328 children with complete prospective exposure data and 1,246 F2-isoprostane measurements (mean 3.8 measurements per child) used for analysis (Figure 2). There were 14 children who developed IA and the measurements at clinic visits at which a child was determined to be islet autoantibody positive are not included in these analyses.
Figure 2.
Flow chart for formation of analysis cohort
F2-Isoprostane Measurement
Urine specimens were stored at −70°C until shipment to the Vanderbilt University Eicosanoid Core Laboratory for the analysis of F2-isprostanes. F2-isoprostanes were measured as previously described(35,36). Briefly, after addition of the internal standard, [2H4]-8-iso-PGF2α, F2-IsoPs were extracted with C18 and silica Sep-Pak cartridges. The extracted F2-isoprostanes were then converted to the corresponding pentaflurobenzyl esters and purified by TLC. They were then converted to the trimethylsilyl ether derivatives and subsequently analyzed by gas chromatography (GC)/negative ion chemical ionization (NICI)-mass spectrometry (MS)(37,38). Creatinine is measured to correct for the concentration of the urine. Analysis of blinded duplicated pairs sent to the Eicosanoid Core Laboratory showed an intraclass coefficient (ICC) of 0.93. F2-isoprostane concentrations are reported as nanograms per milligram (ng/mg) of urine creatinine. For children who were not toilet-trained, we collected urine samples by placing cotton balls in the diaper. This mode of urine collection (cotton ball vs. direct stream catch) had no effect on F2-isoprostane measurements(39).
Infant Diet Measurement
Data on infant diet were collected during telephone or face-to-face interviews at 3, 6, 9, 12, and 15 months of age. At each interview, mothers were asked to report the date of introduction and frequency of intake (i.e., number of servings per day) of all milks, formulas, and foods the infants consumed during the previous 3 months. Exclusive breastfeeding duration was determined by the reported age at which the infant was exposed to any foods or liquids other than breast-milk or water. Breast-milk months is a relative quantity of breast-milk based on the proportion of breast-milk to formula over the first 9 months of life. For example, for infants exclusively breastfed for the first 9 months, the proportion of breast-milk to formula for each month was 1.0 and the number of breast-milk months summed to 9.0 breast-milk months. For infants who received both breast-milk and formula, the total number of servings of breast-milk for each month was divided by the total number of servings of formula and breast-milk for that month, and these were summed over the first 9 months to arrive at the number of breast-milk months. Based on previous work showing a protective effect of breastfeeding when introducing cereals (for IA)(26) or gluten (for celiac disease)(23), we created 3 additional breastfeeding variables to represent whether the child was breastfed at the time of introduction to any solid foods, cereals, and foods containing gluten (wheat/barley).
We created an overall variable of age at first exposure to any solid foods, as well as six variables that were components of this solid foods variables that included age at exposure to cereal (wheat/barley/oats/rice), wheat/barley, rice/oats, fruit (not including fruit juice), vegetables, and meat. There were no reports of introducing rye in the infant diet in DAISY children. Juices were not included in the fruit variable because we were interested in solid food introductions. The study was an observational study; therefore, no dietary advice was given to the participating families.
Environmental Tobacco Smoke
Exposure to maternal smoke in utero was collected via a mailed questionnaire when the child was 8–12 weeks old. The child’s exposure to environmental tobacco smoke (ETS) was assessed via questionnaire every 3 months during the first 15 months of life and annually starting at age 2 years. Children who had a mother or father who smoked or who were exposed at least once per week to other adults who smoked were coded as “exposed” to ETS. Seifert et al. (2002) demonstrated a correlation of 0.8 between responses to this ETS questionnaire and urine cotinine concentrations (a marker of tobacco smoke inhalation) in the DAISY population(40).
Statistical Analysis
We analyzed the relationship between F2-isoprostane concentrations and timing of infant diet exposures using a linear mixed modeling approach in the DAISY subcohort. We tested each infant diet exposure for association with F2-isoprostane concentrations in separate models adjusted for age, age2, HLA-DR3/4,DQB1*0302, first-degree relative with T1D, sex, maternal education, maternal age, and exposure to maternal cigarette smoke in utero. As seen previously(20), the relationship between age and F2-isoprostanes was non-linear, and the best fit of the model required the inclusion of both age and age2. The following linear mixed models were tested for each of the individual predictors for the best fit based on the lowest Akaike information criteria: a random intercept only; a random slope for age only; a random intercept and a random slope for age; and a random intercept, random slope for age, and a random slope for age2. Linear mixed models with both a random intercept and a random slope for age represented the best fit to the data. The mixed model provides a regression coefficient, a standard error, and a P-value for each variable to indicate its contribution toward explaining variation in F2-isoprostane concentrations.
Acknowledgments
We thank the dedicated and talented staff of the DAISY study for their clinical, data, and laboratory support, and all the DAISY children and families who generously volunteer their time and knowledge.
Funding source: This research was supported by the National Institutes of Health grants DK32493, DK49654, DK32083, DK050979, DK57516, and by the Juvenile Diabetes Research Foundation grant 17-2013-535.
Footnotes
Financial disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of interest: The authors have no conflicts of interest to disclose.
Contributor’s Statements
Brittni N. Frederiksen: Dr. Frederiksen carried out the analyses, drafted the manuscript, and approved the final manuscript as submitted.
Jennifer Seifert: Ms. Seifert coordinated and supervised data collection, critically reviewed the manuscript, and approved the final manuscript as submitted.
Ginger L. Milne: Dr. Milne collected all the F2-isoprostane data, critically reviewed the manuscript, and approved the final manuscript as submitted.
Miranda Kroehl and Molly M. Lamb: Drs. Kroehl and Lamb contributed to the analysis and interpretation of data, critically reviewed the manuscript, and approved the final manuscript as submitted.
Marian Rewers and Jill Norris: Drs. Rewers and Norris conceptualized and designed the study, designed the data collection instruments, critically reviewed the manuscript, and approved the final manuscript as submitted.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References
- 1.Huh SY, Rifas-Shiman SL, Taveras EM, Oken E, Gillman MW. Timing of Solid Food Introduction and Risk of Obesity in Preschool-Aged Children. Pediatrics. 2011;127:e544–51. doi: 10.1542/peds.2010-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearce J, Taylor MA, Langley-Evans SC. Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. Int J Obes. 2013;37:1295–306. doi: 10.1038/ijo.2013.99. 2005. [DOI] [PubMed] [Google Scholar]
- 3.Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA J Am Med Assoc. 2005;293:2343–51. doi: 10.1001/jama.293.19.2343. [DOI] [PubMed] [Google Scholar]
- 4.Ivarsson A, Myléus A, Norström F, et al. Prevalence of Childhood Celiac Disease and Changes in Infant Feeding. Pediatrics. 2013;131:e687–94. doi: 10.1542/peds.2012-1015. [DOI] [PubMed] [Google Scholar]
- 5.Frederiksen B, Kroehl M, Lamb MM, et al. Infant exposures and development of type 1 diabetes mellitus: The Diabetes Autoimmunity Study in the Young (DAISY) JAMA Pediatr. 2013;167:808–15. doi: 10.1001/jamapediatrics.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chmiel R, Beyerlein A, Knopff A, Hummel S, Ziegler A-G, Winkler C. Early infant feeding and risk of developing islet autoimmunity and type 1 diabetes. Acta Diabetol. 2014 doi: 10.1007/s00592-014-0628-5. [DOI] [PubMed] [Google Scholar]
- 7.Fergusson DM, Horwood LJ, Shannon FT. Early solid feeding and recurrent childhood eczema: a 10-year longitudinal study. Pediatrics. 1990;86:541–6. [PubMed] [Google Scholar]
- 8.Grimshaw KEC, Maskell J, Oliver EM, et al. Introduction of Complementary Foods and the Relationship to Food Allergy. Pediatrics. 2013;132:e1529–38. doi: 10.1542/peds.2012-3692. [DOI] [PubMed] [Google Scholar]
- 9.Kleinman RE. American Academy of Pediatrics recommendations for complementary feeding. Pediatrics. 2000;106:1274. [PubMed] [Google Scholar]
- 10.SECTION ON BREASTFEEDING. Breastfeeding and the Use of Human Milk. Pediatrics. 2012;129:e827–41. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 11.Agostoni C, Decsi T, Fewtrell M, et al. Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2008;46:99–110. doi: 10.1097/01.mpg.0000304464.60788.bd. [DOI] [PubMed] [Google Scholar]
- 12.Clayton HB, Li R, Perrine CG, Scanlon KS. Prevalence and reasons for introducing infants early to solid foods: variations by milk feeding type. Pediatrics. 2013;131:e1108–14. doi: 10.1542/peds.2012-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granot E, Kohen R. Oxidative stress in childhood–in health and disease states. Clin Nutr Edinb Scotl. 2004;23:3–11. doi: 10.1016/s0261-5614(03)00097-9. [DOI] [PubMed] [Google Scholar]
- 14.Chelchowska M, Ambroszkiewicz J, Gajewska J, Laskowska-Klita T, Leibschang J. The effect of tobacco smoking during pregnancy on plasma oxidant and antioxidant status in mother and newborn. Eur J Obstet Gynecol Reprod Biol. 2011;155:132–6. doi: 10.1016/j.ejogrb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Noakes PS, Thomas R, Lane C, et al. Association of maternal smoking with increased infant oxidative stress at 3 months of age. Thorax. 2007;62:714–7. doi: 10.1136/thx.2006.061630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friel JK, Diehl-Jones B, Cockell KA, et al. Evidence of oxidative stress in relation to feeding type during early life in premature infants. Pediatr Res. 2011;69:160–4. doi: 10.1203/PDR.0b013e3182042a07. [DOI] [PubMed] [Google Scholar]
- 17.Friel JK, Martin SM, Langdon M, Herzberg GR, Buettner GR. Milk from mothers of both premature and full-term infants provides better antioxidant protection than does infant formula. Pediatr Res. 2002;51:612–8. doi: 10.1203/00006450-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Lipińska J, Lipińska S, Stańczyk J, et al. Reactive oxygen species and serum antioxidant defense in juvenile idiopathic arthritis. Clin Rheumatol. 2014 doi: 10.1007/s10067-014-2571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadeem A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol. 2003;111:72–8. doi: 10.1067/mai.2003.17. [DOI] [PubMed] [Google Scholar]
- 20.Kauffman LD, Sokol RJ, Jones RH, Awad JA, Rewers MJ, Norris JM. Urinary F2-isoprostanes in young healthy children at risk for type 1 diabetes mellitus. Free Radic Biol Med. 2003;35:551–7. doi: 10.1016/s0891-5849(03)00333-2. [DOI] [PubMed] [Google Scholar]
- 21.Buescher ES, McIlheran SM. Antioxidant properties of human colostrum. Pediatr Res. 1988;24:14–9. doi: 10.1203/00006450-198807000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Goldman AS, Thorpe LW, Goldblum RM, Hanson LA. Anti-inflammatory properties of human milk. Acta Paediatr Scand. 1986;75:689–95. doi: 10.1111/j.1651-2227.1986.tb10275.x. [DOI] [PubMed] [Google Scholar]
- 23.Ivarsson A, Hernell O, Stenlund H, Persson LÅ. Breast-feeding protects against celiac disease. Am J Clin Nutr. 2002;75:914–21. doi: 10.1093/ajcn/75.5.914. [DOI] [PubMed] [Google Scholar]
- 24.Peters U, Schneeweiss S, Trautwein EA, Erbersdobler HF. A case-control study of the effect of infant feeding on celiac disease. Ann Nutr Metab. 2001;45:135–42. doi: 10.1159/000046720. [DOI] [PubMed] [Google Scholar]
- 25.Szajewska H, Chmielewska A, Pieścik-Lech M, et al. Systematic review: early infant feeding and the prevention of coeliac disease. Aliment Pharmacol Ther. 2012;36:607–18. doi: 10.1111/apt.12023. [DOI] [PubMed] [Google Scholar]
- 26.Norris JM, Barriga K, Klingensmith G, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA J Am Med Assoc. 2003;290:1713–20. doi: 10.1001/jama.290.13.1713. [DOI] [PubMed] [Google Scholar]
- 27.Virtanen SM, Räsänen L, Ylönen K, et al. Early introduction of dairy products associated with increased risk of IDDM in Finnish children. The Childhood in Diabetes in Finland Study Group. Diabetes. 1993;42:1786–90. doi: 10.2337/diab.42.12.1786. [DOI] [PubMed] [Google Scholar]
- 28.Virtanen SM, Takkinen H-M, Nevalainen J, et al. Early introduction of root vegetables in infancy associated with advanced ß-cell autoimmunity in young children with human leukocyte antigen-conferred susceptibility to Type 1 diabetes. Diabet Med. 2011;28:965–71. doi: 10.1111/j.1464-5491.2011.03294.x. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler A-G, Schmid S, Huber D, Hummel M, Bonifacio E. Early Infant Feeding and Risk of Developing Type 1 Diabetes–Associated Autoantibodies. JAMA J Am Med Assoc. 2003;290:1721–8. doi: 10.1001/jama.290.13.1721. [DOI] [PubMed] [Google Scholar]
- 30.Virtanen SM, Kenward MG, Erkkola M, et al. Age at introduction of new foods and advanced beta cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes. Diabetologia. 2006;49:1512–21. doi: 10.1007/s00125-006-0236-1. [DOI] [PubMed] [Google Scholar]
- 31.Qiao Y, Sun J, Ding Y, Le G, Shi Y. Alterations of the gut microbiota in high-fat diet mice is strongly linked to oxidative stress. Appl Microbiol Biotechnol. 2013;97:1689–97. doi: 10.1007/s00253-012-4323-6. [DOI] [PubMed] [Google Scholar]
- 32.Palma GD, Capilla A, Nova E, et al. Influence of milk-feeding type and genetic risk of developing coeliac disease on intestinal microbiota of infants: the PROFICEL study. PloS One. 2012;7:e30791. doi: 10.1371/journal.pone.0030791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: Diabetes Autoimmunity Study in the Young (DAISY) Diabetologia. 1996;39:807–12. doi: 10.1007/s001250050514. [DOI] [PubMed] [Google Scholar]
- 34.Norris JM, Yin X, Lamb MM, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA J Am Med Assoc. 2007;298:1420–8. doi: 10.1001/jama.298.12.1420. [DOI] [PubMed] [Google Scholar]
- 35.Morrow JD, Roberts LJ., 2nd Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol. 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 36.Milne GL, Gao B, Terry ES, Zackert WE, Sanchez SC. Measurement of F2- isoprostanes and isofurans using gas chromatography-mass spectrometry. Free Radic Biol Med. 2013;59:36–44. doi: 10.1016/j.freeradbiomed.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Awad JA, Roberts LJ, 2nd, Burk RF, Morrow JD. Isoprostanes–prostaglandin-like compounds formed in vivo independently of cyclooxygenase: use as clinical indicators of oxidant damage. Gastroenterol Clin North Am. 1996;25:409–27. doi: 10.1016/s0889-8553(05)70255-7. [DOI] [PubMed] [Google Scholar]
- 38.Morrow JD, Roberts LJ., 2nd Mass spectrometry of prostanoids: F2-isoprostanes produced by non-cyclooxygenase free radical-catalyzed mechanism. Methods Enzymol. 1994;233:163–74. doi: 10.1016/s0076-6879(94)33019-0. [DOI] [PubMed] [Google Scholar]
- 39.Seifert J, Ross C, Deutsch J, Awad J, Norris J. Validation of methods for the collection of urine in infants and toddlers. Urol Nurs. 2002;22:113–7. [PubMed] [Google Scholar]
- 40.Seifert JA, Ross CA, Norris JM. Validation of a five-question survey to assess a child’s exposure to environmental tobacco smoke. Ann Epidemiol. 2002;12:273–7. doi: 10.1016/s1047-2797(01)00264-2. [DOI] [PubMed] [Google Scholar]


