Abstract
BACKGROUND
The AKT inhibitor MK-2206 at a dose of 60 mg every other day was evaluated in gastric/gastroesophageal junction cancers.
METHODS
Patients who had progressed after first-line treatment were eligible. Pertinent eligibility criteria included adequate organ function, a fasting serum glucose level ≤ 150 mg/dL, and less than grade 2 malabsorption or chronic diarrhea. MK-2206 was given orally (60 evaluable patients required). The primary endpoint was overall survival, and a median survival of 6.5 months (power, 89%; significance level, 0.07) was considered encouraging for further investigation.
RESULTS
Seventy patients were included in the final analyses. The median age was 59.8 years (range, 30.4–86.7 years); 70% were male, 89% were white, and 7% were Asian. There were 2 deaths possibly related to the study drug (cardiac arrest and respiratory failure). Grade 4 adverse events included hyperglycemia, anemia, and lung infection (1 each). Grade 3 adverse events occurred in < 5% of patients except for fatigue (6%). Other adverse events (all grades) included anemia (17%), anorexia (30%), diarrhea (26%), fatigue (50%), hyperglycemia (30%), nausea (40%), vomiting (22%), dry skin (19%), maculopapular rash (30%), and acneiform rash (13%). The response rate was 1%, the median progression-free survival was 1.8 months (95% confidence interval, 1.7–1.8 months), and the median overall survival was 5.1 months (95% confidence interval, 3.7–9.4 months)
CONCLUSIONS
MK-2206 as second-line therapy was well tolerated by an unselected group of patients with gastric/gastroesophageal junction cancers, but it did not have sufficient activity (response rate, 1%; overall survival, 5.1 months) to warrant further testing in this population.
Keywords: AKT inhibitor, gastric cancer, gastroesophageal junction, MK-2206, phase 2 study
INTRODUCTION
Gastric cancer is a common cause of cancer deaths worldwide and results in approximately 800,000 deaths per year.1 Early diagnosis is uncommon, and many patients have metastatic disease at the time of diagnosis. The median survival of patients with advanced unresectable gastric cancer is in the range of 7 to 9 months.2 A number of combination chemotherapy regimens are used with wide regional and country-specific variations, and there is no general consensus regarding the optimal first-line chemotherapy regimen for the treatment of advanced gastric and gastroesophageal junction (GEJ) adenocarcinomas. Docetaxel, cisplatin, and fluorouracil (DCF) constitute a Food and Drug Administration–approved regimen in the United States because of the improved clinical benefit in comparison with cisplatin and fluorouracil. However, DCF is associated with significant toxicity, with grade 3/4 neutropenia seen in 82% of patients and febrile neutropenia seen in 29%.3 Combination chemotherapy regimens such as DCF and epirubicin, cisplatin, and infusional 5-fluorouracil require central venous access and an ambulatory infusion pump; this adds to the complexity of care and potentially increases the risk of infection and thrombosis.3 Despite the development of new regimens substituting capecitabine for 5-fluorouracil and oxaliplatin for cisplatin (ie, epirubicin, cisplatin, and capecitabine; epirubicin, oxaliplatin, and fluorouracil; and epirubicin, oxaliplatin, and capecitabine), the outcomes of patients with advanced gastric and GEJ adenocarcinomas have shown only modest improvement in the last decade, and median survival is still less than 12 months.3,4 Recent targeting of Her2/neu, which is overexpressed in 10% to 25% of cases, was shown to be effective in gastric cancer. The addition of trastuzumab to chemotherapy (cisplatin plus 5-fluorouracil) was explored in a phase 3 randomized trial of Her2/neu-positive gastric cancer patients (n = 594), and it significantly improved overall survival (OS) in comparison with chemotherapy alone (13.8 vs 11.1 months, P = .0046); it is a standard-of-care regimen for these patients.5 However, other targeted agents, such as epidermal growth factor receptor inhibitors (cetuximab and panitumumab) and antiangiogenic inhibitors (bevacizumab), have failed to improve survival when they have been combined with chemotherapy.6–8
A number of other biomarkers are being evaluated for targeted therapy in gastric and GEJ cancers. The phosphoinositide 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway appears important, and evidence of AKT activation is seen in approximately 30% of tumor biopsies.9 Phosphatase and tensin homolog (PTEN) overexpression, seen in approximately 10% of patients, appears to be a poor prognostic marker and is associated with invasion and chemoresistance.9,10 The mTOR inhibitor everolimus (RAD001) was first evaluated in a phase 2 trial of previously treated gastric and GEJ tumors (n = 53), with an encouraging median survival of 10.1 months.11 However, a phase 3 trial randomized 653 patients to best supportive care or everolimus therapy and failed to show a benefit from everolimus12; this indicates that other methods of inhibiting the PI3K/AKT/mTOR pathways are necessary.
MK-2206 is the first allosteric inhibitor of AKT to enter clinical development.13,14 MK-2206 has demonstrated AKT inhibition and antiproliferative activity as a single agent and in combination with other agents in multiple human cancer cell lines.13,14 Phase 1 studies established 2 dosing regimens for further evaluation: 60 mg every other day and 150 mg weekly.15 Chronic dosing was well tolerated in early studies, with common side effects (mostly grade 1/2) being skin rash, nausea, pruritus, hyperglycemia, and diarrhea.15
At the time of the study initiation and during the accrual period, there was no standard therapy available for advanced gastric/GEJ cancer patients who had progressed on a first-line regimen. A significant number of patients have an adequate performance status after first-line therapy, and the development of a well-tolerated and effective regimen was the objective of this phase 2 trial with the targeted agent MK-2206.
MATERIALS AND METHODS
Patient Eligibility
The protocol was approved by the institutional review board at each participating site, and written consent was obtained from all patients before enrollment. The study was registered with ClinicalTrials.gov before patients were enrolled (NCT01260701). Eligible patients (Zubrod performance status of 0–1) had histologically or cytologically proven gastric or GEJ adenocarcinoma. Patients had advanced, surgically inoperable, measurable disease (according to Response Evaluation Criteria in Solid Tumors 1.1). Evidence of disease progression after first-line treatment or recurrence within 6 months after adjuvant therapy was required. Previous adjuvant radiotherapy was permitted, with or without concurrent chemotherapy. Patients had evidence of adequate hematologic, renal, hepatic, and coagulation function. Acceptable cardiac function required a QTcF (by Fridericia’s calculation) < 450 milliseconds (for males) or < 470 milliseconds (for females) and an absence of a history of congenital long QT syndrome, the use of concomitant medications that could prolong the QTc interval, New York Heart Association class III or IV heart failure, a history of myocardial infarction within 6 months before registration, and uncontrolled dysrhythmias and poorly controlled angina.
A fasting blood sugar level ≤ 150 mg/dL and a hemoglobin A1c level < 7% were also required. Pertinent exclusion criteria were the use of warfarin, brain metastasis, grade 2 or higher malabsorption or chronic diarrhea, chemotherapy or radiotherapy within the 3 weeks (6 weeks for nitrosoureas or mitomycin C) before registration, and prior treatment with a PI3K, AKT, or mTOR inhibitor.
Drug Administration
MK-2206 at the dose of 60 mg every other day was administered 2 hours before or 2 hours after a meal. Treatment was continued until the progression of disease or unacceptable toxicity. One cycle of therapy was 28 days. A treatment delay for >3 weeks for any reason also resulted in study discontinuation. Therapy was held for grade 3 or higher drug-related adverse events until recovery to grade 1 or lower, and therapy was resumed with a dose reduction. Two dose reductions to 45 and 30 mg were permitted. In the case of prolonged or medically concerning grade 2 toxicity (ie, diarrhea and rash), therapy was interrupted until recovery to grade 1 or lower, and it was resumed at the same dose.
Study Evaluations
The patient history was taken, and a physical examination, an electrocardiogram, and laboratory tests (including fasting blood sugar and hemoglobin A1c) were performed before study entry and then every 2 weeks during the study. A radiological evaluation of disease was performed every 8 weeks. The Common Terminology Criteria for Adverse Events (version 4.0) were used for toxicity and serious adverse event reporting.
Study Statistics
The primary objective of this study was to estimate the OS for patients with advanced gastric/GEJ adenocarcinomas treated with MK-2206. In comparison with a historical control OS of 5 months, a median OS of 6.5 months was considered promising for MK-2206 (the study had a power of 89% and a significance level of 0.07). The secondary objectives were to estimate the progression-free survival, to estimate the response rate (according to Response Evaluation Criteria in Solid Tumors 1.1), and to assess the frequency and severity of toxicities associated with this regimen. The study required 60 evaluable patients. An interim analysis was performed for the first 24 eligible patients. A 4-month OS ≥ 50% was required in this group to proceed to the next stage.
RESULTS
The study accrued 75 patients; 70 were evaluable for the study, and 5 were not evaluable because of ineligibility at the baseline (n = 4) or patient withdrawal after registration (n = 1). The accrual period was 28 months (January 2011 to May 2013) at 22 Southwest Oncology Group sites. The interim analysis for the first 24 eligible patients met the criteria of 4-month OS ≥ 50%, and the study proceeded to complete accrual. The median age was 59.8 years (range, 30.4–86.7 years). The study population consisted of males (70%), females (30%), whites (89%), and Asians (7%; Table 1). Postprotocol therapy was administered to 30 patients (43%).
TABLE 1.
Patient Characteristics
| Registered patients, No. | 75 |
|---|---|
| Evaluable patients, No. | 70 |
| Sex, No. (%) | |
| Male | 49 (70) |
| Female | 21 (30) |
| Age, years | |
| Median | 59.8 |
| Range | 30–87 |
| Race, No. (%) | |
| White | 62 (89) |
| Asian | 5 (7) |
| Unknown | 3 (4) |
| Performance status (Zubrod), No. (%) | |
| 0 | 30 (43) |
| 1 | 40 (57) |
| Disease status, No. (%) | |
| Progressed | 58 (83) |
| Recurrent | 12 (17) |
| Prior radiation, No. (%) | |
| No | 50 (71) |
| Yes | 20 (29) |
| Extensive (≥50%) | 3 (4) |
| Limited (<50%) | 16 (23) |
| Other (NOS) | 1 (1) |
Abbreviation: NOS, not otherwise specified.
Safety and Toxicity
There were 2 deaths during the study possibly related to the study drug (cardiac arrest and respiratory failure). Grade 4 adverse events were hyperglycemia, anemia, and lung infection (1 patient each). Grade 3 adverse events were infrequent and occurred < 5% of patients except for fatigue (6%). Common all-grade toxicities (mostly grade 1/2) included anemia (17%), anorexia (30%), diarrhea (26%), fatigue (50%), hyperglycemia (30%), nausea (40%), and vomiting (22%). Skin toxicities included dry skin (19%), maculopapular rash (30%), and acneiform rash (13%; Table 2).
TABLE 2.
Selected Drug-Related Toxicities (>10%) in All Patients (n = 70) for All Cycles
| Category | Grade 1/2, No. (%) |
Grade 3/4, No. (%) |
|---|---|---|
| AST/ALT | 8 (11) | 2 (3) |
| Abdominal pain | 7 (10) | 0 |
| Alkaline phosphatase | 9 (13) | 2 (3) |
| Anemia | 9 (13) | 3 (4) |
| Anorexia | 18 (26) | 3 (4) |
| Constipation | 10 (14) | 0 |
| Diarrhea | 18 (26) | 0 |
| Dry skin | 13 (19) | 0 |
| ECG QT interval | 7 (10) | 0 |
| Fatigue | 31 (44) | 4 (6) |
| Hyperglycemia | 19 (27) | 2 (3) |
| Hyponatremia | 7 (10) | 2 (3) |
| Lymphopenia | 10 (14) | 1 (1) |
| Nausea | 27 (39) | 1 (1) |
| Pruritus | 15 (21) | 1 (1) |
| Rash, acneiform | 7 (10) | 2 (3) |
| Rash, maculopapular | 19 (27) | 2 (3) |
| Vomiting | 13 (19) | 2 (3) |
| Weight loss | 12 (17) | 0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ECG, electrocardiogram.
Drug-related indicates that the toxicity was scored by an investigator as possibly, probably, or definitely related to therapy. There were 2 deaths (grade 5) possibly related to the study drug (cardiac arrest and respiratory failure). Grade 4 adverse events included hyperglycemia, anemia, and lung infection (1 each).
Efficacy
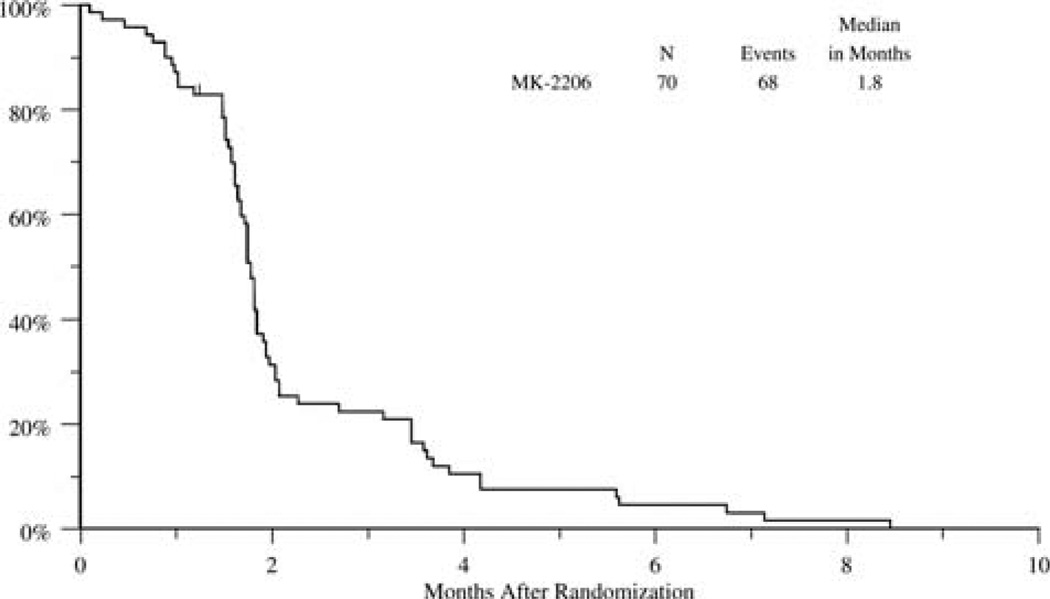
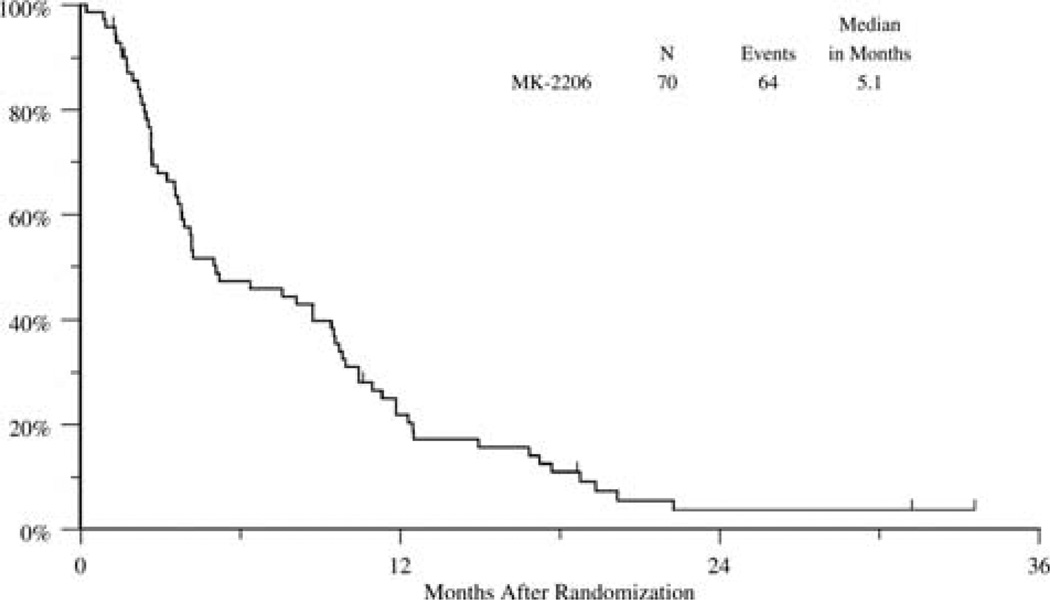
As of August 19, 2014 (the data analysis date), 6 patients were alive. The radiological partial response rate was 1% (n = 1), and stable disease was seen in 20% (n = 14). The median progression-free survival was 1.8 months (95% confidence interval, 1.7–1.8 months), and the median OS was 5.1 months (95% confidence interval, 3.7–9.4 months; Figs. 1 and 2).
Figure 1.
Progression-free survival.
Figure 2.
Overall survival.
DISCUSSION
MK-2206 as second-line therapy was well tolerated with mostly grade 1/2 side effects. There was some indication of modest activity with a radiological partial response in 1 patient and stable disease seen in 20%. The median OS was 5.1 months and did not meet the study efficacy endpoint of 6.5 months. A limitation of our study was that therapy was performed in unselected patients, and tumor samples were not collected. In addition, pharmacokinetic sampling was not performed to determine whether MK-2206 absorption in the gastrointestinal tract was affected by factors such as prior gastrectomy and abdominal radiation. Until recently, there were no standard options for gastric/GEJ patients who had progression on first-line therapy. Recently, randomized phase 3 trials with previously treated patients have shown irinotecan, docetaxel, and ramucirumab to improve survival over best supportive care, and they are now standard options. In these studies, patients randomized to best supportive care alone had a median OS of 3.8 months, patients treated with ramucirumab had an OS of 5.2 months,16 and irinotecan or docetaxel resulted in an OS of 5.3 months.17 In addition, combination therapy can also be considered with ramucirumab and paclitaxel in the second-line setting18 because a recently published randomized study (n = 665) showed a survival advantage for a group assigned to treatment with ramucirumab and paclitaxel versus a placebo plus paclitaxel (median, 9.6 vs 7.4 months; P = .017).
Optimal biomarkers that can be used to select patients for therapy with PI3K/AKT/mTOR inhibitors are still in development. Recent work suggests that the activity of these agents may be greater in tumors with PTEN loss or PIK3CA mutations.19 Though not common (<5%), AKT1 and AKT2 mutations have also been described in gastric cancer.20 Trastuzumab is an important agent for Her2/neu-overexpressing gastric cancer. However, resistance eventually develops with therapy, and recent preclinical studies have shown a strong association between PI3K/PTEN activation and resistance to trastuzumab.21 Thus, future studies with MK-2206 and other related agents in gastric cancer should consider patient stratification by the PI3K/PTEN/AKT status and/or demonstrate progression on trastuzumab. Combination therapy should be evaluated because MK-2206 in preclinical studies has shown an additive or synergistic effect with a number of commonly used chemotherapeutic agents such as erlotinib, lapatinib, doxorubicin, gemcitabine, 5-fluorouracil, docetaxel, and carboplatin in lung NCI-H460 cells and ovarian A2780 tumor cells.14
MK-2206 continues to be actively investigated; there are a wide variety of ongoing or completed phase 2 trials (approximately 40) in multiple tumor types with MK-2206 used as a single agent or in combination with trastuzumab, taxanes, and platinum.22 Results of these studies as well as inhibitors targeting other AKT isoforms13 with correlative biomarkers and patient selection will help future development and aid in patient stratification.
MK-2206 is well tolerated in patients with gastric/gastroesophageal junction cancers, and there is some evidence of activity, but overall survival (5.1 months) is less than anticipated (6.5 months).
Acknowledgments
We thank the patients and families who participated in the study, the Southwest Oncology Group Operations Office, the Statistical Center, and the personnel of participating sites involved in the study.
FUNDING SUPPORT
This study was supported in part by the following Public Health Service cooperative agreement grants awarded by the National Cancer Institute (Department of Health and Human Services): CA180888, CA180819, CA142559, CA11083, CA180830, CA45560, CA35178, CA46282, CA67575, CA20319, CA180834, CA63844, CA35192, and CA35431.
Ramesh R. Ramanathan has received research funding from Merck Pharmaceuticals.
Footnotes
This study was presented in part at the 2014 Annual Meeting of the American Society of Clinical Oncology in Chicago, IL.
CONFLICT OF INTEREST DISCLOSURES
The remaining authors have no conflicts of interest to declare.
REFERENCES
- 1.International Agency for Research on Cancer. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. [Accessed 10 March, 2015]; http://globocan.iarc.fr/Default.aspx. [Google Scholar]
- 2.Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD004064.pub3. CD004064. [DOI] [PubMed] [Google Scholar]
- 3.Ajani JA, Moiseyenko VM, Tjulandin S, et al. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: the V-325 study group. J Clin Oncol. 2007;25:3205–3209. doi: 10.1200/JCO.2006.10.4968. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 5.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 6.Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–499. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 7.Waddell TS, Chau I, Barbachano Y, et al. A randomized multicenter trial of epirubicin, oxaliplatin, and capecitabine (EOC) plus panitumumab in advanced esophagogastric cancer (REAL3) J Clin Oncol. 2012;30 doi: 10.1200/JCO.2010.29.2847. [abstract]. LBA400. [DOI] [PubMed] [Google Scholar]
- 8.Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 9.Han Z, Wu K, Shen H, et al. Akt1/protein kinase Ba is involved in gastric cancer progression and cell proliferation. Dig Dis Sci. 2008;53:1801–1810. doi: 10.1007/s10620-007-9824-2. [DOI] [PubMed] [Google Scholar]
- 10.Oki E, Baba H, Tokunaga E, et al. Akt phosphorylation associates with LOH of PTEN and leads to chemoresistance for gastric cancer. Int J Cancer. 2005;117:376–380. doi: 10.1002/ijc.21170. [DOI] [PubMed] [Google Scholar]
- 11.Doi T, Muro K, Boku N, et al. Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J Clin Oncol. 2010;28:1904–1910. doi: 10.1200/JCO.2009.26.2923. [DOI] [PubMed] [Google Scholar]
- 12.Ohtsu A, Ajani JA, Bai YX, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol. 2013;31:3935–3943. doi: 10.1200/JCO.2012.48.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pal SK, Reckamp K, Yu H, et al. Akt inhibitors in clinical development for the treatment of cancer. Expert Opin Investig Drugs. 2010;19:1355–1366. doi: 10.1517/13543784.2010.520701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirai H, Sootome H, Nakatsuru Y, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 15.Yap TA, Yan L, Patnaik A, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 17.Kang JH, Lee SI, Lim do H, et al. Salvage chemotherapy for pre-treated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30:1513–1518. doi: 10.1200/JCO.2011.39.4585. [DOI] [PubMed] [Google Scholar]
- 18.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 19.Sangai T, Akcakanat A, Chen H, et al. Biomarkers of response to Akt inhibitor MK-2206 in breast cancer. Clin Cancer Res. 2012;18:5816–5828. doi: 10.1158/1078-0432.CCR-12-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soung YH, Lee JW, Nam SW, et al. Mutational analysis of AKT1, AKT2 and AKT3 genes in common human carcinomas. Oncology. 2006;70:285–289. doi: 10.1159/000096289. [DOI] [PubMed] [Google Scholar]
- 21.Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther. 2011;11:263–275. doi: 10.1586/era.10.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. [Accessed September 5, 2014]; ClinicalTrials.gov. http://clinicaltrials.gov/ct2/results?term5MK-2206.