Abstract
Aims
To determine if a low-intensity, clinic-integrated behavioural intervention reduced the incidence of hypoglycaemic events in children with Type 1 diabetes.
Methods
A total of 390 families with children with Type 1 diabetes were enrolled in a 2-year, randomized clinical trial of a behavioural intervention. The intervention was designed to improve diabetes management practices by targeting the family’s diabetes problem-solving skills. Hypoglycaemic events were categorized in two groups: those treated by oral ingestion and those treated by parenteral therapy. Events were self-reported by participants at each clinic visit, which occurred approximately every 3–4 months. Analyses included two-sample t-tests, the mean cumulative function test, and the Cox proportional hazards model for recurrent events to compare the incidence between groups.
Results
Across the entire 2-year study period, the incidence of hypoglycaemic events treated by oral ingestion of glucose-rich foods and events requiring parenteral therapy did not significantly differ between study conditions; however, during the second year of participant enrolment, the incidence of events treated by oral ingestion in the intervention group was 13.6 per 100 person-years compared with 27.3 per 100 patient-years in the control group (P = 0.02). The hazard ratio of these events during the second year was 0.49 (95% CI 0.27–0.90; P = 0.02).
Conclusions
Our findings suggest the need for a long-term (> 1 year) focus on the implementation of interventions targeting diabetes management in young people. Behavioural interventions targeting problem-solving skills could be considered as practical, non-pharmacological strategies to reduce hypoglycaemia in adolescents with Type 1 diabetes.
Introduction
Despite improvements in glucose monitoring and insulin therapy, individuals with Type 1 diabetes mellitus are at an increased risk of deteriorations in glycaemic control during adolescence. The physical and hormonal changes during puberty contribute to this risk by increasing insulin resistance [1,2]. Additionally, psychosocial issues such as autonomy-seeking and increased parent–child conflict, as well as problematic behavioural issues, such as risk-taking and experimentation, present barriers to optimum diabetes management [3]. In an effort to mitigate this deterioration in glycaemic control, previous research has examined modifiable behavioural factors to improve self-management of diabetes. Family factors, such as parental involvement [4,5], responsibility-sharing [6], monitoring [7] and lower family conflict [8,9] are associated with better glycaemic control in adolescents.
Hypoglycaemia is an extremely common acute complication associated with diabetes and has been widely regarded as one of the limiting factors in glycaemic control [10]. Onset of hypoglycaemia not only poses immediate and long-term threats to physical and neurological health, but carries the potential for adverse social, emotional and behavioural consequences as well. Despite advancements in therapy and treatment, hypoglycaemia has remained a continuous threat in young people with Type 1 diabetes, with the most recent US estimate reporting 6.2% of children and young adults experiencing a severe hypoglycaemic event within the past 12 months [11]. Recurrent episodes of hypoglycaemia may increase child and parent fear of hypoglycaemia [12], which can lead to ‘over-compensatory’ behaviour, characterized by intentional over-eating and/or under-administration of insulin to avoid the recurrence of another hypoglycaemic event. Such coping strategies can result in poor glycaemic control and can increase the risk of long-term diabetes-related complications.
Although there is emerging evidence showing the clinical utility of psycho-educational and behavioural interventions for improving and maintaining adherence and glycaemic control [13–18], limited evidence exists as to whether or not such interventions are capable of reducing hypoglycaemia. One intervention targeting education and patient scheduling significantly reduced the incidence of emergency department visits, hospitalizations and hypoglycaemia in a single-centre setting [19].
The aim of the present study was to determine the effect of a low-intensity, clinic-integrated behavioural intervention on the incidence of hypoglycaemia in young people with Type 1 diabetes. This intervention has shown efficacy in improving glycaemic control relative to standard care [20], with this effect observed after 12 months of exposure to the intervention. We hypothesized that the intervention would reduce the risk of having a hypoglycaemic event relative to the risk in the control group.
Participants and methods
Research design
This clinical trial was a multicentre, parallel-group study with equal randomization. Participants were recruited from four large, geographically spread paediatric endocrinology clinics in the USA (Boston, MA; Chicago, IL; Jacksonville, FL; and Houston, TX).
Participants
The inclusion criteria for the children included: age 9--14.9 years, being diagnosed with Type 1 diabetes for ≥ 3 months; a daily insulin usage of ≥ 0.5 μ/kg/day for those diagnosed for ≥ 1 year or 0.2 μ/kg/day for those diagnosed for < 1 year, with ≥ 2 injections or use of insulin pump; most recent HbA1c concentration of > 6.0 and <12.0% for those diagnosed for ≥ 1 year and > 6.0% at any time post-diagnosis for those diagnosed for <1 year; and no other major chronic disease (with the exception of well-controlled thyroid, asthma and coeliac’s disease), cognitive impairments or psychiatric diagnosis. Additional parent/family inclusion criteria included home telephone access, fluency in English, attendance at at least two clinic visits in the past year, and no psychiatric diagnoses in participating parents. Sample size was based on detecting meaningful differences in HbA1c concentration, the primary study outcome, and has been reported in detail previously [20].
Procedures
Child–parent dyads were recruited during routine clinic visits between January 2006 and March 2009. Families were randomized into either an intervention or usual care group, stratified by age (≥9 to <12 years old and ≥12 to <15 years) and HbA1c concentration (≤8.3% and >8.3%). Random permuted blocks were prepared by the data coordinating centre by a person not affiliated with the study’s data collection. A separate randomization list was prepared for each stratum; lists were transferred to a sequence of sealed envelopes, each containing the assignment of intervention or usual care.
Study assessments were conducted during home visits at study baseline and end, by telephone at study mid-point, and at each clinical visit (which typically occurred every 3–4 months). The staff conducting home visits and telephone assessments were hired by the data coordinating centre, not affiliated with the clinical site and were blinded to study assignment. Clinic assessments were conducted by health advisors. The study protocol was approved by the institutional review board of each participating centre.
Treatment conditions
The intervention was designed to improve diabetes management by facilitating constructive collaboration between children and parents and enhancing individual and family problem-solving skills. Grounded in social cognitive theory [21] and self-regulation theory [22], the ‘WE-CAN manage diabetes’ intervention was delivered by specially trained non-professionals (called health advisors) at each routine clinic visit for ~21 months.
Health advisors possessed a bachelor’s or master’s degree in a relevant field, and had received extensive training in diabetes management, study procedures and the intervention process. In addition to on-site training and shadowing of healthcare professionals, health advisors from all clinical sites attended a 2-day workshop that included extensive role-playing about the intervention process with feedback by study investigators and group discussion. Additional post-session training was provided until each site investigator was confident that each advisor had mastered the requisite skills, and periodic review of session audiotapes was used to provide ongoing feedback and ensure fidelity. Annual in-person training occurred throughout the study, and weekly telephone meetings were held with all health advisors and one or more study investigator to address emerging issues and ensure consistency of implementation across the sites. To help facilitate advisor-family rapport, families were typically assigned the same advisor for the duration of their enrolment in the study.
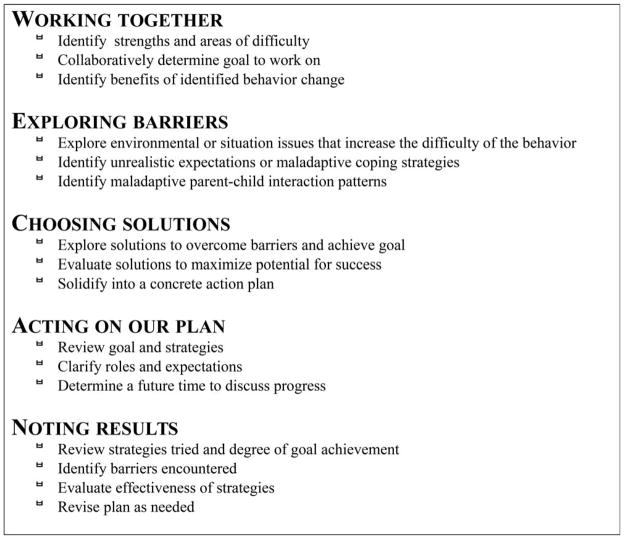
Health advisors facilitated the development of a behavioural plan targeting the diabetes management problem identified by the family. The advantages of this approach are that it is easy to understand, flexible and individualized. Before each visit, each child and their parents were asked to collaboratively identify specific diabetes management problems they were facing. Each intervention session, lasting ~30 min, was structured by the WE-CAN manage diabetes problem-solving approach, a pneumonic representing the steps in the problem-solving process (Fig. 1). The behavioural plan was documented on a paper form developed for the study. At 2 and 6 weeks after each clinic visit, health advisors contacted families to provide general support, assess progress, and help resolve emerging problems.
FIGURE 1.
WE-CAN manage diabetes’ problem-solving process used in the behavioural intervention.
Measures
Information on hypoglycaemia was self-reported by the family at each clinic visit. Hypoglycaemia was categorized into two levels according to the standards set by the Diabetes Complication and Control Trial [23]: (1) events requiring the assistance of another individual and treated through oral ingestion of glucose-rich foods; (2) events requiring parenteral therapy (e.g. glucagon or i.v. glucose) and resulting in seizure and/or loss of consciousness. Demographic variables including age, gender, date of diagnosis, family composition, socioeconomic status, race and ethnicity were obtained by medical record abstraction and parent report. The participant’s diabetes regimen was recorded at each clinic visit.
Analyses
Baseline variables were summarized with means and SD values. The primary null hypothesis to be tested was that there was no significant difference in the incidence of either type of hypoglycaemic events between study conditions. Incidence was calculated for each study condition and presented in episodes per 100 person-years. Events were included in the analysis if they occurred between the participant’s first clinic visit date and their termination date. To adjust for the different lengths in follow-up time across participants, a Cox proportional hazards model for recurrent events [24] was performed to generate a hazards ratio. The mean cumulative function [25] is a statistical technique used for the analysis of recurrent events data. This test was used to summarize the average number of events between study conditions and to visualize graphically the patterns and intensities of events as a function of follow-up time. Data were analysed using SAS software (SAS Institute, Inc, Cary, NC, USA). Null hypothesis were tested with a two-sided significance level of 0.05.
Results
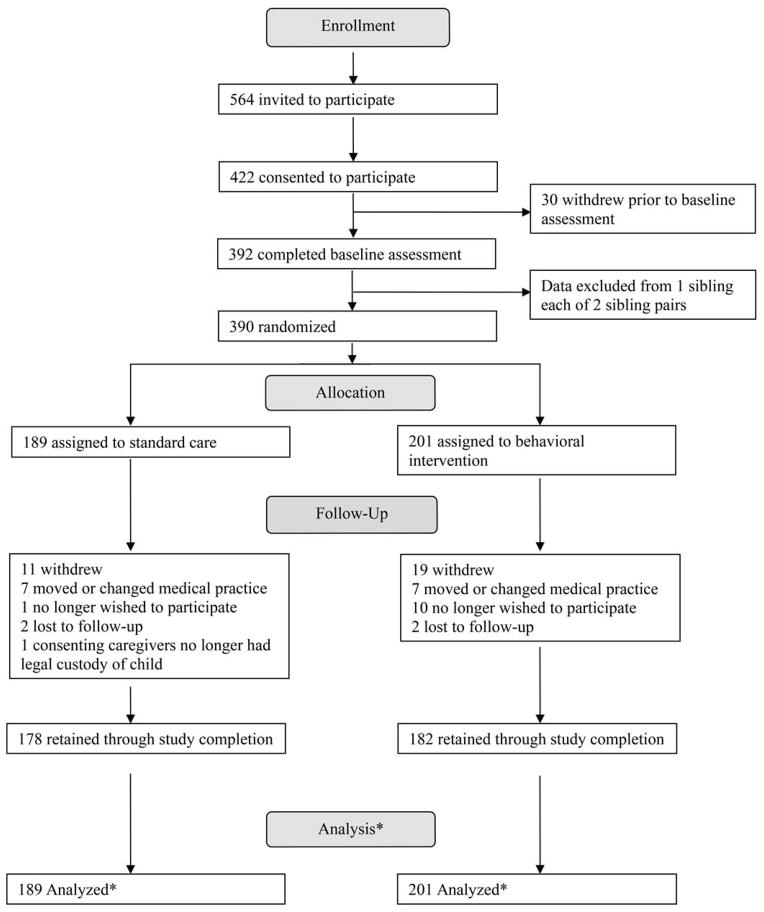
Participant flow is summarized in Figure 2. Of 564 families invited to participate, 422 consented (75%) and 390 were randomized (70%). Participant baseline characteristics are summarized in Table 1. There were no statistically significant differences in baseline demographic characteristics between groups. Participants in the usual care and intervention group were enrolled for a mean ± SD of 734.3 ± 153.8 and 700.6 ± 200.5 days and attended a mean ± SD of 7.4 ± 1.8 and 7.0 ± 2.2 clinic visits, respectively. No statistically significant differences were observed between groups in insulin regimen change, number of days enrolled, or clinic attendance during the study period. Participant retention throughout the study was 92%. No non-diabetes-related adverse outcomes were reported.
FIGURE 2.
Participant flow through study.
Table 1.
Baseline characteristics of participants by study condition
| Control | Intervention | P* | |
|---|---|---|---|
| Mean (SD) age, years | 12.4 (1.7) | 12.5 (1.8) | 0.62 |
| Gender, n (%) | 0.99 | ||
| Male | 96 (50.8) | 102 (50.7) | |
| Female | 93 (49.2) | 99 (49.3) | |
| Race/ethnicity, n (%) | 0.75 | ||
| White | 131 (74.4) | 145 (75.5) | |
| Hispanic | 16 (9.1) | 21 (10.9) | |
| Black | 19 (10.8) | 15 (7.8) | |
| Other | 10 (5.7) | 11 (5.7) | |
| Number of adults in the home, n (%) | 0.95 | ||
| 1 | 25 (14.0) | 26 (13.5) | |
| 2 | 138 (77.1) | 147 (76.6) | |
| ≥3 | 16 (8.9) | 19 (9.9) | |
| Family income, n (%) | 0.21 | ||
| <$50,000 | 37 (22.0) | 50 (27.3) | |
| $50,000–99,999 | 74 (44.0) | 64 (35.0) | |
| $100,000+ | 57 (34.0) | 69 (37.7) | |
| Mean (SD) duration of diabetes, years | 4.9 (3.2) | 4.8 (3.3) | 0.88 |
| Regimen, n (%) | 0.75 | ||
| Pump | 62 (32.8) | 69 (34.3) | |
| Injection | 127 (67.2) | 132 (65.7) | |
| Mean (SD) HbA1c | 8.3 (1.1) | 8.4 (1.2) | 0.58 |
Test for group differences using ANOVA for continuous and chi-squared test for categorical variables.
Of the 390 participants (families) enrolled, 299 (76.7%) reported no hypoglycaemic event requiring assistance with oral treatment, 47 (12.1%) experienced one event, and 44 experienced ≥ 2 events (11.2%). For events requiring parenteral therapy, 349 (89.5%) participants had no events, 31 (7.9%) had one event, and eight (2.0%) had ≥ 2 events. There were no significant differences in the incidence of hypoglycaemic events in person-years when analysed across the entire study period (Table 2).
Table 2.
Incidence of hypoglycaemic events by treatment condition across the entire study duration
| Control (n = 189) | Intervention (n = 201) | P * | |
|---|---|---|---|
| Hypoglycaemic events treated by oral ingestion | |||
| Number of events | 97 | 69 | |
| Total months of follow-up | 4602 | 4670 | |
| Incidence per 100 person-years | 25.3 | 17.7 | 0.10 |
| Hypoglycaemic events requiring parenteral therapy | |||
| Number of events | 25 | 35 | |
| Months of follow-up | 4602 | 4670 | |
| Incidence per 100 person-year | 6.5 | 9.0 | 0.28 |
Test for group differences using ANOVA.
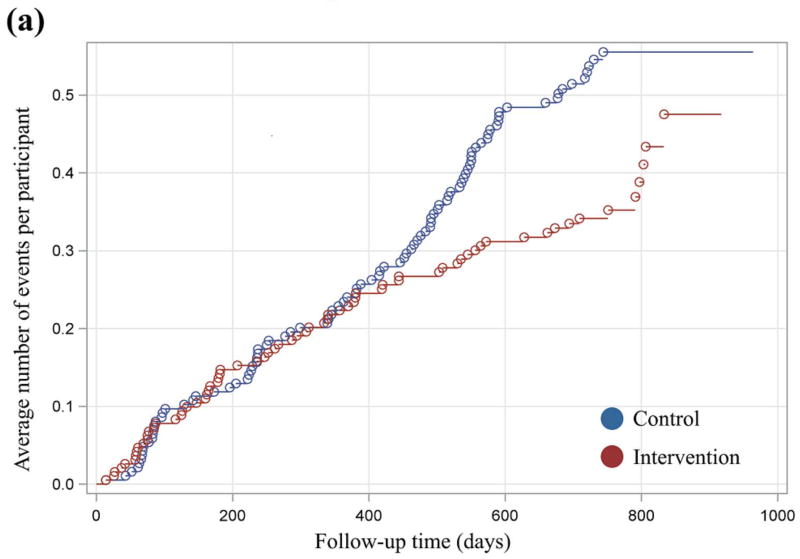
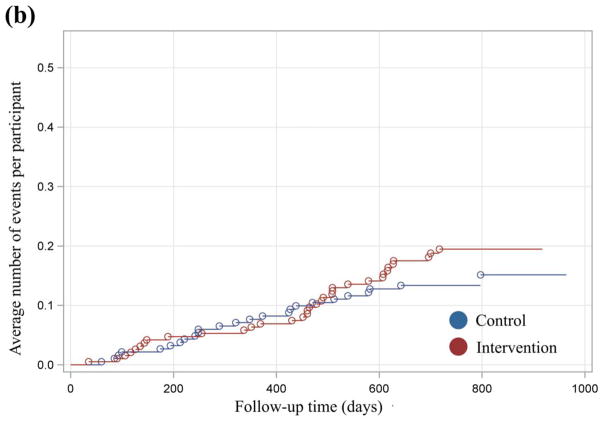
Mean cumulative function graphs, which were plotted to evaluate qualitatively the patterns of events across time, indicated a divergence between treatment groups with regard to events requiring assistance with oral treatment after 12 months of exposure (Fig. 3). For these events, both groups increased in similar fashion until ~400 days. From 400 days to the end of follow-up, a reduction in the average number of events experienced by the intervention group relative to the control group was observed. This trend persisted and increased in magnitude until the end of follow-up. By contrast, the observed differences in the plot for hypoglycaemia requiring parenteral therapy were less definitive and consistent.
FIGURE 3.
(a) xxx. (b) xxx.
Based on these findings and previous documentation of an intervention effect on glycaemic control after 12 months of exposure, separate analyses of year 2 data were conducted. Analysis of year 2 data indicated a significantly reduced incidence of events requiring assistance with oral treatment among participants in the intervention group (13.6/100 person-years) compared with the control group (27.3/100 person-years; P= 0.02; Table 2). The hazard ratio of 0.49 indicates that, at any time during the second year of exposure, participants in the intervention group were 51% less likely to experience a hypoglycaemic event relative to the control group (P = 0.02; Table 3). There was no difference between groups in the incidence of events requiring parenteral therapy (P = 0.29).
Table 3.
Incidence of hypoglycaemic events by treatment condition during year 2
| Control (n = 179) | Intervention (n = 183) | P * | |
|---|---|---|---|
| Hypoglycaemic events treated by oral ingestion | |||
| Number of events | 54 | 27 | |
| Total months of follow-up | 2376 | 2388 | |
| Incidence per 100 person-years | 27.3 | 13.6 | 0.015 |
| Hypoglycaemic events requiring parenteral therapy | |||
| Number of events | 11 | 23 | |
| Months of follow-up | 2376 | 2388 | |
| Incidence per 100 person-year | 5.6 | 11.6 | 0.29 |
Test for group differences using ANOVA.
Discussion
In addition to maintaining targeted levels of HbA1c, one of the long-standing and underemphasized challenges of diabetes management interventions is reducing hypoglycaemic events. To date, little evidence exists as to whether or not behavioural interventions targeting children with Type 1 diabetes can reduce the occurrence of this acute complication. In the present randomized clinical trial, the intervention significantly reduced the incidence of hypoglycaemic events requiring the assistance of oral treatment by ~50% during the second year of enrolment, at which time an improvement in glycaemic control relative to the control group was also observed [20]. This effect was achieved with minimal participant burden, approximately four intervention sessions or 2 h of contact per year.
The strengths of the present study include its large sample size, recruitment from multiple, geographically diverse clinical sites and the integration of the intervention into routine clinical care. The intervention was delivered by research assistants not involved with the patients’ clinical care, decreasing the likelihood of differences in the provision of healthcare between groups. A limitation of the study was the scope and setting in which the participants were recruited. The study was conducted in large, well-staffed, urban paediatric centres; it is unknown whether or not the intervention would yield the same findings in rural, lower-resource settings; however, rates of hypoglycaemia in the present study are similar to those reported by the Juvenile Diabetes Research Foundation, which indicates an incidence of severe hypoglycaemia (including events treated by oral ingestion and parenteral therapy) for children aged 8–14 years of 26.4 per 100 person-years [29]. As hypoglycaemia was not a primary study outcome, the power to detect between-group differences in hypoglycaemia was not evaluated a priori, and data on events were collected by parent and child retrospective report at the time of each clinic visit.
The intervention did not reduce the incidence of events requiring parenteral therapy. These events occurred at a low frequency and precipitating factors may be multifactorial (e.g. illness, accidental over-administration of insulin, vigorous physical activity). Such events may result from more complex behavioural and physiological pathways, making them more difficult to prevent.
The timing of the intervention effect on hypoglycaemia requiring assistance with oral treatment is consistent with the expectation that repeated exposure to intervention sessions would build and refine the participant’s problem-solving ability. Other explanations for the timing of the intervention effect include improved rapport between health advisors and participants, enhanced skillsets of health advisors and honing in on the most salient problems to address during sessions. The delayed effect of the intervention during the second year of enrolment suggests behavioural interventions should be implemented with a long-term focus. Future research could investigate whether or not low-intensity interventions lasting > 2 years, perhaps spanning the period of adolescence, can further improve health outcomes.
In the context of intervention studies for young people with Type 1 diabetes, no clear consensus exists as to which intervention method optimizes health outcomes. An education-based intervention conducted by Svoren et al. [19] is the only study that significantly reduced the incidence of acute complications in a single-centre clinical trial. This 2-year trial employed case managers who facilitated appointment scheduling, with and without supplementation of psycho-educational modules. The annual incidence of hypoglycaemic events treated via oral therapy in the intervention arm employing both care ambassadors and modules was 41.1 events per 100 person-years, while the incidence in the control arm was 53.5 events per 100 person-years. The incidence of events treated via parenteral therapy in the intervention and control group was 4.2 and 11.8 events per 100 person-years, respectively. While the results provided by Svoren et al. [26] are compelling, education-based interventions, although effective in improving knowledge, may not be equally effective in promoting behavioural changes that last over extended periods of time. It is theoretically reasonable that the problem-solving approach used in the present study, which seeks to modify behaviour directly, could hold potential for effecting long-term changes in routine management behaviours [27]. Additional research is needed to determine the long-term effects of both education and problem-solving based approaches in improving health outcomes in this population.
Novel technological methods, such as continuous glucose monitoring, have shown promise in reducing the incidence of hypoglycaemic events in young people. In a study that tested continuous glucose monitoring as an intervention strategy to reduce hypoglycaemia in children aged 8–14 years, the incidence of hypoglycaemia was reduced from 26.4 to 13.0 per 100 person-years [28]. This magnitude of effect was similar to the findings of the present study, which showed a 50% reduction in the incidence of events treated with enteral administration during the second year of enrolment. Future research could investigate the utility of combining a problem-solving approach with continuous glucose monitoring for reducing hypoglycaemia and enhancing glycaemic control.
The results of the present study are noteworthy when coupled with the previous finding that the intervention significantly improved glycaemic control during the same timeframe [20]. Efforts to improve management and reduce blood glucose levels through intensive insulin therapy can unintentionally increase the risk of hypoglycaemia [29]. The simultaneous improvement in both of these areas speaks to the promise of this intervention approach and the clinical utility of using behavioural interventions as a strategy for enhancing the management of diseases with complex regimens.
In conclusion, children with Type 1 diabetes participating in a low-intensity, clinic-integrated behavioural intervention had a reduced incidence of hypoglycaemic episodes. Future effectiveness research is needed to determine the potential utility of this intervention approach outside the context of a randomized trial. Given the timing of the intervention effect observed in the present study, further research should also explore longer-term implementations (> 2 years) that span the pre-adolescent and adolescent developmental period. Researchers and clinical staff could consider behavioural interventions as practical, non-pharmacological strategies to improve health outcomes associated with Type 1 diabetes.
Table 4.
Hazard ratios of events treated by oral ingestion and requiring parenteral therapy calculated from year 2 data
| Hazard ratio | 95% CI | P* | |
|---|---|---|---|
| Hypoglycaemic events treated by oral ingestion | 0.49 | 0.27–0.90 | 0.022 |
| Hypoglycaemic events requiring parenteral therapy | 1.37 | 0.68–2.7 | 0.38 |
Test for group differences with the Cox proportional hazards regression model.
What’s new?
Hypoglycaemia is a common and serious acute complication associated with Type 1 diabetes mellitus. There has been limited research examining whether interventions targeting management can reduce the incidence of hypoglycaemia in young people with Type 1 diabetes.
The findings from the present study show that a behavioural intervention targeting problem-solving skills reduced the risk of hypoglycaemic events treated by oral ingestion during the second year of enrolment.
Given the simultaneous improvement in hypoglycaemic risk and glycaemic control (a previous finding), behavioural interventions could be considered as strategies to improve diabetes management in young with Type 1 diabetes.
Acknowledgments
Funding sources
This study was supported by the intramural research programme of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, under the following contracts: N01-HD-4-3364, Joslin Diabetes Center, Boston, MA; N01-HD-4-3361, Nemours Children’s Clinic, Jacksonville, FL; N01-HD-4-3362, Texas Children’s Hospital, Houston, TX; N01-HD-4-3363, Children’s Memorial Hospital, Chicago, IL; and N01-HD-3-3360, James Bell Associates, Arlington, VA.
The authors acknowledge the research staff at each clinical centre, the families who participated in this study and Dr Kaigang Li for his guidance on data management.
Footnotes
Competing interests
None declared.
References
- 1.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty: A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. 1986;315:215–219. doi: 10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- 2.Susman-Stillman A, Hyson DM, Anderson FS, Collins WA. Adolescent psychosocial development and adherence to treatment for insulin-dependent diabetes mellitus. In: McNamara JA Jr, Trotman CA, editors. Creating the Compliant Patient. Vol. 33. Ann Arbor, MI: Center for Human Growth and Development The University of Michigan; 1997. pp. 73–101. [Google Scholar]
- 3.Hamilton J, Daneman D. Deteriorating diabetes control during adolescence: physiological or psychosocial? J Pediatr Endocrinol Metab. 2002;15:115–126. doi: 10.1515/jpem.2002.15.2.115. [DOI] [PubMed] [Google Scholar]
- 4.Anderson B, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: Relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. J Pediatr. 1997;130:257–265. doi: 10.1016/s0022-3476(97)70352-4. [DOI] [PubMed] [Google Scholar]
- 5.Wysocki T, Taylor A, Hough BS, Linscheid TR, Yeates KO, Naglieri JA. Deviation from developmentally appropriate self-care autonomy: association with diabetes outcomes. Diabetes Care. 1996;19:119–125. doi: 10.2337/diacare.19.2.119. [DOI] [PubMed] [Google Scholar]
- 6.Helgeson VS, Reynolds KA, Siminerio L, Escobar O, Becker D. Parent and adolescent distribution of responsibility for diabetes self-care: links to health outcomes. J Pediatr Psychol. 2008;33:497–508. doi: 10.1093/jpepsy/jsm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis DA, Podolski C-L, Frey M, Naar-King S, Wang B, Moltz K. The role of parental monitoring in adolescent health outcomes: impact on regimen adherence in youth with type 1 diabetes. J Pediatr Psychol. 2007;32:907–917. doi: 10.1093/jpepsy/jsm009. [DOI] [PubMed] [Google Scholar]
- 8.Anderson BJ, Vangsness L, Connell A, Butler D, Goebel-Fabbri A, Laffel B. Family conflict, adherence, and glycaemic control in youth with short duration type 1 diabetes. Diabet Med. 2002;19:635–642. doi: 10.1046/j.1464-5491.2002.00752.x. [DOI] [PubMed] [Google Scholar]
- 9.Wysocki T. Associations among teen-parent relationships, metabolic control, and adjustment to diabetes in adolescents. J Pediatr Psychol. 1993;18:441–452. doi: 10.1093/jpepsy/18.4.441. [DOI] [PubMed] [Google Scholar]
- 10.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- 11.Cengiz E, Xing D, Wong JC, Wolfsdorf JI, Haymond MW, Rewers A, et al. Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D Exchange clinic registry. Pediatr Diabetes. 2013;14:447–454. doi: 10.1111/pedi.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnard K, Thomas S, Royle P, Noyes K, Waugh N. Fear of hypoglycaemia in parents of young children with type 1 diabetes: A systematic review. 2010:10. doi: 10.1186/1471-2431-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grey M, Boland EA, Davidson M, Li J, Tamborlane WV. Coping skills training for youth with diabetes mellitus has long-lasting effects on metabolic control and quality of life. J Pediatr. 2000;137:107–113. doi: 10.1067/mpd.2000.106568. [DOI] [PubMed] [Google Scholar]
- 14.Delamater AM, Bubb J, Davis SG, Smith JA, Schmidt L, White NH, et al. Randomized prospective study of self-management training with newly diagnosed diabetic children. Diabetes Care. 1990;13:492–498. doi: 10.2337/diacare.13.5.492. [DOI] [PubMed] [Google Scholar]
- 15.Laffel LM, Vangsness L, Connell A, Goebel-Fabbri A, Butler D, Anderson BJ. Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with type 1 diabetes. J Pediatr. 2003;142:409–416. doi: 10.1067/mpd.2003.138. [DOI] [PubMed] [Google Scholar]
- 16.Nansel TR, Iannotti RJ, Simons-Morton BG, Plotnick LP, Clark LM, Zeitzoff L. Long-term maintenance of treatment outcomes: “Diabetes Personal Trainer” intervention for youth with type 1 diabetes. Diabetes Care. 2009;32:807–809. doi: 10.2337/dc08-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Taylor A, et al. Effects of behavioral family systems therapy for diabetes on adolescents’ family relationships, treatment adherence, and metabolic control. J Pediatr Psychol. 2006;31:928–938. doi: 10.1093/jpepsy/jsj098. [DOI] [PubMed] [Google Scholar]
- 18.Anderson B, Brackett J, Ho J, Laffel L. An office-based intervention to maintain parent-adolescent teamwork in diabetes management: Impact on parent involvement, family conflict, and subsequent glycemic control. Diabetes Care. 1999;22:713–721. doi: 10.2337/diacare.22.5.713. [DOI] [PubMed] [Google Scholar]
- 19.Svoren BM, Butler D, Levine BS, Anderson BJ, Laffel LMB. Reducing acute adverse outcomes in youth with type 1 diabetes: a randomized, controlled trial. Pediatrics. 2003;112:914–922. doi: 10.1542/peds.112.4.914. [DOI] [PubMed] [Google Scholar]
- 20.Nansel TR, Iannotti RJ, Liu A. Clinic-integrated behavioral intervention for families of youth with type 1 diabetes: Randomized clinical trial. Pediatrics. 2012;129:e866–e873. doi: 10.1542/peds.2011-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 22.Leventhal H, Leventhal EA, Cameron L. Representations, procedures, and affect in illness self-regulations: a perceptual-cognitive model. In: Baum A, Revenson TA, Singer JE, editors. Handbook of Health Psychology. Mahwah, NJ: Erlbaum; 2001. pp. 19–47. [Google Scholar]
- 23.Lorenz RA, Santiago JV, Siebert C, Cleary PA, Heyse S. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. 1991;90:450–459. [PubMed] [Google Scholar]
- 24.Andersen PK, Gill RD. Cox’s Regression Model for Counting Processes: A Large Sample Study. Ann Statistics. 1982;10:1100–1120. [Google Scholar]
- 25.Nelson WB. Recurrent events data analysis for product repairs, disease recurrences, and other applications. 10. SIAM; 2003. [Google Scholar]
- 26.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-Management education for adults with type 2 Diabetes A meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25:1159–1171. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]
- 27.Ewart CK. A social problem-solving approach to behavior change in coronary heart disease. 1990 [Google Scholar]
- 28.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17–22. doi: 10.2337/dc09-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Care D. Adverse events and their association with treatment regimens in the Diabetes Control and Complications Trial. Diabetes Care. 1995;18:1415–1427. doi: 10.2337/diacare.18.11.1415. [DOI] [PubMed] [Google Scholar]