Abstract
Synaptic plasticity has long been known to involve three key elements of neuropil, the presynapse, the postsynapse and adjacent glia. Here we review the role of the extracellular matrix in synaptic plasticity as a necessary component forming the tetrapartite synapse. We describe the role of matrix metalloproteinases as enzymes sculpting extracellular proteins and thereby creating an extracellular signaling domain required for synaptic plasticity. Specifically we focus on the role of the tetrapartite synapse in mediating the effects of addictive drugs at corticostriatal synapses, and conclude that the extracellular signaling domain and its regulation by matrix metalloproteinases is critical for developing and expressing drug seeking behaviors.
Keywords: extracellular matrix, addiction, nucleus accumbens, matrix metalloproteinase, integrin
1. Introduction
Drug addiction has large negative consequences for individuals, families and society (Volkow et al., 2011). Significant effort has gone towards determining the mechanisms of synaptic plasticity that underlie the transition from recreational drug use to dependence/addiction, including substantial research on drug-induced neuroadaptations in glutamatergic inputs to the nucleus accumbens and the dysregulation of glutamate homeostasis (Gipson et al., 2014; Kalivas, 2009; Russo et al., 2010). While enduring, constitutive imbalances in glutamate homeostasis are induced by all addictive drugs (Kalivas, 2009), this dysregulation is manifested in different ways following chronic exposure to different drugs. For example, in the case of cocaine and nicotine, there is prolonged potentiation of glutamatergic synapses in the nucleus accumbens core (NAcore)(Gipson et al., 2013b; Kourrich et al., 2007; Moussawi, 2011), whereas these synapses are depotentiated following withdrawal from chronic heroin exposure (Shen et al., 2011). A major glutamatergic afferent into the accumbens arises from the medial prefrontal cortex. This projection can be partially parsed into two pathways, one from the prelimbic cortex (PL) terminating in the NAcore that promotes the initiation of drug seeking for all drugs tested (LaLumiere and Kalivas, 2008; McLaughlin and See, 2003; Rocha and Kalivas, 2010; Stefanik et al., 2013b; Willcocks and McNally, 2013), and a second from the infralimbic cortex (IL) with terminals in the nucleus accumbens shell (NAshell) that promotes extinction of drug seeking for cocaine or alcohol (Gass et al., 2014; Peters et al., 2008; Stefanik et al., 2013a), but not heroin (Millan et al., 2011; Peters et al., 2013; Willcocks and McNally, 2013).
Data have recently emerged indicating that reinstatement induced by drug-associated cues to all tested classes of addictive drug, including cocaine, heroin and nicotine, is accompanied by a rapid, transient synaptic potentiation (t-SP) of glutamatergic synapses in the NAcore, characterized by enlargement of dendritic spine head diameter (dh) and increase in the AMPA:NMDA ratio (A:N; measure of changing AMPA receptor function) (Gipson et al., 2013a; Gipson et al., 2013b; Shen et al., 2014b). Because this is a shared component of reinstatement to multiple drugs, it is viewed as a particularly promising point of pharmacotherapeutic intervention for the prevention of relapse. In the past decade it has been made clear that the matrix metalloproteinases (MMPs) are a family of extracellular proteases that strongly modulate synaptic plasticity (Ethell and Ethell, 2007). Hippocampal long-term potentiation is dependent on MMP activity and their cleavage of molecules that signal through integrin cell adhesion receptors (Huntley, 2012). Physiologically, MMPs are required for a number of tasks that depend on synaptic plasticity, such as fear conditioning and spatial learning (Meighan et al., 2006; Nagy et al., 2007). Cocaine, nicotine, and heroin reinstatement are each characterized by the rapid induction of extracellular matrix-remodeling matrix-metalloproteinase (MMP) activity in the NAcore, and inhibiting MMP activity prevents reinstatement and the associated synaptic plasticity (Smith et al., 2014). The emergence of data implicating extracellular matrix proteolysis in synaptic plasticity mediating addiction constitutes a paradigm shift away from the tripartite synapse, and towards a tetrapartite synapse, in which all four elements of synaptic architecture are considered in modeling the addicted synapse, including the presynaptic, postsynaptic, glial, and extracellular compartments (Figure 1). The tetrapartite synapse is a term coined by Dityatev and Rusakov (2011), and in this review we focus on the role of MMPs in synaptic plasticity, with most attention dedicated to their role specifically in the constitutive and transient synaptic plasticity contributing to drug addiction.
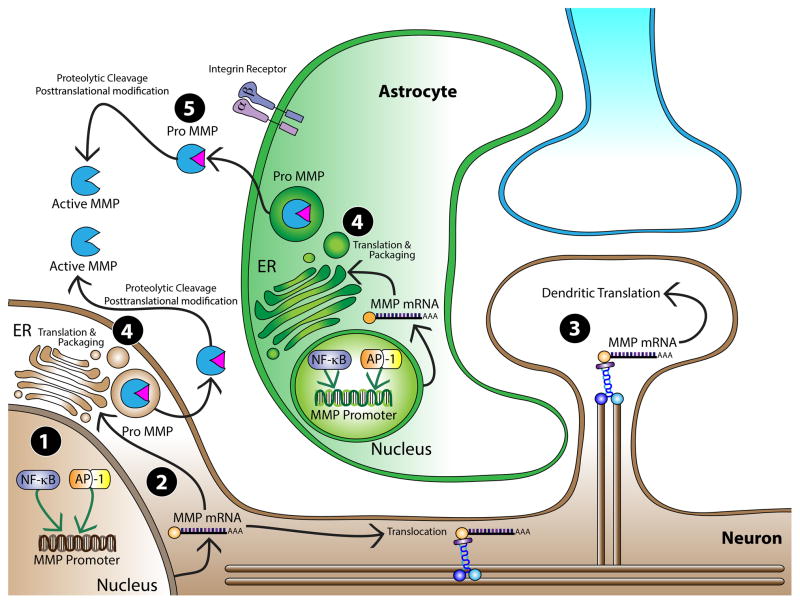
Figure 1. MMP expression and secretion in neuronal and non-neuronal cells.
Shown here is a graphical representation of MMP expression and secretion mechanisms in neurons (brown) and astrocytes (green). 1) In both neurons (brown) and astroglia (green) MMP transcription is generally positively regulated by transcription factors NF-κ-1 (orange/yellow). 2) After MMP mRNA is transcribed it can be transported to the endoplasmic reticulum (ER). 3) Alternatively, MMP mRNA can be translocated to dendritic spine heads where it is locally translated and secreted. 4) Once translocated from the nucleus (in neurons or in astrocytes), MMP mRNA is translated at the endoplasmic reticulum and packaged and released in a pro-form. 5) Once outside of the cell, pro-MMPs are activated in the extracellular space by proteolytic cleavage or by posttranslational modification.
2. MMP Structure and Function
MMPs are Zn2+-dependent endopeptidases that degrade extracellular matrix (ECM) as well as cell-surface molecules to promote cellular reorganization. These proteins were originally discovered for their role in tumor cell invasion (Himelstein et al., 1994), angiogenesis (Yu and Stamenkovic, 2000), and wound healing (Agren et al., 1994), and more recently their contributions to synaptic and neuronal reorganization have been elucidated (Huntley, 2012; Meighan et al., 2006). Twenty-three distinct MMPs have been identified in the human genome, 16 of which are soluble proteins, and 7 transmembrane or GPI-anchored proteins (Huntley, 2012), and these multidomain proteins can be further divided into subgroups based on shared domains, inserts, and substrate recognition motifs. All MMPs contain an N-terminal secretory signal peptide, an autoinhibitory propeptide, and all have similar catalytic regions. Most also contain a C-terminal hemopexin domain, which is attached to the catalytic domain via a flexible hinge linker. The specific structure of the hemopexin domain varies between MMPs, and confers substrate and protein binding specificity to each enzyme (Overall, 2002). Hemopexin domains often dictate subcellular localization by binding to cell surface proteins or ECM molecules, and because of the flexible nature of the hinge region, the catalytic domain can move to process substrates freely while the hemopexin domain is tethered (Collier et al., 2001). The most studied MMPs in the brain are MMPs-2, -3 and -9 (Verslegers et al., 2013). MMP-2 and MMP-9 are also referred to as Gelatinase A & B, respectively, so named for their ability to proteolytically process gelatin. Their unique ability to bind to gelatin is due to fibronectin type II (FNII) repeats within the enzymes’ catalytic domain. FNII repeats recognize and bind to ECM glycoproteins that contain Arg-Gly-Asp (RGD) domains (e.g. fibronectin, laminin, thrombospondin), which are the endogenous ligands for the integrin family of cell adhesion receptors (Verslegers et al., 2013). Thus, MMPs-2/9 are able to recognize and expose integrin-signaling domains, giving them a crucial role in neuron-ECM adhesion. MMP-3 is also called Stromelysin-1, and differs from the gelatinases in that it lacks FNII repeats, and contains a different C-terminal hemopexin domain (Sternlicht and Werb, 2001a). In addition to ECM molecules, MMPs proteolytically process a variety of growth factors, such as transforming growth factor beta (TGFβ; (Yu and Stamenkovic, 2000) brain-derived neurotrophic factor (BDNF; (Mizoguchi et al., 2011a), cell surface glycoproteins (e.g. β-dystroglycan; (Michaluk et al., 2007), cell adhesion molecules/receptors (e.g. SynCAM2; (Bajor et al., 2012), and many other proteins positioned to regulate signaling between the intra- and extra-cellular space (Huntley, 2012).
Another major role for MMPs in the brain is in regulating the blood brain barrier (BBB), and these proteinases are sensitive to a variety of neuroinflammatory signals. Neuroinflammation describes the immune response of central nervous system tissue, normally isolated immunologically from the peripheral system by the BBB. The BBB is maintained through the concordance of neural, glial, vascular and extracellular components and can be degraded by the aberrant action of MMPs, allowing peripheral components to exert effects on CNS tissue (Kousik et al., 2012). Chronic exposure to drugs of abuse, particularly psychostimulant drugs, alters the integrity of the BBB, allowing for entry of viral and bacterial agents from the periphery that contribute to drug-induced neurotoxicity and neuroinflammation (Clark et al., 2013; Kousik et al., 2012). The neuroinflammatory action of psychostimulants can occur through a variety of mechanisms including through the disruption of tight junction channels at the BBB interface, the activation of microglia and the resulting release of pro-inflammatory cytokines, and by the aberrant activation of enzymes that regulate the remodeling of the extracellular matrix (Kousik et al., 2012). Studies show that proinflammatory cytokines and their downstream signaling pathways potently upregulate MMP production in astrocytes and miocroglia (Gottschall and Deb, 1996; O'Shea et al., 2014). Specifically, neuron/glia co-culture studies show that pro-inflammatory cytokine release increases expression of the two main inducible MMPs, MMP-3 and MMP-9, further demonstrating the link between neuroinflammation and MMP activation (Candelario-Jalil et al., 2009).
Neuroinflammation in response to drug exposure is a growing topic of addiction research because the pharmacological reversal of drug-induced neuroinflammation in animal models of addiction can inhibit drug seeking (Scofield and Kalivas, 2014). Among the most studied examples of addictive substances that impact the integrity of the BBB and invoke a neurotoxic and neuroinflammatory responses are methamphetamine, cocaine and 3,4-methylenedioxy-N-methylamphetamine (MDMA).
3. MMP Synthesis, Release and Activation
3.1 Transcriptional regulation
For MMPs to appropriately participate in the neuronal processes including cellular reorganization and remodeling in synaptic plasticity and memory, they must be appropriately expressed, localized and temporally activated (Sternlicht and Werb, 2001b). As such, the regulation of MMP expression and activity is particularly complex and involves several well-regulated mechanisms and signaling cascades (Dzwonek et al., 2004). At the transcriptional level, MMPs and related proteins are tightly regulated with the exception of MMP-2, MMP-14 (MT1-MMP) and tissue inhibitor of metalloproteinases 2 (TIMP-2), which are less tightly restricted by transcriptional control and are co-regulated by the same transcription factors (Lohi et al., 2000). This is likely due to the fact that MMP-2 is more constitutively expressed than other MMPs. Moreover, the positive co-regulation of pro-MMP-2, MT1-MMP, and TIMP-2 also reflects the role that these three factors play in forming a complex to activate pro-MMP-2 (discussed in detail below). Apart from MMP-2, MMPs are regulated at the transcriptional level by phorbol esters, integrin derived signals, extracellular matrix proteins and stress signals (Kheradmand et al., 1998; Sternlicht and Werb, 2001b). As discussed above, MMP expression is regulated by interferons, interleukins, and growth factors, which typically induce expression of c-fos and c-jun immediate early genes whose protein products dimerize to form AP-1 (Sternlicht and Werb, 2001b) (See Figure 1). Generally AP-1 serves as a critical positive regulator of MMP expression and the promoter regions of several MMP genes contain canonical AP-1 binding sites, (Gottschall and Deb, 1996; Sternlicht and Werb, 2001b). In addition, AP-2, Sp1, Sp3 and NF-κB sites are found in several MMP promoters, speaking to the coordinated regulation of these species (Sternlicht and Werb, 2001b). As an example, MMP-9 is positively regulated by both AP-1 and NF-κB at the transcriptional level (Huntley, 2012), and post-transcriptionally nitric oxide (NO) levels regulate the stability of MMP-9 mRNA (Dzwonek et al., 2004). Interestingly, a role for microRNAs (miRs) has been established for both regulating MMP-2/9 expression and in drug addiction. Micro-RNAs are small, non-coding RNAs that can each regulate the translation of many mRNAs. In the dorsal striatum, miR-212 upregulates Raf1 activity and thus CREB signaling, decreasing motivation to take cocaine (Hollander et al., 2010). While the regulation of miRs and MMPs in the brain has not been established, MMP-9 mRNA has a potential miR-212 binding site according to a TargetScan screen (targetscan.org; version 6.2), while there was no identified binding site for miR-212 on MMP-2 mRNA. MicroRNA regulation of MMP gene translation has been shown to be physiologically relevant in systems outside of the brain, most notably miR-29a regulation of MMP-2 is important in proteolysis during thoracic aortic aneurysm (Jones et al., 2011).
3.2 Release and post-transcriptional regulation
MMPs are expressed and secreted as inactive pro-enzymes, also known as zymogens. Enzymatic inactivation is maintained by the interaction of a pro-domain cysteine residue with the catalytic Zn2+. When this interaction is broken, Zn2+ is able to fully coordinate with 3 cysteine residues in the active site, a process known as the “cysteine switch” (Loffek et al., 2011). Zymogens are processed into active MMPs through proteolytic cleavage or posttranslational modification, both of which occur through variety of mechanisms. This makes the steady state level of MMP expression at the mRNA level a relatively poor index of activity due to the large amount of regulation of pro-MMP proteins already present in the neural parenchyma (Huntley, 2012). Beyond MMP-2, other zymogens can be cleaved by activated MMPs in the extracellular space or by serine proteases that cleave peptide bonds within the MMP pro-domain (Sternlicht and Werb, 2001b). These proteases include plasmin, tissue plasminogen activator and urokinase-type plasminogen activator, which are also important mediators of the transition from pro-MMP to an active MMP molecule (Candelario-Jalil et al., 2009).
In keeping with the activity dependent and inducible nature of MMP expression, evidence exists for the translocation of MMP-9 mRNA to the dendritic arbor with a preference for dendrites actively engaged in synaptic transmission (Dzwonek et al., 2004). Notably, the MMP-9 mRNA contains specific sequence elements implicated in the translocation of other mRNAs, suggesting that this translocation plays an important role in rapidly inducible action of MMP-9 (Dzwonek et al., 2004). Furthermore, studies show that dendritic MMP-9 translation is activity dependent and contributes to the rapid increase in MMP-9 activity seen with increased excitatory neurotransmission (Dziembowska et al., 2012).
3.3 Regulation through protein-protein interactions
The activity of MMPs is regulated by a family of secreted proteins called the tissue inhibitors of metalloproteinases (TIMPs), which promote growth and act to regulate cell cycle in a variety of cell types (Mizoguchi et al., 2011b). TIMPs display substantial sequence complementarity to their MMP counterparts, and form reversible noncovalent bonds with MMPs to inactivate them (Sternlicht and Werb, 2001b). In biological systems, levels of TIMP expression are tuned to act in concert with levels of MMP expression and activation to precisely orchestrate the appropriate glycoprotein turnover rate (El Hajj et al., 2014). The TIMP family contains four members, TIMP1-4. Interestingly, members of the TIMP family differ in their ability to inhibit specific MMPs. Relatively little is known about TIMP-3 and TIMP-4. TIMP-3 is expressed at very low levels, but mRNA has been detected in cortex, cerebellum, olfactory bulb, and brain stem (Dzwonek et al, 2004). TIMP-4 is expressed specifically by cerebellar purkinje neurons, and by neurons in specific brain stem regions (Dzwonek et al, 2004).
TIMP-1 expression is found primarily in neurons (apart from Bergman glial cells), and to date has been anatomically localized in the cortex, hippocampus, cerebellum, substantia nigra and hypothalamus (Dzwonek et al., 2004). The apparent expression pattern of TIMP1 is restricted to neuronal cell bodies in these regions, with the exception of the hippocampus where dendritic localization is also observed (Rivera et al., 1997). TIMP1 expression is sensitive to several stimuli and is induced by neuronal depolarization and in certain pathological conditions including electroconvusive seizures (ECS), where the expression of TIMP1 is upregulated in both the cortex and hippocampus (Newton et al., 2003). In addition, in a cell culture model using rat cardiac fibroblasts, exposure to alcohol for 48 hours induced expression of TIMP1 (El Hajj et al., 2014). However, in the serum of human heroin addicts, levels for TIMP1 were lower than control individuals, with ratios of serum levels of MMP-2/TIMP-1, MMP-9/TIMP-1 higher in the heroin group (Kovatsi et al., 2013).
TIMP-2 has been the most studied of the TIMPs. Its expression is predominantly restricted to neurons and is abundant in brain, yet is anatomically restricted to the cortex, nucleus accumbens and cerebellum (Dzwonek et al., 2004). Unlike TIMP-1, TIMP-2 is not upregulated by neuronal activity or in pathological conditions (Dzwonek et al., 2004). However, methamphetamine exposure upregulates TIMP2 in the frontal cortex and nucleus accumbens (Mizoguchi et al., 2011b). Interestingly, when the upregulation of TIMP-2 is thwarted with antisense RNA inhibition, methamphetamine locomotor sensitization is increased (Mizoguchi et al., 2011b). As discussed above for TIMP-1, heroin exposure also increases the MMP-2/TIMP-2 ratio in serum (Kovatsi et al., 2013) and in a cell culture model of human gingival fibroblasts, exposure to nicotine exposure redistributes TIMP2 to the cell surface (Zhou et al., 2007). In a series of experiments using cultured Kupffer cells (stellate macrophages isolated from the liver) from ethanol fed rats, TIMP-2 expression was elevated when compared to cultures made from control rats (Aziz-Seible et al., 2011). In a recent report we show that MMP-9 and TIMP-2 protein levels are increased in the nucleus accumbens core following cocaine-primed reinstatement of cocaine seeking (Smith et al., 2014). However, the induction of TIMP-2 protein levels did not inhibit the increased activity of MMPs observed following cocaine-primed reinstatement (Smith et al., 2014).
Paradoxically, apart from inhibiting the action of MMPs, TIMP proteins also form complexes with MMPs and can facilitate their activation. For example, TIMP-2 interacts with the membrane bound MT1-MMP to facilitate the formation of active MMP-2 from proMMP-2 (Shofuda et al., 1998), while MT2-MMP can activate pro-MMP-2 in a TIMP-2-independent manner (Morrison and Overall, 2006). In either a TIMP-2 dependent or independent activation, MMP-2 activated in this way activates MMP-9. In addition, TIMP-1 interacts with pro-MMP-9 and MMP-3. In this complex of proteins, residues of the C-terminal domain of pro-MMP-9 and TIMP-1 physically interact. This tertiary complex can be modified to release pro-MMP-9, leaving TIMP-1 and MMP-3 bound (Dzwonek et al., 2004).
4. MMP contributions to reward and addiction
Addiction can be described as a pathological form of learning, wherein drug associated cues and/or contexts become strong conditioned stimuli that promote a drug seeking response, even after protracted abstinence (Hyman et al., 2006). A role for MMPs in plasticity accompanying learning and memory was posited in 2003, when Wright and colleagues found that ethanol decreased performance in a Morris water maze, and also impaired MMP-9 activity in the hippocampus (Wright et al., 2003). It has since been established that MMP-9 is activated by and required for the maintenance phase of long-term potentiation (LTP; (Nagy et al., 2006), and application of auto-active MMP-9 onto a slice is able to drive enlargement of dendritic spines and potentiate excitatory field potentials, even in the absence of LTP-inducing high frequency stimulation (Wang et al., 2008). Given their role in LTP, it is not surprising that MMPs are involved in many forms of learning and memory, including spatial memory (Meighan et al., 2006), fear conditioning (Brown et al., 2009), avoidance learning (Nagy et al., 2007), and memory related to contextual cues (Brown et al., 2007).
Human post-mortem data indicate that MMPs may have clinical relevance in addiction. A functional polymorphism in the MMP-9 gene is associated with higher risk for alcoholism, and MMP-9 is elevated in the serum of alcohol abusers (Samochowiec et al., 2010; Sillanaukee et al., 2002). Also, cocaine abusers had decreased MMP-9 activity in the hippocampus (Mash et al., 2007), and heroin users have significantly higher circulating MMP-2 and MMP-9 than do non-drug-using subjects (Kovatsi et al., 2013). While these clinical data are interesting, most knowledge about MMPs in reward and addiction comes from animal models.
4.1 Cocaine
Early work showed that intracerebroventricular (i.c.v.) infusion of a broad-spectrum MMP inhibitor (FN-439) blocked acquisition of cocaine conditioned place preference (CPP) when infused prior to daily conditioning sessions, and also blocked cocaine-primed reinstatement when infused immediately prior to this session (Brown et al., 2007). MMP-9, but not MMP-2 activity was increased in the medial prefrontal cortex (mPFC) following reinstatement of CPP (Brown et al., 2008). In a self-administration reinstatement paradigm, it was shown that MMP-2 activity is constitutively upregulated in the NAcore following extinction, and that acutely inhibiting MMP-2 reversed the persistent synaptic potentiation induced by cocaine self-administration and extinction. Following extinction there is no increase in MMP-9 activity, but MMP-9 is activated upon initiation of cue-induced reinstatement. Moreover, blocking MMP-9 activity also prevents the rapid, transient synaptic potentiation (measured by AMPA currents and dendritic spine morphology) that correlates with magnitude of drug seeking (Smith et al., 2014). When reinstatement is instead initiated by a 10mg/kg priming injection of cocaine, there is rapid suppression of MMP-2 activity, followed by a delayed (45 min) induction of MMP-9 activity (Smith et al., 2014). This differential time course of MMP activation in cued versus drug-primed reinstatement is paralleled by behavioral time course data indicating that cue-induced reinstatement peaks within the first 15 minutes following initiation, whereas cocaine-primed reinstatement peaks by 45 minutes into the session (Shen et al., 2014a). Together, these data indicate that MMP activity and motivation to seek cocaine follow similar time courses and may be intrinsically linked.
While largely untested, there are many putative mechanisms by which MMP activity may translate into to the transient synaptic potentiation associated with reinstated cocaine seeking (measured as increased AMPA:NMDA ratio and dendritic spine head diameter). The integrin family of cell adhesion receptors is particularly interesting in this regard. In a self-administration paradigm, 1 day following the last of 10 daily 2-hour sessions, the β3-integrin subunit is downregulated, with no change in the β1 subunit. Following 3 weeks of extinction training, the β3 subunit is upregulated by 500%, again with no change in the β1 subunit (Wiggins et al., 2011). Injecting a synthetic RGD peptide daily, prior to self-admin sessions prevented the enduring β3 subunit upregulation, and also attenuated cocaine primed reinstatement (Wiggins et al., 2011). In addition to cleaving matrix glycoproteins to expose RGD domains that signal through a5β3 and a5β1 integrin receptors (Sternlicht and Werb, 2001a), MMPs are able to cause integrins to shed subunits that themselves contain RGD domains. For example MMP-2 activity may induce cell motility through proteolytic shedding of the β1 subunit (Kryczka et al., 2012). Integrins signal primarily through integrin-linked kinase (ILK), and ILK can directly phosphorylate cofilin in order to stimulate actin polymerization and dendritic spine head growth (Kim et al., 2008). ILK also phosphorylates Ser845 of GluA1 AMPA subunits, stimulating insertion of the receptor into the synapse, which may increase AMPA:NMDA ratio (Chen et al., 2010). Behaviorally, inhibiting ILK attenuates locomotor sensitization to cocaine, as well as the increased AMPA receptor insertion that accompanies sensitization (Chen et al., 2008), but it has not yet been tested whether inhibiting ILK during extinction training following self-administration can reduce AMPA:NMDA ratio. See figure 2 for a schematic outlining MMP signaling within the extracellular matrix.
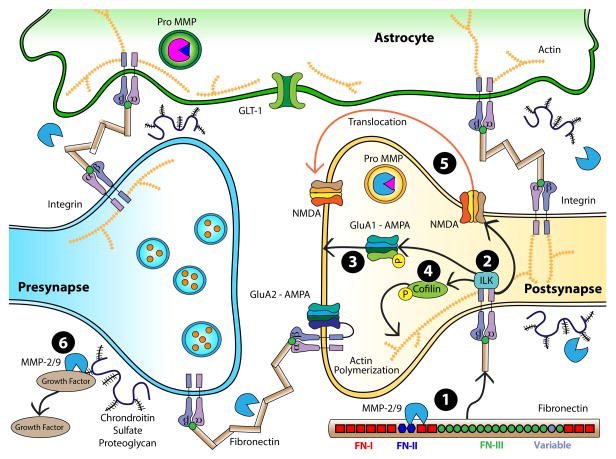
Figure 2. Overview of signaling in the extracellular matrix.
1) MMPs-2 and -9 signal within the ECM by proteolytically processing RGD-containing glycoproteins such as fibronectin. The brown rectangles shown here represent fibronectin, with an enlarged fibronectin molecule shown at the bottom right corner with labeled fibronectin type-1, type-2 and type-3 domains. MMPs-2 and -9 recognize FN-II repeats, allowing them to bind to and cleave fibronectin in order to expose RGD domains. 2) Putative mechanisms by which addictive drug-induced MMP activity alters synaptic strength include signaling through integrin-linked kinase (ILK). Stimulation of ILK then promotes 3) phosphorylation of GluA1 S845 to increase AMPA receptor insertion, 4) phosphorylation of cofilin which stimulates actin polymerization and 5) stimulates NMDA receptor lateral diffusion into the synapse. 6) In addition to fibronectin, MMPs can also proteolytically process chondroitin sulfate proteoglycans, acting to liberate growth factors such as BDNF, TGFb, NGF, and TNFa that may also act to influence synaptic plasticity.
4.2 Methamphetamine
Repeated methamphetamine (METH; 2 mg/kg) exposure induces MMP-2 and MMP-9 activity in the NAcore within 2h of the last injection, and MMP-2 or MMP-9 KO mice show decreased dopamine release, and impaired behavioral sensitization in response to a METH challenge injection. Furthermore, addition of recombinant MMP-2 potentiated METH-induced dopamine release (Mizoguchi, 2007). Acutely, a large METH dose (40 mg/kg) induces MMP-9 activity within 6 hours, and this leads to proteolytic shedding of the ectodomain of intracellular adhesion molecule 5 (ICAM5), producing a soluble fragment that can signal through β1-integrin subunits to cause cofilin phosphorylation (Conant et al., 2011). Soluble ICAM5 ectodomains have also been shown to increase frequency of mEPSCs and GluA1 Ser845 phosphorylation and surface expression, without affecting expression of GluA2 (Lonskaya et al., 2013).
Data from human imaging studies and animal models of addiction clearly demonstrate METH is potently neurotoxic (Panenka et al., 2013). Specifically, METH exposure disrupts the BBB by causing alterations in tight junction proteins (Ramirez et al., 2009) and also enhances release of pro-inflammatory cytokines including interleukin 6 and 8 (Shah et al., 2012), which may activate MMP-9 and cause aberrant degradation of the BBB (Yao et al, 2006). The importance of the neuroinflammatory response to METH exposure in the treatment of addiction has been underscored by the fact that in the laboratory setting, the systemic administration of glial modulator drugs that inhibit the release of pro-inflammatory factors has been shown to also inhibit METH seeking in several animal models of addiction (Beardsley et al., 2010; Snider et al., 2012; Snider et al., 2013). Furthermore, METH exposure increased the expression of MMP-1 and MMP-9, which can act to degrade tight junction proteins producing structural changes to the basement BBB membrane that contribute to the neuroinflammatory response (Conant et al., 2004). In addition, studies show that METH exposure decreases expression of MMP-9 substrate laminin, indicating that MMPs may degrade the BBB by attacking the basal lamina (O'Shea et al., 2014).
4.3 Opiates
MMP-9 expression and activity are increased by acute morphine treatment, and MMP-9 is required for the development of morphine tolerance (Nakamoto et al., 2012). In the spinal cord, MMP-9 inhibition blocks morphine-induced phosphorylation of NMDA receptors, ERK1/2, and cAMP response element binding proteins, and behavioral signs of morphine withdrawal (Liu et al., 2010). Following extinction of heroin self-administration, two constituents of perineuronal nets (PNNs), tenascin R (TNR) and brevican (bcan) were downregulated in both the mPFC and accumbens, indicating increased proteolytic degradation by MMPs. Furthermore, i.c.v. infusion of a broad spectrum MMP inhibitor restored PNN composition, and attenuated cue-induced heroin reinstatement (Van Den Oever, 2010). Interestingly, we did not find an increase in MMP-2 or MMP-9 activity using in vivo zymography following extinction of heroin self-administration, but there was an induction of activity following 15 minutes of cue-induced reinstatement (Smith et al., 2014). These data indicate that a third protease, possibly MMP-3, is responsible for regulating the composition of perineuronal nets (PNNs), which are exclusively localized around GABAergic fast-spiking interneurons (FSIs). PNNs are hypothesized to be selectively localized around these interneurons because their largely anionic composition is protective against oxidative stress which results from the relatively higher metabolic requirements of fast spiking interneurons (Cabungcal et al., 2013).
4.4 Nicotine
Very little about the role of MMPs in nicotine addiction has been established. MMP activity in the accumbens core following nicotine exposure parallels cocaine: following extinction training there is a constitutive induction of gelatinolytic fluorescence, and following reinstatement there is a further induction of activity (Smith et al., 2014). This is the only observation regarding MMPs in the nucleus accumbens following nicotine exposure; another laboratory has examined these enzymes in the hippocampus and mPFC following nicotine CPP. Natarajan and colleagues (2013) induced conditioned place preference with nicotine injections, and measured MMP-2, -3, and -9 expression following each of 5 days of acquisition of CPP, and following re-exposure to the drug-paired context after 5 days of abstinence. Inhibition of MMP activity via daily i.c.v. FN-439 infusion prior to conditioning blocked the acquisition of place preference. Following 5 days of conditioning, both MMP-2 and MMP-9 were both significantly increased in the hippocampus, but not in the mPFC, while MMP-3 remained unchanged. Following 5 days of abstinence from nicotine in the home cage, when re-exposed to the CPP apparatus there was no change in MMP-2 or MMP-9 expression, but MMP-3 expression was increased in both nicotine and saline treated rats. This indicates a broad role for MMP-3 in reactivation of contextual memories, but does not imply a drug-specific effect (Natarajan et al., 2013).
4.5 Alcohol
Acute ethanol intoxication decreases MMP-9 activity in the hippocampus, and impairs spatial memory formation without interfering with MMP-2 activity (Wright et al., 2003). In the chronic intermittent ethanol vapor model in which rats are exposed to ethanol vapor for 14 hours per day for 4 weeks (with a target daily BAC of 200mg/dL), animals undergo repeated cycles of intoxication and withdrawal. Following 4 weeks of vapor exposure, rats allowed to self-administer ethanol during acute (6h) withdrawal display an escalation of self-administration that is indicative of a dependence-like behavioral phenotype (Walker, 2012). Chronic i.c.v. infusion of broad spectrum MMP inhibitor FN-439 during these 4 weeks blocks escalation of ethanol intake (Smith et al., 2011). Interestingly, acute FN-439 infusion only prior to post-vapor self-administration sessions also blocks this escalation, although once a rat experienced one session of post-vapor self-administration without the presence of FN-439, drinking escalated during the next session. Furthermore, in rats that received aCSF rather than FN-439 and escalated immediately following vapor exposure, acute FN-439 was not thereafter effective. This indicates that MMPs contribute to the negative reinforcement learning that occurs from ethanol consumption during acute withdrawal (Smith et al., 2011).
4.6 Broad framework relating MMPs to addiction
While modification of MMP activity has been implicated in multiple forms of synaptic plasticity, at least some of these neuroadaptations appear to be specific to the addicted synapse. Particularly relevant to pharmacotherapeutic goals for treating addiction is evidence that reinstatement to multiple classes of drugs induces MMP activity and subsequent enlargement of dendritic spine head diameter and increased AMPA/NMDA ratio (Table 1). While reinstatement to all 3 tested classes of drug caused a transient induction of MMP activity, only the psychostimulants (i.e. cocaine and nicotine) caused constitutive activation of MMP-2, whereas heroin did not. Given MMP-2/9’s role in dendritic spine formation and maturation (Tian et al., 2007), these data are consistent with the stimulants causing constitutive dendritic spine head enlargement, whereas heroin does not. However, upregulation of MMP-2 activity is very interesting as an addiction-specific phenotype because MMP-2 is typically constitutively active and does not play a role in activity-dependent synaptic potentiation in the hippocampus during LTP underlying non-pathological learning and memory (Nagy et al., 2006). Likewise, cocaine experience did not change MMP-2 expression in the PFC (Brown et al., 2008), but in the accumbens we show that following extinction of cocaine self-administration, constitutive upregulation of MMP-2 activity is necessary for the growth of new dendritic spines, whereas transient induction of MMP-9 by cues is responsible for potentiating pre-existing spines. Indeed, MMP-9 activity in the hippocampus promotes enlargement of dendritic spine head diameter and potentiation of field potentials (Wang et al., 2008). Most previous attention has been paid to activity-dependent induction of MMP-9 activity, while MMP-2 is mostly considered to be developmentally important and its role in adult synaptic physiology has not been well studied. It is not understood how the induction of MMP-2 activity by cocaine occurs, but unpublished observations in our laboratory indicate there may be a role for glutamatergic regulation of neuronal nitric oxide synthase (nNOS) and subsequent S-Nitrosylation of MMP-2 by nitric oxide.
Table 1. Summary of both constitutive and transient drug-induced neuroplasticity in the nucleus accumbens.
Consititutive neuroplasticity is measured after a period of withdrawal from daily drug use, while transient neuroplasticity is measured at 15 min following cue-induced reinstatement of drug seeking. All measurements were made in the accumbens core. Spine head refers to a measure of spine head diameter in medium spiny neurons (MSNs). AMPA/NMDA ratios were measured in MSNs, and MMP activity quantified using a FITC-quenched gelatin substrate microinjected into the accumbens core. Finally, GLT-1 was quantified using both Western blots and measurement of Na+-dependent 3H-glutamate uptake. These data are derived from: (Knackstedt et al., 2010; Smith et al., 2014; Gipson et al., 2013; Gipson et al., 2014; Shen et al., 2011; Shen et al., 2014)
| Spine Head | AMPA/NMDA | MMP Activity | GLT-1 | |
|---|---|---|---|---|
| Extinguished | ||||
| Cocaine | ↑ | ↑ | ↑ | ↓ |
| Heroin | ↓ | ↓ | No Change | ↓ |
| Nicotine | ↑ | ↑ | ↑ | ↓ |
| Reinstated | ||||
| Cocaine | ↑ | ↑ | ↑ | N/A |
| Heroin | ↑ | ↑ | ↑ | N/A |
| Nicotine | ↑ | ↑ | ↑ | N/A |
4.7 Addiction conceptualized as a return to early development?
Because of specific alterations in synaptic receptor content following chronic exposure to drugs of abuse, it has been suggested that many of these changes constitute a “juvenilization” of synapses, or a return to conditions similar to those in early postnatal development (Dong and Nestler, 2014; Gipson et al., 2014). Specifically, chronic cocaine exposure causes formation of GluA2-lacking AMPA receptors that are permeable to calcium in the accumbens (McCutcheon et al., 2011), and increased accumbens extrasynaptic GluN2B-containing NMDA receptors following chronic exposure to cocaine (Brown et al., 2011; Schumann and Yaka, 2009), heroin (Shen et al., 2011), and nicotine (Gipson et al., 2013b). Both of these receptors are much more highly expressed in early development than in the adult brain, and this is thought to contribute to the higher level of ongoing synaptic plasticity in the developing brain. MMPs are also highly developmentally regulated. Both MMP-2 and MMP-9 mRNA are highly expressed in the brain during early development (7D postnatal), after which expression is drastically decreased (Ayoub et al., 2005; Bednarek et al., 2009). Both MMP-2 and MMP-9 are required for outgrowth of both axons and dendrites in the developing brain (Gonthier et al., 2009; Saygili et al., 2011; Tian et al., 2007). Indeed, MMP-2 is capable of inducing neurite outgrowth via proteolytically activating pro-nerve growth factor (NGF), and MMP-9 proteolytically activates pro-brain derived neurotrophic factor (BDNF), which bind to the TrkA and TrkB receptors, respectively, in order to stimulate neural growth (Lee R., 2001; Saygili et al., 2011). Interestingly, chronic cocaine exposure increased dendritic spine density in the nucleus accumbens, and this was completely reversed by acute administration of an MMP-2 inhibitor, indicating that MMP-2 drives increased neurite outgrowth following extinction of cocaine self-administration (Smith et al., 2014). Taken together, new data implicating MMPs-2 and -9 in addiction-related plasticity consistent with the hypothesis that drug addiction may reopen a critical period for plasticity that mimics that which is seen during development.
5. Conclusions
The importance of each component of the tripartite synapse in plasticity has long been recognized. Here we reviewed recent data that implicates signaling within the extracellular matrix in many of the processes required for synaptic potentiation or depression, posing the tetrapartite synapse as the primary integrated unit of synaptic plasticity. MMPs are particularly interesting in this regard because of the large variety of substrates that are well positioned to regulate both morphological reorganization and synaptic receptor content. Of these substrates, proteolytic processing of ECM glycoproteins to expose RGD domains that signal through integrin receptors, as well as liberating latent growth factors within extracellular microdomains are particularly promising as potential new targets for pharmacotherapeutics to reverse or block maladaptive neuroadaptations following chronic drug abuse.
Acknowledgments
This work was supported in part by USPHS grants DA03906, DA12513 and DA15369.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ayoub AE, et al. Developmental expression of matrix metalloproteinases 2 and 9 and their potential role in the histogenesis of the cerebellar cortex. J Comp Neurol. 2005;481:403–15. doi: 10.1002/cne.20375. [DOI] [PubMed] [Google Scholar]
- Aziz-Seible RS, et al. Ethanol feeding potentiates the pro-inflammatory response of Kupffer cells to cellular fibronectin. Alcohol Clin Exp Res. 2011;35:717–25. doi: 10.1111/j.1530-0277.2010.01389.x. [DOI] [PubMed] [Google Scholar]
- Bajor M, et al. Synaptic cell adhesion molecule-2 and collapsin response mediator protein-2 are novel members of the matrix metalloproteinase-9 degradome. J Neurochem. 2012;122:775–88. doi: 10.1111/j.1471-4159.2012.07829.x. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, et al. The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse. Eur J Pharmacol. 2010;637:102–8. doi: 10.1016/j.ejphar.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek N, et al. Ontogeny of MMPs and TIMPs in the murine neocortex. Pediatr Res. 2009;65:296–300. doi: 10.1203/PDR.0b013e3181973aee. [DOI] [PubMed] [Google Scholar]
- Brown TE, et al. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learning & Memory. 2007;14:214–223. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, et al. Increase in matrix metalloproteinase-9 levels in the rat medial prefrontal cortex after cocaine reinstatement of conditioned place preference. Synapse. 2008;62:886–9. doi: 10.1002/syn.20562. [DOI] [PubMed] [Google Scholar]
- Brown TE, et al. Inhibition of matrix metalloproteinase activity disrupts reconsolidation but not consolidation of a fear memory. Neurobio Learn & Mem. 2009;91:66–72. doi: 10.1016/j.nlm.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, et al. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci. 2011;31:8163–74. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabungcal JH, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A. 2013;110:9130–5. doi: 10.1073/pnas.1300454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–94. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, et al. Neural plasticity and addiction: integrin-linked kinase and cocaine behavioral sensitization. J Neurochem. 2008;107:679–89. doi: 10.1111/j.1471-4159.2008.05619.x. [DOI] [PubMed] [Google Scholar]
- Chen Q, et al. Integrin-linked kinase is involved in cocaine sensitization by regulating PSD-95 and synapsin I expression and GluR1 Ser845 phosphorylation. J Mol Neurosci. 2010;40:284–94. doi: 10.1007/s12031-009-9218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KH, Wiley CA, Bradberry CW. Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotox Res. 2013;23:174–88. doi: 10.1007/s12640-012-9334-7. [DOI] [PubMed] [Google Scholar]
- Collier IE, et al. Substrate recognition by gelatinase A: the C-terminal domain facilitates surface diffusion. Biophys J. 2001;81:2370–7. doi: 10.1016/S0006-3495(01)75883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, et al. Human immunodeficiency virus type 1 Tat and methamphetamine affect the release and activation of matrix-degrading proteinases. J Neurovirol. 2004;10:21–8. doi: 10.1080/13550280490261699. [DOI] [PubMed] [Google Scholar]
- Conant K, et al. Methamphetamine-associated cleavage of the synaptic adhesion molecule intercellular adhesion molecule-5. J Neurochem. 2011;118:521–32. doi: 10.1111/j.1471-4159.2010.07153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Nestler EJ. The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol Sci. 2014;35:374–83. doi: 10.1016/j.tips.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziembowska M, et al. Activity-dependent local translation of matrix metalloproteinase-9. J Neurosci. 2012;32:14538–47. doi: 10.1523/JNEUROSCI.6028-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzwonek J, Rylski M, Kaczmarek L. Matrix metalloproteinases and their endogenous inhibitors in neuronal physiology of the adult brain. FEBS Lett. 2004;567:129–35. doi: 10.1016/j.febslet.2004.03.070. [DOI] [PubMed] [Google Scholar]
- El Hajj EC, et al. Alcohol modulation of cardiac matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs favors collagen accumulation. Alcohol Clin Exp Res. 2014;38:448–56. doi: 10.1111/acer.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. Journal of Neuroscience Research. 2007;85:2813–23. doi: 10.1002/jnr.21273. [DOI] [PubMed] [Google Scholar]
- Gass JT, et al. Enhancement of extinction learning attenuates ethanol-seeking behavior and alters plasticity in the prefrontal cortex. J Neurosci. 2014;34:7562–74. doi: 10.1523/JNEUROSCI.5616-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, et al. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013a;77:867–72. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, et al. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A. 2013b;110:9124–9. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Kalivas PW. Rapid, transient synaptic plasticity in addiction. Neuropharmacology. 2014;76(Pt B):276–86. doi: 10.1016/j.neuropharm.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonthier B, et al. A PKC-dependent recruitment of MMP-2 controls semaphorin-3A growthpromoting effect in cortical dendrites. PLoS One. 2009;4:e5099. doi: 10.1371/journal.pone.0005099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschall PE, Deb S. Regulation of matrix metalloproteinase expressions in astrocytes, microglia and neurons. Neuroimmunomodulation. 1996;3:69–75. doi: 10.1159/000097229. [DOI] [PubMed] [Google Scholar]
- Hollander JA, et al. Striatal microRNA controls cocaine intake through CREB signalling. Nature. 2010;466:197–202. doi: 10.1038/nature09202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13:743–57. doi: 10.1038/nrn3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jones JA, et al. Selective microRNA suppression in human thoracic aneurysms: relationship of miR-29a to aortic size and proteolytic induction. Circ Cardiovasc Genet. 2011;4:605–13. doi: 10.1161/CIRCGENETICS.111.960419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kheradmand F, et al. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. 1998;280:898–902. doi: 10.1126/science.280.5365.898. [DOI] [PubMed] [Google Scholar]
- Kim YB, et al. Cell adhesion-dependent cofilin serine 3 phosphorylation by the integrin-linked kinase.c-Src complex. J Biol Chem. 2008;283:10089–96. doi: 10.1074/jbc.M708300200. [DOI] [PubMed] [Google Scholar]
- Kourrich S, et al. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–8. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousik SM, Napier TC, Carvey PM. The effects of psychostimulant drugs on blood brain barrier function and neuroinflammation. Front Pharmacol. 2012;3:121. doi: 10.3389/fphar.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovatsi L, et al. Alterations in serum MMP and TIMP concentrations following chronic heroin abuse. Toxicol Mech Methods. 2013;23:377–81. doi: 10.3109/15376516.2012.758681. [DOI] [PubMed] [Google Scholar]
- Kryczka J, et al. Matrix metalloproteinase-2 cleavage of the beta1 integrin ectodomain facilitates colon cancer cell motility. J Biol Chem. 2012;287:36556–66. doi: 10.1074/jbc.M112.384909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–7. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RKP, Teng KK, Hempstead BL. Regulation of Cell Survival by Secreted Proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Liu WT, et al. Spinal matrix metalloproteinase-9 contributes to physical dependence on morphine in mice. J Neurosci. 2010;30:7613–23. doi: 10.1523/JNEUROSCI.1358-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffek S, Schilling O, Franzke CW. Series "matrix metalloproteinases in lung health and disease": Biological role of matrix metalloproteinases: a critical balance. Eur Respir J. 2011;38:191–208. doi: 10.1183/09031936.00146510. [DOI] [PubMed] [Google Scholar]
- Lohi J, et al. Structural analysis and promoter characterization of the human membrane-type matrix metalloproteinase-1 (MT1-MMP) gene. Gene. 2000;242:75–86. doi: 10.1016/s0378-1119(99)00549-1. [DOI] [PubMed] [Google Scholar]
- Lonskaya I, et al. Soluble ICAM-5, a product of activity dependent proteolysis, increases mEPSC frequency and dendritic expression of GluA1. PLoS One. 2013;8:e69136. doi: 10.1371/journal.pone.0069136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, et al. Gene expression in human hippocampus from cocaine abusers identifies genes which regulate extracellular matrix remodeling. PLoS One. 2007;2:1187. doi: 10.1371/journal.pone.0001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, et al. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenteradministered cocaine. J Neurosci. 2011;31:5737–43. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Meighan SE, et al. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J Neurochem. 2006;96:1227–41. doi: 10.1111/j.1471-4159.2005.03565.x. [DOI] [PubMed] [Google Scholar]
- Michaluk P, et al. Beta-dystroglycan as a target for MMP-9, in response to enhanced neuronal activity. J Biol Chem. 2007;282:16036–41. doi: 10.1074/jbc.M700641200. [DOI] [PubMed] [Google Scholar]
- Millan EZ, Marchant NJ, McNally GP. Extinction of drug seeking. Behav Brain Res. 2011;217:454–62. doi: 10.1016/j.bbr.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, et al. Matrix metalloproteinase-9 contributes to kindled seizure development in pentylenetetrazole-treated mice by converting pro-BDNF to mature BDNF in the hippocampus. J Neurosci. 2011a;31:12963–71. doi: 10.1523/JNEUROSCI.3118-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Nabeshima T. Matrix metalloproteinases contribute to neuronal dysfunction in animal models of drug dependence, Alzheimer's disease, and epilepsy. Biochem Res Int. 2011b;2011:681385. doi: 10.1155/2011/681385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi HYK, Mouri A, Niwa N, Mizuno T, Noda, Nitta A, Itohara S, Banno Y, Nabeshima T. Role of matrix metalloproteinase and tissue inhibitor of MMP in methamphetamine-induced behavioral sensitization and reward: implications for dopamine receptor down-regulation and dopamine release. J Neurochem. 2007;102:1548–1560. doi: 10.1111/j.1471-4159.2007.04623.x. [DOI] [PubMed] [Google Scholar]
- Morrison CJ, Overall CM. TIMP independence of matrix metalloproteinase (MMP)-2 activation by membrane type 2 (MT2)-MMP is determined by contributions of both the MT2-MMP catalytic and hemopexin C domains. J Biol Chem. 2006;281:26528–39. doi: 10.1074/jbc.M603331200. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaineinduced synaptic potentiation provides enduring protection from relapse. Proc Nat Acad Sci. 2011;106:385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, et al. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26:1923–34. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Huntley GW. The extracellular matrix protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory. Learning & Memory. 2007;14:655–64. doi: 10.1101/lm.678307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto K, et al. Involvement of matrix metalloproteinase-9 in the development of morphine tolerance. Eur J Pharmacol. 2012;683:86–92. doi: 10.1016/j.ejphar.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Natarajan R, Harding JW, Wright JW. A role for matrix metalloproteinases in nicotine-induced conditioned place preference and relapse in adolescent female rats. J Exp Neurosci. 2013;7:1–14. doi: 10.4137/JEN.S11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton SS, et al. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J Neurosci. 2003;23:10841–51. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea E, et al. Current preclinical studies on neuroinflammation and changes in blood-brain barrier integrity by MDMA and methamphetamine. Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Overall CM. Molecular determinants of metalloproteinase substrate specificity: matrix metalloproteinase substrate binding domains, modules, and exosites. Mol Biotechnol. 2002;22:51–86. doi: 10.1385/MB:22:1:051. [DOI] [PubMed] [Google Scholar]
- Panenka WJ, et al. Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend. 2013;129:167–79. doi: 10.1016/j.drugalcdep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–53. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Pattij T, De Vries TJ. Targeting cocaine versus heroin memories: divergent roles within ventromedial prefrontal cortex. Trends Pharmacol Sci. 2013;34:689–95. doi: 10.1016/j.tips.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Ramirez SH, et al. Methamphetamine disrupts blood-brain barrier function by induction of oxidative stress in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29:1933–45. doi: 10.1038/jcbfm.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera S, et al. Tissue inhibitor of metalloproteinases-1 (TIMP-1) is differentially induced in neurons and astrocytes after seizures: evidence for developmental, immediate early gene, and lesion response. J Neurosci. 1997;17:4223–35. doi: 10.1523/JNEUROSCI.17-11-04223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. The European journal of neuroscience. 2010;31:903–9. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–76. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samochowiec A, et al. Functional polymorphism of matrix metalloproteinase-9 (MMP-9) gene in alcohol dependence: family and case control study. Brain res. 2010;1327:103–6. doi: 10.1016/j.brainres.2010.02.072. [DOI] [PubMed] [Google Scholar]
- Saygili E, et al. Sympathetic neurons express and secrete MMP-2 and MT1-MMP to control nerve sprouting via pro-NGF conversion. Cell Mol Neurobiol. 2011;31:17–25. doi: 10.1007/s10571-010-9548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann J, Yaka R. Prolonged withdrawal from repeated noncontingent cocaine exposure increases NMDA receptor expression and ERK activity in the nucleus accumbens. J Neurosci. 2009;29:6955–63. doi: 10.1523/JNEUROSCI.1329-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Kalivas PW. Astrocytic Dysfunction and Addiction: Consequences of Impaired Glutamate Homeostasis. Neuroscientist. 2014 doi: 10.1177/1073858413520347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, et al. Involvement of metabotropic glutamate receptor 5, AKT/PI3K signaling and NFkappaB pathway in methamphetamine-mediated increase in IL-6 and IL-8 expression in astrocytes. J Neuroinflammation. 2012;9:52. doi: 10.1186/1742-2094-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, et al. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci U S A. 2011;108:19407–12. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, et al. Prelimbic cortex and ventral tegmental area modulate synaptic plasticity differentially in nucleus accumbens during cocaine-reinstated drug seeking. Neuropsychopharmacology. 2014a;39:1169–77. doi: 10.1038/npp.2013.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, et al. Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J Neurosci. 2014b;34:5649–57. doi: 10.1523/JNEUROSCI.4564-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shofuda K, et al. Role of tissue inhibitor of metalloproteinases-2 (TIMP-2) in regulation of progelatinase A activation catalyzed by membrane-type matrix metalloproteinase-1 (MT1-MMP) in human cancer cells. J Biochem. 1998;124:462–70. doi: 10.1093/oxfordjournals.jbchem.a022136. [DOI] [PubMed] [Google Scholar]
- Sillanaukee P, et al. Matrix metalloproteinase-9 is elevated in serum of alcohol abusers. Eur J Clin Invest. 2002;32:225–9. doi: 10.1046/j.1365-2362.2002.00975.x. [DOI] [PubMed] [Google Scholar]
- Smith AC, et al. Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci. 2014 doi: 10.1038/nn.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AW, et al. Plasticity associated with escalated operant ethanol self-administration during acute withdrawal in ethanol-dependent rats requires intact matrix metalloproteinase systems. Neurobiol Learn Mem. 2011;96:199–206. doi: 10.1016/j.nlm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, et al. The glial cell modulators, ibudilast and its amino analog, AV1013, attenuate methamphetamine locomotor activity and its sensitization in mice. Eur J Pharmacol. 2012;679:75–80. doi: 10.1016/j.ejphar.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, Hendrick ES, Beardsley PM. Glial cell modulators attenuate methamphetamine selfadministration in the rat. Eur J Pharmacol. 2013;701:124–30. doi: 10.1016/j.ejphar.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, et al. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2013a;18:50–3. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, et al. Optogenetic inhibition of cocaine seeking in rats. Addiction biology. 2013b;18:50–3. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht M, Werb Z. How Matrix Metalloproteinases Regulat Cell Behavior. Annu Rev Cell Dev Biol. 2001a;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001b;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, et al. Activation of NMDA receptors promotes dendritic spine development through MMPmediated ICAM-5 cleavage. J Cell Biol. 2007;178:687–700. doi: 10.1083/jcb.200612097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Oever M, Lubbers BR, Goriounova NA, Li KW, Van der Schors RC, Loos M, Riga D, Wiskerke J, Binnekade R, Stegeman M, Schoffelmeer ANM, Mansvelder HD, Smit AB, De Vries TJ, Spijker S. Extracellular Matrix Plasticity and GABAergic Inhibition of Prefrontal Cortex Pyramidal Cells Facilitates Relapse to Heroin Seeking. Neuropsychopharm. 2010;35:2120–2133. doi: 10.1038/npp.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslegers M, et al. Matrix metalloproteinase-2 and -9 as promising benefactors in development, plasticity and repair of the nervous system. Prog Neurobiol. 2013 doi: 10.1016/j.pneurobio.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Goldstein RZ. Addiction: pulling at the neural threads of social behaviors. Neuron. 2011;69:599–602. doi: 10.1016/j.neuron.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM. Conceptualizing withdrawal-induced escalation of alcohol self-administration as a learned, plasticity-dependent process. Alcohol. 2012;46:339–48. doi: 10.1016/j.alcohol.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XB, et al. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Nat Acad Sci. 2008;105:19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins A, et al. Integrins modulate relapse to cocaine-seeking. J Neurosci. 2011;31:16177–84. doi: 10.1523/JNEUROSCI.3816-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks AL, McNally GP. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur J Neurosci. 2013;37:259–68. doi: 10.1111/ejn.12031. [DOI] [PubMed] [Google Scholar]
- Wright JW, et al. Ethanol-induced impairment of spatial memory and brain matrix metalloproteinases. Brain Res. 2003;963:252–61. doi: 10.1016/s0006-8993(02)04036-2. [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-B and promotes tumor invasion and angiogenesis. Genes & Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Olson BL, Windsor LJ. Nicotine increases the collagen-degrading ability of human gingival fibroblasts. J Periodontal Res. 2007;42:228–35. doi: 10.1111/j.1600-0765.2006.00937.x. [DOI] [PubMed] [Google Scholar]