Abstract
Stress fibers are actomyosin-based bundles whose structural and contractile properties underlie numerous cellular processes including adhesion, motility and mechanosensing. Recent advances in high-resolution live-cell imaging and single-cell force measurement have dramatically sharpened our understanding of the assembly, connectivity, and evolution of various specialized stress fiber subpopulations. This in turn has motivated interest in understanding how individual stress fibers generate tension and support cellular structure and force generation. In this review, we discuss approaches for measuring the mechanical properties of single stress fibers. We begin by discussing studies conducted in cell-free settings, including strategies based on isolation of intact stress fibers and reconstitution of stress fiber-like structures from purified components. We then discuss measurements obtained in living cells based both on inference of stress fiber properties from whole-cell mechanical measurements (e.g., atomic force microscopy) and on direct interrogation of single stress fibers (e.g., subcellular laser nanosurgery). We conclude by reviewing various mathematical models of stress fiber function that have been developed based on these experimental measurements. An important future challenge in this area will be the integration of these sophisticated biophysical measurements with the field’s increasingly detailed molecular understanding of stress fiber assembly, dynamics, and signal transduction. This article is part of a Special Issue entitled: Mechanobiology.
Keywords: Stress fiber, Biomechanical property, Mechanobiology, Subcellular laser ablation
The ability of a eukaryotic cell to adhere, spread, migrate and resist deformation depends on the ability of the cell to generate force against the surrounding extracellular matrix (ECM). These forces are not only essential for structural regulation in individual cells but can also control morphological changes in tissue during development [1]. Changes in the magnitude and direction of these forces at either the cell or tissue length scale can contribute to the development of diseases such as atherosclerosis, osteoporosis and cancer. The cytoskeleton, an interconnected network of filamentous proteins consisting of actin filaments (F-actin), microtubules, intermediate filaments, and their associated molecular motors and other accessory proteins, acts as a physical and biochemical link between the cell and the ECM [2]. The cytoskeleton senses, generates and mediates coordinated forces to maintain tensional homeostasis and control normal cell and tissue function [3,4]. It has been shown to contribute to cellular contractility and matrix reorganization in both highly simplified two-dimensional culture paradigms as well as more complex, three-dimensional microenvironments [5], reflecting the need to study how cytoskeletal components generate, transmit and withstand forces over time as a means of understanding how cells behave in vivo.
Stress fibers represent an important component of the cytoskeleton. Stress fibers are bundles of actin filaments with alternating polarity held together by various crosslinking proteins such as α-actinin and zyxin. Often, but not always, stress fibers also contain non-muscle myosin II (NMMII) bipolar filaments. Although stress fibers can resemble myofibrils in their composition, they exhibit a less organized structure; if sarcomeres are present, they are not as regular as myofibrils and actin filaments are not uniformly located along the fiber length [6]. Contraction of NMMII produces a force along the length of the fiber that is transmitted through cellular adhesions to the extracellular matrix (ECM) allowing the fibers to be in isometric tension.
Stress fibers are physiologically important in processes that require cellular contraction such as wound healing and exocrine gland secretion [7]. For example, during wound healing, tension borne by specific stress fibers within fibroblasts can induce recruitment of α-smooth muscle actin to these fibers, which in turn permits even greater generation of tensile force [8,9]. This tension generation can activate latent Transforming Growth Factor β1 (TGF β1) within the matrix, promoting these fibroblasts to differentiate into myofibroblasts that drive tissue compaction [10]. This increased stress fiber-driven contractility therefore initiates a positive feedback loop of increased tension generation and myofibroblast differentiation that contributes to eventual wound closure [11,12]. Epithelial cells that line the wound site also develop actomyosin cables that contract to facilitate wound closure [13]. These principles are not limited to wound healing; epithelial cells around exocrine glands also form stress fibers whose contraction promotes secretion. In the presence of oxytocin, myosin is activated, leading to increased contraction around the mammary gland and eventual secretion of milk [14,15].
The growing appreciation of the physiological importance of stress fibers has spurred significant interest in quantifying the mechanical properties of stress fibers and the roles they may play in supporting cellular structure, motility and tissue processes. Thus, a rich variety of tools have recently emerged to study the mechanical and structural properties of stress fibers and determine new physical models of how stress fibers contract and contribute to the overall mechanics of the cell. In this review, we will discuss both in vitro (cell-free) and in vivo (live-cell) tools available to study the mechanical properties of stress fibers and how these tools have been used to advance our understanding of stress fiber biomechanics. Several excellent reviews have covered broad aspects of stress fiber structure and function [2,16,17]. Here, we will concentrate on current biomechanical models of stress fiber structure and function and end with a discussion of unanswered questions in the field.
1. Stress fiber composition
Stress fibers are generally defined as bundles of 10–30 thin filaments, composed of F-actin crosslinked by actin-binding proteins such as α-actinin, fascin and filamin. These thin filament bundles are frequently but not always interleaved with thick filaments composed primarily of non-muscle myosin 11 (NMMII) motors, the key force generating component in stress fibers (Fig. 1A). NMMII is a hexamer that consists of two essential light chains (ELCs), two regulatory light chains (RLCs) and two heavy chains. The heavy chains contain the head domain, which is a globular structure that can both directly engage F-actin and hydrolyze ATP to provide the free energy required to power the contractile sliding of the thick filaments against the thin filaments, thus creating tension within the stress fiber (Fig. 1B and C). Phosphorylation of the RLC facilitates this process by allowing myosin to uncoil and assemble into linear thick filaments, as well as enhancing its ATPase activity [18].
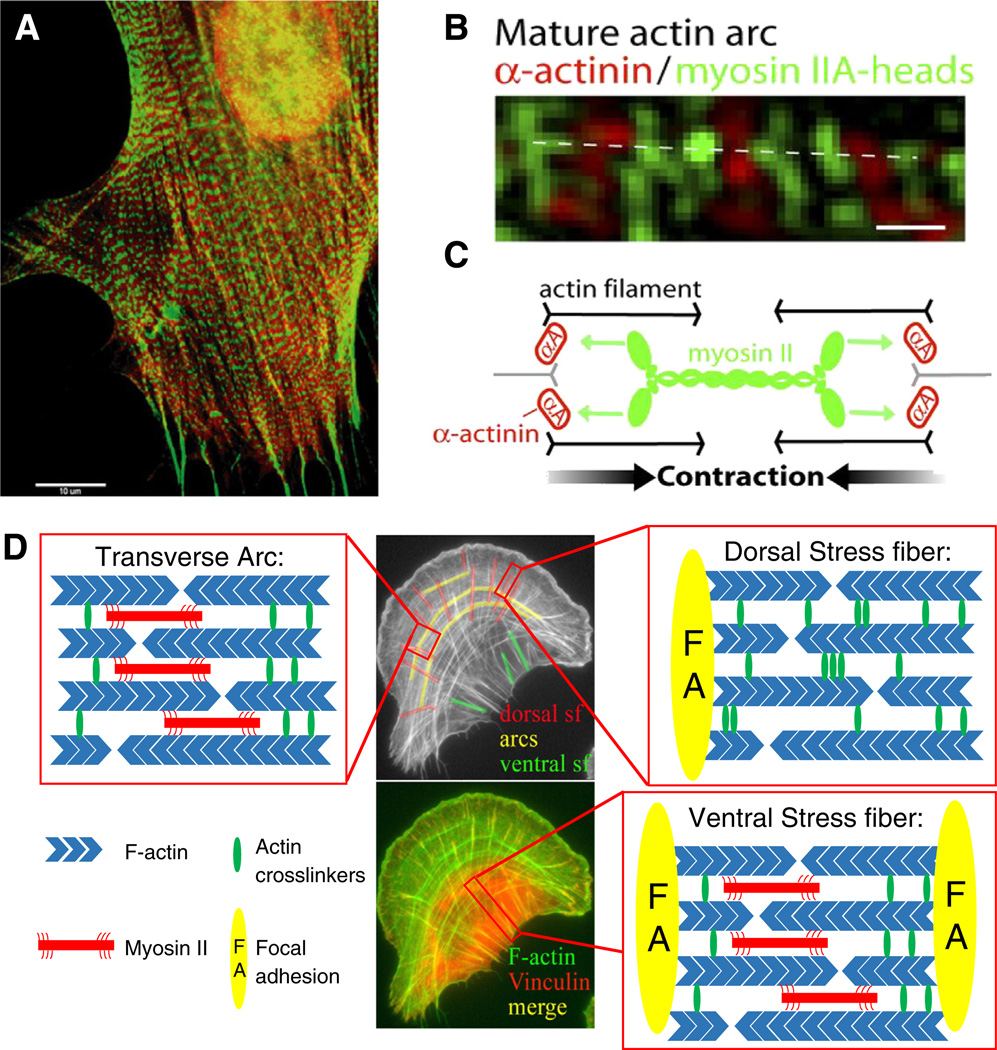
Fig. 1.
Stress fiber structure and composition. (A) A gerbil fibroma cell in stained for myosin light chain (red) and α-actinin (green) to illustrate the periodic localization of proteins along the stress fiber [30]. (B) Structured illumination microscopy of the nanoscale organization of myosin IIA minifilaments in actin fibers using U20S cells expressing α-actinin mApple (green) and myosin IIA-mEGFP [31]. (C) Schematic showing how the interaction of myosin minifilaments can lead to sarcomere contraction of stress fibers [31]. (D) Schematic of the three stress fiber subtypes indicated in a U20S human osteosarcoma cell stained for F-actin (green) and the focal adhesion marker vinculin (red). Boxed regions highlight the structural differences between the three stress fiber subtypes.
There are three NMMII isoforms in mammalian cells: NMMIIIIA, IIB and IIC, with the isoform specified by the heavy chain. NMMIA and IIB are the predominant isoforms, and much effort has been devoted to understand their differential biophysical properties and contributions to cell mechanics and motility. Much less is known about the in vivo function of NMMIIC, although it appears to play central roles in specific physiological contexts. For instance, NMMIIC is critical for the outgrowth of neuronal processes, can modulate neuronal cell adhesion [19] and is important in the cytokinesis and motility of cancer cells [20].
Within polarized cells, there is an overall differential distribution of NMMIIA and IIB with an observable NMMIIA-rich state in the front and a NMMIIB-rich state in the rear [21]. The differential localization of NMMII isoforms is thought to correspond to distinct mechanical functions: NMMIIB found at the cell rear promotes directional migration by preventing protrusion formation and properly positioning the Golgi apparatus, the nucleus and microtubules, whereas NMMIIA found at the cell front contributes to nascent adhesion formation [18,22,23]. The application of new superresolution imaging methodologies has introduced important complexities to this picture; two-color total internal reflection fluorescence/structured-illumination microscopy imaging of NMMIIA, IIB and 11C revealed that IIA and IIB as well as IIA and IIC co-assemble to form heterotypic filaments within single stress fibers, implying that the differences in regional localization may reflect a continuum of isoform-rich states [21]. In addition to their differential localization, myosin isoforms also exhibit varying motor functions. NMMIIA has a higher rate of ATP hydrolysis and can slide actin filaments more rapidly than IIB. NMMIIB has a higher duty ratio and can stay bound to actin longer, which has led to the notion that IIB may drive sustained contraction [24]. Given the different force-generating properties of myosin isoforms, one might predict that stress fibers made up of different compositions of myosin isoforms would exhibit different mechanical properties and may have different biological functions within a cell.
Stress fiber contractility depends on the polarity of the actin filaments. Since NMMII motors move towards the barbed ends of actin filaments, successive actin filaments must have opposite polarity to allow for NMMII sliding and subsequent contraction. Actin filaments in stress fibers can exhibit different patterns of polarity — some stress fibers exhibit the expected opposite polarity, others seem to be randomly oriented, whereas stress fibers of motile cells have regions of both uniformity and randomness [25]. An important open question is how actomyosin networks with randomly oriented polarities that form at the leading edge evolve into more mature stress fibers with sarcomeric orientations. Recent actomyosin reconstitution studies have offered an elegant solution to this problem. In an actomyosin complex with mixed orientations, the sarcomeric regions generate tension whereas the non-sarcomeric regions generate compression. These internal tensile and compressive forces cause the regions with non-sarcomeric orientations to buckle and sever, effectively selecting these regions out and enriching the sarcomeric fraction [26]. The contractile ring formed during cytokinesis represents an example of a structure that is not formally classified as a stress fiber but has been successfully computationally modeled by applying analogous concepts [27]. It is likely that similar approaches may be fruitful in dissecting the function of other actin bundles, such as those found in filopodia [2,28] or retraction fibers during cell division [29].
2. Stress fiber connectivity to the extracellular matrix and force transmission
Stress fibers can directly couple to the cell-ECM interface through focal adhesions, which contain a variety of actin binding proteins such as zyxin and paxillin, or indirectly by inserting themselves into (i.e., branching from) other stress fibers. When the tension within stress fibers is balanced by the mechanical resistance of the structure(s) to which they are attached, stress fibers are under isometric tension. Changes in isometric tension play a central role in sensing and adapting to extracellular forces, and thus stress fibers may be regarded as mesoscale mechanosensors [32]. For example, endothelial and vascular smooth muscle cells commonly respond to cyclic stretch by reorganizing their stress fiber networks perpendicular to the axis of stretch [33]. The strains induced by cyclic stretch can promote localization of specific proteins to stress fibers to facilitate remodeling [34], and stress fiber rupture can initiate a zyxin-mediated repair process [35]. Indeed, even strains that are not sufficient to induce rupture may be sensed by sarcomeres within stress fibers; when a specific region of a stress fiber is extensionally strained beyond a threshold, surrounding sarcomeres compensate by contracting to preserve stress fiber length, after which zyxin and α-actinin are recruited to the sites of high strain to form new sarcomeres [36,37]. These findings suggest the presence of a tensional homeostasis mechanism that is based on sarcomere communication and provide evidence for the mechanosensitivity of stress fibers.
The formation and contractility of stress fibers are also associated with stem cell differentiation, in that cells grown on micropatterned islands of fibronectin that promoted increased stress fiber and focal adhesion formation also exhibited increased osteoblastic differentiation [38]. More recently, Zemel and colleagues computationally investigated the effect of stress fiber alignment and cellular shape in stem cell differentiation [39]. This study revealed that maximal stress fiber polarization occurs at an optimal ECM rigidity analogous to the optimal stiffness for stem cell differentiation, suggesting that fiber orientation may play an important intermediary role in converting matrix stiffness cues into differentiation programs. These studies strongly hint at the importance of stress fiber mechanosensing to cellular phenotype and illustrate the power of microfabrication techniques for manipulating stress fiber alignment and stem cell differentiation.
3. Classification of stress fibers
A widely-used classification scheme describes three different stress fiber subtypes – transverse arcs, dorsal stress fibers, and ventral stress fibers – based on their molecular composition, connectivity to focal adhesions, assembly mechanisms and dynamics, and biological function [40] (see Fig. 1D). As the name would suggest, transverse arcs are curved stress fibers found parallel to the leading edge and are assembled from shorter actin filaments that originate in the lamellipodium [40]. Transverse arcs are contractile and do not attach to focal adhesions at all. Dorsal stress fibers assemble in a myosin-independent fashion from nascent F-actin bundles within the lamellar compartment of the leading edge of the cell and are generally regarded as non-contractile [41]. Dorsal stress fibers extend vertically upwards from their origin in a focal adhesion, insert into transverse arcs, and thereby mechanically link transverse arcs and focal adhesions at the front of the cell. Recent evidence makes a compelling case that this organization allows transverse arcs to distribute tension to the cell-ECM interface via their connectivity to transverse arcs and flatten the lamellar region by “levering down” dorsal stress fibers using focal adhesions as a fulcrum [31]. Dorsal fibers may also act as a structural template to facilitate maturation of integrin-based focal adhesions found at the leading edge [42,43]. While the molecular mechanism of assembly of these structures remains incompletely understood, palladin was recently found to be specifically required for the assembly of dorsal stress fibers and essential for the generation of a fully functional stress fiber network in both two dimensional and three dimensional matrices [44]. Ventral stress fibers, the third category, are NMMII-rich and generate strong traction forces at the cell base. These traction forces are particularly important for detaching the trailing edge of the cell during directional migration, and by extension for the establishment of front-to-rear polarity [45]. Ventral stress fibers are thought to assemble through the fusion of transverse arcs and dorsal stress fibers or by fusion of two dorsal stress fibers; consequently, they are attached to focal adhesions at both ends and are optimally positioned to tense the ECM [40]. The radial architecture and contractile activity of specific fiber subpopulations have been implicated in driving cell-scale symmetry-breaking and establishment of chirality[46].
4. In vitro micromanipulation
4.1. Studying single actin filaments and extracted stress fibers
There have been several notable efforts to measure mechanical properties of stress fibers in cell-free preparations. Initial efforts focused on the use of glass needles, microcantilevers, and microneedles to probe reconstituted single actin filaments, with the notion of scaling up these measurements to estimate properties of bundles [47–49]. While these measurements have been highly instructive, stress fibers are bundles of individual actin filaments reinforced by a variety of binding partners and motor proteins, which makes it inherently challenging to infer the mechanical properties of a bundle of crosslinked actin filaments from those of a single actin filament.
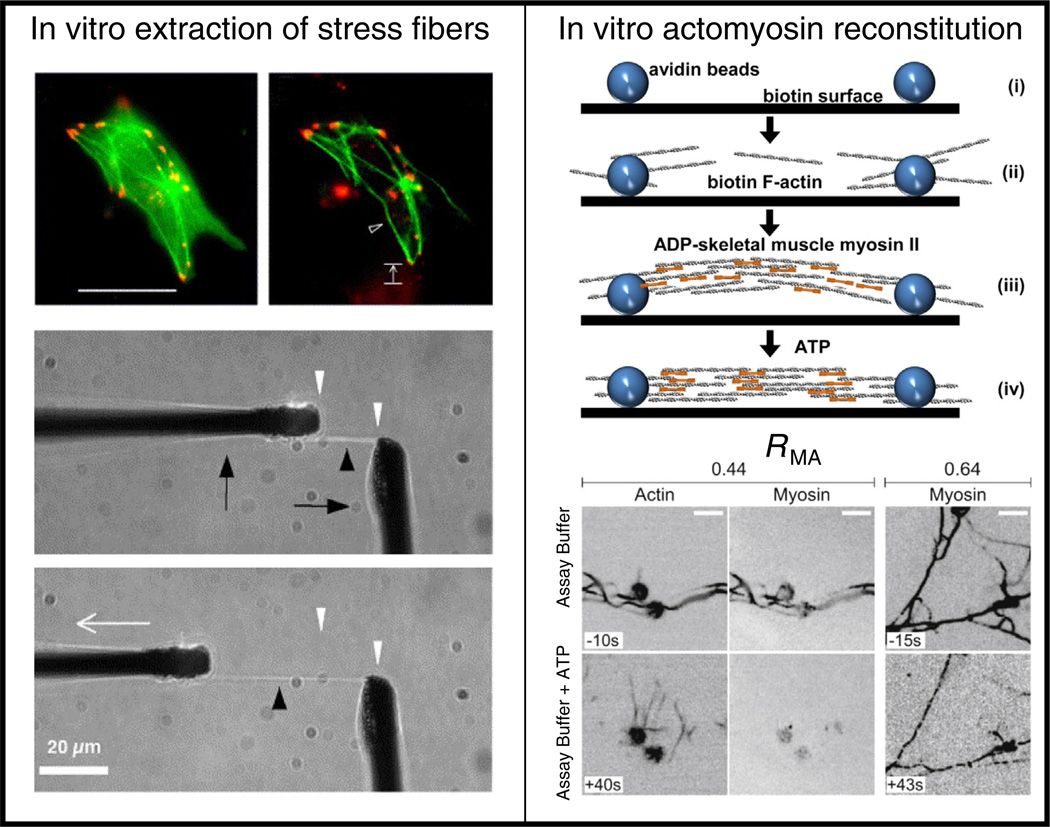
Thus, more recently, strategies have been developed to extract and mechanically manipulate intact stress fibers from living cells. For example, Katoh and colleagues used low ionic strength solution and detergent extractions to “de-roof fibroblasts adherent to two-dimensional substrates, leaving behind matrix adhesions and their associated stress fibers [50] (see Fig. 2). Notably, these isolated stress fibers retained their contractility, with the magnitude of contractility dependent on the concentration of ATP, and produced length contractions of up to 20% [50]. The mechanical properties of isolated stress fibers were further investigated by performing tensile tests with the use of microcantilevers [51]. The longitudinal elastic modulus of an isolated stress fiber increased nonlinearly with strain (approximately 1.45 MPa for smooth muscle cells and 300 kPa for endothelial cells assuming uniform structure and 0.05 µm2 cross sectional area). Additionally, the force required to stretch a single stress fiber from its zero stress length to its original length was approximately 10 nN, which is comparable with local traction forces of single adhesion sites [52]. This suggests that stress fibers can bear substantial stresses and could account for traction observed at the cell-matrix interface.
Fig. 2.
In vitro tools used to study stress fiber mechanical properties. Left: In vitro extraction of stress fibers. SMCs expressing GFP-actin before and after stress fiber extraction treatment and detachment from the substrate to obtain isolated stress fibers that can then be used in mechanical tests such as tensile stretching [51]. Right: In vitro actomyosin reconstitution. Schematic illustrating bundle assembly and contraction of in vitro reconstituted actomyosin fibers [60]. Addition of ATP allows for myosin contraction which results in an isometric tension within the reconstituted fibers as illustrated in the images [57].
4.2. Reconstitution of actomyosin cables
Another powerful approach used to study the mechanical organization and function of stress fibers is in vitro reconstitution of minimal actomyosin structures. In principle, this approach combines the control associated with the use of purified materials while capturing some of the complexity that might be found in intact stress fibers. Actomyosin reconstitution involves polymerization of F-actin in the presence of myosin thick filaments and subsequent measurement of contraction and force generation. For over 60 years, this approach has been used to identify the minimal components necessary for reconstructing contractility and their dependence to contractility [53–55]. Studies have concentrated on the effect of actin crosslinkers such as fascin, filamin A and α-actinin on the mechanical properties of an actomyosin network [56]. Until recently, mechanical studies on reconstituted actomyosin networks were performed in a three dimensional gelatinous state and not the highly organized and anisotropic state characteristic of the cellular actomyosin network. In particular, a major challenge in the field had been the assembly of actomyosin complexes into two-dimensional bundles that could begin to mimic stress fibers.
This need has motivated recent efforts to reconstitute actomyosin bundles in vitro, with the goal of measuring the mechanical properties of the assembled structures and understanding how specific molecular components contribute to mechanics and function. In one strategy, to promote assembly of actomyosin bundles, F-actin was attached on neutravidin beads bound to a coverslip. Thick filaments of smooth muscle myosin were subsequently added, allowing the myosin heads to bind to F-actin, resulting in the formation of actomyosin bundles of varying lengths (5 to 50 µm) (see Fig. 2) [57,58]. At a low density of myosin, smooth muscle myosin thick filaments stabilized actin filaments and formed bundles with no measurable contraction. At a higher density and with the addition of ATP, the presence of myosin filaments was sufficient to elicit contraction and generate tension, even in the absence of actin crosslinkers or sarcomere formation [57]. More recently, these bundles have been used to study the effects of the isoform composition of myosin filaments (nonmuscle vs. skeletal muscle vs. smooth muscle) to contraction rates as well as the mechanism of self-organization to sarcomere-like structures and its regulation by myosin-mediated forces [59,60].
In addition to exploring how the mechanical properties of stress fibers depend on their molecular composition, actomyosin reconstitution can also be used to dissect the molecular mechanisms through which focal adhesions associate with the actomyosin cytoskeleton [61]. Recon-stitution of purified actin, myosin and vinculin on talin-micropatterned surfaces led to new insight into how vinculin associates with talin within a focal adhesion and the role of the cytoskeleton in reinforcing this association. Activation of vinculin by a stretched talin protein leads to a positive feedback loop that reinforces the actin-talin-vinculin association, revealing a molecular switch mechanism that controls the connection between adhesion complexes and the actomyosin network [61]. Recently, actomyosin bundles were reconstituted in lipid vesicles to investigate effects of spatial confinement on bundle assembly and contractile function [62]. Remarkably, increasing the myosin concentration facilitated the assembly of an equatorial actin ring and then contracted the ring, reminiscent of constrictions formed at the cleavage furrow during cytokinesis.
Reconstitution and extraction approaches have complementary strengths and limitations. Actomyosin reconstitution provides a more simplified and controlled system to study the contractility of stress fibers than extraction of stress fibers from fixed cells. Simply by including or excluding specific molecular components and controlling their relative stoichiometry, it is possible to identify functionally important proteins and quantitatively dissect how these proteins contribute to contractility. In contrast, isolated stress fibers are a complex multi-component system made up of different proteins that can vary based on the extraction method used, making it hard to identify how each protein contributes to contractility in a quantitative manner. On the other hand, isolated stress fibers retain key components found in cellular stress fibers and offer the opportunity to study how stimulation of specific signaling systems influences contractility. For example, stress fibers subjected to glycerol extraction have been reported to contract both in the presence and absence of Ca2+ whereas fibers extracted using Triton X-100 (presumably a different subset of fibers) only contract in the presence of Ca2+[63–65]. Triton X-100 dissolves RhoA and Rho-associated coiled-coil containing protein kinase (ROCK) leading to isolated stress fibers that can only contract via the Myosin Light Chain Kinase (MLCK) pathway, providing direct evidence that stress fiber contraction is dually regulated with two different modes of contraction — a rapid, extensive contraction induced by MLCK and a slower contraction controlled by RhoA/ROCK [65].
5. In vivo macromanipulation
5.1. Whole-cell stretch experiments to explore cytoskeletal mechanics
Complementary to the above approaches, there has been much interest and effort to understand the mechanics of stress fibers in their cellular context, without having to remove or rebuild them. Initial efforts to do this have sought to infer the properties of cytoskeletal structures from measurements of whole-cell mechanics. Whole-cell tensile and compression tests, including those involving stretched/released silicone culture substrates and micropipette-based stretching of single cells, have been used to study how stress fibers bear and dissipate externally applied loads [66,67]. Osteoblasts seeded on a stretched silicone rubber were fixed 5 min after constant strain release with an observable 20% pre-strain on each stress fiber [68]. Stress fibers of human aortic endothelial cells seeded on an initially pre-stretched silicone substrate buckled in a non-uniform manner with a calculated pre-strain distribution of 15–26% when tension was released. The variability suggests the presence of heterogeneity in cytoskeletal tension and stiffness within individual stress fibers [66]. The presence of buckling was surprising since it suggested that stress fibers are not only mechanically constrained at their ends via focal adhesions but may also be constrained along the length of the fiber, potentially due to smaller adhesions or other cytoskeletal constituents such as microtubules. Nocodazole-induced disintegration of the microtubule network decreased the buckling frequency, suggesting a possible coupling between stress fibers and microtubules. A later study explored the connection of microtubules with the surrounding elastic cytoskeleton and provided evidence that the coupling of the two networks mechanically reinforces microtubules, allowing them to withstand high compressive forces and buckle at short wavelengths, without necessarily requiring discrete adhesion points between the microtubule and surrounding network [69], Given the heterogeneity of sarcomere contraction observed within a single fiber [30] as well as the buckling heterogeneity observed within the whole cell, it is challenging to interpret from these studies how a single stress fiber contributes to the overall cellular tension, or whether the mechanical differences in different fiber populations arise due to differences in fiber architecture or differences in myosin activation.
5.2. Tools to correlate cellular mechanical properties to stress fiber mechanics
Perhaps most relevant to stress fiber function, several tools have been developed over the past two decades to quantify traction force generated by cells against the ECM. Coupled with molecular manipulations, these techniques may be used to determine how specific components contribute to traction force generation. For example, in traction force microscopy (TFM), traction stresses applied at the cell-ECM interface are recovered by tracking the displacement of fiduciary markers embedded within the underlying matrix. An important limitation of most current TFM strategies is that, with few exceptions [70], they do not incorporate intracellular structure, such that it is difficult to infer how much traction forces measured at specific adhesion sites are being transmitted to stress fibers, and what the molecular mechanism of that force transmission may be [71].
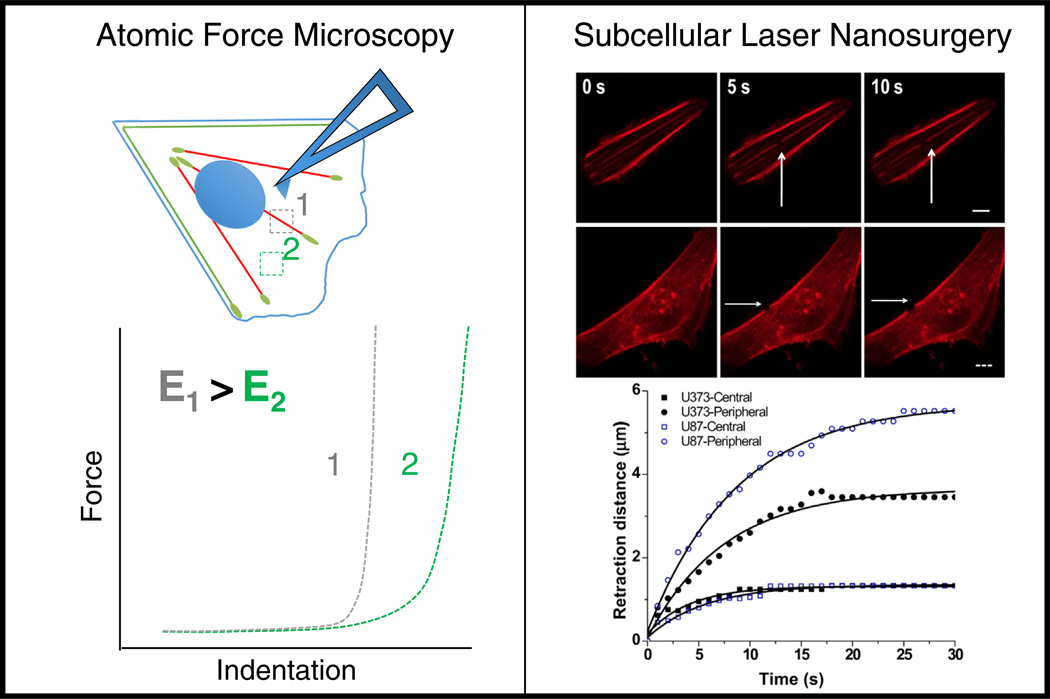
Atomic force microscopy (AFM) is another single-cell technology that has been used to mechanically interrogate living cells and to infer mechanical properties of stress fibers and other cytoskeletal structures (Fig. 3). Because AFM does not require fixation and may be performed in culture medium, it is ideally suited to probe living cells under physiological conditions. In AFM, a microscale cantilever spring may either be scanned across a surface to obtain nanometer-resolution images or used to indent materials at a fixed horizontal position to discern mechanical properties. In the latter modality, AFM indentation curves may be fit using models of indentational elasticity (e.g., Hertz) to obtain a Young’s modulus; repetition of this measurement at different locations along the sample permits construction of elasticity maps. When combined with fluorescence imaging of labeled intracellular structures, elasticity mapping can be used to determine the local elastic modulus of a variety of superficial cellular structures, including stress fibers. For example, AFM elasticity mapping and topographical imaging combined with fluorescence imaging of the cytoskeleton showed that stress fibers colocalize with regions of higher stiffness [72]. These approaches may also be coupled with small-molecule inhibitors of cytoskeletal function to dissect the contribution of specific networks to cellular elasticity [73]. In one study, 3T3 and NRK fibroblasts were incubated with cytochalasin or latrunculin, which potently inhibit actin polymerization. Subsequent correlation of AFM elasticity maps with fluorescence images of the actin cytoskeleton revealed that these treatments both disaggregated stress fibers and reduced cell rigidity, underscoring the important contributions of the actin cytoskeleton to cellular elastic properties. Even with the use of highly specific cytoskeletal inhibitors, however, it is impossible to dissect the mechanical contributions of individual stress fibers within a cell.
Fig. 3.
In vivo tools used to study stress fiber mechanical properties. Left: atomic force microscopy may be used to measure the stiffness of individual stress fibers within the cell. The AFM tip probes the cell surface at different locations (positions 1 and 2). The resulting force-indentation data may be fit to extract local elastic modulus values, thus allowing stiffness comparisons between the fiber and the surrounding cytoskeletal cortex [75]. Right: Subcellular laser ablation allows for selective photodisruption of peripheral or central stress fibers. By following the retraction of the fiber over time and fitting it to the Kelvin–Voigt model of viscoelasticiry, one can deduce effective viscoelastic properties of the severed fiber [79].
While the ability to correlate images and mechanical measurements is an important advancement, it nevertheless still represents an indirect probe of the elasticity of subcellular structures and reflects the collective contributions of other structural elements. Moreover, living cells violate key assumptions of the theories commonly used to obtain Young’s moduli from AFM stiffness measurements, in that cells are neither homogeneous, nor isotropic, nor linearly elastic, nor infinitely thick.
5.3. Imaging and mechanically indenting individual stress fibers using atomic force microscopy
More recently, AFM has been used to investigate the mechanical properties of individual stress fibers within the cell providing an even more direct assessment of the mechanical properties of a stress fiber using a non-Hertzian approach [74,75]. AFM was used to perform nanoindentations in an 8 × 6 array on a region of the cell surface that included a single stress fiber. Based on differences in the AFM contact mode images, stress fiber localization was determined and its presence was correlated with elasticity measurements. The authors demonstrated that for strains up to 12%, an individual stress fiber had approximately linear stress-strain properties with a calculated transverse elastic modulus of approximately 12 kPa at both peripheral and central regions within the same stress fiber [75]. The addition of calyculin-A, a drug that increases myosin activation through inhibition of myosin light chain (MLC) phosphatase, led to heterogeneous changes in stiffness along the same stress fiber. This highlights the critical role of myosin force generation in determining the stress fiber mechanical stiffness as well as the dynamic and nonuniform contractile nature of stress fibers.
It should be noted that the elastic modulus of an individual stress fiber reported by AFM was much smaller than the elastic modulus of isolated stress fibers, with the difference potentially arising from the differences in the experimental systems, the intrinsic anisotropy of the stress fiber, and/or the presence of other cellular components that may physically interact with stress fibers in the cell but are absent when the stress fiber is isolated. Additionally, an important caveat with AFM measurements of stress fibers is that AFM can only examine the exterior surface of a living cell, so that deep structures cannot be indented without also indenting the overlying superficial structures, which may then contribute to measured elastic moduli.
6. In vivo micromanipulation: subcellular laser nanosurgery
Subcellular laser nanosurgery has emerged as a tool that allows one to directly study the viscoelastic properties of individual stress fibers within the cell interior. Subcellular laser microsurgery has been used since the late 1970s to study the mechanical properties of an individual stress fiber directly. In 1979, Strahs and Berns used an attenuated Nd:YAG laser microbeam with various wavelengths to ablate a single stress fiber in non-muscle cells [76]. The authors observed that after ablation, the fiber ends retracted and repaired over time. Incubation of cells with cytochalasin B prior to laser ablation led to increased retraction distances, possibly due to disruption of fiber attachment points with the membrane, and decreased repair of the fiber ends [77]. The method described could create a break ranging from 0.25–2 µn in length without affecting cell motility, shape and matrix adhesion. Interestingly, these early studies revealed that not all stress fibers retracted upon incision, suggesting that different fibers bear different levels of tension and presumably exert different contractile forces, presaging the now well-validated notion that some stress fibers primarily play structural roles (e.g., dorsal fibers) whereas others are highly contractile (e.g., ventral fibers) [78].
The precision of laser nanosurgery has advanced with improvements in laser technology and optics. Building from the early work of Berns and coworkers, intracellular nanosurgery systems have been developed in which femtosecond laser pulses are focused by a high-numerical aperture objective and confined to a very small focal volume, thus inducing multiphoton absorption and plasma mediated ablation of biological material within <300 nm [80–82]. This capability has been leveraged to sever individual stress fiber bundles within the cell and follow the retraction over time [83] (Fig. 3). For example, severing a stress fiber in a living endothelial cell causes the fiber to retract in parallel with the axis of the fiber; fitting the retraction kinetics with a Kelvin-Voigt model revealed that the fiber behaves as a viscoelastic cable that has an elastic component and a viscous dissipative element [84]. Subcellular laser nanosurgery can be combined with TFM to determine how single stress fibers distribute load across focal adhesions and throughout the cell. Studies with TFM revealed that the tension released by a single stress fiber after ablation contributed to ECM strain across the entire cell-ECM interface, with higher strains found at focal adhesions near the ablated stress fiber [83].
In addition to its utility for probing the mechanics of stress fibers, femtosecond laser nanosurgery may also be used to investigate how tension affects the localization and function of specific mechanosensory proteins at the cell-ECM interface. For example, the focal adhesion protein zyxin had been implicated as a mechanosensor based on its ability to localize to stress fibers upon stretch or shear [85], yet the biophysical basis of this connection had remained a mystery. To address this question, Lele and co-authors combined laser nanosurgery with fluorescence recovery after photobleaching (FRAP) to study how stress fiber tension influences the binding kinetics of zyxin to associated focal adhesions. These studies revealed that dissipation of tension significantly increased the off-rate with which zyxin engaged focal adhesions, suggesting a mechanism through which cell-scale forces could influence molecular-scale localization [86,87].
Taking this approach one step further, laser nanosurgery has been combined with molecular biosensors of tension to determine how individual stress fibers contribute to tension across specific mechanosensory proteins within focal adhesions. One study used laser nanosurgery to sever stress fibers while simultaneously applying a vinculin-based fluorescence resonance energy transfer (FRET) tension sensor [88] to show that tension released from a single stress fiber increased or decreased vinculin tension within a given focal adhesion in a manner that depended on the directional alignment of that adhesion with the incised stress fiber [89]. Combining laser nanosurgery with other techniques has the potential to map cytoskeletal mechanics within the cell and show direct connections of tension transmission between individual molecules at the nanoscale, individual contractile elements in the micron scale and traction forces exerted by whole cells.
Combining laser nanosurgery with pharmacological and genetic manipulation of signaling has enabled more precise determination of how specific myosin activators contribute to tension generation. For example, inhibition of the myosin activators ROCK and MLCK significantly altered the rate and extent of stress fiber retraction, providing evidence that myosin motors contribute heavily to stress fiber elasticity [79,83]. This capability has been used to map elastic properties of stress fibers within the cell and implicate specific myosin activators in controlling tension within different cellular compartments [79]. Inhibition of ROCK and MLCK abolished the retraction of central and peripheral stress fibers respectively, and this is consistent with earlier findings [64,65]. Moreover, each stress fiber population displayed different viscoelastic properties and structural contributions, with incision of peripheral stress fibers producing more viscous retraction and leading to whole-cell contraction [79].
The ability to combine subcellular laser nanosurgery with other techniques such as the ones described above makes it a very powerful tool that allows one to probe not only the mechanical and force transducing properties of stress fibers but also determine how stress fiber molecular activation and architecture contribute to their contractility and mechanical behavior.
7. Developing biomechanical models of stress fibers using subcellular laser nanosurgery
Despite the importance of stress fibers in multiple biological processes, the molecular mechanisms that contribute to tension in stress fibers remain unclear. Part of the reason is that stress fibers in cultured cells are often found under isometric tension (i.e., constant length) and thus offer little opportunity to observe kinetic properties that may shed light on the origins of tension. Because laser nanosurgical incision of stress fibers releases this tension and provides access to retraction kinetics, these measurements have greatly promoted efforts to develop biomechanical models of stress fiber contraction [83,86,90,91].
While macroscale, continuum descriptions of stress fibers (e.g., Kelvin-Voigt) continue to be useful to broadly describe viscoelastic properties [79,83,89], there has been increasing interest in creating models that incorporate various levels of microscale detail, including sarcomeres. After laser ablation, the retraction of stress fibers of Swiss 3T3 fibroblasts was observed to be restricted to the proximity of the cut with new adhesions forming at the retraction end over time. The presence of focal adhesion markers along the fiber suggests that stress fibers are attached to focal adhesions along their entire length and that after ablation, zyxin is further recruited to those sites to form new adhesions [86]. To mathematically model these data, one framework treated each sarcomeric unit as a viscoelastic unit, taking into consideration fiber internal viscosity and elasticity, with the addition of an elastic element that symbolizes the fiber’s longitudinal crosslinking to the ECM via adhesions [86]. In this model, stress fibers undergo a viscoelastic retraction that scales inversely with external crosslinking and, as a result, only sarcomeres close to the ablated site retract while others remain unaffected, leading to a highly non-uniform contraction of sarcomeres. More recently, this model was analytically solved and it was determined that friction between the cytoplasm and the stress fiber is insignificant and can be disregarded as a parameter that contributes to the viscoelastic retraction observed [92].
In contrast, Stachowiak and co-authors developed a model with no external crosslinks but high frictional coupling between the stress fiber and the cytoplasm [91]. These substantial external drag forces act on the recoiling fiber and slow it down over time, leading to power-law fiber retraction kinetics with highly non-uniform contraction of individual sarcomeres. Sarcomeres close to the ablated side retract faster than those further away due to the increased drag force. Since there are no external crosslinks, all sarcomere-like structures eventually collapse to a minimum size determined by the overlapping distance of myosin and actin filaments. More recently, the authors explored the relationship between stress fiber recoil after natural rupture (not induced by laser nanosurgery) and the overlapping distance of myosin and actin filaments more deeply [93]. Experimental data obtained by observing the natural rupture rate of stress fibers (0.03 breaks/min per cell) further suggested the absence of external crosslinks, supporting the model described above [35]. In addition, a new model was proposed where after severing, actin overlap within sarcomeres is negligible, allowing all sarcomeres to contract with minimal actin disassembly. As the sarcomeres contract further, however, they reach a minimum contraction distance where the overlapping distance of myosin and actin filaments builds stresses of around 3.3 pN per sarcomere. The model suggests that the presence of this stress is enough to activate a mechanosensing feedback loop that will increase actin disassembly, causing actin filaments to shorten and the stress to be relieved [93]. Both these models argue against external crosslinking and, as a result, all sarcomeres eventually contract [91,93].
Unlike the high non-uniformity of sarcomeric contraction observed in the two models discussed above, another study reported a two-phase sarcomeric contraction after stress fiber ablation in endothelial cells: an instantaneous initial decrease, followed by a linear contraction of sarcomeres at constant speed [90]. Inhibition of myosin with blebbistatin (a myosin ATPase inhibitor) after severing of the fiber yielded no elastic recovery, in agreement with the results obtained by Katoh et al., who showed that isolated stress fibers cannot relax after contracting in a myosin dependent manner [50]. These observations would seemingly argue that stress fiber tension generation cannot be fully accounted for by models that formulate stress fibers as elastic elements in parallel with viscous elements [79,83,86,89]. Based on these data, a model was proposed in which stress fiber tension is determined only by myosin contractility within each individual sarcomere-like unit. Due to the near instantaneous initial retraction, there is no external viscosity or crosslinking that limits the rate of contraction after severing.
The disparate predictions of these models may reflect the fact that they are built from data obtained from heterogeneous combinations of cells, matrix formulations, and stress fiber populations (see Table 1). This exemplifies a broader challenge in interpreting subcellular laser nanosurgery data, as stress fibers vary dramatically in their length, connectivity, and position within the cell, making it challenging to reconcile data obtained in living cells with idealized models of single stress fibers. Constraining stress fiber geometry with single-cell micropatterning may represent a potential solution to this problem.
Table 1.
Overview of stress fiber biomechanical models based on data obtained from subcellular laser nanosurgery.
| Viscoelastic model | Progressive linear collapse model | Progressive inhomogeneous collapse model | |
|---|---|---|---|
| Stress fiber retraction | Decreasing exponential | Decreasing exponential | Decreasing exponential |
| Sarcomere retraction | Decreasing exponential | Linear | Time dependent 1/2 power law |
| Do all sarcomeres collapse? | No – only ones close to ablated side | Yes | Yes |
| Viscosities considered | Internal + external | Internal | Internal + external |
| External Crosslinking | Yes | No | No |
| Tension generation | Borne entirely due to myosin contractility | Borne due to the elasticity of the fiber and myosin contractility | Borne due to the elasticity of the fiber and myosin contractility |
8. Conclusions
Advances in molecular biology, imaging and biochemistry have provided a rich diversity of tools and insights into the contractile mechanisms of stress fibers. Each of these approaches has its complementary strengths and limitations. Reconstitution of actomyosin bundles from purified proteins provides a simplified in vitro system that allows one to quantitatively study how specific proteins contribute to bundle assembly and contractility. While this approach yields unmatched control over molecular composition, it inevitably cannot capture all elements that would be found in cellular stress fibers. In a similar way, stress fibers extracted from cells are presumably structurally complete but lack connections to the cytoplasm and other cytoskeletal networks that may contribute in unforeseeable ways to the mechanics of the structure. This in turn has motivated the development of strategies for measuring stress fiber mechanics in living cells, including AFM and subcellular laser nanosurgery, both of which may be combined with advanced fluorescence methodologies to yield insight into the origins and functional contributions of stress fiber tension.
Despite these important advances, a number of key questions and challenges remain. First, how are the mechanical properties of whole stress fibers connected to their microscale, sarcomeric organization? The spatial orientation of actin filaments across stress fiber subtypes as well as within individual stress fibers varies between motile and non-motile cells [94]. For example, as described earlier, within the same stress fiber of a migrating cell, barbed ends of actin filaments present at the ends of a stress fiber are directed towards focal adhesions whereas the middle regions of the same stress fiber have a mixed actin filament orientation, suggesting that the middle region is highly contractile [25]. Conversely, stress fibers of non-motile cells often exhibit a sarcomeric-like pattern throughout the length of the fiber. How do these structural differences translate to force generation and how do they affect the overall function of the fiber in cell migration? Do geometric parameters such as stress fiber length and width play a role?
Another important question centers around the compartmentalization of stress fiber populations within the cell, and how this compartmentalization is associated with differential regulatory control of NMMII activation in peripheral and central stress fibers via MLCK and ROCK respectively [79,95]. In addition to the studies discussed earlier, inhibition of ROCK blocked myosin phosphorylation and formation of focal adhesions at the center in gerbil fibroma cells whereas inhibition of MLCK resulted in loss of myosin phosphorylation at the periphery of the cell [96]. It is currently not clear how this compartmentalization originates – are ROCK and MLCK found at different regions in the cell or are ROCK and MLCK preferentially activated at different regions of the cell? Evidence has begun to emerge to support the idea that ROCK and MLCK preferentially promote phosphorylation of NMMIIA or IIB [97]. Taken together with the differences in mechanochemical function between the two myosin isoforms, findings such as these begin to explain regional differences in stress fiber contractility in different parts of the cell. Clearly, much more work is required to determine the specific roles of NMMIIA and IIB and their differential regulation mechanism.
It is also unclear how site-specific phosphorylation of NMMII contributes to force generation, and how this in turn contributes to overall stress fiber contractile properties. As described earlier, each RLC has multiple phosphorylation sites that control myosin activity, with phosphorylation of serine 19 activating the motor domain and phosphorylation of threonine 18 and serine 19 enhancing ATPase activity [98]. Mono-phosphorylation of RLC has been observed throughout the cell whereas di-phosphorylation is found at the rear of the cell where MLCK seems to preferentially activate myosin [99]. Do ROCK and MLCK phosphorylate different sites on the RLC and how does the force generated from a mono-phosphorylated myosin motor differ quantitatively from that of a di-phosphorylated myosin motor? From current evidence, it would seem that differential control of stress fibers via ROCK and MLCK translates to differential mechanical properties of stress fibers. It will be extremely interesting to examine in more detail the molecular mechanisms responsible for this preferential control and determine functional contributions of each myosin activator to cell-scale force-dependent properties such as cell-ECM tensional homeostasis and cellular migration.
Finally, much remains to be learned about the differential mechanics and structural contributions of stress fibers in the context of migration. As discussed earlier, our understanding of the assembly mechanisms and evolution of stress fibers during migration has advanced dramatically over the past decade due to the application of increasingly sophisticated live-cell imaging technologies. It will be interesting to explore further the molecular mechanisms that allow stress fibers to evolve from one type to the next and investigate how their mechanical properties change over time during migration. Superresolution imaging, which has already proven fruitful for visualizing the organization of structural and motor proteins within stress fibers [21], could be combined in creative ways with reconstituted actomyosin systems and subcellular laser nanosurgery to provide answers.
Supplementary Material
Acknowledgments
EK gratefully acknowledges the support of the Howard Hughes Medical Institute International Student fellowship. SK gratefully acknowledges support from the N1H Physical Sciences-Oncology Center Award (1U54CA143836); the NSF (CAREER Award CMMI 1055965) and the NIH(1R21EB016359).
Footnotes
This article is part of a Special Issue entitled: Mechanobiology.
Transparency document
The Transparency document associated with this article can be found, in the online version.
The authors do not disclose any conflicts of interest.
References
- 1.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev. Mol. Cell Biol. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 2014;94:235–263. doi: 10.1152/physrev.00018.2013. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463:485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev. Mol. Cell Biol. 2011;12:308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraning-Rush CM, Carey SP, Califano JP, Smith BN, Reinhart-King CA. The role of the cytoskeleton in cellular force generation in 2D and 3D environments. Phys. Biol. 2011;8:015009. doi: 10.1088/1478-3975/8/1/015009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naumanen P, Lappalainen P, Hotulainen P. Mechanisms of actin stress fibre assembly. J. Microsc. 2008;231:446–454. doi: 10.1111/j.1365-2818.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- 7.Pellegrin S, Mellor H. Actin stress fibres. J. Cell Sci. 2007;120:3491–3499. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]
- 8.Tomasek JJ, Haaksma CJ, Schwartz RJ, Howard EW. Whole animal knockout of smooth muscle alpha-actin does not alter excisional wound healing or the fibroblast-to-myofibroblast transition. Wound Repair Regen. 2013;21:166–176. doi: 10.1111/wrr.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coffin JM, et al. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J. Cell Biol. 2006;172:259–268. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-betal from the extracellular matrix. J. Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown Ra. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 12.Darby IA, Laverdet B, Bonte F, Desmouliere A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet Investig. Dermatol. 2014;7:301–311. doi: 10.2147/CCID.S50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans ND, Oreffo ROC, Healy E, Thurner PJ, Man YH. Epithelial mechanobiology, skin wound healing, and the stem cell niche. J. Mech. Behav. Biomed. Mater. 2013;28:397–409. doi: 10.1016/j.jmbbm.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Gudjonsson T, Adriance MC, Sternlicht MD, Petersen OW, Bissell MJ. Myoepithelial cells: their origin and function in breast morphogenesis and neoplasia. J. Mammary Gland Biol. Neoplasia. 2005;10:261–272. doi: 10.1007/s10911-005-9586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sopel M. The myoepithelial cell: its role in normal mammary glands and breast cancer. Folia Morphol. (Warsz) 2010;69:1–14. [PubMed] [Google Scholar]
- 16.Burridge K, Wittchen ES. The tension mounts: stress fibers as force-generating mechanotransducers. J. Cell Biol. 2013;200:9–19. doi: 10.1083/jcb.201210090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tojkander S, Gateva G, Lappalainen P. Actin stress fibers-assembly, dynamics and biological roles. J. Cell Sci. 2012;125:1855–1864. doi: 10.1242/jcs.098087. [DOI] [PubMed] [Google Scholar]
- 18.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wylie SR, Chantler PD. Myosin IIC: a third molecular motor driving neuronal dynamics. Mol. Biol. Cell. 2008;19:3956–3968. doi: 10.1091/mbc.E07-08-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takaoka M, Saito H, Takenaka K, Miki Y, Nakanishi A. BRCA2 phosphorylated by PLK1 moves to the midbody to regulate cytokinesis mediated by nonmuscle myosin IIC. Cancer Res. 2014;74:1518–1528. doi: 10.1158/0008-5472.CAN-13-0504. [DOI] [PubMed] [Google Scholar]
- 21.Beach JR, et al. Nonmuscle myosin II isoforms coassemble in living cells. Curr. Biol. 2014;24:1160–1166. doi: 10.1016/j.cub.2014.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raab M, et al. Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. J. Cell Biol. 2012;199:669–683. doi: 10.1083/jcb.201205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vicente-Manzanares M, Newell-Litwa K, Bachir AI, Whitmore LA, Horwitz AR. Myosin IA/IIB restrict adhesive and protrusive signaling to generate front-back polarity in migrating cells. J. Cell Biol. 2011;193:381–396. doi: 10.1083/jcb.201012159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De La Cruz EM, Ostap EM. Relating biochemistry and function in the myosin superfamily. Curr. Opin. Cell Biol. 2004;16:61–67. doi: 10.1016/j.ceb.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Cramer LP, Siebert M, Mitchison TJ. Identification of novel graded polarity actin filament bundles in locomoting heart fibroblasts: implications for the generation of motile force. J. Cell Biol. 1997;136:1287–1305. doi: 10.1083/jcb.136.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murrell MP, Gardel ML. F-actin buckling coordinates contractility and severing in a biomimetic actomyosin cortex. Proc. Natl. Acad. Sci. 2012;109:20820–20825. doi: 10.1073/pnas.1214753109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bidone TC, Tang H, Vavylonis D. Dynamic network morphology and tension buildup in a 3D model of cytokinetic ring assembly. Biophys. J. 2014;107:2618–2628. doi: 10.1016/j.bpj.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winkelman JD, Bilancia CG, Peifer M, Kovar DR. Ena/VASP Enabled is a highly processive actin polymerase tailored to self-assemble parallel-bundled F-actin networks with Fascin. Proc. Natl. Acad. Sci. 2014;111:4121–4126. doi: 10.1073/pnas.1322093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchison TJ. Actin based motility on retraction fibers in mitotic PtK2 cells. Cell Motil. Cytoskeleton. 1992;22:135–151. doi: 10.1002/cm.970220207. [DOI] [PubMed] [Google Scholar]
- 30.Peterson LJ, et al. Simultaneous stretching and contraction of stress fibers in vivo. Mol. Biol. Cell. 2004;15:3497–3508. doi: 10.1091/mbc.E03-09-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burnette DT, et al. A contractile and counterbalancing adhesion system controls the 3D shape of crawling cells. J. Cell Biol. 2014;205:83–96. doi: 10.1083/jcb.201311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burridge K, Wittchen ES. The tension mounts: stress fibers as force-generating mechanotransducers. J. Cell Biol. 2013;200:9–19. doi: 10.1083/jcb.201210090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res. 2006;98:176–185. doi: 10.1161/01.RES.0000200162.94463.d7. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman LM, Jensen CC, Chaturvedi A, Yoshigi M, Beckerle MC. Stretch-induced actin remodeling requires targeting of zyxin to stress fibers and recruitment of actin regulators. Mol. Biol. Cell. 2012;23:1846–1859. doi: 10.1091/mbc.E11-12-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.a Smith M, et al. A zyxin-mediated mechanism for actin stress fiber maintenance and repair. Dev. Cell. 2010;19:365–376. doi: 10.1016/j.devcel.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapin LM, Blankman E, Smith MA, Shiu Y-T, Beckerle MC. Lateral communication between stress fiber sarcomeres facilitates a local remodeling response. Biophys. J. 2012;103:2082–2092. doi: 10.1016/j.bpj.2012.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chapin LM, Edgar LT, Blankman E, Beckerle MC, Shiu Y-T. Mathematical modeling of the dynamic mechanical behavior of neighboring sarcomeres in actin stress fibers. Cell. Mol. Bioeng. 2014;7:73–85. doi: 10.1007/s12195-013-0318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.a Kilian K, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zemel a, Rehfeldt F, Brown aEX, Discher DE, Safran Sa. Optimal matrix rigidity for stress-fibre polarization in stem cells. Nat Phys. 2010;6:468–473. doi: 10.1038/nphys1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 2006;173:383–394. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovac B, Teo JL, Mäkelä TP, Vallenius T. Assembly of non-contractile dorsal stress fibers requires α-actinin-1 and Rac1 in migrating and spreading cells. J. Cell Sci. 2013;126:263–273. doi: 10.1242/jcs.115063. [DOI] [PubMed] [Google Scholar]
- 42.Oakes PW, Beckham Y, Stricker J, Gardel ML. Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J. Cell Biol. 2012;196:363–374. doi: 10.1083/jcb.201107042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsons JT, Horwitz AR, Schwartz Ma. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev. Mol. Cell Biol. 2010;11:633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gateva G, Tojkander S, Koho S, Carpen O, Lappalainen P. Palladin promotes assembly of non-contractile dorsal stress fibers through VASP recruitment. J. Cell Sci. 2014;127:1887–1898. doi: 10.1242/jcs.135780. [DOI] [PubMed] [Google Scholar]
- 45.Vicente-Manzanares M, a Koach M, Whitmore L, Lamers ML, Horwitz AF. Segregation and activation of myosin IIB creates a rear in migrating cells. J. Cell Biol. 2008;183:543–554. doi: 10.1083/jcb.200806030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tee YH, et al. Cellular chirality arising from the self-organization of the actin cytoskeleton. Nat Cell Biol. 2015;17:445–457. doi: 10.1038/ncb3137. [DOI] [PubMed] [Google Scholar]
- 47.Kojima Ishijimaa H, Yanagida T. Direct measurement of stiffness of single actin filaments with and without tropomyosin by in vitro nanomanipulation. Proc. Natl. Acad. Sci. U. S. A. 1994;91:12962–12966. doi: 10.1073/pnas.91.26.12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akiyoshi K, Toshio Y. Force measurements by micromanipulation of a single actin filament by glass needles. Nature. 1988;334:74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- 49.Liu X, Pollack GH. Mechanics of F-actin characterized with microfabricated cantilevers. Biophys. J. 2002;83:2705–2715. doi: 10.1016/S0006-3495(02)75280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katoh K, Kano Y, Masuda M, Onishi H, Fujiwara K. Isolation and contraction of the stress fiber. Mol. Biol. Cell. 1998;9:1919–1938. doi: 10.1091/mbc.9.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deguchi S, Ohashi T, Sato M. Tensile properties of single stress fibers isolated from cultured vascular smooth muscle cells. J. Biomech. 2006;39:2603–2610. doi: 10.1016/j.jbiomech.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 52.Tan JL, et al. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc. Natl. Acad. Sci. U. S. A. 2002;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spicer SS. Gel formation caused by adenosinetriphosphate in actomyosin solutions. J. Biol. Chem. 1951;190:257–267. [PubMed] [Google Scholar]
- 54.Ebashi S, Ebashi F. A new protein component participating in the superprecipitation of myosin B. J. Biochem. 1964;55 doi: 10.1093/oxfordjournals.jbchem.a127933. [DOI] [PubMed] [Google Scholar]
- 55.Weber A, Winicur S. The role of calcium in the superprecipitation of actomyosin. J. Biol. Chem. 1961;236:3198–3202. [PubMed] [Google Scholar]
- 56.Bendix PM, et al. A quantitative analysis of contractility in active cytoskeletal protein networks. Biophys. J. 2008;94:3126–3136. doi: 10.1529/biophysj.107.117960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thoresen T, Lenz M, Gardel ML. Reconstitution of contractile actomyosin bundles. Biophys. J. 2011;100:2698–2705. doi: 10.1016/j.bpj.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murrell M, Thoresen T, Gardel M. Reconstitution of contractile actomyosin arrays. Methods Enzymol. 2014;540:265–282. doi: 10.1016/B978-0-12-397924-7.00015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thoresen T, Lenz M, Gardel ML. Thick filament length and isoform composition determine self-organized contractile units in actomyosin bundles. Biophys. J. 2013;104:655–665. doi: 10.1016/j.bpj.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stachowiak MR, et al. Self-organization of myosin II in reconstituted actomyosin bundles. Biophys. J. 2012;103:1265–1274. doi: 10.1016/j.bpj.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ciobanasu C, Faivre B, Le Clainche C. Actomyosin-dependent formation of the mechanosensitive talin-vinculin complex reinforces actin anchoring. Nat Commun. 2014;5:3095. doi: 10.1038/ncomms4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miyazaki M, Chiba M, Eguchi H, Ohki T, Ishiwata S. Cell-sized spherical confinement induces the spontaneous formation of contractile actomyosin rings in vitro. Nat Cell Biol. 2015;17:480–489. doi: 10.1038/ncb3142. [DOI] [PubMed] [Google Scholar]
- 63.Katoh K, Kano Y, Noda Y. Rho-associated kinase-dependent contraction of stress fibres and the organization of focal adhesions. J. R Soc. Interface. 2011;8:305–311. doi: 10.1098/rsif.2010.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Katoh K, et al. Rho-kinase—mediated contraction of isolated stress fibers. J. Cell Biol. 2001;153:569–584. doi: 10.1083/jcb.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katoh K, Kano Y, Amano M, Kaibuchi K, Fujiwara K. Stress fiber organization regulated by MLCK and Rhokinase in cultured human fibroblasts. Am. J. Physiol. Cell Physiol. 2001;280:1669–1679. doi: 10.1152/ajpcell.2001.280.6.C1669. [DOI] [PubMed] [Google Scholar]
- 66.Costa KD, Hucker WJ, Yin FC-P. Buckling of actin stress fibers: a new wrinkle in the cytoskeletal tapestry. Cell Motif Cytoskeleton. 2002;52:266–274. doi: 10.1002/cm.10056. [DOI] [PubMed] [Google Scholar]
- 67.Nagayama K, Matsumoto T. Contribution of actin filaments and microtubules to quasi-in situ tensile properties and internal force balance of cultured smooth muscle cells on a substrate. Am. J. Physiol. Cell Physiol. 2008;295:1569–1578. doi: 10.1152/ajpcell.00098.2008. [DOI] [PubMed] [Google Scholar]
- 68.Sato K, Adachi T, Matsuo M, Tomita Y. Quantitative evaluation of threshold fiber strain that induces reorganization of cytoskeletal actin fiber structure in osteoblastic cells. J. Biomech. 2005;38:1895–1901. doi: 10.1016/j.jbiomech.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 69.Brangwynne CP, et al. Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J. Cell Biol. 2006;173:733–741. doi: 10.1083/jcb.200601060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soiné JRD, et al. Model-based traction force microscopy reveals differential tension in cellular actin bundles. PLoS Comput Biol. 2014 doi: 10.1371/journal.pcbi.1004076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang JH-C, Lin J-S. Cell traction force and measurement methods. Biomech. Model. Mechanobiol. 2007;6:361–371. doi: 10.1007/s10237-006-0068-4. [DOI] [PubMed] [Google Scholar]
- 72.Haga H, et al. Elasticity mapping of living fibroblasts by AFM and immunofluorescence observation of the cytoskeleton. Ultramicroscopy. 2000;82:253–258. doi: 10.1016/s0304-3991(99)00157-6. [DOI] [PubMed] [Google Scholar]
- 73.Rotsch C, Radmacher M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: an atomic force microscopy study. Biophys. J. 2000;78:520–535. doi: 10.1016/S0006-3495(00)76614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Costa KD, Sim AJ, Yin FC-P. Non-Hertzian approach to analyzing mechanical properties of endothelial cells probed by atomic force microscopy. J. Biomech. Eng. 2006;128:176–184. doi: 10.1115/1.2165690. [DOI] [PubMed] [Google Scholar]
- 75.L Lu, Oswald SJ, Ngu H, Yin FC-P. Mechanical properties of actin stress fibers in living cells. Biophys. J. 2008;95:6060–6071. doi: 10.1529/biophysj.108.133462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berns W, Strahs R, Biology C. Laser microirradiation of stress fibers and intermediate filaments in non-muscle cells from cultured rat heart. Exp. Cell Res. 1979;119:31–45. doi: 10.1016/0014-4827(79)90332-x. [DOI] [PubMed] [Google Scholar]
- 77.Koonce MP, Strahs KR, Berns MW. Repair of laser-severed stress fibers in myocardial non-muscle cells. Exp. Cell Res. 1982;141:375–384. doi: 10.1016/0014-4827(82)90226-9. [DOI] [PubMed] [Google Scholar]
- 78.Berns MW. History of laser scissors. Methods Cell Biol. 2007;82 doi: 10.1016/S0091-679X(06)82001-7. [DOI] [PubMed] [Google Scholar]
- 79.Tanner K, Boudreau A, Bissell MJ, Kumar S. Dissecting regional variations in stress fiber mechanics in living cells with laser nanosurgery. Biophys. J. 2010;99:2775–2783. doi: 10.1016/j.bpj.2010.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vogel A, Venugopalan V. Mechanisms of pulsed laser ablation of biological tissues. Chem Rev. 2003;103:577–644. doi: 10.1021/cr010379n. [DOI] [PubMed] [Google Scholar]
- 81.Heisterkamp A, et al. Pulse energy dependence of subcellular dissection by femtosecond laser pulses. Opt Express. 2005;13:3690–3696. doi: 10.1364/opex.13.003690. [DOI] [PubMed] [Google Scholar]
- 82.Ronchi P, Terjung S, Pepperkok R. At the cutting edge : applications and perspectives of laser nanosurgery in cell biology. Biol. Chem. 2012;393:235–248. doi: 10.1515/hsz-2011-0237. [DOI] [PubMed] [Google Scholar]
- 83.Kumar S, et al. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys. J. 2006;90:3762–3773. doi: 10.1529/biophysj.105.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berkeley F. Sanjay Kumar Department and University: Manuel Thery Département et Université: Institut de Recherche en Technologies et Sciences pour Address: Budget: Funding Requested From UC or French Partner None Beginning: None End: N/A [Google Scholar]
- 85.Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J. Cell Biol. 2005;171:209–215. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Colombelli J, et al. Mechanosensing in actin stress fibers revealed by a close correlation between force and protein localization. J. Cell Sci. 2009;122:1928–1928. doi: 10.1242/jcs.042986. [DOI] [PubMed] [Google Scholar]
- 87.Lele TP, Pendse JAY, Kumar S, Salanga M, Karavitis J. Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J. Cell. Physiol. 2006;207:187–194. doi: 10.1002/jcp.20550. [DOI] [PubMed] [Google Scholar]
- 88.Grashoff C, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chang C-W, Kumar S. Vinculin tension distributions of individual stress fibers within cell-matrix adhesions. J. Cell Sci. 2013;126:3021–3030. doi: 10.1242/jcs.119032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Russell RJ, Xia S-L, Dickinson RB, Lele TP. Sarcomere mechanics in capillary endothelial cells. Biophys. J. 2009;97:1578–1585. doi: 10.1016/j.bpj.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stachowiak MR, O’Shaughnessy B. Recoil after severing reveals stress fiber contraction mechanisms. Biophys. J. 2009;97:462–471. doi: 10.1016/j.bpj.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Besser A, Colombelli J, Stelzer EHK, Schwarz US. Viscoelastic response of contractile filament bundles. Phys. Rev. E. 2011;83:051902. doi: 10.1103/PhysRevE.83.051902. [DOI] [PubMed] [Google Scholar]
- 93.Stachowiak MR, et al. A mechanical-biochemical feedback loop regulates remodeling in the actin cytoskeleton. Proc. Natl. Acad. Sci. U. S. A. 2014;111 doi: 10.1073/pnas.1417686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Deguchi S, Sato M. Biomechanical properties of actin stress fibers of non-motile cells. Biorheology. 2009;46:93–105. doi: 10.3233/BIR-2009-0528. [DOI] [PubMed] [Google Scholar]
- 95.Kazuo Katoh Kano Yumino Amano Mutsuki KKKF. Stress Fiber Organization Regulated by MLCK and Rho-kinase in Cultured Human Fibroblasts. 2001 doi: 10.1152/ajpcell.2001.280.6.C1669. [DOI] [PubMed] [Google Scholar]
- 96.Totsukawa G, et al. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J. Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sandquist JC, Swenson KI, Demali KA, Burridge K, Means R. Rho kinase differentially regulates phosphorylation of nonmuscle myosin II isoforms A and B during cell rounding. J. Biol. Chem. 2006;281:35873–35883. doi: 10.1074/jbc.M605343200. [DOI] [PubMed] [Google Scholar]
- 98.Umemoto S, Bengur aR, Sellers JR. Effect of multiple phosphorylations of smooth muscle and cytoplasmic myosins on movement in an in vitro motility assay. J. Biol. Chem. 1989;264:1431–1436. [PubMed] [Google Scholar]
- 99.Vicente-Manzanares M, Horwitz AR. Myosin light chain mono- and di-phosphorylation differentially regulate adhesion and polarity in migrating cells. Biochem. Biophys. Res. Commun. 2010;402:537–542. doi: 10.1016/j.bbrc.2010.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.