Abstract
Although iron is commonly used to correct iron deficiency anemia (IDA) in chronic kidney disease (CKD) its effect on kidney function is unclear. To assess this, we randomly assigned patients with Stage 3 and 4 CKD and IDA to either open-label oral ferrous sulfate (69 patients to 325 mg three times daily for 8 weeks) or intravenous iron sucrose (67 patients to 200 mg every 2 weeks, total 1 gram). The primary outcome was the between group difference in slope of measured glomerular filtration rate (mGFR) change over two years. The trial was terminated early on the recommendation of an independent Data and Safety Monitoring Board based on little chance of finding differences in mGFR slopes, but a higher risk of serious adverse events in the intravenous iron treatment group. mGFR declined similarly over two years in both treatment groups (oral −3.6 mL/min/1.73m2, intravenous − 4.0 mL/min/1.73m2, between group difference − 0.35 mL/min/1.73m2 (95% confidence interval −2.9 to 2.3). There were 36 serious cardiovascular events among 19 participants assigned to the oral iron treatment group and 55 events among 17 participants of the intravenous iron group (adjusted incidence rate ratio 2.51 (1.56−4.04). Infections resulting in hospitalizations had a significant adjusted incidence rate ratio of 2.12 (1.24−3.64). Thus, among non-dialyzed patients with CKD and IDA, intravenous iron therapy is associated with an increased risk of serious adverse events, including those from cardiovascular causes and infectious diseases.
Keywords: Anemia, chronic kidney disease, iron, adverse effects, randomized controlled trial
Introduction
It is estimated that there are approximately 8 million individuals in the United States with moderate to severe chronic kidney disease (CKD) [1]. Anemia often occurs during moderate (stage 3) CKD, primarily from reduced erythropoietin production but also due to iron deficiency [2]. Enhanced erythropoiesis after therapy with recombinant human erythropoietin may lead to functional iron deficiency that often necessitates therapy with intravenous iron [3]. Although partial correction of anemia reduces the need for blood transfusions, toxicity due to the participation of elemental iron in causing cell damage and in generating oxidative stress has raised concern of potential health risks which remain incompletely understood [4;5]. There is a paucity of information on the safety of this therapy as only one-half of the randomized clinical trials of oral and intravenous iron in adults and children reported adverse events, and even fewer described those that were serious [6].
Oxidative stress plays an important role in the pathogenesis and progression of CKD [7;8]. Iron increases biological markers of oxidative stress [9] in cell cultures [10], animal models [11], and among end-stage renal disease (ESRD) patients treated with hemodialysis [12–14]. Among patients with CKD not on dialysis, intravenous iron can generate oxidative stress and downstream effects such as endothelial damage and kidney injury [15;16]. Thus iron-induced injury may lead to an accelerated course of renal [7] and cardiovascular disease [17;18]. Research recommendations emphasize the need to evaluate the long-term risks of intravenous iron therapy among CKD patients [19].
We hypothesized that among patients with moderate to severe CKD and iron deficiency anemia, compared to oral iron, infusion of intravenous iron will result in greater decline in kidney function. We report here the primary results of the randomized trial to evaluate intravenous and oral iron in chronic kidney disease (REVOKE).
Results
Between August 15, 2008 and October 20, 2014 we randomized 136 subjects with iron deficiency anemia and chronic kidney disease not on dialysis to either oral iron sulfate or intravenous iron sucrose. The trial flow is shown in the appendix (Figure S1).
The clinical characteristics of study participants at baseline are shown in Table 1. Of note, compared to the oral iron group, the intravenous iron group was younger (p=0.02), had less baseline cardiovascular disease (p=0.04), and past history of hospitalization due to infection (p=0.05). Overall, the mean hemoglobin concentration at baseline was 10.6 g/dL, transferrin saturation 17.3% and serum ferritin 153 ng/mL. At baseline, erythopoiesis stimulating agents were used by only 8.1% of the participants. Mean mGFR was 34.5 mL/min/1.73 m2 and proteinuria had a geometric mean of 0.5 g/g creatinine. The median follow up (interquartile range) of all participants was 24.0 months (11.0–24.3) and did not differ by treatment group assignment.
Table 1.
Baseline characteristics of the study sample, overall and by treatment group assignment
| Clinical characteristic | Oral iron (n=69) | Intravenous Iron (n=67) | All subjects (n=136) |
|---|---|---|---|
| Age (y) | 67.8 ± 11.5 | 63.2 ± 10.7 | 65.5 ± 11.3 |
| Male sex n(%) | 54 (78.3%) | 50 (74.6%) | 104 (76.5%) |
| Blacks n(%) | 18 (26.1%) | 27 (40.3%) | 45 (33.1%) |
| Hispanic n(%) | 0 (0%) | 2 (3%) | 2 (1.5%) |
| Etiology of Chronic Kidney Disease | |||
| Diabetes mellitus n(%) | 30 (43.5%) | 29 (43.3%) | 59 (43.4%) |
| Hypertension n(%) | 20 (29%) | 19 (28.4%) | 39 (28.7%) |
| Ischemic kidney disease n(%) | 5 (7.2%) | 3 (4.5%) | 8 (5.9%) |
| Glomerulonephritis n(%) | 2 (2.9%) | 4 (6%) | 6 (4.4%) |
| Polycystic kidney disease n(%) | 1 (1.4%) | 2 (3%) | 3 (2.2%) |
| Other etiologies n(%) | 3 (4.3%) | 6 (9%) | 9 (6.6%) |
| Diabetes mellitus n(%) | 54 (78.3%) | 47 (70.1%) | 101 (74.3%) |
| Cardiovascular disease n(%) | 43 (62.3%) | 30 (44.8%) | 73 (53.7%) |
| Hospitalized heart failure n(%) | 21 (30.4%) | 16 (23.9%) | 37 (27.2%) |
| Myocardial infarction n(%) | 17 (24.6%) | 17 (25.4%) | 34 (25%) |
| Coronary revascularization n(%) | 21 (30.4%) | 15 (22.4%) | 36 (26.5%) |
| Pacemaker or defibrillator n(%) | 7 (10.1%) | 6 (9%) | 13 (9.6%) |
| Stroke n(%) | 9 (13%) | 6 (9%) | 15 (11%) |
| Peripheral vascular disease n(%) | 12 (17.4%) | 7 (10.4%) | 19 (14%) |
| Hospitalized infectious disease n(%) | 34 (49.3%) | 22 (32.8%) | 56 (41.2%) |
| Skin (eg cellulitis) n(%) | 11 (15.9%) | 4 (6%) | 15 (11%) |
| Bone (eg pyogenic arthritis, osteomyelitis) n(%) | 6 (8.7%) | 3 (4.5%) | 9 (6.6%) |
| Lung (eg pneumonia) n(%) | 16 (23.2%) | 5 (7.5%) | 21 (15.4%) |
| Sepsis n(%) | 6 (8.7%) | 1 (1.5%) | 7 (5.1%) |
| Urinary tract infections n(%) | 7 (10.1%) | 5 (7.5%) | 12 (8.8%) |
| Other infections n(%) | 3 (4.3%) | 4 (6%) | 7 (5.1%) |
| Gastrointestinal bleeding n(%) | 8 (11.6%) | 3 (4.5%) | 11 (8.1%) |
| Past RBC transfusion n(%) | 13 (18.8%) | 12 (17.9%) | 25 (18.4%) |
| Smoking n(%) | |||
| Never smoker n(%) | 13 (18.8%) | 17 (25.4%) | 30 (22.1%) |
| Past smoker n(%) | 44 (63.8%) | 39 (58.2%) | 83 (61%) |
| Active smoker n(%) | 12 (17.4%) | 11 (16.4%) | 23 (16.9%) |
| ACE inhibitor or ARB use n(%) | 45 (65.2%) | 43 (64.2%) | 88 (64.7%) |
| Statin use n(%) | 48 (69.6%) | 44 (65.7%) | 92 (67.6%) |
| Antiplatelet agent use n(%) | 47 (68.1%) | 35 (52.2%) | 82 (60.3%) |
| Erythropoietin agent use n(%) | 5 (7.2%) | 6 (9%) | 11 (8.1%) |
| Seated clinic systolic BP (mmHg) | 131.4 ± 21.9 | 129 ± 18.5 | 130.2 ± 20.3 |
| Seated clinic diastolic BP (mmHg) | 13.4 | 65.7 ± 12.4 | 64.4 ± 13 |
| Proteinuria stratum | |||
| High proteinuria stratum (≥3 g/g) | 9 (13%) | 9 (13.4%) | 18 (13.2%) |
| Low proteinuria stratum (<3 g/g) | 60 (87%) | 58 (86.6%) | 118 (86.8%) |
| Hemoglobin (g/dL) | 10.5 ± 1 | 10.7 ± 1 | 10.6 ± 1 |
| Transferrin saturation (%) | 17.3 ± 6.7 | 17.4 ± 5.1 | 17.3 ± 5.9 |
| Serum ferritin (ng/mL) | 133 ± 155 | 173 ± 138 | 153 ± 148 |
| Serum albumin (g/dL) | 3.5 ± 0.5 | 3.5 ± 0.6 | 3.5 ± 0.5 |
| estimated GFR (mL/min/1.73m2) | 34.7 ± 10 | 34.3 ± 10.2 | 34.5 ± 10 |
| Log urinary protein/creatinine (mg/mg) | −.9 ± 1.4 | −.6 ± 1.5 | −.7 ± 1.4 |
Hemoglobin control between groups and interventions to maintain hemoglobin
Figure 1a shows hemoglobin levels at baseline and over time in all participants. At baseline, mean hemoglobin was 10.5 g/dL in the oral iron group and 10.7 g/dL in the IV iron group. Hemoglobin levels improved over time in both groups, and no statistically significant difference between mean levels in the treatment groups was noted during follow-up.
Figure 1.
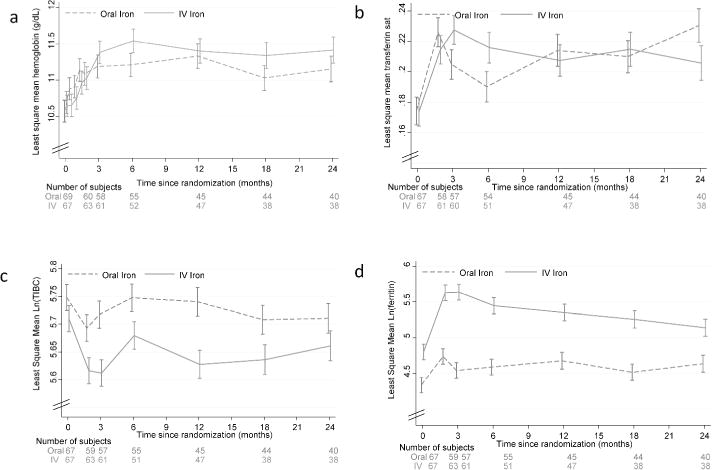
Time course of hemoglobin and iron parameters
Hemoglobin change from baseline to 3 months in the oral iron group was 0.61 g/dL and in the IV iron group 0.69 g/dL (difference + 0.08 (95% CI −0.34 to +0.51, p = 0.72). Difference at 6 months (0.22 g/dL, p=0.3), 12 months (−0.04 g/dL, p=0.85) and 24 months (0.15 g/dL, p=0.56) were also not statistically significant.
Transferrin saturation change from baseline to 3 months in the oral iron group was 0.03 and in the IV iron group 0.05 (difference + 0.024 (95% CI −0.004 to +0.052, p = 0.10). Difference at 6 months (0.026, p=0.08), 12 months (−0.04 g/dL, p=0.85) and 24 months (− 0.024 g/dL, p=0.14) were also not statistically significant.
Log total iron binding capacity change from baseline to 3 months in the oral iron group was −0.031 (p=0.13) and in the IV iron group −0.098 (p<0.001) (difference − 0.067 (95% CI −0.122 to −0.012, p = 0.02). Differences in change from baseline at 6 months (−0.030, p=0.31), 12 months (−0.075 g/dL, p=0.015) and 24 months (−0.01, p=0.74) were small. Log ferritin change from baseline to 3 months in the oral iron group was −0.20 (p=0.01) and in the IV iron group 0.84 (p<0.001) (difference 0.63 (95% CI 0.41 to 0.85, p < 0.001). Differences between groups in change from baseline at 6 months (0.39, p=0.001), 12 months (0.21g/dL, p=0.085) and 24 months (0.04, p=0.77) diminished.
Changes in transferrin saturation, total iron binding capacity, and serum ferritin are shown in figure 1b–d. Serum ferritin concentration was significantly higher in the IV iron group only from baseline to 6 months.
Beyond the first 8 weeks of repletion therapy, intravenous iron administration was given to two subjects in the oral iron group (doses of 250 mg and 1000 mg) of which one was given during a hospitalization. In contrast, 9 subjects in the IV iron group received additional IV iron at a median dose of 1729 mg (IQR 838 mg to 2000 mg). In comparison, oral iron was prescribed after the 8 week randomized treatment period to 7 subjects in the IV iron group for median of 20 days (IQR 12.3 to 20) and beyond the first 8 weeks to 36 subjects for a median of 60 days (IQR 30 to 90) (p value for difference in medians = 0.001)
The average ESA use over the course of two years was similar in the two groups. In the oral iron group 22 subjects required ESA for average of 61 weeks (SD 39) with a geometric mean cumulative dose of darbepoetin of 483 mcg. In the IV iron group 16 subjects required ESA for average of 54 weeks (SD 41) with a geometric mean cumulative dose of darbepoetin of 614 mcg.
Twelve study participants in each group received blood transfusions. Of those who needed packed red blood cell transfusions, the mean number of units needed over 2 years was 5.3 (range 2 to 20 units) in the oral group and 3.5 (range 1 to 7) in the IV group (t=0.99, p=0.3)
Early Termination of the Trial
The trial was stopped early on the unanimous recommendation of the DSMB based on an increase in the serious adverse event rate in participants assigned to IV iron treatment compared to oral iron therapy and little difference in mGFR between treatment groups. Given the persisting signal of safety, but little chance of finding the projected difference in measured GFR between groups, they unanimously recommended termination of the trial.
Rate of decline in kidney function
Figure 2a shows the modeled iothalamate measured GFR slopes in the two groups adjusted for baseline log urinary protein/creatinine ratio. Iothalamate GFR declined similarly over time in both groups (oral iron −3.6 mL/min/1.73m2 per year, IV iron − 4.0 mL/min/1.73m2 per year, between group difference −0.35 mL/min/1.73m2 per year (95% confidence interval (CI) −2.9 to 2.3, p=0.79). After additional adjustment for age, sex, black race, ACE/ARB use, and cardiovascular disease the rate of change in GFR became more similar between groups (oral iron −3.8 mL/min/1.73m2 per year, IV iron − 3.9 mL/min/1.73m2 per year, between group difference −0.11 mL/min/1.73m2 per year (95% confidence interval (CI) −2.7 to 2.5, p=0.94) (Figure 2b).
Figure 2.
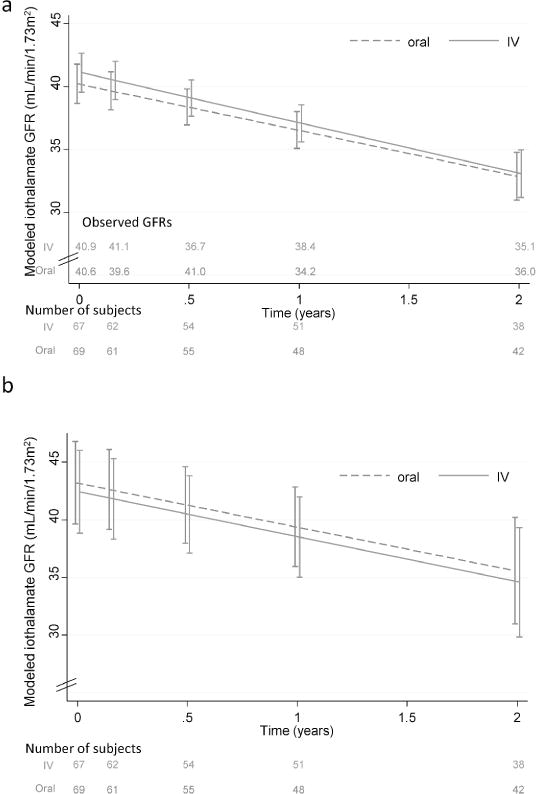
Time course of change in measured GFR using plasma iothalamate clearance. Plasma iothalamate clearances were measured at 5 time points over 2 years (baseline, 8 weeks, 6 months, 1 year and 2 years). Error bars indicate one standard error of the modeled mean at each time point. The numbers at the bottom each of the figures denote the number of subjects with measurements in each of the two groups. Observed means for each group are shown just above the x-axis and were similar to modeled means. Figure 2a shows the modeled slopes over 2 years adjusted for baseline log urine protein/creatinine ratio. This was the primary end point of the study. Slope for oral iron −3.6 mL/min/1.73m2 per year, IV iron − 4.0 mL/min/1.73m2 per year, between group difference −0.35 mL/min/1.73m2 per year (95% confidence interval (CI) −2.9 to 2.3, p=0.79). Figure 2b is a model further adjusted for age, sex, race, ACE or ARB use, and cardiovascular disease. This was the secondary end point: slope for oral iron −3.8 mL/min/1.73m2 per year, IV iron − 3.9 mL/min/1.73m2 per year, between group difference by 0.11 mL/min/1.73m2 per year (95% confidence interval (CI) −2.7 to 2.5, p=0.94).
The small baseline difference in proteinuria was not statistically significant. There was significant increase in proteinuria over time (p=0.04) in both treatment groups, however, there was no significant difference between groups (Figure S2).
Results of Kidney Disease Quality of Life
None of the domains of the KDQOL Questionnaire demonstrated any significant change over time or a significant interaction between treatment groups over time (Figure 3a–d).
Figure 3.
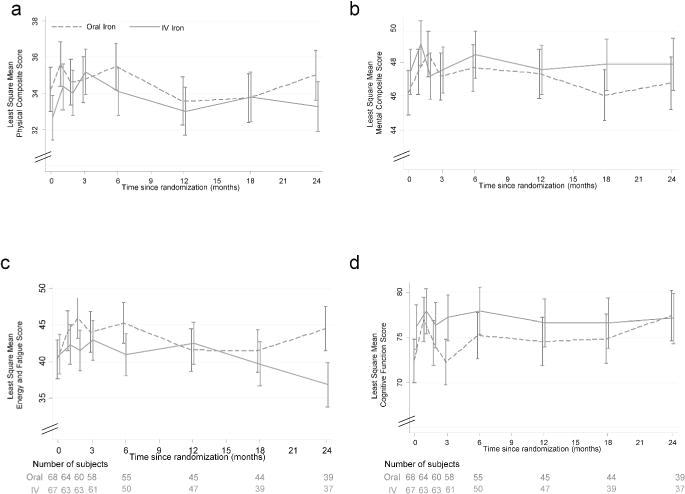
Time course of kidney disease quality of life by treatment group Least square mean estimates of health related quality of life scores are shown over time by two treatment groups. Error bars are one standard error of the mean. None of the comparisons were statistically different over time or between groups over time. Higher scores mean denote a higher health related quality of life. Other domains of the KDQOL (data not shown) were also not significant.
Serious adverse events
Table 2 shows the serious adverse events between groups over the course of the trial. There were 6 deaths in the IV group and 4 in the oral iron group. A total of 104.5 patient-years (PY) of follow-up was obtained in the oral iron treatment group and 101 PY of follow up in the intravenous iron treatment group. Serious adverse events in the oral iron group occurred in 40 subjects who had 176 events (168.4/100 PY); in the intravenous iron group they occurred in 37 subjects who had 201 events (199/100 PY), unadjusted incidence rate ratio (IRR) 1.18 (95% CI 0.97–1.45, p=0.106). Adjusted IRR was 1.60 (1.28 – 2.00), p<0.0001.
Table 2.
Serious adverse events reported following randomization
| Event type | Oral Iron (n=69) | IV Iron (n=67) | Incidence rate ratio IV/Oral (95% CI) | p | Adjusted incidence rate ratio IV/Oral (95% CI) | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subjects (n) | Events (n) | Incidence rate (events/100 PY) | Subjects (n) | Event s (n) | Incidence rate (events/1 00 PY) | |||||
| Overall SAEs | 40 | 176 | 168.4 | 37 | 201 | 199 | 1.18 (0.97 – 1.45) | 0.106 | 1.60 (1.28 – 2.00) | <0.0001 |
| Infections | 11 | 27 | 25.8 | 19 | 37 | 36.6 | 1.42 (0.86 – 2.33) | 0.168 | 2.12 (1.24 – 3.64) | 0.006 |
| Skin | 6 | 6 | 5.7 | 7 | 11 | 10.9 | 1.90 (0.70 – 5.13) | >0.2 | 3.79 (1.32 – 10.87) | 0.013 |
| Bone | 2 | 7 | 6.7 | 3 | 4 | 4 | 0.59 (0.17 – 2.02) | >0.2 | ||
| Lung | 4 | 4 | 3.8 | 8 | 11 | 10.9 | 2.85 (0.91 – 8.94) | 0.073 | 4.35 (1.23 – 15.39) | 0.022 |
| UTI | 3 | 5 | 4.8 | 3 | 5 | 4.9 | 1.03 (0.30 – 3.57) | >0.2 | 2.37 (0.60 – 9.34) | >0.2 |
| Sepsis | 1 | 2 | 1.9 | 5 | 5 | 4.9 | 2.59 (0.50 – 13.33) | >0.2 | 122.15 (0.89 – 16819.84) | 0.056 |
| Other | 2 | 3 | 2.9 | 1 | 1 | 1 | 0.34 (0.04 – 3.32) | >0.2 | ||
| Cardiovascular | 19 | 36 | 34.4 | 17 | 55 | 54.4 | 1.58 (1.04 – 2.41) | 0.033* | 2.51 (1.56 – 4.04) | <0.001 |
| CHF | 9 | 15 | 14.3 | 9 | 28 | 27.7 | 1.93 (1.03 – 3.62) | 0.040* | 2.07 (1.04 – 4.11) | 0.038 |
| Angina | 2 | 2 | 1.9 | 2 | 2 | 2 | 1.03 (0.15 – 7.35) | >0.2 | ||
| MI | 8 | 9 | 8.6 | 8 | 9 | 8.9 | 1.03 (0.41 – 2.61) | >0.2 | 1.25 (0.41 – 3.82) | >0.2 |
| Stroke | 0 | 0 | 0 | 2 | 2 | 2 | 2.0e+07 (0.00 – .) | >0.2 | ||
| Arrhythmia | 4 | 4 | 3.8 | 4 | 5 | 4.9 | 1.29 (0.35 – 4.82) | >0.2 | ||
| PVD | 1 | 2 | 1.9 | 2 | 3 | 3 | 1.55 (0.26 – 9.29) | >0.2 | ||
| Other | 4 | 4 | 3.8 | 5 | 6 | 5.9 | 1.55 (0.44 – 5.50) | >0.2 | ||
| Renal | 18 | 29 | 27.7 | 14 | 28 | 27.7 | 1.00 (0.59 – 1.68) | >0.2 | 1.39 (0.78 – 2.47) | >0.2 |
| AKI | 15 | 22 | 21 | 12 | 21 | 20.8 | 0.99 (0.54 – 1.80) | >0.2 | ||
| Hyperkalemia | 5 | 6 | 5.7 | 2 | 4 | 4 | 0.69 (0.19 – 2.44) | >0.2 | ||
| Other | 1 | 1 | 1 | 3 | 3 | 3 | 3.10 (0.32 –29.84) | >0.2 | ||
| Cancer-related | 4 | 4 | 3.8 | 4 | 8 | 7.9 | 2.07 (0.62 – 6.87) | >0.2 | ||
| Other | 31 | 69 | 66 | 25 | 61 | 60.4 | 0.91 (0.65 – 1.29) | >0.2 | ||
| PRBC transfusion | 12 | 17 | 16.3 | 12 | 19 | 18.8 | 1.16 (0.60 – 2.22) | >0.2 | ||
| GI Bleed | 5 | 7 | 6.7 | 0 | 0 | 0 | NA | NA | ||
| Hyperglycemia | 1 | 1 | 1 | 2 | 2 | 2 | 2.07 (0.19 – 22.82) | >0.2 | ||
| Hypoglycemia | 3 | 5 | 4.8 | 0 | 0 | 0 | NA | NA | ||
| Diabetic retinopathy | 1 | 2 | 1.9 | 1 | 5 | 4.9 | 2.59 (0.50 – 13.33) | >0.2 | ||
| Hypertensive crisis | 1 | 1 | 1 | 3 | 5 | 4.9 | 5.17 (0.60 – 44.28) | 0.134 | ||
| Urinary retention | 2 | 3 | 2.9 | 2 | 3 | 3 | 1.03 (0.21 – 5.13) | >0.2 | ||
| Miscellaneous | 21 | 33 | 31.6 | 20 | 27 | 26.7 | 0.85 (0.51 – 1.41) | >0.2 | ||
| ESRD | 7 | 7 | 6.7 | 6 | 6 | 5.9 | 0.89 (0.30 – 2.64) | >0.2 | 1.04 (0.25 – 4.24) | >0.2 |
| Death | 4 | 4 | 3.8 | 6 | 6 | 5.9 | 1.55 (0.44 – 5.50) | >0.2 | 1.60 (0.28 – 9.07) | >0.2 |
| CV related | 2 | 2 | 1.9 | 2 | 2 | 2 | 1.03 (0.15 – 7.35) | >0.2 | ||
| Non-CV related | 2 | 2 | 1.9 | 4 | 4 | 4 | 2.07 (0.38 – 11.30) | >0.2 | ||
Oral iron exposure 104.5 patient-years (PY), IV iron exposure 101 PY
Adjustments for overall serious adverse events, cardiovascular events, renal events, AKI, hyperkalemia, ESRD, death: age, sex, black race, stratum of proteinuria, baseline estimated GFR, diabetes, cardiovascular disease, tobacco use, systolic BP, statin use, antiplatelet therapy, ACE or ARB use
Adjustments for CHF events: all the above adjustments except cardiovascular disease replaced by history of hospitalization for CHF.
Adjustments for MI events: all the above adjustments except cardiovascular disease replaced by history of myocardial infarction.
Adjustments for infection events: all the above adjustments except dropped systolic BP, statin use, antiplatelet therapy, ACE or ARB use and added history of hospitalized infection
Adjustments for skin infection events: all the adjustments for infection except that history of hospitalized infection replaced by prior history of hospitalized cellulitis.
Adjustments for lung infection events: all the adjustments for infection except that history of hospitalized infection replaced by prior history of hospitalized pneumonia.
Adjustments for urinary infection events: all the adjustments for infection except that history of hospitalized infection replaced by prior history of hospitalized UTI.
Adjustments for sepsis events: all the adjustments for infection except that history of hospitalized infection replaced by prior history of hospitalized sepsis.
Serious adverse events due to infections in the oral iron group occurred 27 times in 11 subjects (25.8/100 PY); in the intravenous iron group they occurred 37 times in 19 subjects (36.6/100 PY; incidence rate ratio (IRR) 1.42 (95% CI 0.86–2.33, p=0.17). Adjusted IRR was 2.12 (1.24 – 3.64), p<0.006. Compared to the oral iron group, the incidence of lung and skin infections were increased between 3–4 fold in the intravenous iron group.
Cardiovascular events in the oral iron group occurred 36 times in 19 subjects (34.4/100 PY); in the intravenous iron group they occurred 55 times in 17 subjects (54.4/100 PY; incidence rate ratio (IRR) 1.58 (95% CI 1.04–2.41, p=0.033). Adjusted IRR was 2.51 (1.56 – 4.04), p<0.001. Compared to the oral iron group, the incidence of hospitalized heart failure was increased about 2 fold in the intravenous iron group. Supplementary figures S3–5 show that the distribution of the events was such that it was not one or two subjects in one group who influenced the outcomes.
Overall, gastrointestinal adverse events particularly diarrhea were more common among participants randomized to oral iron (Table S1, appendix). Gout on the other hand was more frequent among those randomized to IV iron.
Discussion
Among anemic patients with iron deficiency and CKD enrolled in the REVOKE trial intravenous iron therapy we failed to confirm that intravenous iron accelerates the decline in kidney function. However, intravenous iron was associated with an increased frequency of overall serious adverse events as well as cardiovascular and infectious complications. Specifically, intravenous iron administration was also associated with an increased incidence of the hospitalizations due to congestive heart failure, pneumonias and serious skin infections (requiring administration of antibiotics in the hospital) which became apparent after adjustment for the more favorable clinical and demographic characteristics at baseline among participants assigned to intravenous iron therapy (younger age, lower prevalence of cardiovascular disease and hospitalized infections). Treatment with either oral or intravenous iron-repletion therapy produced statistically and clinically significant improvements in hemoglobin that were sustained over the 24 months of the trial.
In a meta-analysis comparing randomized trials of oral to intravenous iron on hemoglobin response among patients with chronic kidney disease not on dialysis Rozen-Zvi et al reported 6 studies [20]. Five of these 6 trials were short-term, 1 to 3 months, and compared to oral iron, the mean increase in hemoglobin with intravenous iron was 0.31 (95% CI 0.09 to 0.53) g/dL. However, one of the studies included in this meta-analysis was 6 months long and had a mean decline in hemoglobin of 0.52 g/dL associated with intravenous iron administration [21]. In the largest randomized clinical trial comparing IV to oral iron in iron deficient anemic patients with CKD, the Ferinject® assessment in patients with Iron deficiency anemia and Non-Dialysis-dependent Chronic Kidney Disease (FIND-CKD) investigators randomized 626 patients in 193 centers in 1:1:2 ratio to ferric carboxymaltose targeting ferritin to high level (400 – 600 ng/mL), lower level (100–200 ng/mL) or oral iron with the primary end-point of time to initiation of other anemia management (ESA, other iron therapy or blood transfusion) or hemoglobin (Hb) trigger of two consecutive values <10 g/dL during Weeks 8–52. The investigators reported the mean change in Hgb from baseline to 52 weeks as 1.0 g/dL in oral iron group, 0.9 g/dL when IV ferric carboxymaltose targeted ferritin to 100–200 ng/mL and 1.4 g/dL (p=0.26) when IV ferric carboxymaltose targeted ferritin to 400–600 ng/mL (p=0.014) [22]. Although statistically significant, the difference in hemoglobin of 0.4 g/dL between oral iron and high dose IV iron observed in that study should be interpreted cautiously because the oral ferrous sulfate administration was only 100 mg twice daily which is much below the recommended intake of ferrous sulfate 325 mg three times daily used in our trial. Given the short duration of most of the clinical trials comparing oral with intravenous administration of iron the long-term safety of these modes of administration of supplemental iron could not be assessed. Accordingly, guidelines have no recommendation on the preferred mode of iron administration in such patients [19].
We showed that compared to oral iron-based therapy, intravenous iron therapy was associated with greater risk of infections and cardiovascular complications. Although assignment to the intravenous iron treatment group resulted in greater increments in both transferrin saturation and serum ferritin concentration, suggesting a better repletion of iron stores, there was little difference in mean hemoglobin increments in the long term. Overall, there was an increase in proteinuria noted, however between group differences over time in baseline proteinuria were not observed. These findings confirm no increase in basal level of proteinuria over several weeks among patients receiving intravenous iron [16].
The adverse events observed in our randomized trial are biologically plausible. Iron promotes growth of even common bacteria such as Staphylococcus epidermidis [23]. In addition, the inflammatory response to infection is enhanced [24;25] and phagocytic function of neutrophils has been shown to be impaired by iron [26]. Compared to oral iron, a greater iron saturation and a higher serum ferritin concentration was seen in the IV iron group which may increase the likelihood for the generation of free iron. Free iron induces the generation of the hydroxyl ion via the Haber-Weiss Fenton reaction, quenching of nitric oxide, endothelial dysfunction and may accelerate atherosclerosis [27]. Repeated administration of iron sucrose results in post infusion proteinuria [16]; if this results in impaired sodium handling by the kidney it may explain excess heart failure hospitalizations seen in our study.
Our findings may not be generalizable to patients with kidney failure requiring hemodialysis patients or those with heart failure. As an example, among patients with heart failure randomized to either ferric carboxymaltose or placebo and followed for six months, cardiovascular disorders (sum of cardiac, vascular and neuro disorders) were incident in 11.6/100 PY in iron group and 25.6/100 PY in the placebo group [28]. This is in sharp contrast to 52.5 events/100 PY in IV iron group and 33.5/100 PY in the oral group in our study. Infections were seen with an incidence of 1.4/100 PY in iron group and none in the placebo group which again is in contrast to 36.6 events/100 PY in IV iron group and 25.8/100 PY in the oral group in our study. In another study, heart failure patients with iron deficiency with or without anemia 86% of participants came from Russia, Ukraine, and Poland. Participants were randomized to ferric carboxymaltose (FCM) or placebo and followed for one year had serious adverse event rate of about one-quarter that reported in our study [29]. Because compared to the general population, patients with CKD are at elevated risk for cardiovascular and infectious illnesses, intravenous iron may exacerbate this risk.
Neither of the above studies was done in the CKD population. However, despite about a year long trial, our safety data are difficult to compare to even the FIND–CKD study for several reasons [22]. First, they excluded patients whose CKD was progressing rapidly and they could reach ESRD within 12 months. Second, adverse events and serious adverse events are reported up to the point at which another anemia therapy was initiated and/or the randomized study medication was discontinued. In other words, if ESA was initiated or patient transfused, the study stopped reporting serious adverse events. Third, serious adverse events were reported if they occurred in <1% of the patients. Even so, the investigators reported serious adverse events in 25.3, 24.0 and 18.9% of patients in the high-ferritin IV iron, low-ferritin IV iron, and oral iron groups, respectively. Thus, compared to oral iron group, IV iron SAE was between 27% and 34% higher. Fourth, multiple events within patients were not reported. In other words, multiple CHF events in one patient would only be reported once. REVOKE counted each event as a separate SAE. In fact, the number of patients who had SAEs in REVOKE were similar between oral and IV iron groups. Indeed in our study, we found that exposure to IV iron increased the frequency—not the number of participants—with serious adverse events.
Although our results should not be extrapolated to hemodialysis patients, it is notable that among hemodialysis patients intravenous iron is being increasingly used [30;31]. This is presumably so in an effort to maintain hemoglobin concentration while sparing erythropoietin to reduce cost; this has provoked an increase in serum ferritin concentration and transferrin saturation. Our study raises the need for further research regarding the safety of this practice.
Among the trials conducted to date to evaluate role of route of therapy to replenish iron, ours is the first to directly measure GFR and is one of the longest. Also randomizing a reasonably large number of subjects at one center with all adverse event ascertainments done by one investigator is a notable strength of this trial. There are limitations to consider including an open-label design although this likely did not affect measurement of GFR or occurrence of all cause adverse events. Second, there were slight imbalances in the clinical and demographic characteristics of the treatment groups at baseline, however, they were less favorable (older age, more cardiovascular and infectious disease history) among the oral iron group. Third, we did not have a placebo group. Thus, we did not study whether no iron is even safer than oral iron in this group of patients, but that would be considered unethical. Taken together, our findings raise the urgent need for long term safety of intravenous iron in vulnerable populations such as CKD.
The primary question posed by our study as to whether intravenous iron accelerates the decline in GFR in patients with CKD was not conclusively answered; however, the likelihood of finding a 50% difference in mGFR at two years was very low. However, among patients with iron deficiency anemia and moderate to advanced CKD, compared to oral iron, intravenous iron sucrose appears to result in a higher risk for infections and cardiovascular complications over the long-term. Oral iron may be the preferred initial mode of treatment for iron deficiency anemia in stage 3 and 4 CKD.
Methods
The randomized trial to evaluate intravenous and oral iron in chronic kidney disease (REVOKE) was an open-label, parallel group, active control, single center randomized trial designed to compare the safety and efficacy of an oral iron therapy with an intravenous iron treatment, each administered over the first 8 weeks after randomization. The study was conducted between August 2008 and November 2014 with study participants recruited from Eskenazi Hospital and the Veterans Administration (VA) Hospital both located in Indianapolis, IN, USA. Initially constituted by the National Institutes of Health, an independent data and safety monitoring board (DSMB) reviewed the information on safety and study progress at least annually. During the latter part of the study a new DSMB was established by Indiana University. The study was approved by the Institutional Review Board of Indiana University and the Research and Development Committee of the Roudebush VA Medical Center, Indianapolis. All study participants provided written informed consent.
Participants
Study participants were required to be at least 18 years of age, have an estimated GFR (eGFR) by the 4-component MDRD formula of >20 and ≤ 60 ml/min/1.73m2 using IDMS-calibrated creatinine [32], anemia and iron deficiency. Anemia was defined as blood hemoglobin concentration <12 g/dL and iron deficiency as either a serum ferritin concentration of <100 ng/mL or serum transferrin saturation of <25%. Other exclusions are listed in the appendix.
Study design
Randomization
Eligible participants were randomized in a 1:1 ratio, using permuted blocks, to either oral iron or intravenous iron using concealed opaque envelopes. The randomization sequence was computer generated by a statistician.
Study intervention
Iron deficiency was treated over 8 weeks beginning at time of randomization for both treatment groups. Participants were seen at weeks 0, 2, 4, 6, and 8 after randomization. Those randomized to the IV iron group received iron sucrose 200 mg intravenously over 2 hours at each of these 5 visits. Participants randomized to oral iron were counseled to take ferrous sulfate 325 mg three times daily for 8 weeks to provide at least the minimum dose of 200 mg elemental iron per day. Subsequently, in-person visits were conducted at 3, 6, 12, 18 and 24 months after randomization. The planned duration of the study was 24 months and mGFR was obtained at baseline, 8 weeks, 6 months, 12 months, and 24 months after randomization.
An interim medical history, review of a list of medications and all adverse events related or unrelated to the randomized treatment were obtained at each in-person study visit.
Measurement of glomerular filtration rate
Plasma clearance of iothalamate was measured by administering an intravenous bolus of 5 mL of iothalamate meglumine (Conray 60, Malinkrodt, St Louis, MO) and sampling 2 mL of blood at 0, 5, 10, 20. 30, 45, 60, 90, 120, 150, 180, 240 and 300 minutes after injection. Iothalamate was measured by high performance liquid chromatography. Plasma clearance was calculated using a two-pool model using validated pharmacokinetic software (Winnonlin). Coefficient of variation for repeated measurement of plasma iothalamate clearance three days apart in the same subjects was 5.7% [33].
Other measurements
Hemoglobin and serum chemistries were measured at biweekly intervals for the first 8 weeks after randomization and with every research visit thereafter. Iron stores were determined at baseline, 8 weeks and then at every GFR visit thereafter. Quality of life was measured by the Kidney Disease Quality of Life (KDQOL) instrument [34] at baseline, and periodic intervals thereafter. Proteinuria was estimated using measurements of urinary protein and creatinine prior to iron administration at baseline and at periodic intervals thereafter.
Anemia was managed by protocol using iron and erythropoiesis stimulating agents (see appendix). Rescue therapy with additional iron therapy is also described in the appendix.
Outcomes
The primary outcome was the between treatment group difference in the slope of mGFR from baseline to two years adjusted for the log of baseline urinary protein/creatinine ratio. Further adjustment of the primary outcome was also made for age, sex, race (Black vs non-Black), ACE/ARB use, and the presence or absence of cardiovascular disease (all determined at baseline) and was a pre-specified secondary outcome. Another secondary outcome was the between group percent change in proteinuria from baseline to 8 weeks. Other outcomes were the difference between hemoglobin response between treatment groups and change in KDQOL.
Statistical Analysis
We assumed a mean rate of decline in GFR of 4 mL/min/1.73 m2 per year in the oral iron group and a 50% greater decline in the IV iron group and a cumulative rate of drop-out of 25%. We established a recruitment target of 100 patients for each treatment group with a minimum duration of follow-up of two years to achieve 82% power to detect our hypothesized difference in decline in kidney function at the 5% level of significance.
The analysis of the primary outcome was intention to treat, if the patient received at least one dose of the randomized drug (which was the case for each subject). A linear mixed model was used with GFR as the outcome variable. Fixed effects were indicator variables for time (treated as a continuous variable), treatment, and their interaction. Random effects were subject and time with unstructured covariance; statistical inference was made using the maximum likelihood estimator. To avoid biasing the slopes we imputed GFR to be 10 mL/min/1.73m2 in case of death or ESRD. ESRD was defined as kidney transplantation or the receipt of dialysis for at least 30 days unless death occurred. Similar mixed models were used for analyzing the KDQOL domains and log transformed urine protein/creatinine ratio, except that time was treated as a categorical variable.
An adverse event was deemed serious if it resulted in hospitalization or a visit to the emergency room where an intervention was made to avert hospitalization. The nature and number of all serious adverse events was adjudicated by RA who was unaware of treatment assignment at the time of ascertainment. The duration of participation in the study per subject, which according to the trial design could be up to 24 months, was determined. The event rate was calculated by treatment group assignment. Incidence rate ratio by treatment was then determined along with the 95% confidence intervals using the Poisson distribution assumption.
Adjudicated cardiovascular events included the following: myocardial infarction, stroke, hospitalization for congestive heart failure, hospitalized angina, arrhythmias, cardiac arrest, coronary revascularization, and peripheral vascular interventions. Adverse events reported are those during the course of 24 months of participation in the trial.
All analyses were conducted using Stata version 11.2 (Stata Corp, College Station, TX). The P values reported are two-sided and taken to be significant at <0.05.
RA was solely responsible for the decision to submit the manuscript and takes full responsibility for the accuracy and completeness of the reported data and analyses.
Supplementary Material
Acknowledgments
Supported in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (U01-DK71633) and Indiana Institute for Medical Research
Footnotes
Disclosure statement
RA served on a Data Safety Monitoring Board of a study sponsored by Amgen. He has also served as a consultant for Celegene, Takeda, Bayer, and Daiichi Sankyo, Inc. Other authors have nothing to disclose.
Contributions of authors:
Rajiv Agarwal – literature search, study design, obtain funding, supervision of the study data analysis, data interpretation, writing
Maria K Pappas – patient recruitment and study procedures, data collection, data entry and summarizing data.
John W Kusek – convening the data safety monitoring board, critical revision of the manuscript.
Reference List
- 1.Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994) Arch Intern Med. 2001;161:1207–1216. doi: 10.1001/archinte.161.9.1207. [DOI] [PubMed] [Google Scholar]
- 2.Fishbane S, Pollack S, Feldman HI, Joffe MM. Iron indices in chronic kidney disease in the National Health and Nutritional Examination Survey 1988–2004. Clin J Am Soc Nephrol. 2009;4:57–61. doi: 10.2215/CJN.01670408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nissenson AR, Strobos J. Iron deficiency in patients with renal failure. Kidney Int Suppl. 1999;69:S18–S21. doi: 10.1046/j.1523-1755.1999.055suppl.69018.x. [DOI] [PubMed] [Google Scholar]
- 4.Besarab A. Iron and cardiac disease in the end-stage renal disease setting. Am J Kidney Dis. 1999;34:S18–S24. doi: 10.1053/AJKD034s00018. [DOI] [PubMed] [Google Scholar]
- 5.Besarab A, Frinak S, Yee J. An indistinct balance: the safety and efficacy of parenteral iron therapy. J Am Soc Nephrol. 1999;10:2029–2043. doi: 10.1681/ASN.V1092029. [DOI] [PubMed] [Google Scholar]
- 6.Albaramki J, Hodson EM, Craig JC, Webster AC. Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database Syst Rev. 2012;1:CD007857. doi: 10.1002/14651858.CD007857.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Haugen E, Nath KA. The involvement of oxidative stress in the progression of renal injury. Blood Purif. 1999;17:58–65. doi: 10.1159/000014377. [DOI] [PubMed] [Google Scholar]
- 8.Klahr S. Oxygen radicals and renal diseases. Miner Electrolyte Metab. 1997;23:140–143. [PubMed] [Google Scholar]
- 9.Agarwal R, Warnock D. Issues related to iron replacement in chronic kidney disease. Semin Nephrol. 2002;22:479–487. doi: 10.1053/snep.2002.35972. [DOI] [PubMed] [Google Scholar]
- 10.Zager RA, Johnson AC, Hanson SY, Wasse H. Parenteral iron formulations: a comparative toxicologic analysis and mechanisms of cell injury. Am J Kidney Dis. 2002;40:90–103. doi: 10.1053/ajkd.2002.33917. [DOI] [PubMed] [Google Scholar]
- 11.Shah SV. Role of iron in progressive renal disease. Am J Kidney Dis. 2001;37:S30–S33. doi: 10.1053/ajkd.2001.20736. [DOI] [PubMed] [Google Scholar]
- 12.Handelman GJ, Walter MF, Adhikarla R, et al. Elevated plasma F2-isoprostanes in patients on long-term hemodialysis. Kidney Int. 2001;59:1960–1966. doi: 10.1046/j.1523-1755.2001.0590051960.x. [DOI] [PubMed] [Google Scholar]
- 13.Spittle MA, Hoenich NA, Handelman GJ, et al. Oxidative stress and inflammation in hemodialysis patients. Am J Kidney Dis. 2001;38:1408–1413. doi: 10.1053/ajkd.2001.29280. [DOI] [PubMed] [Google Scholar]
- 14.Salahudeen AK, Oliver B, Bower JD, Roberts LJ. Increase in plasma esterified F2-isoprostanes following intravenous iron infusion in patients on hemodialysis. Kidney Int. 2001;60:1525–1531. doi: 10.1046/j.1523-1755.2001.00976.x. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal R, Vasavada N, Sachs NG, Chase S. Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney Int. 2004;65:2279–2289. doi: 10.1111/j.1523-1755.2004.00648.x. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal R, Leehey DJ, Olsen SM, Dahl NV. Proteinuria induced by parenteral iron in chronic kidney disease–a comparative randomized controlled trial. Clin J Am Soc Nephrol. 2011;6:114–121. doi: 10.2215/CJN.06020710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drueke T, Witko-Sarsat V, Massy Z, et al. Iron therapy, advanced oxidation protein products, and carotid artery intima-media thickness in end-stage renal disease. Circulation. 2002;106:2212–2217. doi: 10.1161/01.cir.0000035250.66458.67. [DOI] [PubMed] [Google Scholar]
- 18.Becker BN, Himmelfarb J, Henrich WL, Hakim RM. Reassessing the cardiac risk profile in chronic hemodialysis patients: a hypothesis on the role of oxidant stress and other non-traditional cardiac risk factors. J Am Soc Nephrol. 1997;8:475–486. doi: 10.1681/ASN.V83475. [DOI] [PubMed] [Google Scholar]
- 19.Kidney Disease Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 20.Rozen-Zvi B, Gafter-Gvili A, Paul M, et al. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: systematic review and meta-analysis. Am J Kidney Dis. 2008;52:897–906. doi: 10.1053/j.ajkd.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 21.Stoves J, Inglis H, Newstead CG. A randomized study of oral vs intravenous iron supplementation in patients with progressive renal insufficiency treated with erythropoietin. Nephrol Dial Transplant. 2001;16:967–974. doi: 10.1093/ndt/16.5.967. [DOI] [PubMed] [Google Scholar]
- 22.Macdougall IC, Bock AH, Carrera F, et al. FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant. 2014;29:2075–2084. doi: 10.1093/ndt/gfu201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parkkinen J, von Bonsdorff L, Peltonen S, et al. Catalytically active iron and bacterial growth in serum of haemodialysis patients after i.v. iron-saccharate administration. Nephrol Dial Transplant. 2000;15:1827–1834. doi: 10.1093/ndt/15.11.1827. [DOI] [PubMed] [Google Scholar]
- 24.Zager RA, Johnson AC, Hanson SY. Parenteral iron therapy exacerbates experimental sepsis. Rapid communication. Kidney Int. 2004;65:2108–2112. doi: 10.1111/j.1523-1755.2004.00742.x. [DOI] [PubMed] [Google Scholar]
- 25.Zager RA, Johnson AC, Hanson SY, Lund S. Parenteral iron compounds sensitize mice to injury-initiated TNF-alpha mRNA production and TNF-alpha release. Am J Physiol Renal Physiol. 2005;288:F290–F297. doi: 10.1152/ajprenal.00342.2004. [DOI] [PubMed] [Google Scholar]
- 26.Sengoelge G, Kletzmayr J, Ferrara I, et al. Impairment of transendothelial leukocyte migration by iron complexes. J Am Soc Nephrol. 2003;14:2639–2644. doi: 10.1097/01.asn.0000087087.61306.4a. [DOI] [PubMed] [Google Scholar]
- 27.Zheng H, Huang X, Zhang Q, Katz SD. Iron sucrose augments homocysteine-induced endothelial dysfunction in normal subjects. Kidney Int. 2006;69:679–684. doi: 10.1038/sj.ki.5000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anker SD, Comin CJ, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 29.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miskulin DC, Zhou J, Tangri N, et al. Trends in anemia management in US hemodialysis patients 2004–2010. BMC Nephrol. 14(264):2013. doi: 10.1186/1471-2369-14-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brookhart MA, Schneeweiss S, Avorn J, et al. Comparative mortality risk of anemia management practices in incident hemodialysis patients. JAMA. 2010;303:857–864. doi: 10.1001/jama.2010.206. [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal R, Vasavada N, Chase SD. Liquid chromatography for iothalamate in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;785:345–352. doi: 10.1016/s1570-0232(02)00960-1. [DOI] [PubMed] [Google Scholar]
- 34.Hays RD, Kallich JD, Mapes DL, et al. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3:329–338. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


