Abstract
Interactions between cells and the extracellular matrix (ECM) are known to play critical roles in regulating cell phenotype. The identity of ECM ligands presented to mesenchymal stem cells (MSCs) has previously been shown to direct the cell fate commitment of these cells. In order to enhance osteogenic differentiation of MSCs, alginate hydrogels were prepared that present the DGEA ligand derived from collagen I. When presented from hydrogel surfaces in 2D, the DGEA ligand did not facilitate cell adhesion, while hydrogels presenting the RGD ligand derived from fibronectin did encourage cell adhesion and spreading. However, the osteogenic differentiation of MSCs encapsulated within alginate hydrogels presenting the DGEA ligand was enhanced when compared with unmodified alginate hydrogels and hydrogels presenting the RGD ligand. MSCs cultured in DGEA-presenting gels exhibited increased levels of osteocalcin production and mineral deposition. These data suggest that the presentation of the collagen I-derived DGEA ligand is a feasible approach for selectively inducing an osteogenic phenotype in encapsulated MSCs.
Keywords: mesenchymal stem cells, DGEA peptide, osteogenesis, alginate hydrogels, ECM-mimicking ligands
Introduction
While the process of bone fracture repair is generally efficient, a subset of cases, including traumatic injury, tumor resection, and infection, results in poor healing or nonunion 1. The present gold standard for repair of critical size defects is autologous bone grafting, in which host bone is harvested from another site and used to replace the bone in the defect site 2. Complications from autograft procedures, including donor site morbidity, pain, and increased risk of infection, have led to the widespread use of allogeneic grafting of bone obtained from human cadavers 2,3. Given the risk of donor to recipient disease transmission and immune rejection of the graft, recent research has focused on developing synthetic bone graft substitutes, however, these substitutes have not yet demonstrated clinical efficacy 1,4.
An alternative strategy to heal critical sized bone defects is to deliver or recruit osteoprogenitor cells to the defect site, and to locally direct their proliferation and differentiation 1. Mesenchymal stem cells (MSCs) are a promising source of cells for bone regeneration, as MSCs can be obtained from the patient in need of the therapy with relative ease 5,6. These bone marrow-derived stem cells are characterized by their ability to differentiate into bone, cartilage, and adipose tissue 7. Consequently, directing the differentiation of MSCs post-transplantation using ECM cues may provide clinical benefit. Present strategies in bone regeneration and tissue engineering utilize delivery of soluble growth factors, such as bone morphogenetic proteins (BMPs), to control the fate of transplanted cells 1. Because these factors can diffuse out of the defect site, they must be administered in high doses. Clinical use of BMPs as monotherapies to enhance spinal fusion has revealed that supraphysiological doses of BMPs are both costly 8 and often result in undesirable secondary effects in surrounding tissues 9,10.
In addition to soluble cues such as growth factors, insoluble cues provided by the extracellular matrix (ECM) have been demonstrated to influence the differentiation of MSCs. For instance, mechanical properties such as substrate stiffness can modulate the phenotype of MSCs, with relatively stiff substrates yielding enhanced osteogenic differentiation 11,12. In addition, previous studies have reported that the identity of the ECM material can contribute to the lineage specification of MSCs. For instance, culturing MSCs in laminin-5 has been reported to inhibit chondrogenesis 13,14, and MSCs cultured on laminin-1 were shown to adopt a neuronal-like phenotype, extending fine, neurite-like processes 15. Hydrogels composed of collagen II, a major component of cartilage ECM, enhanced the chondrogenic differentiation of MSCs 16, while collagen I, a major component of bone, has been shown to enhance osteogenesis 17. Therefore, we hypothesized that presenting an ECM ligand derived from collagen I to MSCs would result in increased osteogenesis. RGD peptides, initially derived from the ECM protein fibronectin, are routinely used in hydrogel systems to facilitate cell adhesion and spreading 18–20. Another peptide sequence, DGEA, found in collagen I, is known to bind the α2β1 integrin pair 21. Previous studies utilizing biomaterials functionalized with a different collagen I-derived, integrin-binding ligand, GFOGER, have shown enhanced bone defect healing and implant osseointegration in vivo22–24. Thus, presenting MSCs with the integrin-binding DGEA ligand may also improve osteogenic differentiation. In this study, a peptide containing the DGEA motif was covalently bound to alginate via carbodiimide chemistry. Alginate hydrogels presenting the DGEA peptide were used to assess the ability of the DGEA peptide to facilitate cell adhesion and induce osteogenesis in MSCs.
Materials and Methods
Materials
Alginate polymers were purchased from FMC Biopolymer (Princeton, NJ). Dulbecco’s Modified Eagle Medium (DMEM), Dulbecco’s phosphate buffered saline (PBS), trypsin/EDTA, fetal bovine serum (FBS), Hoechst 33258, normal goat serum (NGS), penicillin-streptomycin, fluorescein-phalloidin, PrestoBlue Cell Viability Reagent, and dialysis tubing were purchased from Invitrogen (Carlsbad, CA). Sulfo-N-hydroxysuccinimide (Sulfo-NHS) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC)were purchased from Thermo Scientific (Waltham, MA). Phenol red-free DMEM was purchased from MediaTech (Manassas, VA). RGD peptide (H2N-GGGGRGDSP-OH, MW 758.74) was purchased from Commonwealth Biotechnologies (Alexandria, Virginia). DGEA peptide (H2N-GGGGDGEASP-OH, MW 802.75) was purchased from Peptide 2.0 (Chantilly, VA). Mesenchymal stem cell (MSC) growth kit (low serum), consisting of L-alanyl-L-glutamine and MSC supplement, was purchased from ATCC Primary Cell Solutions (Manassas, VA). Protected amino acids, pre-loaded polystyrene resins, and 1-(bis(dimethylamino)methylene)-1H-benzotriazolium hexafluorophosphate 3-oxide (HBTU) for solid-phase peptide synthesis were purchased from Peptides International (Louisville, KY). N-methylpyrrolidinone (NMP), piperidine, diisopropylethylamine (DIEA), and trifluoroacetic acid (TFA) were purchased from Advanced ChemTech. Unless otherwise noted, other reagents were purchased from Sigma (St. Louis, MO).
Peptide Synthesis
For initial studies, the DGEA peptide (H2N-GGGGDGEASP-OH) was synthesized by solid phase Fmoc chemistry as described previously 25. Briefly, polystyrene resin pre-loaded with Fmoc-protected proline was swelled in dichloromethane and NMP prior to beginning the synthesis. Peptide synthesis was performed on a CSBio CS336X peptide synthesizer. Twenty percent piperidine was used for Fmoc deprotection, and working concentrations of 0.32 M HBTU and 0.2 M DIEA were used to facilitate amino acid coupling. A double coupling protocol was utilized, followed by capping with acetic anhydride. After the final Fmoc deprotection step, the resin was washed sequentially with NMP, dichloromethane, and methanol and allowed to dry. The peptide was cleaved from the resin using 95% TFA with 2.5% water and 2.5% TIS as scavengers. The cleavage solution was concentrated in vacuo, and the peptide was recovered by precipitation in cold diethyl ether. The peptide was purified by reverse-phase high-performance liquid chromatography (HPLC) on an Agilent 1100 Series Purification HPLC, using an acidic mobile phase (0.1% TFA) and a gradient from 20% to 50% acetonitrile on an Agilent C18 Zorbax column. Fractions containing the desired peptides were verified by LC-MS (Agilent 1290 LC/MS System) to have a purity of >90% and then were combined and lyophilized to afford the peptide as a white powder.
Preparation of Alginate Polymers
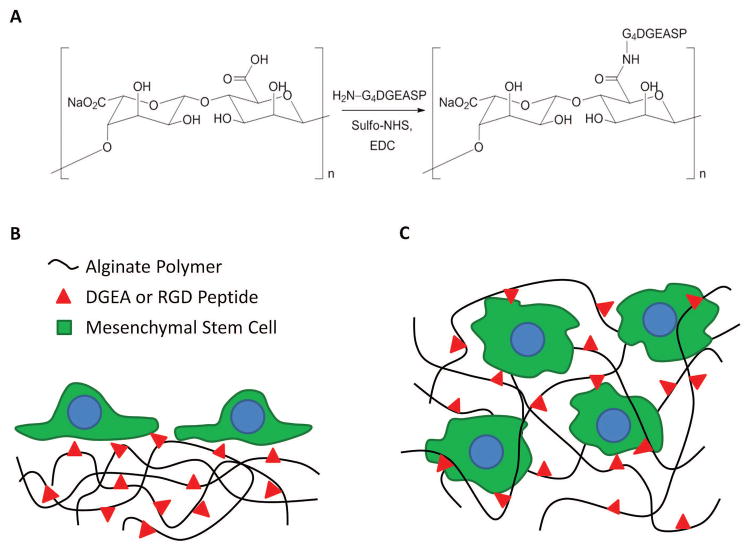
High guluronic acid (GA) content alginate (average MW ~ 165 kDa, >60% GA content) was purified by dialyzing a 1% (w/v) solution against deionized water in 3500 MWCO dialysis tubing. The alginate was subsequently decolored with activated charcoal, sterile (0.22 μm) filtered, and lyophilized. Alginate to be modified with RGD or DGEA peptides was then dissolved to 1% (w/v) in a 0.1 M MES and 0.3 M sodium chloride buffer solution at pH 6.5. High purity (>98%), commercially synthesized DGEA and RGD peptides were used for all cell culture studies. Peptide conjugation via carbodiimide chemistry was performed following a previously published procedure 20. Briefly, sulfo-NHS and EDC were added in a 1:2 molar ratio, followed immediately by addition of the peptide. Sufficient peptide was added to yield a theoretical degree of substitution of 5 peptides per polymer chain, based on a previous study that determined reaction efficiency using 125I labeled peptides 20. The reaction was allowed to proceed for 20 hours before being quenched by the addition of hydroxylamine. The reaction mixtures were dialyzed against a decreasing concentration of sodium chloride to remove salts and any unbound peptide. The alginate was then sterile (0.22 μm) filtered and lyophilized.
Cell Culture
Clonally derived murine mesenchymal stem cells (D1; ATCC) were maintained in high glucose DMEM supplemented with 10% FBS and 0.1% penicillin/streptomycin at 37° C and 5% CO2. D1 cells between passages 21 and 29 were used for experiments. Primary rat MSCs were isolated from bone marrow aspirates of Lewis rat femurs and maintained in high glucose DMEM supplemented with 10% FBS, 0.1% penicillin/streptomycin, L-alanyl-L-glutamine (1:100) and MSC supplement (1:50) at 37° C and 5% CO2. Rat MSCs were used between passages 2 and 6.
For experiments assessing the osteogenic differentiation of rMSCs, the cell culture medium was additionally supplemented with 100 nM dexamethasone, 10 mM β-glycerophosphate, and 50 mg/ml ascorbic acid. Additionally, one experimental condition included human BMP-2, recombinantly expressed in E. coli (courtesy of Walter Sebald, Wurzburg, Germany), at a concentration of 500 ng/ml. This concentration was chosen because previous studies have shown 500 ng/mL to be an effective dose of E. coli-derived BMP-2 to elicit an osteogenic response in primary human MSCs26. Cell culture medium was replenished every two days.
Preparation of Alginate Hydrogels
Sterile alginate, either unmodified or conjugated with RGD or DGEA peptides, was dissolved to 2% (w/v) in phenol red-free, serum-free DMEM (SF DMEM). Prior to casting gels, alginate was diluted to 1% (w/v) with SF DMEM. For 3D cell encapsulation studies, the cells were introduced to the alginate solution at this step, at a final concentration of 2×106 cells/ml. Prior to encapsulation, the cells were washed twice with PBS to remove any loosely bound serum proteins. Hydrogels were formed by mixing the alginate solution (with or without cells) with 4% (v/v) of a 1.22 M calcium sulfate slurry and casting between two glass plates separated by 1 mm for 45 minutes at room temperature. This crosslinking method has been previously optimized to yield homogeneous alginate gels with elastic moduli conducive to osteogenic differentiation of MSCs (~20 kPa)12. No effects on MSC phenotype due to the presence of any residual calcium from the crosslinking reaction have been previously observed12. Alginate disks were punched out and transferred to either SF DMEM (for 2D adhesion studies) or DMEM plus 10% FBS (for 3D cell encapsulation and differentiation studies).
Time Lapse Microscopy of Cell Adhesion
D1 cells were seeded onto alginate hydrogels in 8-well cell culture slides (BD Biosciences) at a density of 120,000 cells/cm2 and were maintained at 37°C in SF DMEM for 3 hours. Time lapse images were obtained using a DIC microscope.
Metabolic Activity Assay
D1 cells were seeded onto alginate hydrogels in cell culture slides at a density of 60,000 cells/cm2 and were maintained at 37°C in SF DMEM for 3 days. The cells were incubated for 1 hour in PrestoBlue Cell Viability Reagent, which is reduced by metabolically active cells. The cell media was then transferred to a 96-well black plate and its fluorescence was read using a Biotek Synergy plate reader.
Adhesion assay
Rat MSCs were seeded onto alginate hydrogels in cell culture slides at a density of 330,000 cells/cm2 and were maintained at 37°C in SF DMEM for 7 hours. As a control, cells were also seeded onto the glass surface of the cell culture slides at the same density. To visualize cell nuclei, Hoechst 33258 was added to the media (1:10,000). After 30 minutes of incubation at 37°C, five separate fields of view of the gels for each experimental condition were imaged with a fluorescence microscope. The cell culture slides containing the gels were then agitated on an orbital shaker at 37°C for 30 minutes. The gels were washed with SF DMEM to remove any unbound cells and were imaged again. The number of cells present on the gels before and after washing was calculated by quantifying the number of nuclei present in each field of view using ImageJ.
Immunostaining
To assess cells on peptide modified alginate hydrogels in 2D, D1 cells were seeded onto alginate hydrogels in cell culture slides at a density of 120,000 cells/cm2 and maintained at 37°C in SF DMEM for three hours. The gels were then fixed in a 4% PFA solution in PBS containing divalent cations (cPBS) for 15 minutes at 4°C and permeabilized with 0.1% Triton X-100 in cPBS for 5 minutes at room temperature. The samples were blocked with 2% bovine serum albumin (BSA) and 5% normal goat serum (NGS) in cPBS plus 0.3% Triton X-100 for 45 minutes at room temperature. The samples were then incubated with rabbit anti-phospho-paxillin antibody (1:100, Cell Signaling) in cPBS plus 1% BSA for two hours and washed with cPBS. Finally, the gels were treated with AlexaFluor 546 linked goat anti-rabbit antibody (1:1,000, Invitrogen), fluorescein-conjugated phalloidin (1:50), and Hoechst 33258 (1:5,000) for 1 hour at room temperature and washed with cPBS prior to imaging.
Production of collagen I and osteocalcin by rMSCs encapsulated within alginate hydrogels was also assessed via immunostaining as a metric of osteogenic differentiation. After 30 days in culture, samples were fixed with 4% paraformaldehyde in SF DMEM, supplemented with 0.1% sodium azide, 0.1% Triton X-100, and 0.1% Tween-20 for 30 minutes at 25°C. After washing the gels in 100 mM HEPES (pH 7.4), the gels were incubated in 5% sucrose for 15 minutes at room temperature, followed by an overnight incubation at 4°C in 30% sucrose containing a trace amount of fluorescein free acid. The following day, the gels were incubated in a 1:1 mixture of 30% sucrose and Optimal Cutting Temperature (OCT) media (TissueTek) for 2 hours at room temperature, followed by an incubation in 100% OCT for 30 minutes. Meanwhile, freezing molds (Cryomold, TissueTek) were filled halfway with OCT and frozen on dry ice. The fixed and cryoprotected gels were placed on top of the frozen OCT in the mold, and the molds were filled entirely with OCT and frozen on dry ice for 15 minutes. The samples were sectioned to achieve a thickness of 8–10 μm and mounted on Superfrost Plus slides. For immunostaining, the slides were washed with deioinized water and permeabilized with 0.15% Triton X-100 in cPBS at room temperature for 10 minutes. Samples were blocked with 2% BSA and 5% NGS in cPBS at room temperature for 1 hour. The samples were then incubated in either rabbit anti-collagen I or rabbit anti-osteocalcin antibody (1:100, Abcam) in PBS containing 1% BSA and 0.01% Tween-20 for 3 hours at room temperature, followed by a one hour incubation in AlexaFluor 488 linked goat anti-rabbit antibody (1:500, Invitrogen) in PBS plus 0.01% Tween-20 and Hoechst 33258 (1:5,000). The presence of collagen I and osteocalcin was quantified by dividing the total image area staining positive for collagen I or osteocalcin by the number of cells present in that field of view.
Western Blotting
Rat MSCs cultured in expansion media as described above were recovered from tissue culture flasks by treating with 5 mM EDTA, washed twice, and then resuspended into PBS with 10 mM RGD or DGEA peptide. PBS with no peptide served as a negative control. Samples were incubated at 37°C for 30 minutes, collected by centrifugation, and lysed into Radio Immunoprecipitation Assay (RIPA) buffer (Sigma) with Minitab Protease Inhibitors (Roche). The samples were then centrifuged at 14,000 rpm, and the protein content of the supernatant was determined using the bicinchoninic acid (BCA) assay (Thermo Scientific), using bovine serum albumin (BSA) to generate a standard curve. Twelve μg of protein per sample were loaded onto 4–20% Tris-Glycene gradient gels, separated by SDS-PAGE, and then transferred to nitrocellulose membranes. Membranes were blocked with 3% BSA in Tris-buffered saline with Tween (TBST) for 1 hour. Membranes were then incubated with rabbit anti-pERK primary antibody (1:1000, Cell Signaling) overnight at 4°C and washed with TBST. Membranes were incubated with goat anti-rabbit secondary antibody (Cell Signaling) conjugated to horseradish peroxidase (HRP) for 30 minutes at room temperature and then washed with TBST. Blots were developed using Bioluminescence X-ray film (Kodak) and the Enhanced Chemiluminescence Substrate System (Thermo Scientific). Actin was probed with mouse anti-actin primary antibody (Chemicon) and HRP-conjugated rabbit anti-mouse secondary antibody (Cell Signaling) to serve as a loading control.
Alkaline Phosphatase Staining
After 7 days in culture, rat MSCs encapsulated in alginate hydrogels were fixed with 4% paraformaldehyde in serum-free DMEM, supplemented with 0.1% sodium azide, 0.1% Triton X-100, and 0.1% Tween-20, for 30 minutes at room temperature. The gels were then incubated in 100 mM BaCl2 in 100 mM HEPES, pH 7.4 for 30 minutes to stabilize the alginate network and washed with 100 mM HEPES, pH 7.4. The gels were equilibrated in alkaline buffer (100 mM Tris-HCl, 100 mM NaCl, 0.1% Tween-20, pH 7.4) for 30 minutes at 37°C. Fast Blue staining buffer (alkaline buffer with 100 mM BaCl2, 500 μg/ml Fast Blue, 500 μg/ml NAMP, and 50 mM MgCl2) was then added to gels. After 2 hours of incubation at 37°C, the gels were washed in SF DMEM containing 0.1% sodium azide and 0.1% Tween-20 for 15 minutes at room temperature. Hoechst 33258 at 1 μg/ml in cPBS was added for nuclear counterstaining. The gels were sectioned as described above, mounted on glass microscope slides and visualized both in brightfield (for Fast Blue staining) and in fluorescence (for nuclear counterstain).
Von Kossa Staining
Rat MSCs encapsulated in alginate hydrogels and cultured for 30 days were fixed and cryosectioned as described above. Slides were then washed in deionized water, and a 3% (w/v) silver nitrate solution (PolySciences Inc.) was applied to the sections. Slides were exposed to UV light for 10 minutes and then washed with DI water. The slides were counterstained with Nuclear Fast Red (PolySciences Inc.).
Results
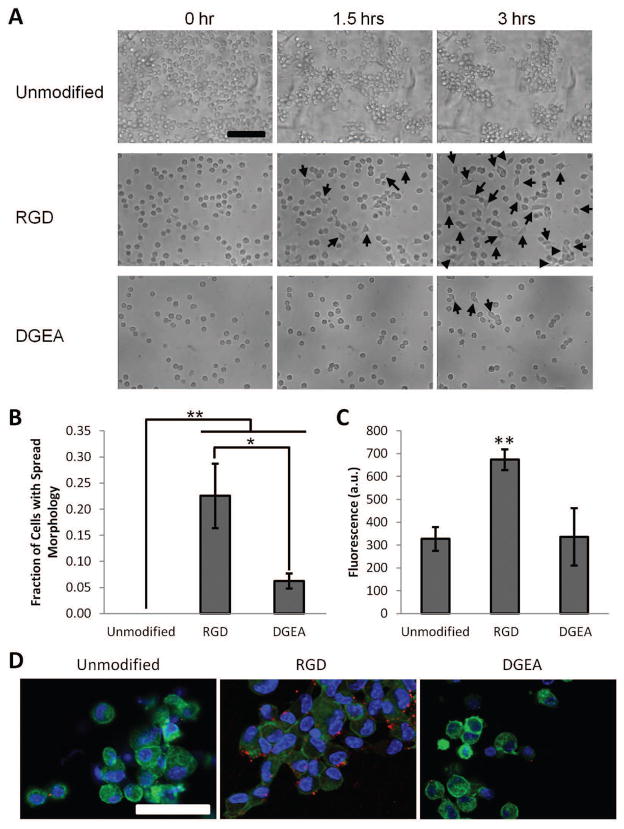
The collagen I mimicking peptide DGEA has previously been reported to bind the α2β1 integrin dimer 21, and thus could serve as a cell adhesion ligand for MSCs. DGEA peptide was conjugated to alginate using previously developed carbodiimide chemistry (Fig. 1). Additionally, alginate modified with RGD peptide (an integrin-binding ligand commonly used to mediate cell adhesion to various polymers 18–20) was prepared to serve as a positive control for MSC adhesion. Unmodified alginate, which is not naturally cell adhesive, served as a negative control. Initial studies to assess the cell adhesive properties of DGEA-conjugated alginate gels utilized a clonally derived murine MSC line (D1), as these cells have been previously shown to express a more stable MSC phenotype than primary bone marrow-derived MSCs12. Under serum-free culture conditions, D1 cells adhered and spread on top of alginate hydrogels modified with RGD peptide, but were generally unable to adhere to unmodified alginate gels, as observed by time lapse microscopy over the course of 3 hours (Fig. 2A,B and Videos S1 and S2). The D1 cells exhibited limited adhesion and spreading on alginate gels modified with DGEA peptide (Fig. 2A,B and Video S3). As cell viability is closely linked to adhesion in MSCs, the viability of D1 cells seeded on peptide-modified hydrogels was assessed with the Presto Blue assay, which measures cellular metabolic activity. D1 cells seeded on RGD-modified gels exhibited significantly higher metabolic activity than cells on either unmodified or DGEA-modified gels after three days in culture, indicating higher cell viability for cells presented with RGD peptide (Fig. 2C).
Figure 1. Alginate Hydrogels Presenting ECM-Mimicking Ligands.
(A) DGEA and RGD peptides were conjugated to alginate via carbodiimide chemistry. Schematics depicting presentation of DGEA and RGD peptide from (B) hydrogel surfaces for 2D cell adhesion studies and (C) cell-encapsulating hydrogels for 3D differentiation studies.
Figure 2. Characterization of D1 Mouse MSC Adhesion to RGD and DGEA Peptide Modified Alginates.
(A). DIC images of D1 MSCs seeded onto unmodified alginate, RGD-modified alginate, or DGEA-modified alginate hydrogels. Cells were maintained in serum free DMEM and imaged over the course of three hours post-seeding. Arrows denote the appearance of spread cells. Scale bar: 100 μm. (B). Fraction of seeded D1 cells adopting a spread morphology three hours post-seeding onto unmodified alginate, RGD-modified alginate, or DGEA-modified alginate hydrogels. *p<0.05, **p<0.01, Student’s t-test, n = 3–4. Error bars are ±SD. (C). Metabolic activity of D1 MSCs seeded onto unmodified alginate, RGD-modified alginate, or DGEA-modified alginate hydrogels, as measured by the PrestoBlue assay, after 3 days in culture. Increased fluorescence corresponds to increased metabolic activity. **p<0.01, Student’s t-test, n = 3. Error bars are ±SD. (D). Immunofluorescence images of D1 MSCs seeded onto unmodified alginate, RGD-modified alginate, or DGEA-modified alginate hydrogels and cultured for 3 hours. Cell nuclei are labeled in blue (Hoechst 33258), actin filaments in green (phalloidin), and phospho-paxillin in red. Scale bar: 50 μm.
To further investigate the mechanism of cellular interaction with the RGD and DGEA peptides, D1 cells were seeded on alginate hydrogels and maintained in serum free media for 3 hours, as above. The cells were then fixed and stained for cytoskeletal organization (F-actin) and focal adhesion formation (phospho-paxillin). Consistent with the previous adhesion data, actin staining revealed a spread morphology for cells cultured on RGD-modified gels, while those cells cultured on unmodified or DGEA-modified gels exhibited a more rounded morphology (Fig. 2D). Furthermore, formation of focal adhesions was clearly observed in cells cultured on RGD-modified gels, as evidenced by punctate staining for phosopho-paxillin (Fig. 2D). Such focal adhesions were largely absent in cells culture on unmodified or DGEA-modified gels.
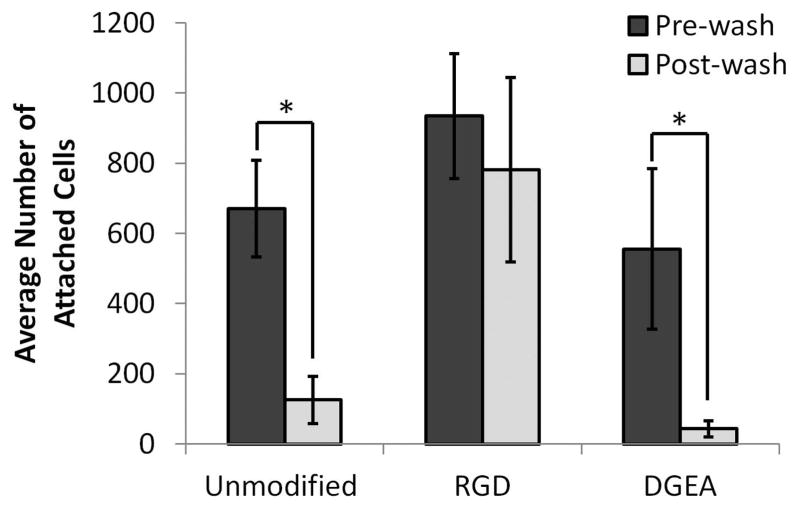
While D1 cells are useful for in vitro mechanistic studies due to their stable MSC phenotype, as an immortalized cell line, D1 cells may not precisely replicate the interactions between native MSCs and DGEA-modified gels. Therefore, the ability of MSCs to adhere in 2D to alginate hydrogels functionalized with DGEA was also assessed using primary rat MSCs (rMSCs). To quantify the number of adherent rMSCs on DGEA modified alginate gels, rMSCs were cultured on unmodified, RGD-modified, or DGEA-modified alginate gels for 7 hours in the absence of serum, and the number of cells present on the gels before and after washing was determined. Consistent with the studies using D1 cells, the seeded rMSCs adhered well to RGD-modified gels, whereas the vast majority of cells were removed from both the unmodified and DGEA-modified gels with washing (Fig. 3). Furthermore, Western blot analysis for phosphorylated ERK (pERK), a protein implicated in integrin-mediated signaling related to cell adhesion and proliferation 27, revealed higher levels of pERK in cells treated with RGD peptide, but not in cells treated with DGEA peptide (Supplemental Fig. S1).
Figure 3. Characterization of Primary Rat MSC Adhesion to RGD and DGEA Peptide Modified Alginates.
Average number of primary rMSCs attached to the surface of unmodified alginate hydrogels, RGD-modified alginate hydrogels, or DGEA-modified alginate hydrogels, before and after washing to remove unbound cells. *p<0.001, Student’s t-test, n = 5. Error bars are ±SD.
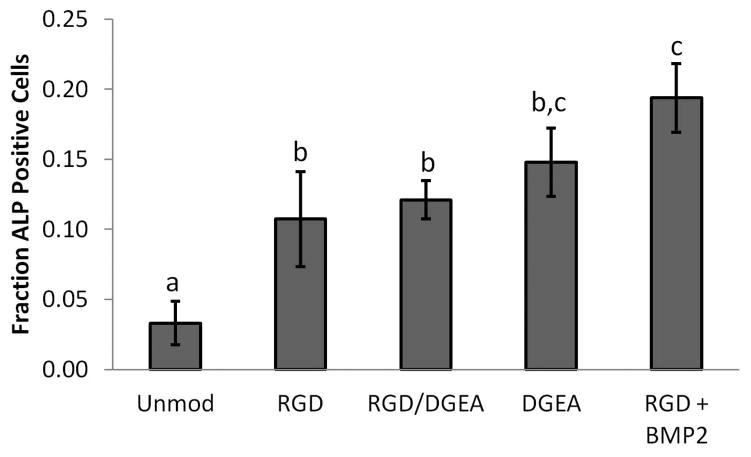
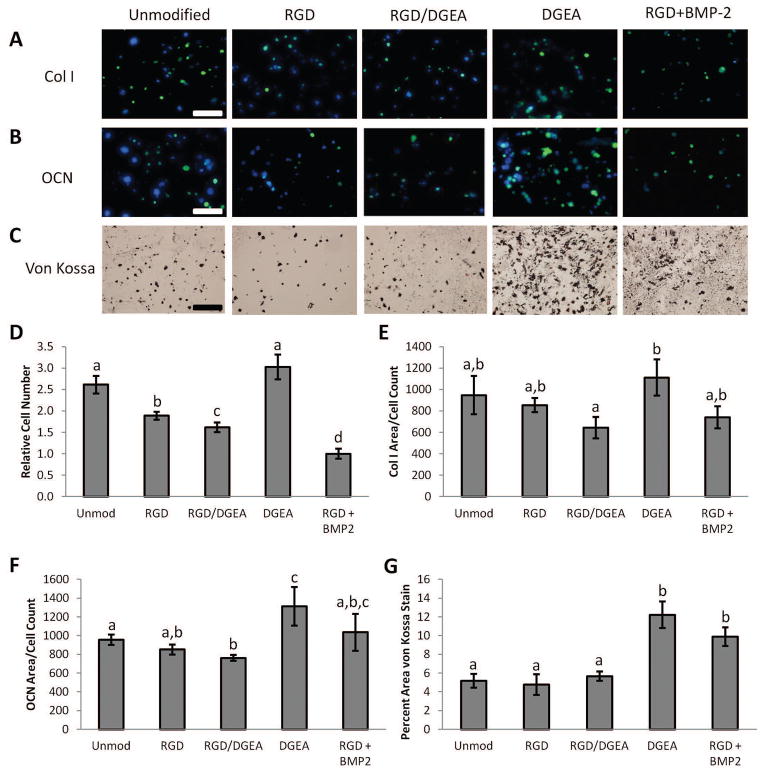
In addition to mediating cell adhesion, integrins play crucial roles in terms of regulating gene expression in cells through a variety of signaling pathways 28. The potential for DGEA-presenting hydrogels to regulate MSC differentiation was investigated by encapsulating rMSCs in alginate hydrogels presenting DGEA, RGD, or both peptides, or in unmodified gels. Gels modified with both peptides were made by mixing DGEA- and RGD-presenting polymers at a 1:1 ratio. Cells cultured in RGD-presenting gels supplemented with BMP-2 were included as a positive control, as such conditions have previously been shown to be conducive for osteogenic differentiation of MSCs 25. The composition of alginate hydrogels used in this study produces gels with elastic moduli of approximately 20 kPa12. Substrate stiffness is known to modulate the interactions between integrins and both RGD12 and DGEA29 ligands, so an elastic modulus of 20 kPa was chosen as alginate hydrogels of this modulus presenting RGD peptides have been previously shown to favor osteogenic differentiation of MSCs12. After 7 days in culture, the fraction of cells positive for alkaline phosphatase (ALP) activity, an early osteogenic marker, was determined by Fast Blue staining. All peptide-presenting conditions exhibited a greater percentage of cells with ALP activity than in unmodified alginate, with the samples supplemented with BMP-2 having the greatest fraction of ALP positive cells (Fig. 4 and Supplemental Fig. S2). After 30 days in culture, more mature markers of osteogenesis were assayed. The relative number of cells in each condition was obtained by quantifying the number of nuclei in sectioned samples and was subsequently used to normalize collagen I and osteocalcin production (Fig. 5D). Production of collagen I and osteocalcin was visualized by immunofluorescence, and mineralization was observed using von Kossa staining. Samples presenting the DGEA peptide exhibited the highest level of staining for collagen I, and significantly higher than samples presenting both RGD and DGEA peptides (Fig. 5A,E). The DGEA-presenting samples had the highest levels of osteocalcin, with significantly more osteocalcin present in these samples than in the unmodified, RGD, and RGD/DGEA samples (Fig. 5B,F). The DGEA-presenting samples also had the greatest amount of mineralization, with a value comparable to the mineralization in samples treated with BMP-2 (Fig. 5C,G).
Figure 4. Alkaline Phospatase Activity in rMSCs Cultured in Peptide-Modified Alginate Hydrogels.
Fraction of rMSCs positive for alkaline phosphatase activity (Fast Blue) after 7 days of culture in alginate hydrogels that were either unmodified or modified with RGD and/or DGEA peptides. Samples treated with recombinant BMP-2 were included as a positive control. Conditions with the same letter are not statistically different from each other, p<0.05, Student’s t-test, n = 4–5 independent samples. Error bars are ±SEM.
Figure 5. Osteogenic Differentiation of rMSCs Cultured in Peptide-Modified Alginate Hydrogels.
Representative images of rMSCs cultured for 30 days in alginate hydrogels that were either unmodified or modified with RGD and/or DGEA peptides. Recombinant BMP-2, the present clinical standard for inducing bone formation, was included as an additional condition. Cells were stained for (A) collagen I deposition (green), (B) osteocalcin production (green), and (C) mineral deposition (von Kossa). Hoechst 33258 was included as a nuclear counterstain in (B) and (C). Scale bars: 100 μm. (D). Relative number of cells in hydrogels after 30 days in culture as determined by counting Hoechst-stained nuclei. (E). Area of images positive for Col I per cell count. (F). Area of images positive for OCN per cell count. (G). Percent area of images positive for mineralization as indicated by von Kossa staining. In (D–G), conditions with the same letter are not statistically different from each other, p<0.05, Student’s t-test, n = 4–8 independent samples. Error bars are ±SEM.
Discussion
Specific integrin engagement by ECM-mimicking peptides is an attractive strategy for directing the differentiation of mesenchymal stem cells. Collagen I, a major component of bone, has previously been shown to enhance osteogenesis in MSCs 17. Furthermore, previous reports have demonstrated that functionalizing biomaterial scaffolds with another collagen-derived ligand, GFOGER, enhances bone defect healing and implant osseointegration in vivo22–24. The DGEA peptide, derived from the α2β1 integrin-binding domain of collagen I, is therefore a potential target ligand to stimulate osteogenesis. The DGEA peptide is known to specifically bind the α2β1 integrin pair, and it has been investigated as a targeted imaging probe for cancers known to overexpress α2β1 integrins30. Because of this peptide’s integrin binding ability, we anticipated that it could serve as an adhesion ligand for MSCs. However, both clonally derived mouse MSCs and primary rat MSCs were unable to adhere to the surface of alginate gels presenting the DGEA peptide, whereas alginate gels presenting the same density of RGD peptides did facilitate adhesion. Therefore, the DGEA peptide does not appear to serve as an adhesion ligand for MSCs at the 2D density presented in this study. These results are consistent with previously published studies demonstrating limited adhesion of rat calvarial osteoblasts and MC3T3-E1 osteoblasts on hydrogel surfaces presenting DGEA peptide31,32. Previous studies reporting cell adhesion mediated by the DGEA peptide utilized peptide adsorbed on hydroxyapatite in the presence of FBS 33,34. Serum adsorption onto the peptide-coated surfaces could have resulted in non-specific adhesion in this earlier work. Other studies demonstrating enhanced osteogenesis using the DGEA ligand have presented the ligand from peptide amphiphile nanofibrous networks35,36. Ligand presentation from these networks occurs at a much higher local density than from hydrogel surfaces, as the DGEA epitopes are localized to the surface of the nanofibers as opposed to homogenously distributed throughout the gel. This increased local ligand density may enhance ligand clustering and promote adhesion in these peptide amphiphile materials. Previous studies have also shown that naïve MSCs express fibronectin-binding integrins at greater levels than collagen-binding integrins, but upregulate collagen-binding integrins during differentiation 37, providing a possible mechanism for the inability of the DGEA peptide to serve as an adhesion ligand for naïve MSCs.
While naïve MSCs may not present sufficient α2β1 integrins to cluster and form stable focal adhesions at the 2D ligand density investigated in this study, integrin engagement by DGEA may still mediate a signaling response that results in changes in gene expression and differentiation 28. Encapsulating the MSCs within alginate hydrogels for the differentiation assays physically entraps the cells in the material, preventing weakly adherent cells from being removed by media changes. This confinement within the hydrogel thus allows for increased opportunities for the α2β1 integrin pairs that are expressed on the cell surface to engage the DGEA ligands, while also providing the cells more time to potentially upregulate α2β1 integrin expression. Previous work has shown that the clustering of RGD ligands in hydrogels in 3D can alter the expression of integrins in mouse MSCs 38, and a similar response may lead to an upregulation of collagen-binding integrins in response to DGEA. At the ligand concentration investigated in this study, the total number of accessible ligands available to a spread MSC on a hydrogel surface and to an MSC encapsulated within a hydrogel is similar, on the order of 106 ligands per cell (see Supplemental Information for calculations). This estimate assumes that a cell can interact with a ligand in the hydrogel network within the length of the extracellular domain of an integrin (approximately 18 nm)39. Presenting the MSCs with ligands from a 3D matrix is significantly different from presenting the same ligands from a 2D surface. The results obtained from the 2D adhesion experiments do not necessarily indicate how the MSCs will interact with the ligands in 3D, and in the current study, the limited adhesion observed on DGEA-modified surfaces in 2D was a poor predictor for the ability of the DGEA ligand to induce an osteogenic response in 3D.
Presenting MSCs encapsulated in hydrogels with DGEA ligands alone resulted in significantly increased osteocalcin production and mineral deposition. Therefore, while the DGEA peptide did not mediate cell adhesion in 2D at the density investigated in this study, presentation of the DGEA ligand in hydrogels in 3D elicited a more osteogenic phenotype from encapsulated MSCs. Hydrogels presenting both RGD and DGEA peptides simultaneously were included to assess whether RGD-mediated adhesion would have a synergistic effect on osteogenic induction in response to the DGEA peptide. Interestingly, the presentation of both ligands in the same gel did not exhibit an additive effect for either cell adhesion nor expression of osteogenic markers. Rather, expression of mature osteogenic markers in these gels is comparable to that in the unmodified alginate and RGD conditions (Fig. 5). A possible explanation for these results is that by adhering to the RGD peptides through the higher expressed RGD-binding integrins, the initially weak association with the DGEA ligands did not result in upregulation of the collagen-binding integrins. In this scenario, the interaction with the RGD ligands present may dominate, and the MSCs would behave as if they were only presented with RGD. The RGD ligand was originally derived from the ECM protein fibronectin, and previous studies have demonstrated enhanced MSC proliferation and maintenance of differentiation capacity when cultured on naturally derived ECMs containing fibronectin 13,40. These previous studies suggest that fibronectin plays a role in maintaining an undifferentiated MSC phenotype, providing a possible explanation for the lower expression of mature osteogenic markers observed in hydrogels presenting the RGD ligand compared to the DGEA ligand. Future studies should investigate the binding interactions between MSC integrins and the RGD and DGEA ligands in hydrogels in 3D to test whether the cells preferentially bind one ligand over the other, and if this preference changes over the duration of the experiment. Future work should also address the mechanism by which the DGEA ligand enhances osteogenic phenotype in MSCs. Western blot analysis suggests that while the RGD peptide leads to ERK activation, the DGEA peptide may elicit an ERK-independent signaling response. One signaling pathway that should be considered is the Jnk pathway, which has been implicated in inducing differentiation in response to integrin binding 27.
The ability to utilize specific interactions between cells and extracellular matrix ligands to control cell fate has significant implications for the fields of tissue engineering and regenerative medicine. Maintaining control over cell phenotype post-transplantation remains a major challenge in the clinical translation of stem cell therapies. Present strategies that utilize soluble factors can result in off target effects and serious complications for patients as a result of these factors diffusing out of the implant site 1. By employing specific cues derived from the natural extracellular matrix, the signals provided to direct stem cell differentiation remain localized to the material in which the cells are delivered, potentially eliminating the off target effects associated with growth factor therapies. In particular, off-label use of BMP-2, which is FDA-approved for use in lumbar spinal fusion procedures, has been shown to cause ectopic bone formation, leading to airway compromise and neurological impairment 10,41,42. In the present study, presenting MSCs with DGEA peptide in hydrogels in 3D significantly enhanced the osteogenic phenotype of these cells. Therefore, future studies should investigate if DGEA presenting hydrogels can induce osteogenesis in transplanted MSCs in vivo, as a potential alternative to co-delivery of soluble growth factors.
Conclusion
The use of hydrogels presenting ECM-mimicking ligands to direct the differentiation of MSCs holds promise as a means to exert control over cell fate post-transplantation. Alginate hydrogels were prepared that presented the RGD ligand, derived from fibronectin, or the DGEA ligand, derived from collagen I. MSCs adhered to and spread on hydrogel surfaces presenting the RGD ligand, but not on gels presenting the DGEA ligand. However, when MSCs were encapsulated within alginate hydrogels, cells presented with the DGEA ligand exhibited a more osteogenic phenotype than cells presented with the RGD ligand, as evidenced by increased production of osteocalcin and increased mineral deposition. These results suggest that ECM ligand presentation may allow for localized control of stem cell differentiation.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Praveen Arany for assistance with Western blotting. This project was supported by funding from the NIH (R01 DE013033).
References
- 1.Mehta M, Schmidt-Bleek K, Duda GN, Mooney DJ. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv Drug Delivery Rev. 2012;64(12):1257–1276. doi: 10.1016/j.addr.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeCoster TA, Gehlert RJ, Mikola EA, Pirela-Cruz MA. Management of posttraumatic segmental bone defects. J Am Acad Orthop Surg. 2004;12:28–38. doi: 10.5435/00124635-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Muscolo DL, Ayerza MA, Aponte-Tinao LA. Massive allograft use in orthopedic oncology. Orthop Clin North Am. 2006;37(1):65–74. doi: 10.1016/j.ocl.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Parikh SN. Bone graft substitutes: past, present, future. J Postgrad Med. 2002;48(2):142–148. [PubMed] [Google Scholar]
- 5.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 6.Pountos I, Corscadden D, Emery P, Giannoudis PV. Mesenchymal stem cell tissue engineering: Techniques for isolation, expansion and application. Injury. 2007;38(Supplement 4):S23–S33. doi: 10.1016/s0020-1383(08)70006-8. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 8.Garrison K, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, Song F. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health Technology Assessment. 2007;11(30) doi: 10.3310/hta11300. [DOI] [PubMed] [Google Scholar]
- 9.Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. J Am Med Assoc. 2009;302:58–66. doi: 10.1001/jama.2009.956. [DOI] [PubMed] [Google Scholar]
- 10.Shields LB, Raque GH, Glassman SD, Campbell M, Vitaz T, Harpring J, Shields CB. Adverse effects associated with high dose rhBMP-2 use in anterior cervical spine fusion. Spine (Philadelphia) 2006;31:542–547. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 11.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 12.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindner U, Kramer J, Behrends J, Driller B, Wendler NO, Boehrnsen F, Rohwedel J, Schlenke P. Improved proliferation and differentiation capacity of human mesenchymal stromal cells cultured with basement-membrane extracellular matrix proteins. Cytotherapy. 2010;12(8):992–1005. doi: 10.3109/14653249.2010.510503. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto J, Kariya Y, Miyazaki K. Regulation of proliferation and chondrogenic differentiation of human mesenchymal stem cells by laminin-5 (laminin-332) Stem Cells. 2006;24(11):2346–2354. doi: 10.1634/stemcells.2005-0605. [DOI] [PubMed] [Google Scholar]
- 15.Mruthyunjaya S, Manchanda R, Godbole R, Pujari R, Shiras A, Shastry P. Laminin-1 induces neurite outgrowth in human mesenchymal stem cells in serum/differentiation factors-free conditions through activation of FAK–MEK/ERK signaling pathways. Biochem Biophys Res Commun. 2010;391:43–48. doi: 10.1016/j.bbrc.2009.10.158. [DOI] [PubMed] [Google Scholar]
- 16.Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93(6):1152–1163. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 17.Tsai K-S, Kao S-Y, Wang C-Y, Wang Y-J, Wang J-P, Hung S-C. Type I collagen promotes proliferation and osteogenesis of human mesenchymal stem cells via activation of ERK and Akt pathways. J Biomed Mater Res, Part A. 2010;94A:673–682. doi: 10.1002/jbm.a.32693. [DOI] [PubMed] [Google Scholar]
- 18.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 19.LeBaron RG, Athanasiou KA. Extracellular matrix cell adhesion peptides: functional applications in orthopedic materials. Tissue Eng. 2000;6(2):85–103. doi: 10.1089/107632700320720. [DOI] [PubMed] [Google Scholar]
- 20.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 21.Staatz WD, Fok KF, Zutter MM, Adams SP, Rodriguez BA, Santoro SA. Identification of a tetrapeptide recognition sequence for the alpha 2 beta 1 integrin in collagen. J Biol Chem. 1991;266:7363–7367. [PubMed] [Google Scholar]
- 22.Reyes CD, Petrie TA, Burns KL, Schwartz Z, García AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007;28(21):3228–3235. doi: 10.1016/j.biomaterials.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wojtowicz AM, Shekaran A, Oest ME, Dupont KM, Templeman KL, Hutmacher DW, Guldberg RE, García AJ. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials. 2010;31(9):2574–2582. doi: 10.1016/j.biomaterials.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shekaran A, García JR, Clark AY, Kavanaugh TE, Lin AS, Guldberg RE, García AJ. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials. 2014;35(21):5453–5461. doi: 10.1016/j.biomaterials.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madl CM, Mehta M, Duda GN, Heilshorn SC, Mooney DJ. Presentation of BMP-2 mimicking peptides in 3D hydrogels directs cell fate commitment in osteoblasts and mesenchymal stem cells. Biomacromolecules. 2014;15:445–455. doi: 10.1021/bm401726u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JH, Jang S-J, Koo T-Y, Suh CW, Lee EN, Lee K-M, Lee HS, Baek H-R. Expression, purification and osteogenic bioactivity of recombinant human BMP-2 derived by Escherichia coli. Tissue Eng. Regener. Med. 2011;8(1):8–15. [Google Scholar]
- 27.Legate KR, Wickstrom SA, Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- 28.Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 29.Chia HN, Vigen M, Kasko AM. Effect of substrate stiffness on pulmonary fibroblast activation by TGF-β. Acta Biomater. 2012;8:2602–2611. doi: 10.1016/j.actbio.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Huang CW, Li Z, Cai H, Shahinian T, Conti PS. Novel α(2)β(1) integrin-targeted peptide probes for prostate cancer imaging. Mol Imaging. 2011;10(4):284–294. doi: 10.2310/7290.2010.00044. [DOI] [PubMed] [Google Scholar]
- 31.Harbers GM, Healy KE. The effect of ligand type and density on osteoblast adhesion, proliferation, and matrix mineralization. J Biomed Mater Res, Part A. 2005;75A(4):855–869. doi: 10.1002/jbm.a.30482. [DOI] [PubMed] [Google Scholar]
- 32.Alsberg E, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. Cell-interactive alginate hydrogels for bone tissue engineering. J Dent Res. 2001;80(11):2025–2029. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 33.Hennessy KM, Pollot BE, Clem WC, Phipps MC, Sawyer AA, Culpepper BK, Bellis SL. The effect of collagen I mimetic peptides on mesenchymal stem cell adhesion and differentiation, and on bone formation at hydroxyapatite surfaces. Biomaterials. 2009;30:1898–1909. doi: 10.1016/j.biomaterials.2008.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Culpepper BK, Phipps MC, Bonvallet PP, Bellis SL. Enhancement of peptide coupling to hydroxyapatite and implant osseointegration through collagen mimetic peptide modified with a polyglutamate domain. Biomaterials. 2010;31:9586–9594. doi: 10.1016/j.biomaterials.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ceylan H, Kocabey S, Gulsuner HU, Balcik OS, Guler MO, Tekinay AB. Bone-like mineral nucleating peptide nanofibers induce differentiation of human mesenchymal stem cells into mature osteoblasts. Biomacromolecules. 2014;15(7):2407–2418. doi: 10.1021/bm500248r. [DOI] [PubMed] [Google Scholar]
- 36.Anderson JM, Vines JB, Patterson JL, Chen H, Javed A, Jun H-W. Osteogenic differentiation of human mesenchymal stem cells synergistically enhanced by biomimetic peptide amphiphiles combined with conditioned medium. Acta Biomater. 2011;7(2):675–682. doi: 10.1016/j.actbio.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goessler UR, Bugert P, Bieback K, Stern-Straeter J, Bran G, Hormann K, Riedel F. Integrin expression in stem cells from bone marrow and adipose tissue during chondrogenic differentiation. Int J Mol Med. 2008;21:271–279. [PubMed] [Google Scholar]
- 38.Lam J, Segura T. The modulation of MSC integrin expression by RGD presentation. Biomaterials. 2013;34:3938–3947. doi: 10.1016/j.biomaterials.2013.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong J-P, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin αVβ3. Science. 2001;294(5541):339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen XD, Dusevich V, Feng JQ, Manolagas SC, Jilka RL. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res. 2007;22(12):1943–1956. doi: 10.1359/jbmr.070725. [DOI] [PubMed] [Google Scholar]
- 41.Smucker J, Rhee J, Singh K, Yoon S, Heller J. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine (Philadelphia) 2006;31:2813–2819. doi: 10.1097/01.brs.0000245863.52371.c2. [DOI] [PubMed] [Google Scholar]
- 42.Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2) Spine J. 2008;8:1011–1018. doi: 10.1016/j.spinee.2007.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.