Abstract
Antithrombin III, encoded by SerpinC1, is a major anti-coagulation molecule in vivo and has anti-inflammatory effects. We found that patients with low antithrombin III activities presented a higher risk of developing acute kidney injury after cardiac surgery. To study this further, we generated SerpinC1 heterozygous knockout rats and followed the development of acute kidney injury in a model of modest renal ischemia/reperfusion injury. Renal injury, assessed by serum creatinine and renal tubular injury scores after 24 h of reperfusion, was significantly exacerbated in SerpinC1+/− rats compared to wild-type littermates. Concomitantly, renal oxidative stress, tubular apoptosis, and macrophage infiltration following this injury were significantly aggravated in SerpinC1+/− rats. However, significant thrombosis was not found in the kidneys of any group of rats. Antithrombin III is reported to stimulate the production of prostaglandin I2, a known regulator of renal cortical blood flow, in addition to having anti-inflammatory effects and to protect against renal failure. Prostaglandin F1α, an assayable metabolite of prostaglandin I2, was increased in the kidneys of the wild-type rats at 3 h after reperfusion. The increase of prostaglandin F1α was significantly blunted in SerpinC1+/− rats, which preceded increased tubular injury and oxidative stress. Thus, our study found a novel role of SerpinC1 insufficiency in increasing the severity of renal ischemia/reperfusion injury.
Keywords: acute kidney injury, ischemia/reperfusion, SerpinC1
Acute kidney injury (AKI) is a severe and common clinical syndrome with adverse outcome.1 AKI mortality is alarmingly high, ranging from 24 to 62%.2 Survivors of AKI have higher long-term risk of developing chronic kidney disease.3 It is therefore important to understand the endogenous modulators of AKI susceptibility and severity.
Antithrombin III (ATIII), encoded by the gene SerpinC1, is a serine protease inhibitor in the coagulation cascade. Protease inhibition by ATIII is profoundly accelerated by its interaction with heparin-like substance on the endothelial cell surface.4 In addition, ATIII exhibits powerful anti-inflammatory effects in part by increasing the production of prostaglandin I2 (PGI2).5 Administration of exogenous ATIII was reported to reduce ischemia/reperfusion injury (IRI) of rat liver and kidney.6, 7, 8
However, it is not known whether endogenous ATIII has a significant role in the development of AKI and whether insufficiency of endogenous ATIII could increase the susceptibility to or severity of AKI. In this present study, we identified an association between low ATIII activities and high incidence of AKI in patients undergoing cardiac surgery. We showed that renal IRI was exacerbated in a newly generated rat model of SerpinC1 insufficiency. The exacerbation of renal IRI in SerpinC1+/− rats appeared to be mediated by oxidative stress and inflammatory mechanisms rather than renal thrombosis.
RESULTS
Patients with low activity of ATIII presented a higher risk for developing AKI after cardiac surgery
We examined 258 cases of cardiac surgery occurring between 1 July 2009 and 30 June 2012 at our hospital. Of these 258 cases, 7 had low ATIII activity before surgery. Of these 7 cases, 5 (or 71.4%) developed AKI after cardiac surgery. Of the 251 cases with normal ATIII activity, 32 (or 12.7%) developed AKI after cardiac surgery. The incidence of AKI was significantly higher in patients with low ATIII activity (Fisher's exact test P=0.0008, odds ratio 16.8; Table 1). In the group of patients with low ATIII activity, there were 4 valve replacement surgeries and 3 coronary bypass grafting surgeries with the average age of 56.9 years and a gender ratio (male/female) of 4:3. In the group of patients with normal ATIII activity, there were 172 valve replacement surgeries and 79 coronary bypass grafting surgeries with the average age of 57.6 years and a gender ratio (male/female) of 117:134. All patients undergoing valve replacement and 10 of the 82 patients undergoing coronary bypass grafting (1 in the low ATIII activity group and 9 in the normal ATIII activity group) were placed on cardiopulmonary bypass. There were no significant differences in surgery type, cardiopulmonary bypass use, diabetes incidence, proteinuria, baseline serum creatinine (Scr), peak Scr, operation time, heart failure, bleeding, and perioperative hypotension between the two groups (Table 1). There was no nephrotoxin use except necessary anticoagulatory agents and diuretics. Moreover, we divided these patients into quartiles based on ATIII activities and found that the lowest quartile had a significantly higher AKI incidence (Table 2), consistent with the analysis based on clinically defined low and normal ATIII activities (Table 1).
Table 1. Incidences of AKI following cardiac surgery in patients with low or normal ATIII activities.
| Normal ATIII (n=251) | Low ATIII (n=7) | P | |
|---|---|---|---|
| Gender (male/female) | 117/134 | 4/3 | >0.05 |
| Age (years) | 57.6±8.3 | 56.9±6.0 | >0.05 |
| ATIII activity (%, median, range) | 98, 76–138 | 59, 48–72 | <0.001 |
| AKI, % | 32, 12.7% | 5, 71.4% | 0.0008 |
| Surgery (valve replacement/coronary bypass grafting) | 172/79 | 4/3 | >0.05 |
| Cardiopulmonary bypass (on-pump/off-pump) | 181/70 | 5/2 | >0.05 |
| Proteinuria pre-op (n, %) | 16, 6.4% | 1, 16.7% | >0.05 |
| Diabetes (n, %) | 46, 18.3% | 2, 28.6% | >0.05 |
| Baseline Scr (μmol/l) | 66.1±19.6 | 57.0±20.4 | >0.05 |
| Peak Scr of AKI patients (μmol/l) | 369.2±109.9 | 343.9±169.7 | >0.05 |
| Dialysis in AKI patients (yes/no) | 10/22 | 3/2 | >0.05 |
| Operation time (min) | 205.3±45.0 | 201.9±31.9 | >0.05 |
| Heart failure (n, %) | 31, 12.4% | 1, 14.3% | >0.05 |
| Bleeding >300 ml (n, %) | 13, 5.2% | 0, 0 | >0.05 |
| Low blood pressure (n, %) | 39, 15.5% | 1, 14.3% | >0.05 |
Abbreviations: AKI, acute kidney injury; ATIII, antithrombin III; pre-op, preoperative; Scr, serum creatinine.
Table 2. AKI incidences in cardiac surgery patients divided into quartiles based on ATIII activities.
| Group | N | ATIII activity | On/off-pump cardiopulmonary bypass | AKI incidence | Dialysis cases/AKI |
|---|---|---|---|---|---|
| Quartile 1 | 72 | 48–90% | 50/22 | 18/72, 25%* | 11/18# |
| Quartile 2 | 59 | 91–97% | 42/17 | 6/59, 10.2% | 0/6 |
| Quartile 3 | 63 | 98–105% | 43/20 | 6/63, 9.5% | 1/6 |
| Quartile 4 | 64 | 105–138% | 51/13 | 7/64, 10.9% | 1/7 |
Abbreviations: AKI, acute kidney injury; ATIII, antithrombin III.
*P<0.05, versus group quartiles 2, 3, and 4; #P<0.05, versus group quartiles 2 and 4.
Renal function following IRI was worsened in rats with SerpinC1 insufficiency
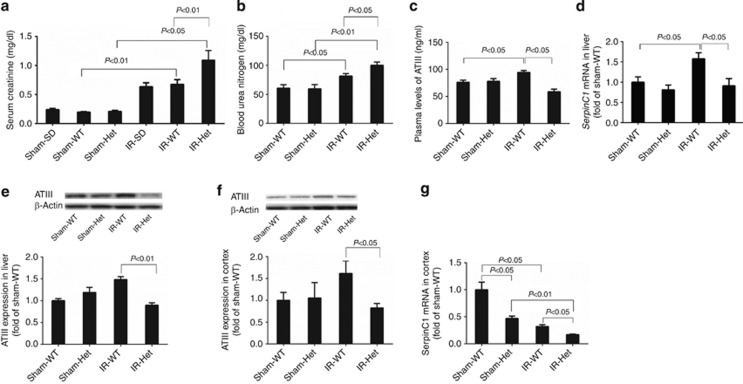
To examine the role of ATIII insufficiency in determining the severity of AKI, we utilized a rat strain with SerpinC1 heterozygous knockout that we just generated. We used a model of modest renal IRI that combined uninephrectomy and 30 min of warm ischemia for the remaining kidney. Following 24 h of reperfusion, Scr was 1.09±0.17 mg/dl in SerpinC1+/− rats that was 63% higher than wild-type littermates (n=6, P<0.01; Figure 1a). Blood urea nitrogen levels were also significantly higher in SerpinC1+/− rats than in wild-type littermates after IRI (Figure 1b). The increase in Scr following IRI was similar between the wild-type littermates and the commonly used, outbred Sprague–Dawley rats (Figure 1a). Scr levels were similar in sham-operated SerpinC1+/− rats, wild-type littermates, and Sprague–Dawley rats (Figure 1a).
Figure 1.
Renal function following ischemia/reperfusion injury (IRI) was worsened in rats with SerpinC1 insufficiency. Rats were subjected to uninephrectomy and 30 min of warm ischemia of the remaining kidney. Blood and tissues were collected 24 h after reperfusion. (a) Serum creatinine. (b) Blood urea nitrogen. (c) Plasma levels of ATIII. (d) SerpinC1 mRNA abundance in liver. (e) ATIII protein abundance in liver. (f) ATIII protein abundance in renal cortex. (g) SerpinC1 mRNA abundance in renal cortex. N=6. ATIII, antithrombin III; Het, SerpinC1+/− rat; IR, ischemia/reperfusion; SD, Sprague–Dawley; Sham, sham operated; WT, wild-type littermate.
Renal IRI led to significant upregulation of ATIII protein abundance in the plasma, liver, and the kidney, as well as ATIII mRNA abundance in the liver in wild-type littermates. This upregulation was abolished in SerpinC1+/− rats (Figure 1c–f). ATIII mRNA in the renal cortex was more abundant in the wild-type rats and downregulated following IRI in both wild-type and SerpinC1+/− rats (Figure 1g). The mutant allele in SerpinC1+/− rats is missing a 29-bp segment in exon 1 that overlaps the start codon of the SerpinC1 coding sequence that should prevent the expression of the native ATIII protein. A second start codon within exon 2 could be used to create a partial protein missing 52 amino acids in the N terminal region of ATIII that would have a predicted molecular weight of ∼50 kDa instead of 55 kDa. The antibody we used recognizes the C terminal of ATIII. However, we did not detect a shorter protein in any of the SerpinC1+/− rats, suggesting that the mutant protein is not expressed or is rapidly degraded. Even if the mutant protein is present, it would completely lack the 32 amino acid signal sequence necessary for secretion and, therefore, would not have the systemic effect of native ATIII.
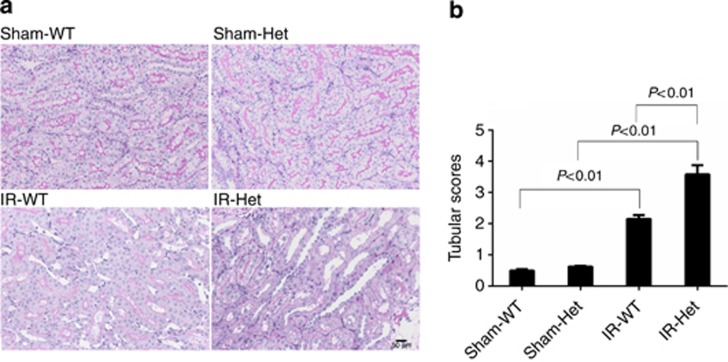
SerpinC1 insufficiency exacerbated renal histological injury in IRI
The pathological findings in SerpinC1+/− rats and wild-type littermates following IRI or sham operation are summarized in Figure 2. Tubular detachment, foamy degeneration, and necrosis were observed in rats of both genotypes following IRI. Tubular injury, however, was significantly more severe in SerpinC1+/− rats than in wild-type littermates (Figure 2).
Figure 2.
SerpinC1 insufficiency exacerbated renal histological injury in ischemia/reperfusion injury (IRI). Rats were subjected to uninephrectomy and 30 min of warm ischemia of the remaining kidney. Kidneys were harvested 24 h after reperfusion. (a) Representative images of periodic acid–Schiff (PAS) staining (× 200). (b) Tubule injury scores. N=6. Het, SerpinC1+/− rat; IR, ischemia/reperfusion; Sham, sham operated; WT, wild-type littermate.
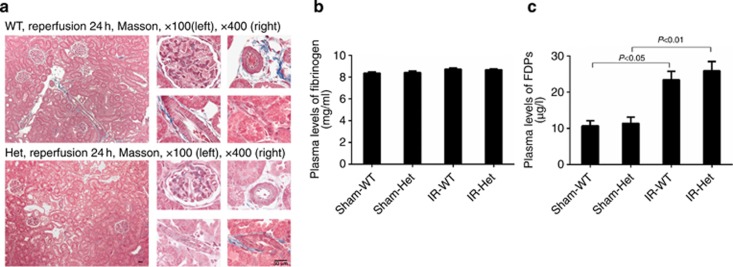
SerpinC1 insufficiency did not result in renal thrombosis
Intuitively, we suspected that SerpinC1 insufficiency might exacerbate renal IRI by causing renal thrombosis. We examined trichrome staining of sections of unflushed kidneys from SerpinC1+/− rats and wild-type littermates and looked for signs of thrombosis in the renal vasculature. No sign of thrombosis was observed in the renal vasculature of either genotype following the IRI (Figure 3a). Plasma levels of fibrinogen and fibrinogen degradation products were not significantly different between wild-type and SerpinC1+/− rats before or after IRI (Figure 3b and c), consistent with the lack of overt thrombosis in SerpinC1+/− rats.
Figure 3.
SerpinC1 insufficiency did not result in renal thrombosis. Rats were subjected to uninephrectomy and 30 min of warm ischemia of the remaining kidney. Unflushed kidneys were harvested 24 h after reperfusion. (a) Several representative images of Masson trichrome staining are shown for a broad region of the kidney (left, × 100) and glomerular capillary, arteriole, and small veins (right, × 400). Red blood cells were observed in the blood vessels, consistent with the kidneys not being flushed before harvesting. Thrombi were not observed. (b) Plasma levels of fibrinogen. (c) Plasma levels of fibrinogen degradation products (FDPs). N=6. Het, SerpinC1+/− rat; IR, ischemia/reperfusion; Sham, sham operated; WT, wild-type littermate.
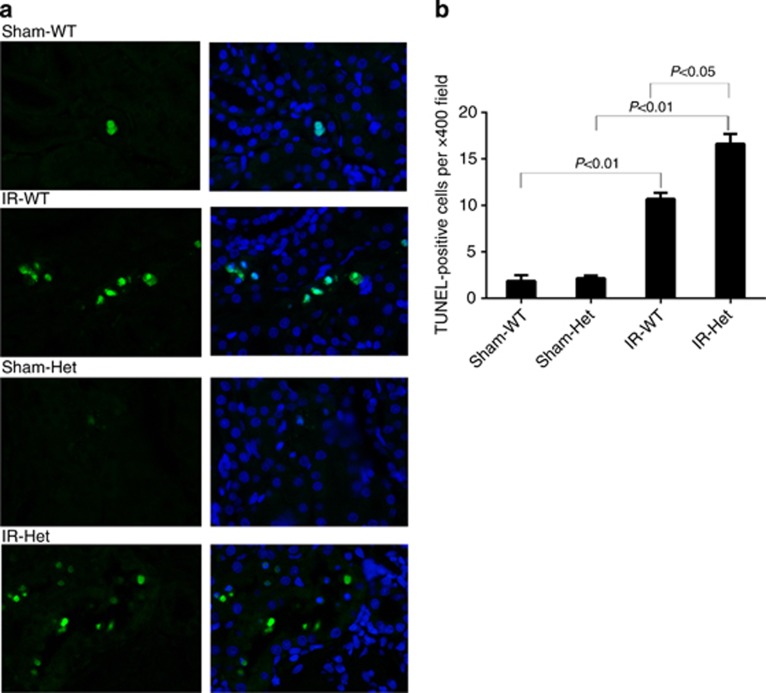
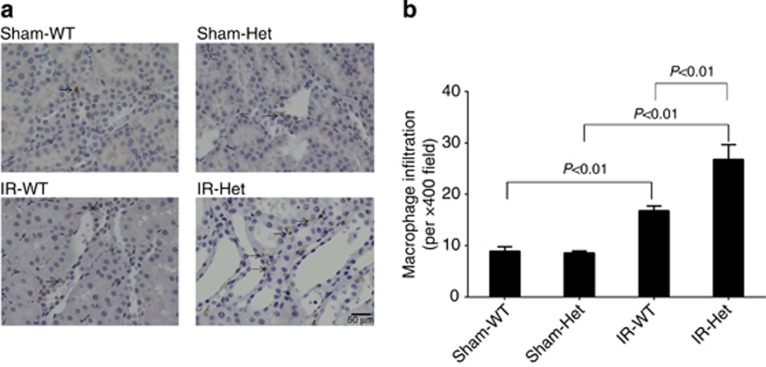
SerpinC1 insufficiency increased renal oxidative stress, tubular apoptosis, and macrophage infiltration in IRI
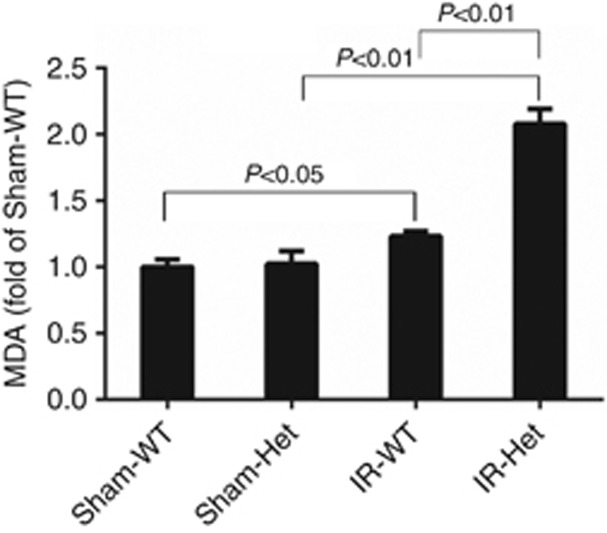
The renal IRI was accompanied by increased renal oxidative stress, tubular apoptosis, and macrophage infiltration in wild-type littermates, assessed by measurements of renal levels of malondialdehyde, TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling)–positive cells, and F4/80-positive cells, respectively (Figures 4, 5, 6). Renal oxidative stress, tubular apoptosis, and macrophage infiltration following IRI were significantly exacerbated in SperinC1+/− rats (Figures 4,5,6).
Figure 4.
SerpinC1 insufficiency increased renal cortical malondialdehyde (MDA) levels in rats with ischemia/reperfusion injury (IRI). Rats were subjected to uninephrectomy and 30 min of warm ischemia of the remaining kidney. Kidneys were harvested 24 h after reperfusion. N=6. Het, SerpinC1+/− rat; IR, ischemia/reperfusion; Sham, sham operated; WT, wild-type littermate.
Figure 5.
SerpinC1 insufficiency increased renal tubular apoptosis in rats with ischemia/reperfusion injury (IRI). Rats were subjected to uninephrectomy and 30 min of warm ischemia of the remaining kidney. Kidneys were harvested 24 h after reperfusion. (a) Representative images of TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) staining (× 400, left) and overlays with 4',6-diamidino-2-phenylindole (DAPI) staining (right). (b) Quantatitive analysis. N=6. Het, SerpinC1+/− rat; IR, ischemia/reperfusion; Sham, sham operated; WT, wild-type littermate.
Figure 6.
SerpinC1 insufficiency increased renal macrophage infiltration in rats with ischemia/reperfusion injury (IRI). Rats were subjected to uninephrectomy and 30 min of warm ischemia of the remaining kidney. Kidneys were harvested 24 h after reperfusion. (a) Representative images (× 200) of immunohistochemistry analysis using an anti-F4/80 antibody. (b) Quantatitive analysis. N=6. Het, SerpinC1+/− rat; IR, ischemia/reperfusion; Sham, sham operated; WT, wild-type littermate.
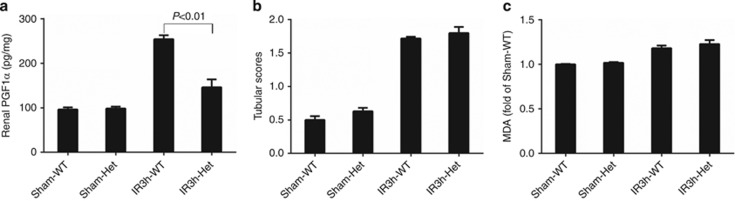
SerpinC1 insufficiency blunted the increase in renal PGI2 at 3 h following ischemia/reperfusion
Renal levels of prostaglandin F1α (PGF1α), a stable metabolite of PGI2, increased in the wild-type group at 3 h after reperfusion. The early increase in PGF1α was significantly blunted in SperinC1+/− rats (Figure 7a). The blunting of the increase in PGF1α in SperinC1+/− rats occurred before any significant exacerbation of tubular injury or oxidative stress. The increases in tubular injury scores and renal levels of malondialdehyde, which were exacerbated in SperinC1+/− rats at 24 h after reperfusion, were similar between SperinC1+/− rats and their wild-type littermates at the 3 h time point (Figure 7b and c).
Figure 7.
SerpinC1 insufficiency blunted the increase in renal prostaglandin (PGI2) following ischemia/reperfusion injury (IRI) before significantly exacerbating tubular injury. Rats were subjected to uninephrectomy and 30 min of warm ischemia of the remaining kidney. Kidneys were harvested 3 h after reperfusion. (a) Renal cortical levels of prostaglandin F1α (PGF1α). (b) Tubule injury score. (c) Renal cortical levels of malondialdehyde (MDA). N=4–5. Het, SerpinC1+/− rat; IR, ischemia/reperfusion; Sham, sham operated; WT, wild-type littermate.
DISCUSSION
This study revealed a novel role of endogenous ATIII levels in modulating the development of AKI and provided mechanistic insights into a new clinical observation. Patients with low levels of ATIII activity appeared to present a higher risk of developing AKI after cardiac surgery. Renal IRI was significantly exacerbated in a newly generated rat gene knockout model of SerpinC1 insufficiency.
The result of this study suggests that it would be clinically valuable to identify patients with low ATIII activities before cardiac surgery or other clinical events that could induce AKI via renal IRI. The study suggests that kidney functions should be monitored more closely, and proactive measures should be taken to prevent or mitigate the development of AKI in these patients.
ATIII, a serine protease inhibitor and glycoprotein, is synthesized in the liver and circulates in the blood.9, 10 ATIII can not only inactivate thrombin and other serine proteases of the coagulation cascade, but also has strong anti-inflammatory effects.11, 12, 13, 14 The mechanisms underlying the anti-inflammatory effects of ATIII include elevation of PGI2, inhibition of nuclear factor (NF)-κB in leukocytes, reduction of leukocyte–endothelial interactions, prevention of microvascular leakage, and inhibition of bacterial growth.12, 13, 14 Infusion of PGI2 has been shown to attenuate renal IRI in previous studies.15, 16 PGI2 is known to regulate renal cortical blood flow in addition to its anti-inflammatory effect and protect against renal failure.17, 18 In this study, SerpinC1+/− rats exhibited more severe oxidative stress, apoptosis, and inflammation than wild-type littermate rats at 24 h after reperfusion. This was preceded by an attenuation of PGI2 increase in the kidney tissues at 3 h after reperfusion. These findings suggest that SerpinC1 insufficiency might increase the severity of AKI in part by preventing compensatory elevation of renal PGI2 shortly after ischemia/reperfusion, leading to worsened renal inflammation and injury as AKI progresses.
It was not practical to generate rats with targeted gene deletion until recently.19 Only a handful of rat strains with targeted deletion of any gene have been reported. The mutant allele of SerpinC1 in this study contained a zinc-finger nuclease–induced deletion of 29 bp that includes the start codon. Interestingly, ATIII protein abundance in the liver and kidney was similar between SerpinC1+/− rats and their wild-type littermates at baseline but was increased only in the wild-type rats after renal IRI. It suggests that one allele of SerpinC1 is sufficient for baseline expression of ATIII in this rat model, whereas the second allele, which is defective in SerpinC1+/− rats, might be needed for the upregulation of ATIII induced by renal IRI. It remains to be determined how renal IRI leads to upregulation of liver ATIII and whether the increase in ATIII in the kidney is due to endogenous expression in the kidney or reflects ATIII levels in residual blood in the harvested kidney. Renal levels of SerpinC1 mRNAs in SerpinC1+/− rats were lower than that in wild-type littermates and were decreased in both strains after renal IRI, suggesting the involvement of post-transcriptional regulatory mechanisms in the renal expression of SerpinC1. The upregulation of ATIII protein following renal IRI could have compensatory effects that limit the severity of AKI, a mechanism that is compromised in SerpinC1+/− rats, leading to greater severity of AKI.
The findings of this study support the importance of several studies that should be performed in the future. First, it would be important to confirm the clinical finding in a larger population of patients with low ATIII activities. The number of subjects with clinically defined low ATIII was small in this study, although the association of low ATIII and high AKI incidence was supported by analysis of patients divided into quartiles based on ATIII levels. ATIII deficiency, presumably hereditary, is found in ∼5% of young patients with venous thrombosis.20 Acquired forms of low ATIII activity can develop as a result of infection or hepatic dysfunction. Of particular interest are patients with modest reductions in ATIII activities that are not sufficient to cause overt thrombosis but could increase the susceptibility to or severity of AKI. Second, although risk stratification can be carried out based on ATIII levels before clinical events that could cause AKI, it remains to be determined what proactive measures could be taken to prevent AKI in patients with low baseline levels of ATIII. ATIII supplementation may not be appropriate for all patients. Third, it would be important to further understand the mechanisms underlying the effect of SerpinC1 insufficiency on AKI. Unlike the patients with low ATIII levels before cardiac surgery, the SerpinC1+/− rats appear to have normal levels of ATIII at baseline but lost the ability to upregulate ATIII following AKI. The relative significance of baseline levels versus compensatory upregulation remains to be examined. The mechanistic links between ATIII, PGI2, inflammation, and renal injury also warrant further investigation.
MATERIALS AND METHODS
ATIII activities and development of AKI in patients undergoing cardiac surgery
Clinical information was reviewed for patients undergoing cardiac surgery from 1 July 2009 to 30 June 2012 in Shanghai Jiao Tong University Affiliated Sixth People's Hospital. Patients with hepatic diseases, endocarditis, and Scr levels of >106 μmol/l before surgery were excluded. ATIII activities in plasma collected before surgery were measured using an automatic coagulation analysis machine (Sysmex CA7000, SIEMENS, Munich, Germany). According to the reference values, ATIII activity at 75–125% of the standard was considered normal, whereas <75% was considered low activity. AKI after cardiac surgery was diagnosed if any one of the following was present: increase in Scr by ≥0.3 mg/dl (≥26.5 μmol/l) within 48 h after surgery; increase in Scr to ≥1.5 times baseline measured within the previous 7 days; or urine volume <0.5 ml/kg per h for 6 h.21 For the diagnosis of heart failure, we adopted American College of Cardiology/American Heart Association (ACC/AHA) 2005 Guideline for the Diagnosis and Management of heart Failure.22 Low blood pressure was defined as blood pressure of <90/60 mm Hg except during the cardiopulmonary bypass period. This survey was approved by the ethics committee of Shanghai Jiao Tong University Affiliated Sixth People's Hospital.
Generation of SerpinC1 knockout rat
SerpinC1 heterozygous knockout rats were established in a congenic model SS.BN-(D13Rat151-D13Rat197)/Mcwi using zinc-finger nucleases targeting exon 1, resulting in a 29 base-pair deletion removing the endogenous translation start codon. The zinc-finger nuclease method for generating gene knockout rats has been described previously.19, 23 Homozygous knockout of SerpinC1 was embryonically lethal. SerpinC1+/− rats and wild-type littermates were produced by breeding SerpinC1+/− rats with SS.BN-(D13Rat151-D13Rat197)/Mcwi rats.
Model of modest renal IRI
Modest renal IRI in rats was induced similar to that described previously.24 Briefly, rats were subjected to right nephrectomy. Left renal ischemia was induced by nontraumatic vascular clamps over the renal artery for 30 min. Reperfusion was established. Rats were killed 3 or 24 h later. The animal protocols were approved by the institutional animal care and use committee of Medical College of Wisconsin.
Biochemical markers of renal function
Commercial kits (BioAssay System, Hayward, CA) were used to measure Scr and blood urea nitrogen.
Plasma levels of ATIII, fibrinogen, and fibrinogen degradation products
Rat antithrombin III and fibrinogen ELISA Kits (GenWay Biotech, San Diego, CA) and rat fibrinogen degradation product ELISA Kit (Cusabio, Wuhan, China) were used to measure plasma levels of ATIII, fibrinogen, and fibrinogen degradation products, respectively, following the vendors' instructions.
Western blot
Western blot was performed similarly to that described previously.25, 26 The primary antibodies used were goat anti-ATIII polyclonal antibody (sc-3253, dilution 1:200; Santa Cruz Biotech) and mouse anti-β-actin monoclonal antibody (A5441, dilution 1:20,000; Sigma-Aldrich). The secondary antibodies were chicken anti-goat IgG-peroxidase antibody (sc-2953, dilution 1:1000; Santa Cruz, Dallas, TX) and sheep anti-mouse IgG-peroxidase antibody (A5906, dilution 1:2000; Sigma-Aldrich, St Louis, MO), respectively. ATIII levels were normalized by β-actin.
Morphological assessments
The formalin-fixed left kidney was embedded in paraffin and cut into 3 μm sections for histological analysis similar to that described previously.27, 28 After hematoxylin–eosin, trichrome, or periodic acid–Schiff staining, the slides were viewed by light microscopy. Renal injury was scored by grading tubular necrosis, loss of brush border, cast formation, and tubular dilatation in 10 randomly chosen, non-overlapping fields. The degree of injury was estimated by the following criteria: 0, none; 1, 0–10% (percentage of area affected); 2, 11–25% 3, 26–45% 4, 46–75% and 5, 76–100%, as described previously.29 In addition, the slides with trichrome staining were examined by light microscopy to evaluate whether there was microthrombosis in the renal vasculature.
Evaluation of oxidative stress
Malondialdehyde levels in renal tissues were determined using a commercial kit (ab118970; Abcam, Cambridge, MA) following the manufacturer's protocol.
Renal apoptosis
TUNEL staining was performed using an in situ cell death detection kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. Apoptotic cells with nuclei staining green fluorescence were counted by fluorescent microscopy. Numbers of TUNEL-positive tubular cells were quantified by counting 10 randomly chosen, non-overlapping fields per slide.
Macrophage infiltration in renal tissues
Immunohistochemistry was performed with an anti-F4/80 antibody (ab74383; Abcam) to identify infiltrated macrophages in renal tissue.
Real-time PCR
Quantitative renal-time PCR was performed as described previously.30, 31 The primers for rat SerpinC1 were as follows: forward: 5′-TTGGGCTGTGCTGTCTGTCA-3′ and reverse: 5′-GGTTCACGGGGATGTCTCG-3′.
PGF1α assay
Renal levels of PGF1α, a stable metabolite of PGI2, were measured using a commercial ELISA kit (Abcam; ab133023) following the vendor's protocol.
Statistical analysis
SPSS (Ver 18.0, Chicago, IL) was used to perform statistical analysis. A one-way analysis of variance with Sidak compensation was used for parametric data and Kruskal–Wallis with Dunn' compensation for nonparametric data. A value of P<0.05 was considered significant.
Acknowledgments
This work was supported by the National Insitutes of Health grants HL082798, HL116264, and HL121233. Part of this work was submitted to ASN 2014 Kidney Week as an abstract.
All the authors declared no competing interests.
References
- 1Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1: 19–32. [DOI] [PubMed] [Google Scholar]
- 2Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 2008; 3: 844–861. [DOI] [PubMed] [Google Scholar]
- 3Wald R, Quinn RR, Luo J et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 2009; 302: 1179–1185. [DOI] [PubMed] [Google Scholar]
- 4Rosenberg RD. Biochemistry of heparin antithrombin interactions, and the physiologic role of this natural anticoagulant mechanism. Am J Med 1989; 87: 2S–9S. [DOI] [PubMed] [Google Scholar]
- 5Horie S, Ishii H, Kazama M. Heparin-like glycosaminoglycan is a receptor for antithrombin III-dependent but not for thrombin-dependent prostacyclin production in human endothelial cells. Thromb Res 1990; 59: 895–904. [DOI] [PubMed] [Google Scholar]
- 6Harada N, Okajima K, Kushimoto S et al. Antithrombin reduces ischemia/reperfusion injury of rat liver by increasing the hepatic level of prostacyclin. Blood 1999; 93: 157–164. [PubMed] [Google Scholar]
- 7Harada N, Okajima K, Uchiba M et al. Antithrombin reduces ischemia/reperfusion-induced liver injury in rats by activation of cyclooxygenase-1. Thromb Haemost 2004; 92: 550–558. [DOI] [PubMed] [Google Scholar]
- 8Ozden A, Sarioglu A, Demirkan NC et al. Antithrombin III reduces renal ischemia-reperfusion injury in rats. Res Exp Med (Berl) 2001; 200: 195–203. [PubMed] [Google Scholar]
- 9Fourrier F. Therapeutic applications of antithrombin concentrates in systemic inflammatory disorders. Blood Coagul Fibrinolysis 1998; 9(Suppl 2): S39–S45. [PubMed] [Google Scholar]
- 10Ostermann H. Antithrombin III in sepsis. New evidences and open questions. Minerva Anestesiol 2002; 68: 445–448. [PubMed] [Google Scholar]
- 11Roemisch J, Gray E, Hoffmann JN et al. Antithrombin: a new look at the actions of a serine protease inhibitor. Blood Coagul Fibrinolysis 2002; 13: 657–670. [DOI] [PubMed] [Google Scholar]
- 12Opal SM. Interactions between coagulation and inflammation. Scand J Infect Dis 2003; 35: 545–554. [DOI] [PubMed] [Google Scholar]
- 13Souter PJ, Thomas S, Hubbard AR et al. Antithrombin inhibits lipopolysaccharide-induced tissue factor and interleukin-6 production by mononuclear cells, human umbilical vein endothelial cells, and whole blood. Crit Care Med 2001; 29: 134–139. [DOI] [PubMed] [Google Scholar]
- 14Dunzendorfer S, Kaneider N, Rabensteiner A et al. Cell-surface heparan sulfate proteoglycan-mediated regulation of human neutrophil migration by the serpin antithrombin III. Blood 2001; 97: 1079–1085. [DOI] [PubMed] [Google Scholar]
- 15Sahsivar MO, Narin C, Kiyici A et al. The effect of iloprost on renal dysfunction after renal I/R using cystatin C and beta2-microglobulin monitoring. Shock 2009; 32: 498–502. [DOI] [PubMed] [Google Scholar]
- 16Canacankatan N, Sucu N, Aytacoglu B et al. Affirmative effects of iloprost on apoptosis during ischemia-reperfusion injury in kidney as a distant organ. Ren Fail 2012; 34: 111–118. [DOI] [PubMed] [Google Scholar]
- 17Lelcuk S, Alexander F, Kobzik L et al. Prostacyclin and thromboxane A2 moderate postischemic renal failure. Surgery 1985; 98: 207–212. [PubMed] [Google Scholar]
- 18Johannes T, Ince C, Klingel K et al. Iloprost preserves renal oxygenation and restores kidney function in endotoxemia-related acute renal failure in the rat. Crit Care Med 2009; 37: 1423–1432. [DOI] [PubMed] [Google Scholar]
- 19Geurts AM, Cost GJ, Freyvert Y et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science 2009; 325: 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Hirsh J, Piovella F, Pini M. Congenital antithrombin III deficiency. Incidence and clinical features. Am J Med 1989; 87: 34S–38S. [DOI] [PubMed] [Google Scholar]
- 21Palevsky PM, Liu KD, Brophy PD et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 2013; 61: 649–672. [DOI] [PubMed] [Google Scholar]
- 22Hunt SA, Abraham WT, Chin MH et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 2005 112: e154–e235. [DOI] [PubMed] [Google Scholar]
- 23Feng D, Yang C, Geurts AM et al. Increased expression of NAD(P)H oxidase subunit p67(phox) in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab 2012; 15: 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Schumer M, Colombel MC, Sawczuk IS et al. Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol 1992; 140: 831–838. [PMC free article] [PubMed] [Google Scholar]
- 25Liang M, Pietrusz JL. Thiol-related genes in diabetic complications: a novel protective role for endogenous thioredoxin 2. Arterioscler Thromb Vasc Biol 2007; 27: 77–83. [DOI] [PubMed] [Google Scholar]
- 26Tian Z, Greene AS, Pietrusz JL et al. MicroRNA-target pairs in the rat kidney identified by microRNA microarray, proteomic, and bioinformatic analysis. Genome Res 2008; 18: 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Mori T, Polichnowski A, Glocka P et al. High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol 2008; 19: 1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Xu X, Kriegel AJ, Liu Y et al. Delayed ischemic preconditioning contributes to renal protection by upregulation of miR-21. Kidney Int 2012; 82: 1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Melnikov VY, Faubel S, Siegmund B et al. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest 2002; 110: 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Liu Y, Taylor NE, Lu L et al. Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension 2010; 55: 974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Mladinov D, Liu Y, Mattson DL et al. MicroRNAs contribute to the maintenance of cell-type-specific physiological characteristics: miR-192 targets Na+/K+-ATPase beta1. Nucleic Acids Res 2013; 41: 1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]