Abstract
Oncolytic measles virus (MV) strains have demonstrated broad spectrum preclinical anti-tumor, including breast cancer. Aurora A kinase controls mitotic spindle formation and plays a critical role in malignant transformation. We hypothesized that, by causing mitotic arrest, the Aurora A kinase inhibitor MLN8237 (alisertib) can increase MV oncolytic effect and efficacy. Alisertib enhanced MV oncolysis in vitro and significantly improved outcome in vivo against breast cancer xenografts. In a disseminated MDA-231-lu-P4 lung metastatic model, the MV/alisertib combination treatment markedly increased median survival to 82.5 days with 20% of the animals being long term survivors vs. 48 days median survival for the control animals. Similarly, in a pleural effusion model of advanced breast cancer, the MV/alisertib combination significantly improved outcome with a 74.5 day median survival versus the single agent groups (57 and 40 days respectively). Increased viral gene expression and IL-24 upregulation were demonstrated, representing possible mechanisms for the observed increase in antitumor effect. Inhibiting Aurora A kinase with alisertib represents a novel approach to enhance measles virus-mediated oncolysis and antitumor effect. Both oncolytic MV strains and alisertib are currently tested in clinical trials, this study therefore provides the basis for translational applications of this combinatorial strategy in the treatment of patients with advanced breast cancer.
Keywords: oncolytic virotherapy, breast cancer, Aurora A inhibitor, alisertib, measles virus, MV-NAP
Introduction
Breast cancer is the most common malignancy in women with more than 230,000 newly diagnosed cases annually in the USA.1 Despite the progress made, metastatic breast cancer is still considered an incurable disease causing almost 40,000 deaths every year. Development of novel drug classes and treatment strategies is critical in order to improve outcome.
Oncolytic virotherapy, using replication competent viruses, is a novel approach in cancer treatment with ongoing phase I/III clinical trials in cancer patients, although breast cancer specific studies have not yet been performed.2 Measles virus (MV) vaccine derivative strains represent promising oncolytic agents with potent anti-tumor efficacy demonstrated in preclinical testing against different human solid tumors, including breast cancer.3–5 MV is a paramyxovirus with negative-stranded RNA genome encoding eight viral proteins.6 MV has a lipoprotein envelope and two surface glycoproteins H and F that mediate attachment and entry into the cells. Attenuated MV strains use CD46, SLAM and nectin-4 as surface receptors in human cells.7–9 MV infection spreads by cell-to-cell fusion causing characteristic cytopathic effect of large syncytia formation and subsequent apoptotic death of infected cells.
In previous studies we have demonstrated that MV has significant oncolytic effect against metastatic human breast cancer xenograft models.3 MV was capable to infect and destroy human breast cancer cells independent of their hormone receptor or HER2-neu status,5 and therefore it is lacking cross-resistance with other therapies commonly employed in breast cancer treatment. Genetically engineered expression of immunomodulatory transgenes, such as the Helicobacter pylori neutrophil-activating protein (NAP), a toll-like receptor 2 agonist,10–12 enhanced MV oncolytic effect and significantly improved median survival in models of lung metastatic and malignant pleural effusion breast cancer models.4 This was in part mediated by NAP-mediated attraction of immune cells and induction of pro-inflammatory cytokine release with anti-proliferative effect on breast cancer cells, such as TNF-α.4 These results encouraged development of combinatorial strategies with drugs that can further augment viral oncolysis.
Aurora A kinase is a member of serine/threonine kinase family with crucial role in cell cycle progression and cytokinesis. Aurora A kinase localizes to centrosomes and its function is regulated by phosphorylation-mediated activation and degradation.13 Aurora A kinase activity controls mitotic spindle formation and G2 to M phase cell cycle transition.14 Aurora A kinase overexpression induces genetic instability, interferes with SMAD5 pathway in promoting tumor invasiveness and distant metastasis formation in estrogen receptor positive (ER+) breast cancer,15 and it is associated with worse outcome in breast cancer patients.16, 17
Small molecule inhibitors of Aurora A kinase have been proposed for treatment of solid tumors and hematological malignancies.18 MLN8237 (alisertib) is an orally administered selective Aurora A kinase inhibitor with potent antiproliferative activity, currently being tested in phase I/phase II clinical trials.19–22 Since alisertib acts as a mitotic inhibitor, we hypothesized that it could augment MV oncolysis and efficacy in the treatment of advanced breast cancer. Of note, alisertib blocks cell cycle progression without direct damaging effect on DNA replication, gene transcription and protein synthesis. Thus, it is not expected that alisertib would negatively interfere with oncolytic virus replication, or increase viral genome mutagenesis.
The experiments presented in this manuscript demonstrate increased therapeutic efficacy of oncolytic MV in combination with the orally administered Aurora A kinase inhibitor alisertib against metastatic breast cancer pleural effusion and lung metastases xenograft models, suggesting that this combinatorial approach should be pursued translationally as a novel strategy for the treatment of breast cancer patients.
Materials and Methods
Cell lines and MV strains
African green monkey Vero cells and human breast cancer MCF-7 and MDA-MB-231 cell lines were purchased from American Type Culture Collection (ATCC). In vivo passaged MDA-MB-231 derivative cells expressing a firefly luciferase (F-lu) reporter expressing MDA231-lu-P3 and P4 cells were isolated and grown as described previously.3 MV encoding green fluorescent protein (MV-GFP) reporter,23 MV expressing human lambda immunoglobulin chain (MV-lambda) reporter gene24 and MVs expressing secretory forms of Helicobacter pylori neutrophil-activating protein transgene MV-s-NAP and MV-lambda-NAP25 are genetically engineered strains deriving from the MV Edmonston vaccine strain platform, and have been constructed as previously described.12 A schematic diagram of these strains is provided in Fig. 1. Propagation of MV strains, evaluation of virus growth kinetics in tumor cells and virus titration on Vero cells were performed as described previously.25 Virus titers were determined in tissue-culture infectious dose 50% (TCID50) according to the Karber’s formula.26
Fig. 1.
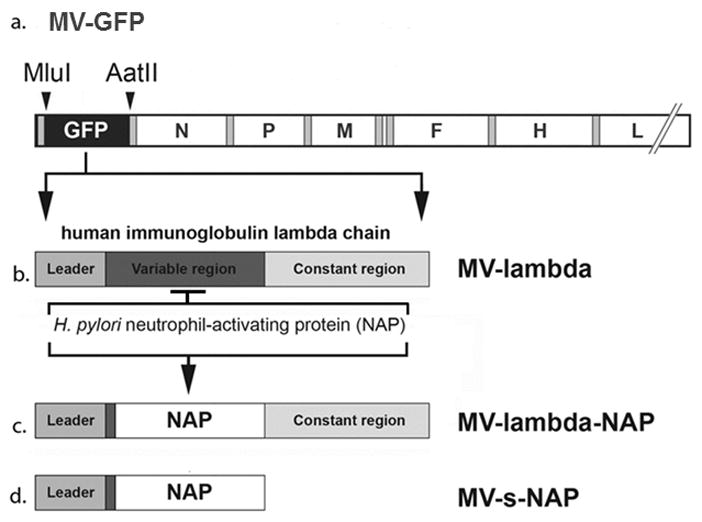
Schematic representation of the recombinant measles virus (MV) strains used in the experiments: MV-GFP, MV-lambda, MV-lambda-NAP and MV-s-NAP. NAP - Helicobacter pylori neutrophil-activating protein.
Cell viability assay
Inhibitory concentration 50% (IC50) of alisertib (Takeda Pharmaceuticals) against breast cancer cells in vitro was determined using MTT cell viability assay (ATCC). Briefly, breast cancer cells (5,000/well) were transferred to 96-well plates (Falcon) and treated with increasing concentrations of alisertib or vehicle control solution. At different time points, plates were incubated with MTT for 4 h and the assay was performed according to the manufacturer’s instructions (ATCC). IC50 was determined using GraphPad Prism 5.0 computer software (GraphPad Software).
For combination treatment, cells were treated with IC50 concentration of alisertib 2 h or 48 h prior to inoculation with MV strains at a multiplicity of infection (MOI) of 0.1 and 1. The anti-tumor effect of combination treatment was compared to those of samples treated with MV or alisertib alone.
Changes in cell morphology upon alisertib treatment and MV induced cytopathic effect in breast cancer cells were evaluated by light and fluorescent microscopy.
Gene microarray and quantitative real-time PCR (QRT-PCR)
MDA-231-P4 breast cancer cells were pre-treated for 48 h with 1 μM of alisertib and subsequently infected with MV-GFP at an MOI=2.0. The total RNA was extracted following 12-h incubation with MV-GFP using RNeasy kit (Qiagen) and gene expression was analyzed using Affymetrix Human U133 Plus 2.0 array (Ilumina). Combined treatment results were compared to those of untreated control cells or cells treated with alisertib or MV alone.
IL-24 gene expression was confirmed by QRT-PCR in MDA231-lu-P4 cells treated as described above. The assay was performed using IL-24-specific primers and One-step RT-PCR master mix reagents kit (Applied Biosystems). Induction of IL-24 expression in the treated cells was calculated as relative to 18S ribosomal RNA levels. Data were analyzed and presented as a fold-change in gene expression vs. control cells.
Immunoblotting for Aurora A kinase expression
Cell lysates from MDA231-lu-P4 or MCF-7 cells were prepared and analyzed by immunoblotting using an Aurora A specific polyclonal antibody (Cell Signaling) as described previously.27 Monoclonal antibody against α-tubulin (Sigma) was used as control to ensure equal protein load.
Expression of lambda chain transgene by MV-lambda infected cells
Breast cancer MDA231-lu-P4 cells were pre-treated with 1 μM of alisertib for 48 h prior to inoculation with MOI=0.1 of MV-lambda in 12-well plates (Nunc). Supernatants were collected and analyzed for lambda chain concentration using human lambda chain specific ELISA (Bethyl Laboratories) as described previously.24 Results were calculated as lambda light chain production by 106 infected cells.
Combination MV/alisertib therapy against metastatic breast cancer xenografts in vivo
Female athymic nude mice (4–5-week-old) were purchased from Harlan (Indianapolis, IN). All animal experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee.
For treatment of lung metastases, 5-week-old female athymic nude mice were engrafted with 106 MDA-231-lu-P4 cells injected i.v. in the tail vein. On day 3 post-engraftment, mice were imaged to ensure lung engraftment using the Xenogen IVIS-200 System (Caliper Life Sciences) as described previously.3 Animals were arranged in groups (n=10–11) with comparable tumor burden as determined by bioluminescence intensity. Alisertib was dissolved in phosphate-buffered saline (PBS) with 20% β-cyclodextrin (Sigma) and mixed 1:1 prior to animal treatment with 2% solution of sodium bicarbonate. Mice received 30 mg/kg daily dose of alisertib in 0.2 ml final volume administered by oral gavage starting on day 5 post-engraftment – five doses per week for three weeks (15 doses total). MV-lambda-NAP was resuspended in 0.1 ml PBS (2×106 TCID50 per injection) and injected i.v. 48 h after the start of the alisertib therapy. MV injection was repeated twice per week during the course of alisertib treatment – six injections of 2×106 TICD50 for a total does of 1.2×107 TCID50 of MV-lambda-NAP. Survival of the treated groups was compared to the control animals treated with the alisertib vehicle and heat-inactivated (incubated at 60°C for 1h).
In the pleural effusion model,4 2×106 MDA-231-lu-P3 cells resuspended in 0.01 ml PBS were implanted by transthoracic (t.t.) injection into the left pleural cavity of 5-week-old athymic mice. On day 7, engraftment of the breast cancer tumor deposits in the pleural space was confirmed by bioluminescence imaging (Xenogen IVIS-200 System) and animals were randomly assigned into groups (n=9–10). Starting on day 7, mice were treated with 30 mg/kg daily dose of alisertib delivered by oral gavage for three weeks as described for the lung metastatic model. Control groups received vehicle solution orally. Mice were treated once per week on day 7, 13 and 19 with 5×105 TCID50 of MV-s-NAP strain in 0.05 ml by t.t. injection. Animals were monitored daily and were euthanized when breathing difficulties and/or 20% weight loss developed.
Statistical analysis
Data statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad Software, San Diego CA). In vivo results were plotted in Kaplan-Meyer curves and group survival was compared by the log-rank test. Prolongation of the median survival was considered statistically significant if p<0.05 between the analyzed groups.
Results
The effect of alisertib treatment on breast cancer cell lines in vitro
Alisertib resulted in a strong inhibitory effect on breast cancer cell proliferation at 5–7 day post-treatment. The drug-induced cell proliferation arrest was associated with accumulation of giant cells with >2–4-fold larger diameter, which became prominent following 48–72 h of incubation (Fig. 2A). Since alisertib inhibits centrosome formation and cell division, these morphologic changes correspond to cells with enlarged mass that cannot successfully complete mitotic division. As expected, the impact of Aurora A kinase inhibition was concentration dependent for both MCF-7 and MDA231-lu-P4 cell lines. IC50 of alisertib for MCF-7 was calculated between 63.3 and 74.8 nM, while the IC50 for the MDA231-lu-P4 line was 220 nM.
Fig. 2.
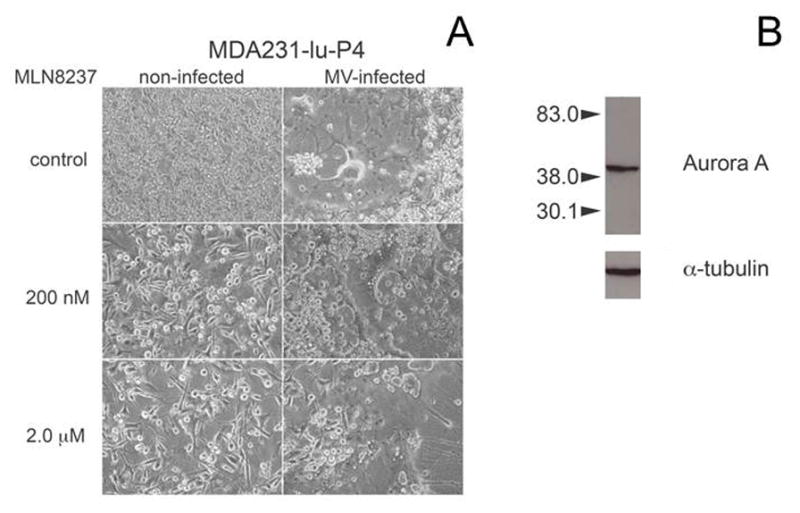
Alisertib effect on breast cancer cell morphology at 72 h post-treatment. MDA231-lu-P4 cells were treated with 200 or 2 μM of alisertib (A), and either infected with MV-GFP at MOI=0.1 or left uninfected. Immunoblotting of Aurora A kinase expression in MDA231-lu-P4 cells (B) demonstrated abundant target expression. Tubulin-specific antibody was used as control.
MLN increased MV oncolytic effect against breast cancer cells
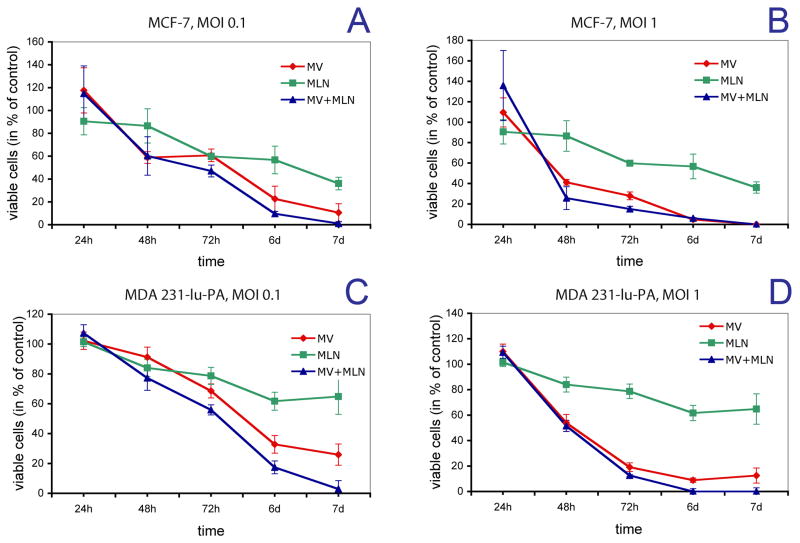
Pretreatment of breast cancer cells in the presence of alisertib at the IC50 concentration, 48 h before infection with MV, improved the in vitro anti-tumor effect of virotherapy. In MCF-7 cells, MV-s-NAP infection at a high MOI (1), completely destroyed the cell monolayers by day 7 and thus the alisertib effect could not be easily detected. In contrast, at a lower MOI (0.1) MV-s-NAP infection reduced MCF-7 cell viability, but did not eradicate the MCF-7 cells (Fig. 3A,B). In this setting, alisertib pre-treatment increased MV-mediated tumor killing resulting in complete eradication of breast cancer cell monolayers. A similar impact of alisertib on the MV anti-tumor effect was observed in MDA231-lu-P4 cells. Pre-treatment with 200 nM alisertib significantly enhanced the anti-tumor effect following infection of MV-s-NAP at both high and low MOIs. At an MOI=1, MV-s-NAP infection at the presence of Aurora A kinase inhibitor resulted in complete elimination of MDA231-lu-P4 cells by day 7, while virus infection alone was able to eliminate approximately 90% of the tumor cells (Fig. 3C,D). Alisertib significantly boosted the MV oncolytic effect at an MOI=0.1, resulting in more 97% cell death vs. 74% for MV alone.
Fig. 3.
Anti-tumor effect of alisertib/MV-s-NAP combination treatment against breast cancer cells. MCF-7 cells were treated with 70 nM alisertib (corresponding to the IC50) and infected with MV-s-NAP at MOI of 0.1 (A) or 1 (B). The cytotoxic effect of alisertib/MV-s-NAP combination on MDA231-lu-P4 cells was evaluated following treatment with 200 nM alisertib (corresponding to the IC50 for this cell line) and infected with MV-s-NAP at MOI of 0.1 (C) or 1 (D). Cell viability at different time points was measured by MTT assay. MLN = alisertib
Combination therapy with alisertib significantly increased MV transgene expression. Alisertib-pretreatment resulted in more than 4-fold increase (p<0.001) of lambda chain (transgene) release per 106 infected cells (Fig. 4A), indicating increased viral proliferation.
Fig. 4.
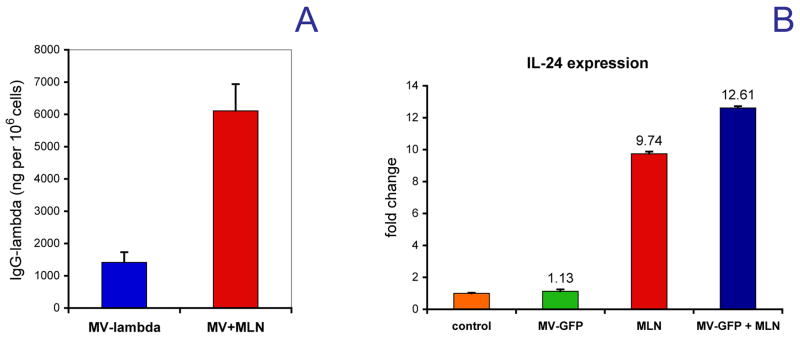
The expression of lambda-immunoglobulin chain reporter in the cell culture supernatants by alisertib-treated and MV-lambda infected MDA231-lu-P4 cells as compared to MV-lambda infection alone (A). Each sample was run in four wells of the 12-well tissue culture plate. Results are presented as human lambda immunoglobulin in ng per 106 infected cells. The IL-24 gene expression was quantified in MDA231-lu-P4 cells pretreated for 48 h with alisertib and and subsequently infected with MV-GFP for 12 h (B). Results are presented as a fold-change in expression vs. control cells ± SD.
Gene microarray data in treated MDA231-lu-PA cells showed that alisertib treatment alone triggered >4-fold overexpression of more than 35 genes, including cytokines, such as interleukin-1α (IL-1α), IL-11 and IL-24. MV infection, however, did not significantly alter the expression profile of alisertib-treated cells (data not shown). One of the alisertib-induced genes, IL-24 is known to exert potential synergistic effect with oncolytic viruses against breast cancer cells.28 MLN-8237-stimulated IL-24 gene transcription in MDA231-lu-P4 cells, which was further confirmed by QRT-PCR (Fig. 4B). Alisertib alone or in combination with MV triggered significant IL-24 expression with a 9.7-fold increase in expression for alisertib treatment alone versus 12.6-fold increase for the alisertib/MV combination following a 24-h incubation. In contrast, MV infection alone had no effect on IL-24 gene expression as compared to the control cells.
Alisertib enhanced MV anti-tumor effect against metastatic breast cancer animal models
Both MV-lambda-NAP and alisertib therapy as single agents significantly (p<0.05) improved survival of mice with disseminated MDA231-lu-P4 metastases to the lungs (Fig. 5). Nevertheless, median survival was only modestly prolonged by 15–20% following single agent treatment: 48 days for the control versus 56 days for the MV-lambda-NAP treated group and 57 days for the alisertib plus inactivated MV treated group respectively. In contrast, the combined treatment approach further improved survival to 82.5 days for mice treated with the alisertib/MV-lambda-NAP combination as compared to the control group (p<0.001). In addition, 20% of the mice in this highly aggressive xenograft model were cured following combination treatment.
Fig. 5.
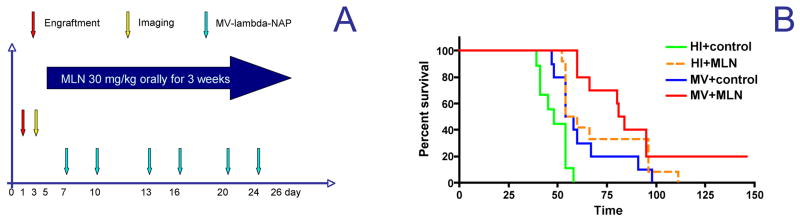
Combination treatment schedule (A) of MDA231-lu-P4 lung metastatic xenografts in athymic nude mice (n=9–10). Data were plotted in Kaplan-Meyer curves and survival outcomes were compared by the log-rank test (B). HI = heat inactivated virus, MLN = alisertib, control=alisertib vehicle control given orally.
In a pleural effusion MDA231-lu-P3 model of metastatic breast cancer, 3 repeat transthoracic injections of 5×105 TCID50 of MV-s-NAP had significant therapeutic efficacy with a more than twice improvement in median survival; 57 days vs. 27.5 days for the control group (p<0.001, Fig. 6). Alisertib treatment alone also prolonged median survival by 12.5 days as compared to control mice (p=0.001). Combination of MV with alisertib further and significantly increased the observed MV anti-tumor effect with a 74.5 day median survival as compared to 57 days with MV-s-NAP single agent therapy (p=0.019) or 40 days with MLN therapy (p=0.005).
Fig. 6.
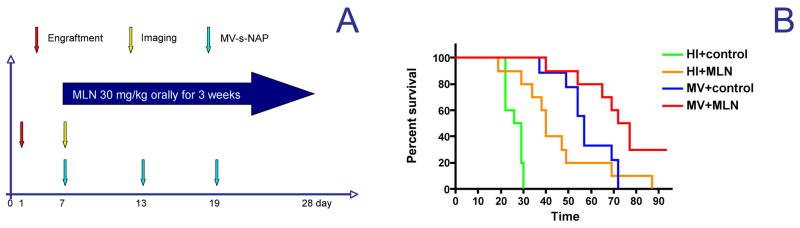
Combination treatment schedule (A) in MDA231-lu-P3 pleural effusion model of metastatic breast cancer in mice (n=10). Data were plotted in Kaplan-Meyer curves and survival outcomes were compared by the log-rank test (B). HI – heat inactivated virus, MLN = alisertib, control=alisertib vehicle control given orally.
These in vivo results further support that the Aurora A inhibitor alisertib not only does not inhibit MV replication in tumor cells, but it can significantly augment the therapeutic efficacy of oncolytic MV-strains in the systemic treatment of advanced breast cancer.
Discussion
Development of combinatorial therapeutic strategies with oncolytic viruses has a higher likelihood of success if agents that target cellular pathways, not interfering with viral replication are employed. We hypothesized that selective molecular targeting of Aurora A kinase using a small molecule inhibitor represents a promising strategy in order to boost oncolytic virus activity, particularly for viruses infecting and replicating in metabolically active and dividing cells. Deregulation of centrosome formation and blockade of mitotic cycle by Aurora A kinase inhibitors would be expected to facilitate viral replication without having an adverse impact on the cellular nucleic acid and protein synthesis machinery, required for replication of intracellular parasites such as viruses.29
Alisertib is an Aurora A kinase inhibitor, results from pharmacokinetics/pharmacodynamics and dose escalation studies in cancer patients have demonstrated excellent safety.30, 31 In preclinical studies, alisertib enhanced the anti-tumor effect of other small molecule cell cycle inhibitors such as histone-deacetylase inhibitors, mTOR inhibitors or chemotherapy agents such as cisplatin, when administered in combination against solid tumors, including breast cancer.32–34 Clinical testing in breast cancer as a single agent and in combinatorial strategies with chemotherapy agents such as paclitaxel and hormonal agents such as Fulvestrant is ongoing. In this study, we compared the combination of alisertib plus oncolytic MV treatment to single agent treatment against aggressive breast cancer xenograft models in athymic mice. In both malignant pleural effusion and lung metastatic models, combination therapy was significantly superior as compared to single agent treatment. The dose of 30 mg/kg for alisertib that we employed in our studies is clinically relevant to the dose employed in humans,35 and significantly enhanced the MV therapeutic effect even in Aurora A kinase overexpressing lines such as MDA-231-lu-P3-P4 derivative cells.
Alisertib at the IC50 concentration boosted MV-mediated cytolysis of breast cancer lines in vitro. Aurora A inhibition led to mitotic arrest and appearance of very large cells that accumulated the cytoplasm of undivided parental cells within 48 h. These cells were viable for 4–5 more days, which represented adequate time for MV to accomplish several replication cycles. Targeting the alisertib-enlarged cells and utilizing their cell machinery for virus replication and virus protein production by oncolytic MV could lead in increased viral gene expression, which could explain the enhancement of the combinatorial therapeutic effect. This hypothesis is supported by the observed >4 fold increase in the lambda chain (transgene) protein production in MV-infected, alisertib-treated cells. In addition, expression of the MV-encoded immunomodulatory transgene could further explain the significantly improved therapeutic outcome in MV-s-NAP or MV-lambda-NAP plus alisertib combination treatment in vivo against metastatic breast cancer in both xenograft models. Previous reports have demonstrated the enhanced therapeutic effect of oncolytic viruses when combined with drugs that disrupt the mitotic cycle or affect the microtubular rearrangement, including Aurora B kinase inhibitors.36–38
Gene microarray analysis revealed overexpression of several cytokines by alisertib-treated MDA231-lu-P4, among them IL-24. IL-24 has been shown to have a tumor suppressive effect on breast cancer by inhibition of angiogenesis and reduction of metastatic spread by the lymphatic route.39 IL-24 is a strong pro-apoptotic inducer causing G2/M cell cycle arrest in breast cancer cells.40 Of note, adenovirus vectors armed with the IL-24 transgene have demonstrated potent oncolytic effect against breast cancer cells, including MDA-MB-231 line.28, 41 Alisertib-triggered IL-24 secretion by MDA231-lu-P4 cells represents one additional mechanistic explanation for the enhanced treatment efficacy of the MV/alisertib combination, but further studies are required to prove this hypothesis.
In conclusion, co-treatment with Aurora A kinase inhibitors significantly enhances MV-mediated antitumor effect on breast cancer lines and xenografts. Based on these preclinical data and early clinical trial data indicating clinical benefit from engineered MV strains in other tumor types such as ovarian cancer42 or multiple myeloma,43 results from this study support a novel translational strategy in the treatment of patients with advanced breast cancer.
Supplementary Material
Acknowledgments
We wish to thank Dr. Jeffery A. Ecsedy from Oncology Translational Medicine, Takeda Pharmaceuticals International Co., Cambridge, Massachusetts for the helpful suggestions about MLN-8237 drug formulation and dosing. This work was supported by Atwater Foundation Grant and NIH grants R01CA 136547, R01CA 154348 and P50CA 116201.
Footnotes
Conflict of Interest
The authors report no conflict of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Bell J, McFadden G. Viruses for tumor therapy. Cell Host Microbe. 2014;15(3):260–5. doi: 10.1016/j.chom.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iankov ID, Msaouel P, Allen C, Federspiel MJ, Bulur PA, Dietz AB, et al. Demonstration of anti-tumor activity of oncolytic measles virus strains in a malignant pleural effusion breast cancer model. Breast Cancer Res Treat. 2010;122(3):745–54. doi: 10.1007/s10549-009-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iankov ID, Allen C, Federspiel MJ, Myers RM, Peng KW, Ingle JN, et al. Expression of immunomodulatory neutrophil-activating protein of Helicobacter pylori enhances the antitumor activity of oncolytic measles virus. Mol Ther. 2012;20 (6):1139–47. doi: 10.1038/mt.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald CJ, Erlichman C, Ingle JN, Rosales GA, Allen C, Greiner SM, et al. A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer. Breast Cancer Res Treat. 2006;99(2):177–84. doi: 10.1007/s10549-006-9200-5. [DOI] [PubMed] [Google Scholar]
- 6.Griffin D. Measles virus. In: Knipe DM, Howley PM, editors. Fields Virology. 4. Lippincott, Williams & Wilkins; Philadelphia, PA: 2001. pp. 1401–1441. [Google Scholar]
- 7.Bjorge L, Hakulinen J, Wahlstrom T, Matre R, Meri S. Complement-regulatory proteins in ovarian malignancies. Int J Cancer. 1997;70(1):14–25. doi: 10.1002/(sici)1097-0215(19970106)70:1<14::aid-ijc3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Muhlebach MD, Mateo M, Sinn PL, Prufer S, Uhlig KM, Leonard VH, et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature. 2011;480(7378):530–3. doi: 10.1038/nature10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406(6798):893–7. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 10.Amedei A, Cappon A, Codolo G, Cabrelle A, Polenghi A, Benagiano M, et al. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J Clin Invest. 2006;116(4):1092–101. doi: 10.1172/JCI27177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans DJ, Jr, Evans DG, Takemura T, Nakano H, Lampert HC, Graham DY, et al. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect Immun. 1995;63(6):2213–20. doi: 10.1128/iai.63.6.2213-2220.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramachandran M, Jin C, Yu D, Eriksson F, Essand M. Vector-encoded Helicobacter pylori neutrophil-activating protein promotes maturation of dendritic cells with Th1 polarization and improved migration. J Immunol. 2014;193(5):2287–96. doi: 10.4049/jimmunol.1400339. [DOI] [PubMed] [Google Scholar]
- 13.Nikonova AS, Astsaturov I, Serebriiskii IG, Dunbrack RL, Jr, Golemis EA. Aurora A kinase (AURKA) in normal and pathological cell division. Cell Mol Life Sci. 2013;70(4):661–87. doi: 10.1007/s00018-012-1073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Horn RD, Chu S, Fan L, Yin T, Du J, Beckmann R, et al. Cdk1 activity is required for mitotic activation of aurora A during G2/M transition of human cells. J Biol Chem. 2010;285(28):21849–57. doi: 10.1074/jbc.M110.141010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Assoro AB, Liu T, Quatraro C, Amato A, Opyrchal M, Leontovich A, et al. The mitotic kinase Aurora--a promotes distant metastases by inducing epithelial-to-mesenchymal transition in ERalpha(+) breast cancer cells. Oncogene. 2014;33(5):599–610. doi: 10.1038/onc.2012.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadler Y, Camp RL, Schwartz C, Rimm DL, Kluger HM, Kluger Y. Expression of Aurora A (but not Aurora B) is predictive of survival in breast cancer. Clin Cancer Res. 2008;14(14):4455–62. doi: 10.1158/1078-0432.CCR-07-5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Zhou YX, Qiao W, Tominaga Y, Ouchi M, Ouchi T, et al. Overexpression of aurora kinase A in mouse mammary epithelium induces genetic instability preceding mammary tumor formation. Oncogene. 2006;25(54):7148–58. doi: 10.1038/sj.onc.1209707. [DOI] [PubMed] [Google Scholar]
- 18.Katayama H, Sen S. Aurora kinase inhibitors as anticancer molecules. Biochim Biophys Acta. 2010;1799(10–12):829–39. doi: 10.1016/j.bbagrm.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cervantes A, Elez E, Roda D, Ecsedy J, Macarulla T, Venkatakrishnan K, et al. Phase I pharmacokinetic/pharmacodynamic study of MLN8237, an investigational, oral, selective aurora a kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012;18(17):4764–74. doi: 10.1158/1078-0432.CCR-12-0571. [DOI] [PubMed] [Google Scholar]
- 20.Dees EC, Cohen RB, von Mehren M, Stinchcombe TE, Liu H, Venkatakrishnan K, et al. Phase I study of aurora A kinase inhibitor MLN8237 in advanced solid tumors: safety, pharmacokinetics, pharmacodynamics, and bioavailability of two oral formulations. Clin Cancer Res. 2012;18(17):4775–84. doi: 10.1158/1078-0432.CCR-12-0589. [DOI] [PubMed] [Google Scholar]
- 21.Friedberg JW, Mahadevan D, Cebula E, Persky D, Lossos I, Agarwal AB, et al. Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas. J Clin Oncol. 2014;32 (1):44–50. doi: 10.1200/JCO.2012.46.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matulonis UA, Sharma S, Ghamande S, Gordon MS, Del Prete SA, Ray-Coquard I, et al. Phase II study of MLN8237 (alisertib), an investigational Aurora A kinase inhibitor, in patients with platinum-resistant or -refractory epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. Gynecol Oncol. 2012;127(1):63–9. doi: 10.1016/j.ygyno.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 23.Duprex WP, McQuaid S, Hangartner L, Billeter MA, Rima BK. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J Virol. 1999;73(11):9568–75. doi: 10.1128/jvi.73.11.9568-9575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iankov ID, Hillestad ML, Dietz AB, Russell SJ, Galanis E. Converting tumor-specific markers into reporters of oncolytic virus infection. Mol Ther. 2009;17(8):1395–403. doi: 10.1038/mt.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iankov ID, Haralambieva IH, Galanis E. Immunogenicity of attenuated measles virus engineered to express Helicobacter pylori neutrophil-activating protein. Vaccine. 2011;29(8):1710–20. doi: 10.1016/j.vaccine.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haralambieva IH, Ovsyannikova IG, Vierkant RA, Poland GA. Development of a novel efficient fluorescence-based plaque reduction microneutralization assay for measles virus immunity. Clin Vaccine Immunol. 2008;15(7):1054–9. doi: 10.1128/CVI.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leontovich AA, Salisbury JL, Veroux M, Tallarita T, Billadeau D, McCubrey J, et al. Inhibition of Cdk2 activity decreases Aurora-A kinase centrosomal localization and prevents centrosome amplification in breast cancer cells. Oncol Rep. 2013;29(5):1785–8. doi: 10.3892/or.2013.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu W, Wei L, Zhang H, Chen J, Qin X. Oncolytic adenovirus armed with IL-24 inhibits the growth of breast cancer in vitro and in vivo. J Exp Clin Cancer Res. 2012;31:51. doi: 10.1186/1756-9966-31-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fielding AK. Measles as a potential oncolytic virus. Rev Med Virol. 2005;15(2):135–42. doi: 10.1002/rmv.455. [DOI] [PubMed] [Google Scholar]
- 30.Falchook G, Kurzrock R, Gouw L, Hong D, McGregor KA, Zhou X, et al. Investigational Aurora A kinase inhibitor alisertib (MLN8237) as an enteric-coated tablet formulation in non-hematologic malignancies: phase 1 dose-escalation study. Invest New Drugs. 2014;32(6):1181–7. doi: 10.1007/s10637-014-0121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macarulla T, Cervantes A, Elez E, Rodriguez-Braun E, Baselga J, Rosello S, et al. Phase I study of the selective Aurora A kinase inhibitor MLN8054 in patients with advanced solid tumors: safety, pharmacokinetics, and pharmacodynamics. Mol Cancer Ther. 2010;9(10):2844–52. doi: 10.1158/1535-7163.MCT-10-0299. [DOI] [PubMed] [Google Scholar]
- 32.Brewer Savannah KJ, Demicco EG, Lusby K, Ghadimi MP, Belousov R, Young E, et al. Dual targeting of mTOR and aurora-A kinase for the treatment of uterine Leiomyosarcoma. Clin Cancer Res. 2012;18(17):4633–45. doi: 10.1158/1078-0432.CCR-12-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiskus W, Hembruff SL, Rao R, Sharma P, Balusu R, Venkannagari S, et al. Co-treatment with vorinostat synergistically enhances activity of Aurora kinase inhibitor against human breast cancer cells. Breast Cancer Res Treat. 2012;135(2):433–44. doi: 10.1007/s10549-012-2171-9. [DOI] [PubMed] [Google Scholar]
- 34.Sehdev V, Peng D, Soutto M, Washington MK, Revetta F, Ecsedy J, et al. The aurora kinase A inhibitor MLN8237 enhances cisplatin-induced cell death in esophageal adenocarcinoma cells. Mol Cancer Ther. 2012;11(3):763–74. doi: 10.1158/1535-7163.MCT-11-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palani S, Patel M, Huck J, Zhang M, Balani SK, Yang J, et al. Preclinical pharmacokinetic/pharmacodynamic/efficacy relationships for alisertib, an investigational small-molecule inhibitor of Aurora A kinase. Cancer Chemother Pharmacol. 2013;72(6):1255–64. doi: 10.1007/s00280-013-2305-8. [DOI] [PubMed] [Google Scholar]
- 36.Heinemann L, Simpson GR, Annels NE, Vile R, Melcher A, Prestwich R, et al. The effect of cell cycle synchronization on tumor sensitivity to reovirus oncolysis. Mol Ther. 2010;18(12):2085–93. doi: 10.1038/mt.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Libertini S, Abagnale A, Passaro C, Botta G, Barbato S, Chieffi P, et al. AZD1152 negatively affects the growth of anaplastic thyroid carcinoma cells and enhances the effects of oncolytic virus dl922-947. Endocr Relat Cancer. 2011;18 (1):129–41. doi: 10.1677/ERC-10-0234. [DOI] [PubMed] [Google Scholar]
- 38.Zeng WG, Li JJ, Hu P, Lei L, Wang JN, Liu RB. An oncolytic herpes simplex virus vector, G47Delta, synergizes with paclitaxel in the treatment of breast cancer. Oncol Rep. 2013;29(6):2355–61. doi: 10.3892/or.2013.2359. [DOI] [PubMed] [Google Scholar]
- 39.Frewer NC, Ye L, Sun PH, Owen S, Ji K, Frewer KA, et al. Potential implication of IL-24 in lymphangiogenesis of human breast cancer. Int J Mol Med. 2013;31 (5):1097–104. doi: 10.3892/ijmm.2013.1319. [DOI] [PubMed] [Google Scholar]
- 40.Zheng M, Bocangel D, Doneske B, Mhashilkar A, Ramesh R, Hunt KK, et al. Human interleukin 24 (MDA-7/IL-24) protein kills breast cancer cells via the IL-20 receptor and is antagonized by IL-10. Cancer Immunol Immunother. 2007;56 (2):205–15. doi: 10.1007/s00262-006-0175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhutia SK, Das SK, Azab B, Menezes ME, Dent P, Wang XY, et al. Targeting breast cancer-initiating/stem cells with melanoma differentiation-associated gene-7/interleukin-24. Int J Cancer. 2013;133(11):2726–36. doi: 10.1002/ijc.28289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galanis E, Atherton PJ, Maurer MJ, Knutson KL, Dowdy SC, Cliby WA, et al. Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res. 2015;75(1):22–30. doi: 10.1158/0008-5472.CAN-14-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell SJ, Federspiel MJ, Peng KW, Tong C, Dingli D, Morice WG, et al. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin Proc. 2014;89(7):926–33. doi: 10.1016/j.mayocp.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.