Comment
A study by de Agular Vallim et al. (MAFG is a transcriptional repressor of bile acid synthesis and metabolism. Cell Metabolism 2015; 21: 298–310)1 identified a liver FXR-induced transcriptional repressor V-Maf Avian Musculoaponeurotic Fibrosarcoma Oncogene Homolog G (MAFG) that directly inhibited the sterol 12α-hydroxylase (CYP8B1) gene in bile acid synthesis pathway and regulated bile acid composition, which may have significant impacts on liver metabolisms and diseases.
Bile acids are physiological agents essential for absorption of lipid-soluble vitamins, dietary fats and sterols in the intestine, and for transport of nutrients and drugs to the liver for metabolism. Bile acids also are signaling molecules that activate a nuclear bile acid receptor FXR (farnesoid X receptor) and a membrane G protein-coupled receptor TGR5 to regulate hepatic lipid, glucose and energy metabolism2. Importantly, FXR and TGR5 agonists have anti-inflammatory function in the gastrointestinal system and activation of these bile acid receptors may protect against inflammation-related diseases such as non-alcoholic fatty liver diseases, diabetes and atherosclerosis. The classic bile acid synthesis pathway is the predominate pathway in human liver and is initiated by cholesterol 7α-hydroxylase (CYP7A1), the rate-limiting enzyme in the pathway, to synthesize two primary bile acids, cholic acid and chenodeoxycholic acid (Fig. 1). Sterol 12α-hydroxylase (CYP8B1) is required for synthesis of cholic acid. Mitochondrial sterol 27-hydroxylase (CYP27A1) catalyzes oxidative cleavage of 3 carbon-steroid side-chain to form C24-bile acids. The alternative pathway is initiated by CYP27A1 and followed by oxysterol 7α-hydroxylase (CYP7B1); these two enzyme activities are expressed in most tissues and are responsible for oxidation of cholesterol. Oxysterols transported to hepatocytes are converted to bile acids. In mouse liver, these two pathways contribute equally for bile acid synthesis, and chenodeoxycholic acid is converted to α- and β-muricholic acids. Bile acids produced in the liver are reabsorbed in the intestine, where gut microbiota modify some primary bile acids to the secondary bile acids, which are re-circulated via enterohepatic circulation to the liver to inhibit CYP7A1 and CYP8B1 gene transcription and bile acid synthesis.
Figure 1. Bile acid synthesis pathways and mechanism of bile acid feedback regulation.
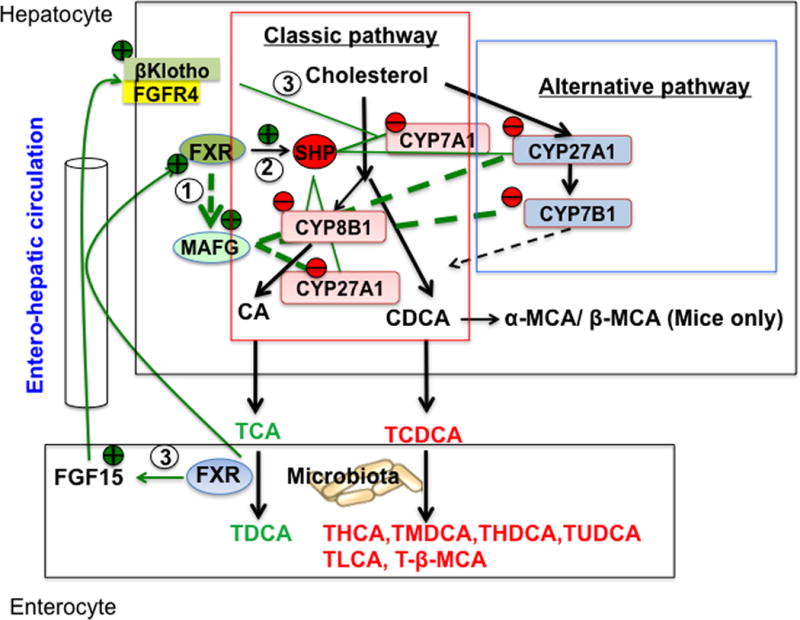
The classic bile acid synthesis pathway is initiated by cholesterol 7α-hydroxylase (CYP7A1) to synthesize two primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA). Cholic acid synthesis requires sterol 12α-hydroxylase (Cyp8B1). Mitochondrial sterol 27-hydroxylase (CYP27A1) catalyzes steroid-side chain oxidative cleavage. In the alternative pathway, side-chain cleavage by CYP27A1 precedes 7α-hydroxylation by oxysterol 7α-hydroxylase (CYP7B1) to synthesize oxidized sterols (oxysterols) in most tissues. In mice, CDCA is converted to α- and β-muricholic acids (MCAs). Most bile acids are conjugated to taurine (T) in mice, and secreted into bile, stored in the gallbladder. After each meal, bile acids are secreted into the intestinal tract for absorption and transport of nutrients. In the intestine, bacteria bile salt hydrolase de-conjugated bile acids and then bacteria 7α-dehydroxylase activity removed a 7α-HO group from CA and CACA to form deoxycholic acid (DCA) and lithocholic acid (LCA), respectively, and other secondary bile acids as indicated. FXR/MAFG signaling directly inhibits CYP8B1 and CYP27A1 in the classic pathway and may also inhibit CYP27A1 and CYP7B1 in the alternative pathway (Pathway 1). In the liver, FXR induces SHP to inhibit transactivation of the CYP7A1/CYP8B1 gene (Pathway 2). In the intestine, FXR induces FGF15, which is transported via portal circulation to the liver to activate a membrane FGF receptor 4 (FGFR4)/β-Klotho signaling to inhibit CYP7A1/CYP8B1 gene transcription (Pathway 3). Abbreviations: CYP7A1, cholesterol 7α-hydroxylase; CYP8B1, sterol 12α-hydroxylase; CYP27A1, sterol 27-hydroxylase; CYP7B1, oxysterol 7α-hydroxylase; CA, cholic acid; CDCA, chenodeoxycholic acid; MCA-muricholic acid; DCA, deoxycholic acid; HCA, hyodeoxycholic acid; MDCA, murideoxycholic acid; HDCA, hyodeoxycholic acid; UDCA, ursodeoxycholic acid; LCA, lithocholic acid; FGF15, fibroblast growth factor 15; FGFR4, FGF receptor 4; FXR, farnesoid X receptor; SHP, small heterodimer partner; MAFG, V-Maf Avian Musculoaponeurotic Fibrosarcoma Oncogene Homolog G.T, tauro-conjugated bile acids.
In search of transcriptional repressors that are induced by FXR and directly inhibit bile acid synthesis in the liver, de Aguiar Vallim et al.1 analyzed chromatin immunoprecipitation sequence (ChIP-seq) data from livers of mice treated with FXR agonists. These investigators performed detailed analyses of several liver FXR-induced transcriptional repressors and identified MAFG as a potential factor that might directly inhibit bile acid synthesis genes. MAFG expression is significantly induced by FXR agonists and by cholic acid feeding in wild type mice, but not in FXR−/− mice. They identified putative FXR binding sites in the Mafg gene. Adenovirus-mediated overexpression of MAFG in mouse liver decreased expression of CYP8B1 and cholic acid in bile acid pool, but not CYP7A1 suggesting that MAFG may regulate bile acid composition (Fig. 1). Microarray gene profiling and pathway analysis of liver mRNA expression confirmed that CYP8B1, CYP27A1 and CYP7B1 and other genes in the bile acid synthesis pathways are major targets of MAFG. They identified a functional MAFG binding site in the Cyp8B1 gene promoter. Furthermore, shRNA knock down of Mafg gene expression de-repressed multiple genes in bile acid synthesis and increased cholic acid levels. Study of heterozygote Mafg deficient mice confirmed the role of MAFG in the regulation of bile acid synthesis genes.
FXR is known to inhibit CYP7A1, CYP8B1, CYP27A1 and CYP7B1 in bile acid synthesis, and to induce bile acid:CoA synthase in bile acid conjugation and bile acid: amino acid transferase in bile acid transport. This study added a new dimension and also complexity to bile acid feedback regulation by an FXR-dependent mechanism that directly inhibited several genes in bile acid synthesis (FXR/MAFG pathway 1, Fig. 1). Previously, two mechanisms have been proposed to mediate bile acid inhibition of bile acid synthesis. In the liver, FXR induces the negative nuclear receptor small heterodimer partner (SHP), which inhibits CYP7A1 and CYP8B1 gene transcription by an indirect mechanism (Pathway 2, Fig. 1). The liver FXR/SHP pathway may be activated by high levels of bile acids in cholestatic livers to inhibit bile acid synthesis as an adaptive response to protect the liver against cholestatic injury2. In the intestine, FXR agonists induces a fibroblast growth factor 15 (FGF15, or FGF19 in human), which activates the liver FGF receptor 4/β-Klotho/signaling pathway to inhibit CYP7A1 and CYP8B1 expression3 (Pathway 3, Fig. 1). This pathway is consistent to a previous report that intra duodenal infusion, but not intravenous infusion of bile acids inhibited CYP7A1 gene transcription suggesting that intestinal factors might be required for bile acid feedback regulation4. Bile acids and FXR agonists induce FGF19 in human, but not FGF15 in mouse hepatocytes. It has been reported that hepatic FGF19 levels are increased and inversely correlated to the reduced CYP7A1 expression levels in cholestatic patients, but not in cholestatic patients with biliary drainage and in non-cholestatic patients4–5. Multiple mechanisms of bile acid feedback regulation are necessary to ensure bile acid homeostasis is maintained and bile acid toxicity is controlled. It should be emphasized that not only bile acid pool size, but also bile acid composition and hydrophobicity, play important roles in regulation of bile acid synthesis and lipid metabolism. Cholic acid is less hydrophobic than chenodeoxycholic acid, but is more efficacious in absorption of dietary cholesterol and fats and in mixed micelle formation with cholesterol and phosphatidylcholine in the gallbladder than other bile acids. Animal fats increased total bile acids and deoxycholic acid, and rapidly increase the abundance of bile-tolerant microorganisms in gut microbiota, and triggered inflammatory bowel disease6–7. On the other hand, Cyp8b1−/− mice are protected against lithogentic diet-induced hypercholesterolemia and atherosclerosis. CYP8B1 is responsible for determining the ratio of 12α- to non-12α-hydroxylated bile acids and hydrophobicity of bile acids in the pool. In type 2 diabetic patients, serum bile acid concentrations and the ratio of 12α- to non-12α-hydroxylated bile acids are increased compared to non-diabetic controls8.
In summary, this study identified an FXR-induced negative transcriptional factor MAFG that may directly inhibit CYP8B1 in cholic acid synthesis, thus alter bile acid composition, but not bile acid pool size in mice. The liver FXR/SHP mechanism and intestine FXR/FGF15 to liver FGFR4 mechanism may regulate the rate of bile acid synthesis under cholestatic or normal physiological state, respectively. The newly discovered FXR/MAGF pathway may regulate bile acid composition. Alteration of bile acid composition and hydrophobicity has been shown to contribute to pathogenesis of non-alcoholic fatty liver disease, cholestatic liver diseases and inflammatory diseases in the intestine2. The underlying mechanism of MAFG inhibition of target gene expression is not clear. The phenotype of MAFG deficient mice has not been studied. It is predicted that Mafg gene deficiency would increase cholic acid and intestinal absorption of dietary cholesterol and fats in mice, and these mice would develop gallstone disease, fatty liver diseases, diabetes, and atherosclerosis on high fat diets. On the other hand, FXR agonists would induce MAFG to suppress CYP8B1 expression and CA synthesis and reduce hypercholesterolemia and gallstone formation in mice. It would be interesting to study whether non-alcoholic steatohepatitis patients treated with FXR agonists will have reduced gallstone disease, diabetes and atherosclerosis. The physiological role of MAFG in regulation of bile acid synthesis and lipid metabolism is not clear and requires further detailed study.
Acknowledgments
This work was supported by NIH grants DK44442 and DK58379 from National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviation list
- CYP7A1
cholesterol 7α-hydroxylase
- CYP8B1
sterol 12α-hydroxylase
- CYP27A1
sterol 27-hydroxylase
- CYP7B1
oxysterol 7α-hydroxylase
- FXR
farnesoid X receptor
- FGF15
fibroblast growth factor 15
- MAFG
V-Maf Avian Musculoaponeurotic Fibrosarcoma Oncogene Homolog G
- SHP
small heterodimer partner
Footnotes
Potential conflict of interest: none
References
- 1.de Aguiar Vallim TQ, Tarling EJ, Ahn H, et al. MAFG is a transcriptional repressor of bile acid synthesis and metabolism. Cell Metab. 2015;21:298–310. doi: 10.1016/j.cmet.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li T, Chiang JY. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66:948–83. doi: 10.1124/pr.113.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–25. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Pandak WM, Heuman DM, Hylemon PB, et al. Failure of intravenous infusion of taurocholate to down-regulate cholesterol 7 alpha-hydroxylase in rats with biliary fistulas. Gastroenterology. 1995;108:533–44. doi: 10.1016/0016-5085(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 5.Schaap FG, van der Gaag NA, Gouma DJ, Jansen PL. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology. 2009;49:1228–1235. doi: 10.1002/hep.22771. [DOI] [PubMed] [Google Scholar]
- 6.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sayin SI, Wahlstrom A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–35. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Haeusler RA, Astiarraga B, Camastra S, et al. Human insulin resistance is associated with increased plasma levels of 12alpha-hydroxylated bile acids. Diabetes. 2013;62:4184–91. doi: 10.2337/db13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]


