Abstract
Background
Antisocial personality disorder (ASPD) and borderline personality disorder (BPD) share genetic and environmental risk factors. Little is known about the temporal stability of these etiological factors in adulthood.
Method
DSM-IV criteria for ASPD and BPD were assessed using structured interviews in 2282 Norwegian twins in early adulthood and again approximately 10 years later. Longitudinal biometric models were used to analyze the number of endorsed criteria.
Results
The mean criterion count for ASPD and BPD decreased 40% and 28%, respectively, from early to middle adulthood. Rank-order stability was 0.58 for ASPD and 0.45 for BPD. The best-fitting longitudinal twin model included only genetic and individual-specific environmental factors. Genetic effects, both those shared by ASPD and BPD, and those specific to each disorder remained completely stable. The unique environmental effects, however, changed substantially, with a correlation across time of 0.19 for the shared effects, and 0.39 and 0.15, respectively, for those specific to ASPD and BPD. Genetic effects accounted for 71% and 72% of the stability over time for ASPD and BPD, respectively. The genetic and environmental correlations between ASPD and BPD were 0.73, and 0.43, respectively, at both time points.
Conclusion
ASPD and BPD traits were moderately stable from early to middle adulthood, mostly due to genetic risk factors which did not change over the 10-year assessment period. Environmental risk factors were mostly transient, and appear to be the main source of phenotypic change. Genetic liability factors were, to a large extent, shared by ASPD and BPD.
Keywords: Antisocial personality disorder, borderline personality disorder, genetics, longitudinal studies
Introduction
Borderline personality disorder (BPD) and antisocial personality disorder (ASPD) frequently co-occur in both clinical (e.g. Becker et al. 2000) and population-based (Eaton et al. 2011; Trull et al. 2013) studies. The disorders have been found to co-aggregate in families (White et al. 2003; Hudson et al. 2014), and results from cross-sectional twin studies suggest that this is largely due to common genetic risk factors (Kendler et al. 2008; Torgersen et al. 2008).
Longitudinal studies indicate that mean levels of BPD and ASPD traits decrease moderately over time from late adolescence throughout adulthood, while rank-order stability is generally relatively high (Johnson et al. 2000; Nestadt et al. 2010; Hopwood et al. 2013; Morey & Hopwood, 2013).
Prior longitudinal twin studies from adolescence to adulthood of both antisocial behavior and ASPD traits (Lyons et al. 1995; Jacobson et al. 2002; Burt et al. 2007; Silberg et al. 2007) and BPD traits (Bornovalova et al. 2009, 2013a) indicate that the contribution of genetic factors increases over time as new genetic effects are expressed, and the influence of shared environment decrease correspondingly often approaching zero in early adulthood. We are, however, not aware of any studies exploring the stability of genetic and environmental influences on BPD and ASPD during adulthood. It also remains unclear to what extent genetic and environmental factors contribute to the longitudinal co-occurrence of these disorders.
In this study we sought to fill these gaps in the literature using data from the Norwegian Institute of Public Health Twin Panel, which includes a population-based sample of young adult twins assessed by interview for DSM-IV personality disorders (PDs) in early adulthood and again approximately 10 years later. Our aims were to estimate the stability of ASPD and BPD traits and their co-occurrence across time and use multivariate biometric models to determine the stability of genetic and environmental influences on BPD and ASPD traits and their association. We specifically explored the extent to which stability and change could be attributed to genetic and/or environmental risk factors shared in common by the two disorders v. factors specific to the individual PDs.
Method
Participants
Data for the present analyses come from a population-based study of psychiatric disorders in Norwegian twins recruited from the Norwegian Institute of Public Health Twin Panel (Harris et al. 2006). Between 1999 and 2004, DSM-IV Axis I and Axis II psychiatric disorders were assessed at interview in 2801 twins (44% of those eligible) born between 1967 and 1979 (wave 1). Zygosity was determined by a combination of questionnaire items and genotyping. The misclassification rate was estimated to be <1.0%, too low to substantially bias results. Several papers describing details of the sample and the measures used in this report have been published (e.g. Kendler et al. 2008; Torgersen et al. 2008). Between 2010 and 2011 a second wave of interviews using the same instruments were conducted. To maximize participation rate, interviews in wave 2 included only a subset of the disorders assessed at wave 1, and were conducted by telephone. After two written reminders and a final telephone contact to non-responders, 2284 twins were interviewed in wave 2 (82.8% of those eligible).
In wave 1, 2793 twins had valid data for PDs, and in wave 2, 2282. The number of complete and incomplete pairs being analyzed by zygosity group is given in Supplementary Table S1.
The participation rate was 86% in monozygotic (MZ) females, 80% in MZ males and 79% in all dizygotic (DZ) groups. Participation in wave 2 was predicted by high education (p < 0.001 adjusted for sex and age), female sex (p = 0.003) and monozygosity (p = 0.001). Non-participants in wave 2 had on average 0.82 more sub-threshold PD criteria than participants (p < 0.001). Of the 10 PDs assessed at wave 1, criteria were significantly higher in non-participants in wave 2 only for ASPD (0.09 criteria difference, p < 0.001) and narcissistic PD (0.09 p = 0.002). BPD did not predict participation (0.05 criteria difference, p = 0.06). Neither the total number of Axis I disorders nor any specific disorder were significantly higher in the non-participation group.
Procedures
A Norwegian version of the comprehensive Structured Interview for DSM-IV Personality (SIDP-IV; Pfohl et al. 1995) was used to assess PDs in both waves. The specific DSM-IV criteria were rated using the following scoring guidelines: 0 = ‘not present’; 1 = ‘sub-threshold’; 2 = ‘present’; and 3 = ‘strongly present’. Behaviors, cognitions, and feelings that predominated for most of the past 5 years were considered to be representative of an individual’s long-term personality. At wave 1, all 10 DSM-IV PDs were assessed, whereas at wave 2 only paranoid, schizotypal, antisocial, borderline, avoidant and obsessive compulsive were included.
Interviewers were mainly senior clinical psychology graduate students and experienced psychiatric nurses. The interviews in wave 1 were largely conducted face-to-face. In wave 2, all interviews were conducted by telephone. Each twin in a pair was interviewed by a different interviewer.
Inter-rater reliability at wave 1 was assessed based on two raters’ scoring of 70 audiotaped interviews. Intraclass correlations for the count of endorsed BPD criteria at the sub-threshold (≥1) and threshold (≥2) level were 0.93 and 0.92, respectively. The polychoric correlation was 0.94. For ASPD the corresponding intraclass correlations at wave 1 were 0.91 and 0.68, respectively, and the polychoric correlation was 0.94. At wave 2, inter-rater reliability was assessed similarly by two interviewers rescoring 95 audio-recorded interviews. Intraclass and polychoric correlations for the number of endorsed criteria at sub-threshold level were 0.63 and 0.84 for ASPD and 0.85 and 0.83 for BPD.
Approval was received from The Norwegian Data Inspectorate and the Regional Ethical Committee, and written informed consent was obtained from all participants after complete description of the study.
Statistical analyses
Two approaches are most commonly applied in analyses of PD stability (Morey & Hopwood, 2013). Absolute stability refers to the average changes of traits. We used the mean number of criteria endorsed for ASPD and BPD at time 1 and time 2. Differential or rank-order stability refers to consistency in the rank ordering of individuals on a given trait over time and was calculated using polychoric correlations between the number of criteria endorsed for each PD at the two time points.
Too few individuals endorsed sufficient criteria exceeding the diagnostic thresholds to permit biometric analyses to be based on fully syndromal PD measures. Using multiple threshold tests, we have previously shown that the 0–3 coding of the ASPD and BPD criteria represents different degrees of severity on a single continuous liability dimension, and that number of criteria endorsed also reflects severity on a single continuum (Kendler et al. 2008; Torgersen et al. 2008). The phenotypes under study here were therefore not ASPD and BPD at the diagnostic level. Instead we counted the number of criteria for each PD scored at either the threshold or sub-threshold level (≥1), resulting in a possible value between 0 and 9 for BPD, and between 0 and 8 for ASPD. However, there were two practical problems in basing the twin analysis on this criteria count. First, statistical inference in SEM analyses is based on the assumption that the variables studied are approximately normally distributed, while the PD criteria counts were highly skewed. Second, optimization in SEM analyses of ordinal variables is known to break down when there are many empty cells in the twin contingency tables. For these reasons, the four count variables (ASPD and BPD at waves 1 and 2) were thresholded so that the final ordinal variable included in the twin analysis only had three levels (criteria counts of 0, 1, ⩾ 2).
In the classical twin model, individual differences in liability are assumed to arise from three latent sources: additive genetic (A), i.e. genetic effects that combine additively, common or shared environment (C) that includes all environmental exposures that contribute to twin similarity, and individual-specific or unique environment (E) which includes all environmental factors that contribute to differences between the twins plus measurement error. Because MZ twins share all their genes and DZ twins share on average 50% of their segregating genes, A contributes twice as much to the resemblance in MZ compared to DZ twins for a particular trait or disorder. Both MZ and DZ twins are assumed to share all their C factors and none of their E factors.
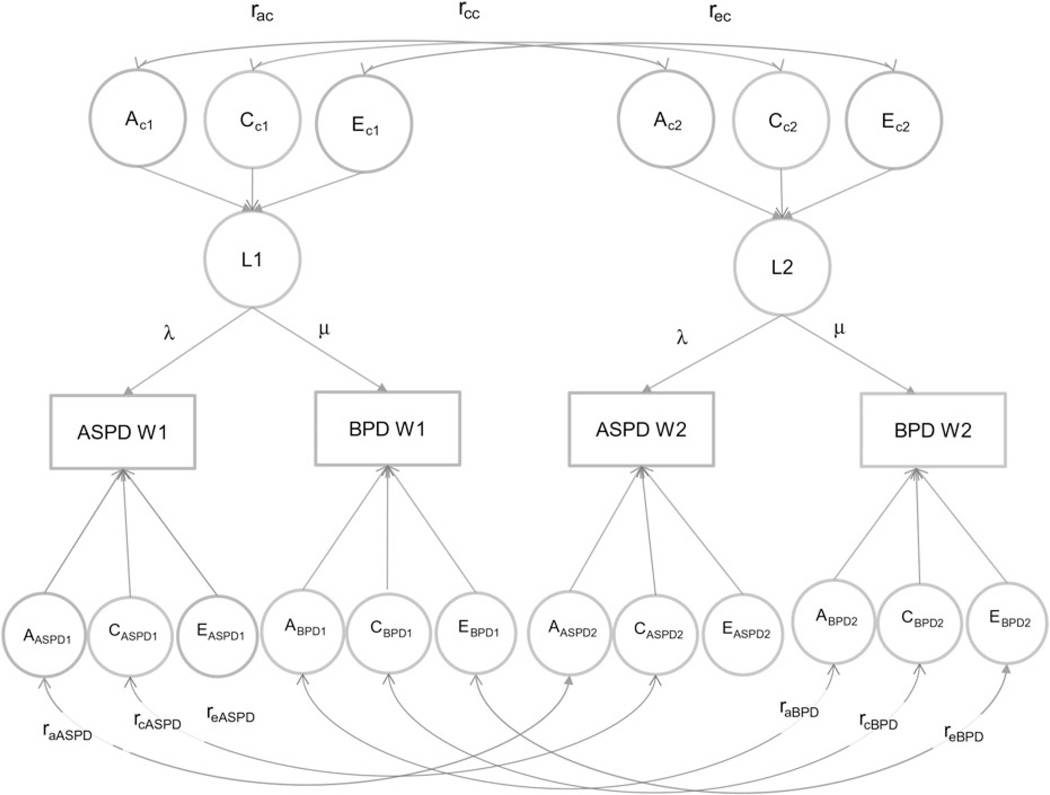
In the present study we fitted longitudinal models that specified one common latent liability factor at each time point influencing both PDs (see Fig. 1). Each latent factor (L1 and L2) was influenced by genetic and environmental factors (AC, CC, EC). The latent factors influenced ASPD and BPD through the pathways λ and µ. Due to restrictions caused by the limited degrees of freedom, we had to restrict λ and µ to be invariant across time. The model also specified specific genetic and environmental effects for each PD at each time point, thus decomposing the genetic and environmental influences on the phenotypes into those common and specific to ASPD and BPD at each time point. The stability of the genetic and environmental factors across time was estimated as correlations separately for the common (rac, rcc, rec) and specific factors (ras, rcs, res). Given the size of our sample, we had insufficient statistical power to detect sex-specific differences in genetic or environmental effects. Our models were therefore run specifying equal parameters, but separate thresholds, for males and females. Previous twin studies, both in our sample and a number of others, have failed to find either quantitative or qualitative gender differences in genetic and environmental influences on ASPD (Rhee & Waldman, 2002) or BPD (Distel et al. 2008; Torgersen et al. 2008).
Fig. 1.
Longitudinal structural equation path model for antisocial and borderline personality disorder traits at two waves. ASPD, Antisocial personality disorder; BPD, borderline personality disorder; A, additive genetic effects; C, shared environmental effects; E, individual environmental effects; subscript c = common; subscript s = specific; L1, latent factor time 1; L2, latent factor time 2; λ, factor loading ASPD; µ, factor loading BPD; W1, wave 1; W2, wave 2; subscript 1, time 1; subscript 2, time 2; ra additive genetic correlation; rc, shared environmental correlation; re, individual environment correlation. Latent additive genetic (A), shared environmental (C) and individual environmental (E) factors have variances fixed at unity (not shown). The latent phenotype factors have no residual variance, but are constrained to have unit variance.
Alternative, more parsimonious sub-models were compared to the full model using the difference in twice the log likelihood (2lnL), which, under certain regularity conditions, is asymptomatically distributed as χ2 with degrees of freedom (df) equal to the difference in number of parameters. According to the principle of parsimony, models with fewer parameters are preferable if they do not result in a significant deterioration of fit. A commonly used index of parsimony is Akaike’s Information Criterion (AIC), calculated as −2lnL − 2 df (Akaike, 1987). Lower AIC values indicate superior fit.
Model fitting was performed using the raw data option in the software package Mx (Neale et al. 2003) which allows for full information maximum likelihood (FILM) estimation of parameters using incomplete data, such as the unmatched ‘singleton’ twins in our sample.
Results
The mean age of the participants was 28.2 (range 19– 36) years at wave 1, and 37.9 (range 30–44) years at wave 2. On average, 9.6 (s.d. = 1.39) years had elapsed between the interviews.
Table 1 shows the mean number of criteria endorsed (⩾1) at waves 1 and 2. The mean (s.d.) score for the twins who had complete information from both waves fell from 0.42 (0.92) to 0.25 (0.67) for ASPD, and from 0.99 (1.45) to 0.71 (1.28) for BPD. The difference, reflecting the absolute stability of the traits, indicated that the magnitude of the decline in mean criteria over 10 years was 40% for ASPD and 28% for BPD. Men endorsed more criteria than females on both occasions for ASPD, and the decline was 43% for males and 36% for females. Women endorsed more criteria on both occasions for BPD, but the mean number of endorsed criteria fell slightly more for men (29%) than for women (27%). The corresponding figures for the whole sample (including those with data from only one wave) were similar.
Table 1.
Mean number of criteria scored at wave 1 and wave 2a
| ASPD wave 1 | ASPD wave 2 | BPD wave 1 | BPD wave 2 | |
|---|---|---|---|---|
| Men | (n = 800) | (n = 800) | (n = 799) | (n = 799) |
| Mean (s.d.) | 0.68 (1.15) | 0.39 (0.83) | 0.83 (1.25) | 0.59 (1.06) |
| Women | (n = 1470) | (n = 1470) | (n = 1470) | (n = 1470) |
| Mean (s.d.) | 0.28 (0.73) | 0.18 (0.55) | 1.07 (1.55) | 0.78 (1.3) |
| All | (n = 2270) | (n = 2270) | (n = 2269) | (n = 2269) |
| Mean (s.d.) | 0.42 (0.92) | 0.25 (0.67) | 0.99 (1.45) | 0.71 (1.28) |
⩾ 1.
ASPD, Antisocial personality disorder traits.
BPD, Borderline personality disorder traits.
s.d., Standard deviation.
The number of cases meeting full criteria for BPD was 11 and seven at waves 1 and 2, respectively. The corresponding numbers for ASPD were nine and three. Supplementary Table S3 shows the frequencies of the three ordinal levels used in the model-fitting analyses.
The rank-order stability of the two PDs, measured as polychoric correlations between the sum of endorsed criteria at waves 1 and 2, was 0.58 [95% confidence interval (CI) 0.53–0.63] for ASPD and 0.45 (95% CI 0.40–0.49) for BPD. The correlations were almost identical for males and females for both PDs (Table 2).
Table 2.
Polychoric correlations between sum of criteria scored for ASPD and BPD at wave 1 and wave 2, (a) men below diagonal, women above, (b) total samplea
| ASPD W1 | BPD W1 | ASPD W2 | BPD W2 | |
|---|---|---|---|---|
| (a) | ||||
| ASPD W1 | 0.56 (0.51–0.61) | 0.59 (0.51–0.66) | 0.35 (0.27–0.42) | |
| BPD W1 | 0.58 (0.52–0.63) | 0.40 (0.33–0.48) | 0.46 (0.41–0.51) | |
| ASPD W2 | 0.51 (0.43–0.59) | 0.32 (0.23–0.41) | 0.54 (0.47–0.60) | |
| BPD W2 | 0.26 (0.17–0.36) | 0.41 (0.33–0.49) | 0.53 (0.45–0.60) | |
| (b) | ||||
| ASPD W1 | – | |||
| BPD W1 | 0.52 (0.48–0.56) | – | ||
| ASPD W2 | 0.58 (0.53–0.63) | 0.34 (0.28–0.40) | – | |
| BPD W2 | 0.28 (0.22–0.34) | 0.45 (0.40–0.49) | 0.50 (0.45–0.55) | – |
> 1.
ASPD, Antisocial personality disorder traits.
BPD, Borderline personality disorder traits.
W1, Wave 1; W2, wave 2.
The phenotypic polychoric correlation between the sum of endorsed criteria for ASPD and BPD was, 0.52 (95% CI 0.48–0.56) at wave 1 and 0.50 (95% CI 0.45–0.55) and wave 2, indicating that despite the absolute reduction in the mean number of criteria endorsed for both PDs, the correlation between ASPD and BPD was quite stable over time (Table 2).
The MZ and DZ within-pair correlations are given in Supplementary Table S2. All the MZ correlations were higher than the DZ correlations suggesting genetic influence. The difference between MZ and DZ correlations for BPD in wave 2 indicates a possible effect of C.
Model fitting
We first fitted a full longitudinal model as depicted in Fig. 1, including both specific and common A, C and E parameters (model 1, Table 3). The parameter estimates with 95% CIs for this model are given in Supplementary Fig. S1. The common A, effects on the latent variable were significant at both waves, whereas the common C effect was significant only in wave 2. None of the specific C effects were significantly different from zero. All E parameters were significant. The magnitude of the total C effect (common + specific) at waves 1 and 2 was 0.05 and 0.14 for ASPD and 0.06 and 0.18 for BPD.
Table 3.
Model fitting results
| Δχ2 | Δ df | p | AIC | |
|---|---|---|---|---|
| 1. Baseline model | – | – | – | |
| 2. Drop all C | 3.16 | 9 | 0.958 | −14.84 |
| 3. Drop all A | 14.85 | 9 | 0.100 | −3.15 |
| 4. Set rac to 1.0 | 3.86 | 10 | 0.953 | −16.14 |
| 5. Set raASPD to 1.0 | 3.86 | 11 | 0.974 | −18.13 |
| 6. Set raBPD to 1.0 | 3.93 | 12 | 0.985 | −20.07 |
| 7. Set common genetic effects equal across time | 3.94 | 13 | 0.992 | −22.05 |
| 8. Set specific genetic effects for ASPD equal across time | 4.17 | 14 | 0.994 | −23.83 |
| 9. Set specific genetic effects for BPD equal across timea | 5.91 | 15 | 0.981 | −24.09 |
| 10. Set rec = 0 | 9.46 | 16 | 0.893 | −22.54 |
Best fit model.
A, Additive genetic effects; C, shared environmental effects; AIC, Akaike’s Information Criterion; ASPD, antisocial personality disorder; BPD, borderline personality disorder; ra, genetic correlation; re, environmental correlation, subscript c=common,
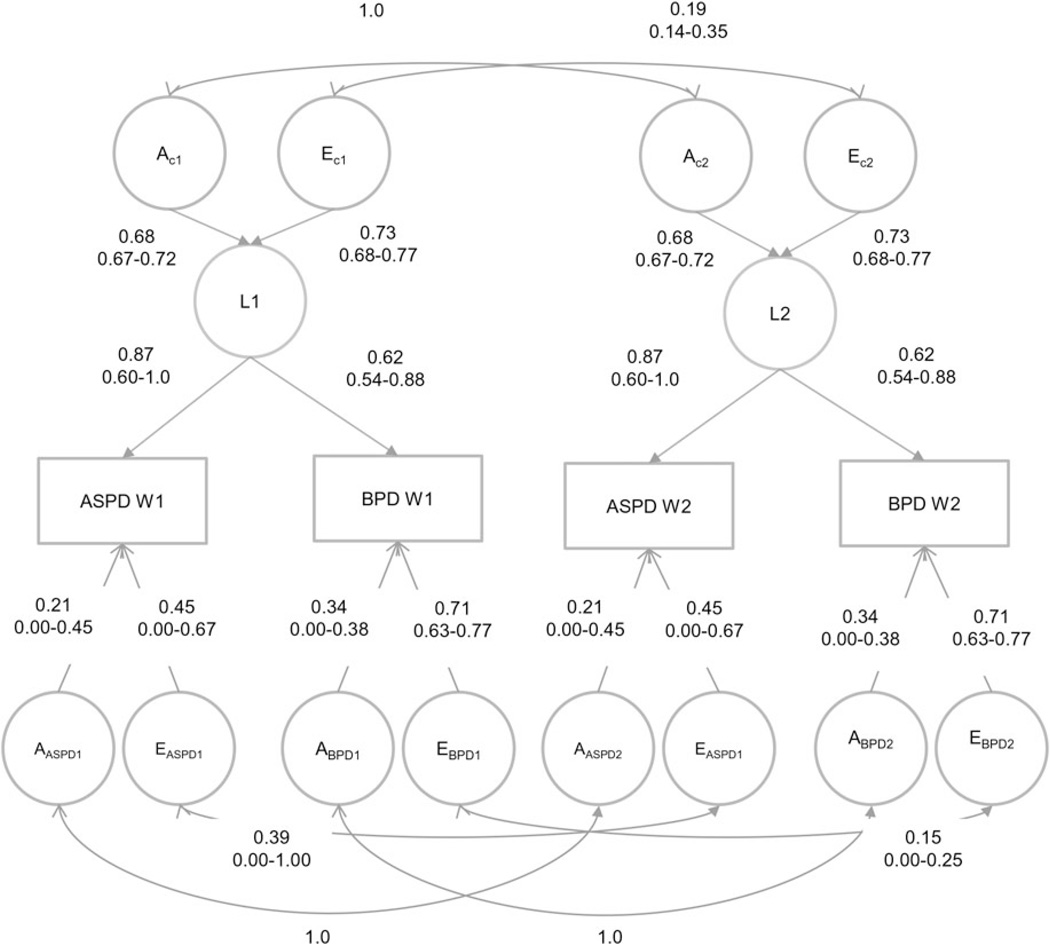
In model 2 and model 3 we set to zero all C and all A paths, respectively. The AE model (model 2) fit the data markedly better than the CE model (model 3) as evidenced by a substantially larger reduction in AIC, and was therefore used as the basis for the subsequent model fitting. We first tested alternative models for the common and specific genetic effects. In model 4 we constrained the correlation between the genetic factors influencing the common latent factors L1 and L2 at the two time points to unity (rac = 1), thus testing if the genetic factors influencing the common latent liability at both wave 1 and wave 2 correlated perfectly. As can be seen in Table 3, this resulted in an improvement in AIC indicating a better fit. Next (model 5) we specified that the specific genetic effects influencing ASPD at the two time points also correlated unity (raASPD = 1). The model fit improved further. In model 6 we did the same with the correlation between the specific genetic factors for BPD (raBPD = 1), again resulting in a slightly better fit according to the AIC values. Further improvement in fit was seen in model 7 when the path coefficients for the common genetic effects (AC1 and AC2) were constrained to be equal at the two time points, and in models 8 and 9 where the specific genetic effects for ASPD and BPD, respectively, were set to be equal across time. In model 10 we tried to further simplify the model by constraining to zero the correlation between environmental factors influencing the common latent factors (rec = 0), which implies that the environmental effects common to both ASPD and BPD are completely distinct at the two time points. This resulted in a deterioration of fit. Model 9 was therefore selected as the best-fitting model. Fig. 2 depicts this model with parameter estimates and 95% CIs.
Fig. 2.
Best fit longitudinal model with parameter estimates and 95% confidence intervals. ASPD, Antisocial personality disorder; BPD, borderline personality disorder; A, additive genetic effects; E, individual environmental effects; subscript c = common; subscript s = specific; L1, latent factor time 1; L2, latent factor time 2; W1, wave 1; W2, wave 2; subscript 1, time 1; subscript 2, time 2.
The genetic influences on ASPD and BPD were identical approximately 10 years apart during early and middle adulthood. Heritability estimates were 0.39 for ASPD and 0.30 for BPD at both time points. Of the genetic influences on ASPD, 89.7% came from the common latent factor shared with BPD, whereas the corresponding figure for BPD was 60%. The heritability of the latent common liability factor was 0.46.
The correlation between the unique environmental influences shared by ASPD and BPD at the two time points was only 0.19, indicating that environmental factors influencing both ASPD and BPD change considerably over time in early to middle adulthood. The correlations between environmental factors specific to each PD were 0.39 for ASPD and 0.15 for BPD. The correlation between the total environmental effects (common + specific) at time 1 and time 2 was 0.26 for ASPD, and 0.16 for BPD indicating that different environmental factors increase the risk for ASPD and BPD in early and middle adulthood At both time points, 66.5% of the environmental influence on ASPD came from factors common to both PDs, whereas BPD received only 28.9% of the total environmental influence from environmental factors that also influence ASPD, indicating that almost three quarters of the environmental influences on BPD were not shared with ASPD.
The rank-order stabilities of both ASPD and BPD from early to middle adulthood were largely due to genetic factors (71% and 72%, respectively). The correlation between genetic risk factors for ASPD and BPD was 0.73 at time 1 and remained stable over time. The correlation between environmental influences on ASPD and BPD was also the same at both time points (re = 0.43). Genetic factors and environmental factors accounted for 46% and 54% of the phenotypic correlation between ASPD and BPD at both times.
Discussion
There are very few previous studies on 10-year stability of ASPD and BPD traits in adulthood. The rank-order stability estimates in our sample (ASPD, 0.59; BPD, 0.41) was very similar to the 10-year rank-order stabilities previously found in a clinical sample (ASPD, 0.60; BPD, 0.36) (Hopwood et al. 2013), and somewhat lower than in a non-clinical sample followed over 4 years (ASPD, 0.65; BPD, 0.50) (Lenzenweger, 1999).
The main finding from the biometric analyses was that the genetic influences on both ASPD and BPD were highly stable over a 10-year period from early to middle adulthood. The correlations between both the common and specific genetic effects on ASPD and BPD at time 1 and 2 did not differ significantly from unity, and the genetic path coefficients were identical at the two time points, indicating that the genes influencing ASPD and BPD, respectively, were identical and the magnitude of genetic effects where the same.
These results are consistent with results from prior studies of normal personality traits during different periods in adulthood which suggest that genetic influences are stable over time (McGue et al. 1993; Johnson et al. 2005; Kandler et al. 2010). They do, however, differ from findings in longitudinal studies of younger twins of both antisocial behavior and ASPD traits (Lyons et al. 1995; Jacobson et al. 2002; Silberg et al. 2007) and BPD traits (Bornovalova et al. 2009, 2013a), which suggest that when individuals mature from adolescence to adulthood the contribution of genetic factors appears to increase over time as new genetic effects appear. By contrast, the influence of C decreases correspondingly, often reaching zero in adulthood. Taken together these findings suggest that genetic effects on ASPD and BPD traits increase through adolescence but stabilize in early adulthood and thereafter remain stable throughout mid-adulthood.
The temporal stability of E on ASPD and BPD was, on the other hand, low, both for the common (0.19) and specific factors (0.34 and 0.15). E includes measurement error, which in the classical twin model cannot be separated from true environmental effects. In our model, however, random error of measurement is restricted to the specific environmental effects, suggesting that the correlation of 0.19 between the common factors reflect only ‘true’ environmental effects. This suggests that the environmental experiences that influence ASPD and BPD in early adulthood is largely unrelated to those that influence the PDs in middle adulthood.
We cannot tell from this study which environmental experiences are involved. A number of previous studies have found associations between childhood adversities and both ASPD and BPD (Johnson et al. 1999; Zanarini, 2000; Beauchaine et al. 2009; Afifi et al. 2011). Although it is not clear if these associations represent a causal relationship (Ball & Links, 2009; Bornovalova et al. 2013b), it is possible that such childhood experiences have a lasting impact (Ehlert, 2013), and thus reflect the stable proportion of the environmental influences identified in our study. Studies of life events in early and middle adulthood associated with ASPD and BPD are rare (Distel et al. 2011; McGowan et al. 2012), but indicate that these represent more transient effects and thus at least partly explain the low environmental correlations.
The heritabilities of ASPD and BPD were modest at both time points. This is in accordance with findings from previous twin studies in adults using models in which the effects of measurement error cannot be separated from non-shared environment which results in an inflation of E and a corresponding deflation of A (Distel et al. 2008; Torgersen et al. 2008). Models in which heritabilities of latent liabilities are corrected for measurement error indicate higher genetic influence (Distel et al. 2010; Kendler et al. 2011b; Reichborn-Kjennerud et al. 2013).
Our best-fitting model did not include C effects. Biometric studies of BPD in adult samples have failed to find significant influence of C (Torgersen et al. 2000, 2008; Distel et al. 2008, 2009; Kendler et al. 2011b). Few twin studies on adult ASPD as defined by DSM have been published (Paris et al. 2013), and none have found significant C effects. The statistical power to detect C effects in our sample was, however, low (Neale et al. 1994; Sullivan & Eaves, 2002), and we cannot with confidence rule it out. In addition, non-additive genetic effects such as dominance and epistasis may have attenuated any effects of the shared environment.
The rank-order temporal stability of both ASPD and BPD traits in early adulthood appears largely due to genetic factors whereas change is determined mostly by environmental factors. This is consistent with results from previous studies of antisocial traits (Lyons et al. 1995; Jacobson et al. 2002; Burt et al. 2007) and BPD traits (Bornovalova et al. 2009, 2013a).
The genetic correlation between ASPD and BPD was high (0.73) and remained stable over 10 years. Within the framework of internalizing and externalizing spectra conceptualization of adult psychopathology, postulating a common liability accounting for the co-occurrence of externalizing disorders (Krueger et al. 2007), our results are consistent with factor analytic studies suggesting that BPD, unlike most mental disorders, loads on both externalizing and internalizing factors (Eaton et al. 2011; Roysamb et al. 2011; Hudson et al. 2014), and results from a recent family study of BPD which suggest that familial factors can to a large extent account for the pattern of co-morbidity seen between BPD and internalizing and externalizing disorders (Hudson et al. 2014). Previous findings from our sample, including a large twin study with 22 Axis I and Axis II disorders, indicate that these familial factors are genetic in nature (Kendler et al. 2008, 2011a). Other twin studies also suggest that familial resemblance for externalizing liability is mainly genetic (Krueger et al. 2002; Kendler et al. 2003; Hicks et al. 2004). Further evidence supporting a relationship between BPD and the externalizing spectrum is the finding of shared genetic risk factors for substance use disorders and BPD both in adolescence (Bornovalova et al. 2013b) and in adulthood (Distel et al. 2012).
The correlation between environmental factors influencing ASPD and BPD was moderate and remained stable over time. This finding is in accordance with previous studies, which indicate that a number of childhood adversities are general risk factors for both ASPD and BPD as well as for other PDs (Johnson et al. 1999; Zanarini, 2000; Cohen, 2008; Beauchaine et al. 2009; Afifi et al. 2011; Paris et al. 2013).
Strengths and limitations
To our knowledge, this is the first population-based longitudinal study of personality disorders based on structured interviews where all DSM criteria were assessed for two non-overlapping periods. Although the time between the interviews was approximately 10 years, the attrition was low with a participation rate of 82.8%.
The following limitations must be taken into consideration in the interpretation of the results. This is a population-based study where few of the participants endorsed a sufficient number of criteria to fulfill categorical diagnoses. To obtain sufficient statistical power to conduct the biometric analyses, we therefore used dimensional measures of the PDs based on number of criteria endorsed at the sub-threshold level or above. We have previously shown that this criterion count indexes the same underlying liability that underlies the fully syndromal diagnoses (Kendler et al. 2008). It is, however, important to note that much of the variance in the data used in these analyses comes from symptoms in individuals who do not meet criteria for a clinical diagnosis. But because our multiple threshold tests indicate that different symptom levels fit on a single continuum, we would expect no qualitative effect differences as mean symptom levels decline. The most probable impact of the low prevalence rates and strongly skewed distribution on the biometric analyses is merely to inflate the CIs.
If the subthreshold criteria are more unreliable than threshold criteria (e.g. due to less temporal stability) this would inflate E estimates at the expense of both A and C. However, we can see no reason why this should selectively bias the relative magnitude of A and C, or mask a true C effect.
Substantial attrition was observed in this sample from the medical birth registry through two questionnaire waves to the first interviews. Detailed previous analyses have, however, shown that cooperation was strongly predicted by female sex, monozygosity and higher educational status, but not by symptoms of psychiatric disorders or substance abuse (Tambs et al. 2009). Attrition from the first to the second wave was low, with a participation rate of 82.2%. There is evidence of somewhat less attrition in the MZF group, but this is unlikely to have influenced the results. The number of single twins increased substantially in wave 2. Given that both twins have to be included to contribute to the biometric analyses, this reduces the statistical power, but is not likely to influence the parameter estimates.
An important limitation is the modest statistical power given our sample size. In particular, our power to detect sex-specific differences in genetic and environmental effects was not sufficient (Sullivan & Eaves, 2002). Although previous twin studies have failed to find either quantitative or qualitative gender differences for antisocial behavior, ASPD or BPD in adulthood (Rhee & Waldman, 2002; Distel et al. 2008; Torgersen et al. 2008), they may exist. Furthermore, our lack of power was also evident in the relatively small differences in AIC value between the best-fitting models, limiting our ability to confidently select the best fitting one.
Our best-fitting model included only genetic and unique environmental effects. Due to the moderate size of our sample and confounding with non-additive genetic effects, we cannot rule out shared environmental effects. However, to our knowledge, previous twin studies of ASPD and BPD in adulthood have not found evidence of shared environmental influences (Torgersen et al. 2000; Distel et al. 2008; Kendler et al. 2008; Bornovalova et al. 2009).
These analyses were limited to the period from early to middle adulthood and can therefore not be generalized to later periods in life. We are not aware of any longitudinal twin studies of ASPD and BPD after middle adulthood, but a study of normal personality in late adulthood found that the genetic influences from 60 to 65 years of age were identical (Johnson et al. 2005).
Conclusion
In a population-based sample of twins, ASPD and BPD traits were moderately stable over a 10-year period from early to middle adulthood. This was largely due to genetic factors which remained highly stable with a genetic correlation over time that did not significantly differ from unity for both disorders. Environmental risk factors were mostly transient, and appear to be the main source of phenotypic change. Genetic liability factors were to a large extent shared by ASPD and BPD at both time points.
Supplementary Material
Acknowledgements
This project was supported by Research Council of Norway grant 196148/V50. Previous collection and analysis of twin data from this project was in part supported by grant MH-068643 from the National Institutes of Health and grants from the Norwegian Research Council, the Norwegian Foundation for Health and Rehabilitation, the Norwegian Council for Mental Health, the Borderline Foundation, and the European Commission. These funding agencies played no role in the design and conduct of the study, its collection, management, analysis, and interpretation of the data or in the preparation, review, or approval of the manuscript. This project was supported by Research Council of Norway grant 196148/V50. Previous collection and analysis of twin data from this project was in part supported by grant MH-068643 from the National Institutes of Health and grants from the Norwegian Research Council, the Norwegian Foundation for Health and Rehabilitation, the Norwegian Council for Mental Health, the Borderline Foundation, and the European Commission.
Footnotes
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291715001117.
Declaration of Interest
None.
References
- Afifi TO, Mather A, Boman J, Fleisher W, Enns MW, Macmillan H, Sareen J. Childhood adversity and personality disorders: results from a nationally representative population-based study. Journal of Psychiatric Research. 2011;45:814–822. doi: 10.1016/j.jpsychires.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Akaike H. Factor-analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Ball JS, Links PS. Borderline personality disorder and childhood trauma: evidence for a causal relationship. Current Psychiatry Reports. 2009;11:63–68. doi: 10.1007/s11920-009-0010-4. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Klein DN, Crowell SE, Derbidge C, Gatzke-Kopp L. Multifinality in the development of personality disorders: a biology x sex x environment interaction model of antisocial and borderline traits. Development and Psychopathology. 2009;21:735–770. doi: 10.1017/S0954579409000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DF, Grilo CM, Edell WS, McGlashan TH. Comorbidity of borderline personality disorder with other personality disorders in hospitalized adolescents and adults. American Journal of Psychiatry. 2000;157:2011–2016. doi: 10.1176/appi.ajp.157.12.2011. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Hicks BM, Iacono WG, McGue M. Stability, change, and heritability of borderline personality disorder traits from adolescence to adulthood: a longitudinal twin study. Development and Psychopathology. 2009;21:1335–1353. doi: 10.1017/S0954579409990186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Hicks BM, Iacono WG, McGue M. Longitudinal twin study of borderline personality disorder traits and substance use in adolescence: developmental change, reciprocal effects, and genetic and environmental influences. Personality Disorders. 2013a;4:23–32. doi: 10.1037/a0027178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Huibregtse BM, Hicks BM, Keyes M, McGue M, Iacono W. Tests of a direct effect of childhood abuse on adult borderline personality disorder traits: a longitudinal discordant twin design. Journal of Abnormal Psychology. 2013b;122:180–194. doi: 10.1037/a0028328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, McGue M, Carter LA, Iacono WG. The different origins of stability and change in antisocial personality disorder symptoms. Psychological Medicine. 2007;37:27–38. doi: 10.1017/S0033291706009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. Child development and personality disorder. Psychiatric Clinics of North America. 2008;31:477–493. doi: 10.1016/j.psc.2008.03.005. vii. [DOI] [PubMed] [Google Scholar]
- Distel MA, Middeldorp CM, Trull TJ, Derom CA, Willemsen G, Boomsma DI. Life events and borderline personality features: the influence of gene-environment interaction and gene-environment correlation. Psychological Medicine. 2011;41:849–860. doi: 10.1017/S0033291710001297. [DOI] [PubMed] [Google Scholar]
- Distel MA, Rebollo-Mesa I, Willemsen G, Derom CA, Trull TJ, Martin NG, Boomsma DI. Familial resemblance of borderline personality disorder features: genetic or cultural transmission? PLoS ONE. 2009;4:e5334. doi: 10.1371/journal.pone.0005334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel MA, Trull TJ, de Moor MM, Vink JM, Geels LM, van Beek JH, Bartels M, Willemsen G, Thiery E, Derom CA, Neale MC, Boomsma DI. Borderline personality traits and substance use: genetic factors underlie the association with smoking and ever use of cannabis, but not with high alcohol consumption. Journal of Personality Disorders. 2012;26:867–879. doi: 10.1521/pedi.2012.26.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel MA, Trull TJ, Derom CA, Thiery EW, Grimmer MA, Martin NG, Willemsen G, Boomsma DI. Heritability of borderline personality disorder features is similar across three countries. Psychological Medicine. 2008;38:1219–1229. doi: 10.1017/S0033291707002024. [DOI] [PubMed] [Google Scholar]
- Distel MA, Willemsen G, Ligthart L, Derom CA, Martin NG, Neale MC, Trull TJ, Boomsma DI. Genetic covariance structure of the four main features of borderline personality disorder. Journal of Personality Disorders. 2010;24:427–444. doi: 10.1521/pedi.2010.24.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton NR, Krueger RF, Keyes KM, Skodol AE, Markon KE, Grant BF, Hasin DS. Borderline personality disorder co-morbidity: relationship to the internalizing-externalizing structure of common mental disorders. Psychological Medicine. 2011;41:1041–1050. doi: 10.1017/S0033291710001662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert U. Enduring psychobiological effects of childhood adversity. Psychoneuroendocrinology. 2013;38:1850–1857. doi: 10.1016/j.psyneuen.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Harris JR, Magnus P, Tambs K. The Norwegian Institute of Public Health twin program of research: an update. Twin Research and Human Genetics. 2006;9:858–864. doi: 10.1375/183242706779462886. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: a twin-family study. Archives of General Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Hopwood CJ, Morey LC, Donnellan MB, Samuel DB, Grilo CM, McGlashan TH, Shea MT, Zanarini MC, Gunderson JG, Skodol AE. Ten-year rank-order stability of personality traits and disorders in a clinical sample. Journal of Personality. 2013;81:335–344. doi: 10.1111/j.1467-6494.2012.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Zanarini MC, Mitchell KS, Choi-Kain LW, Gunderson JG. The contribution of familial internalizing and externalizing liability factors to borderline personality disorder. Psychological Medicine. 2014;44:1–11. doi: 10.1017/S0033291713003140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KC, Prescott CA, Kendler KS. Sex differences in the genetic and environmental influences on the development of antisocial behavior. Development and Psychopathology. 2002;14:395–416. doi: 10.1017/s0954579402002110. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Brown J, Smailes EM, Bernstein DP. Childhood maltreatment increases risk for personality disorders during early adulthood. Archives of General Psychiatry. 1999;56:600–606. doi: 10.1001/archpsyc.56.7.600. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Kasen S, Skodol AE, Hamagami F, Brook JS. Age-related change in personality disorder trait levels between early adolescence and adulthood: a community-based longitudinal investigation. Acta Psychiatrica Scandinavica. 2000;102:265–275. doi: 10.1034/j.1600-0447.2000.102004265.x. [DOI] [PubMed] [Google Scholar]
- Johnson W, McGue M, Krueger RF. Personality stability in late adulthood: a behavioral genetic analysis. Journal of Personality. 2005;73:523–552. doi: 10.1111/j.1467-6494.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- Kandler C, Bleidorn W, Riemann R, Spinath FM, Thiel W, Angleitner A. Sources of cumulative continuity in personality: a longitudinal multiple-rater twin study. Journal of Personality and Social Psychology. 2010;98:995–1008. doi: 10.1037/a0019558. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Czajkowski N, Roysamb E, Tambs K, Torgersen S, Neale MC, Reichborn-Kjennerud T. The structure of genetic and environmental risk factors for DSM-IV personality disorders: a multivariate twin study. Archives of General Psychiatry. 2008;65:1438–1446. doi: 10.1001/archpsyc.65.12.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Knudsen GP, Roysamb E, Neale MC, Reichborn-Kjennerud T. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II disorders. American Journal of Psychiatry. 2011a;168:29–39. doi: 10.1176/appi.ajp.2010.10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Reichborn-Kjennerud T. Borderline personality disorder traits and their relationship with dimensions of normative personality: a web-based cohort and twin study. Acta Psychiatrica Scandinavica. 2011b;123:349–359. doi: 10.1111/j.1600-0447.2010.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzenweger MF. Stability and change in personality disorder features: the Longitudinal Study of Personality Disorders. Archives of General Psychiatry. 1999;56:1009–1015. doi: 10.1001/archpsyc.56.11.1009. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, True WR, Eisen SA, Goldberg J, Meyer JM, Faraone SV, Eaves LJ, Tsuang MT. Differential heritability of adult and juvenile antisocial traits. Archives of General Psychiatry. 1995;52:906–915. doi: 10.1001/archpsyc.1995.03950230020005. [DOI] [PubMed] [Google Scholar]
- McGowan A, King H, Frankenburg FR, Fitzmaurice G, Zanarini MC. The course of adult experiences of abuse in patients with borderline personality disorder and Axis II comparison subjects: a 10-year follow-up study. Journal of Personality Disorders. 2012;26:192–202. doi: 10.1521/pedi.2012.26.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Bacon S, Lykken DT. Personality stability and change in early adulthood: a behavioral genetic analysis. Developmental Psychology. 1993;29:96–109. [Google Scholar]
- Morey LC, Hopwood CJ. Stability and change in personality disorders. Annual Review of Clinical Psychology. 2013;9:499–528. doi: 10.1146/annurev-clinpsy-050212-185637. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6th edn. Richmond VA 23298: Dept. of Psychiatry, Virginia Commonwealth University Medical School; 2003. Box 980126. [Google Scholar]
- Neale MC, Eaves LJ, Kendler KS. The power of the classical twin study to resolve variation in threshold traits. Behavior Genetics. 1994;24:239–258. doi: 10.1007/BF01067191. [DOI] [PubMed] [Google Scholar]
- Nestadt G, Di C, Samuels JF, Bienvenu OJ, Reti IM, Costa P, Eaton WW, Bandeen-Roche K. The stability of DSM personality disorders over twelve to eighteen years. Journal of Psychiatric Research. 2010;44:1–7. doi: 10.1016/j.jpsychires.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J, Chenard-Poirier MP, Biskin R. Antisocial and borderline personality disorders revisited. Comprehensive Psychiatry. 2013;54:321–325. doi: 10.1016/j.comppsych.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality (SIDP-IV) Iowa City: University of Iowa, Department of Psychiatry; 1995. [Google Scholar]
- Reichborn-Kjennerud T, Ystrom E, Neale MC, Aggen SH, Mazzeo SE, Knudsen GP, Tambs K, Czajkowski NO, Kendler KS. Structure of genetic and environmental risk factors for symptoms of DSM-IV borderline personality disorder. JAMA Psychiatry. 2013;70:1206–1214. doi: 10.1001/jamapsychiatry.2013.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychological Bulletin. 2002;128:490–529. [PubMed] [Google Scholar]
- Roysamb E, Kendler KS, Tambs K, Orstavik RE, Neale MC, Aggen SH, Torgersen S, Reichborn-Kjennerud T. The joint structure of DSM-IV Axis I and Axis II disorders. Journal of Abnormal Psychology. 2011;120:198–209. doi: 10.1037/a0021660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberg JL, Rutter M, Tracy K, Maes HH, Eaves L. Etiological heterogeneity in the development of antisocial behavior: the Virginia Twin Study of Adolescent Behavioral Development and the Young Adult Follow-Up. Psychological Medicine. 2007;37:1193–1202. doi: 10.1017/S0033291707000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Eaves LJ. Evaluation of analyses of univariate discrete twin data. Behavior genetics. 2002;32:221–227. doi: 10.1023/a:1016025229858. [DOI] [PubMed] [Google Scholar]
- Tambs K, Ronning T, Prescott CA, Kendler KS, Reichborn-Kjennerud T, Torgersen S, Harris JR. The Norwegian Institute of Public Health twin study of mental health: examining recruitment and attrition bias. Twin Research and Human Genetics. 2009;12:158–168. doi: 10.1375/twin.12.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen S, Czajkowski N, Jacobson K, Reichborn-Kjennerud T, Roysamb E, Neale MC, Kendler KS. Dimensional representations of DSM-IV cluster B personality disorders in a population-based sample of Norwegian twins: a multivariate study. Psychological Medicine. 2008;38:1617–1625. doi: 10.1017/S0033291708002924. [DOI] [PubMed] [Google Scholar]
- Torgersen S, Lygren S, Oien PA, Skre I, Onstad S, Edvardsen J, Tambs K, Kringlen E. A twin study of personality disorders. Comprehensive Psychiatry. 2000;41:416–425. doi: 10.1053/comp.2000.16560. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Verges A, Wood PK, Sher KJ. The structure of DSM-IV-TR personality disorder diagnoses in NESARC: a reanalysis. Journal of Personality Disorders. 2013;27:727–734. doi: 10.1521/pedi_2013_27_107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CN, Gunderson JG, Zanarini MC, Hudson JI. Family studies of borderline personality disorder: a review. Harvard Review of Psychiatry. 2003;11:8–19. doi: 10.1080/10673220303937. [DOI] [PubMed] [Google Scholar]
- Zanarini MC. Childhood experiences associated with the development of borderline personality disorder. Psychiatric Clinics of North America. 2000;23:89–101. doi: 10.1016/s0193-953x(05)70145-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.