Abstract
The endothelium is important not only in regulating vascular tone but also in modulating inflammation. Patients with systemic lupus erythematosus (SLE) have deficits in these endothelial functions. Vitamin D is a nuclear hormone that regulates vascular endothelial nitric oxide synthase activity and expression. Many SLE patients have insufficient levels of vitamin D. The effect of this hormone on vascular endothelial function in SLE patients is not known. This study was designed to determine the effect size of repleting vitamin D levels on endothelial function in patients with SLE and vitamin D deficiency. SLE patients with 25(OH) vitamin D (25(OH)D) levels < 20 ng/ml were randomized to oral vitamin D3 (D3) doses that did or did not raise 25(OH)D levels to ≥ 32 ng/ml. Endothelial function was measured with flow-mediated dilation (FMD) before and after 16 weeks of vitamin D3 supplementation. Half of those who achieved 25(OH)D levels of ≥32 ng/ml experienced increases in FMD, while none of those with continued low 25(OH)D levels did. Those with increases in FMD had significantly higher final 25(OH)D levels. Using the effect size from this study, future studies designed to test the effect of repleting 25(OH)D on FMD in vitamin D deficient SLE patients will require 35 patients in each group. These results suggest a potential role for vitamin D in SLE-related endothelial dysfunction and that an adaptive multi-arm treat-to-target serum level trial design may increase the efficiency and likelihood of success of such a study.
Introduction
Systemic lupus erythematosus (SLE) is a prototypic autoimmune inflammatory disease that is marked by endothelial dysfunction (1). Two of the major causes of morbidity and mortality and SLE (cardiovascular disease and inflammation in vital organs (2)) are modulated by endothelial cell function (3, 4).
Endothelial dysfunction, on the other hand, leads to upregulation of adhesion molecules (1, 2) and release of cytokines and chemokines that are essential for tissue infiltration of inflammatory cells (5). These processes are normally regulated by protective endothelial cell nitric oxide synthase (eNOS) activity in the absence of infection or inflammatory stimuli. Nitric oxide (NO) produced by eNOS is a biosensor response that modifies locally expressed signaling proteins in response to signaling for changes in eNOS activity (6–9). Lack of functional eNOS in murine lupus leads to accelerated vascular plaque formation (10). Thus, strategies to improve eNOS function may reduce inflammation in SLE.
Vitamin D deficiency is seen in patients with lupus, with season, glucocorticoid exposure, darker skin, renal disease, and photosensitivity being the strongest predictors of low levels (11, 12). Low 25(OH) vitamin D (25(OH)D) levels are associated with markers of accelerated atherosclerosis in patients with SLE (13) and in the general population (14, 15). This has physiologic importance because vitamin D, a steroid hormone, regulates production of NO by endothelial cells (16), increases eNOS expression (17), and reduces arterial stiffness (18). This enzyme is essential for normal endothelial function by preventing chemokine release, adhesion molecule expression, and oxidation of LDL (19). Vitamin D repletion in non-SLE patients restores endothelial function (20, 21); however, the descriptive study in SLE patients described above (13) does not address whether repleting vitamin D in SLE patients with deficient levels has therapeutic potential. With the hypothesis that restoring 25(OH)D levels would increase endothelial function in SLE, this pilot study was designed to determine the effect size and variability of restorating endothelial function through 25(OH)D repletion in SLE patients with 25(OH)D deficiency.
Methods
Study Design and Endpoints
We hypothesized that 25(OH) vitamin D3 (D3) repletion would improve endothelial function in 25(OH)D deficient lupus patients. For this pilot study, we opted to use a randomized phase II screening design (22). The screening design provides preliminary comparisons of an experimental treatment to an appropriate control, with the idea that the pilot study would provide valuable information to aid in the design of a definitive Phase III trial. The trial was designed to determine the effect of oral vitamin D3 repletion on flow mediated dilation (FMD) in vitamin D deficient SLE subjects. Potential subjects were be screened for inclusion/exclusion criteria, including serum 25(OH)D levels. FMD measurements were performed at the baseline visit and at the week 16 visit. Participants were randomized 1:1 to receive 1 of 2 daily oral D3 doses. Group 1 (controls) received 400 international units (IU) of D3 daily, a dose that often does not replete deficient vitamin D in African-American lupus patients (23, 24). Group 2 (treatment) received 5,000 IU daily. Neither group received additional calcium supplementation. The primary endpoint was change in FMD after 16 weeks of vitamin D supplementation, comparing patients with repleted 25(OH)D levels (≥32 ng/ml) to patients without repleted levels (<32 ng/ml). The target of 32 ng/ml was chosen given the association with suppression of parathyroid hormone and maintaining bone mineral densitiy (25). Slow recruitment prevented enrollment of the planned 16 patients in each arm during the funded trial period. This would have provided sufficient power to see significant changes in FMD between Groups 1 and 2. The study design was changed to a pilot to determine effect size rather than to demonstrate efficacy. A secondary endpoint was change in 25(OH)D levels in patients who did or did not have positive changes in FMD after 16 weeks of study drug.
Study Population
All human subjects experimentation was conducted in accordance with the guiding principles in the Helsinki Declaration. Institutional Review Board approval was obtained and informed consent obtained from all participants prior to screening. To be enrolled, patients were required to meet at least 4 1997 American College of Rheumatology Classification Criteria for SLE (26), be greater than 18 years old, and be able to provide informed consent. Subjects were recruited from the Medical University of South Carolina rheumatology clinics. To be included, a screening 25(OH)D level ≤ 20 ng/ml was required, as this is well below the 32 ng/ml known to suppress secondary hyperparathyroidism (25). Subjects were who were on immunomodulatory agents (mycophenolate mofetil, leflunomide, methotrexate, mycophenolic acid, azathioprine, cyclosporine, tracrolimus, or hydroxychloroquine) were (if clinically indicated) required to remain on stable doses for 2 months prior to the study and remain on a stable dose throughout the study. They were taking ≤ 20 mg of prednisone or equivalent corticosteroid daily.
To exclude large effects of disease activity on endothelial function, those with an SLE Disease Activity Index (SLEDAI) score of ≥ 4 were excluded. Initially, subjects were excluded if they took > 800 IU of vitamin D, fish oil supplementation, or statins (27, 28). These exclusions significantly hampered recruitment, so they were removed. However, subjects were asked to continue stable doses of these medications throughout the study. Patients who had a clinical cardiovascular event (myocardial infarction, peripheral vascular disease, cerebrovascular event), were taking nitrates, were pregnant, or used tobacco products were excluded. Patients with baseline hypercalcemia (serum calcium > 10.4) or hypercalcuria (calcium/creatinine > 0.8), known hyperparathyroidism, a history of renal stones, a history of cancer not in remission, known chronic viral or mycobacterial infections, chronic kidney disease stage III or greater, or uncontrolled medical disease were excluded.
Patient Recruitment
Subjects were pre-screened for recruitment by automated reporting from the MUSC Clinical Data Warehouse. Report inclusion criteria were an ICD-9 diagnosis code of SLE (710.0) and 25(OH)D level of < 20 ng/ml in standard of care testing.
Randomization and vitamin D3 supplementation
Subjects were randomized once eligibility was confirmed. Randomization tables were constructed using Proc Plan in SAS® and were coordinated by the study biostatistician and project manager. At the baseline visit and week 8 visit, subjects were given a 3 month supply of oral vitamin D capsules (cholecalciferol, D3). The identical appearing capsules (donated by International Vitamin Corporation, Freehold, NJ, with certificates of analysis demonstrating ≥100% levels of active drug) were dispensed by a non-blinded coordinator. Drug was given to the patient by a blinded coordinator, and the investigator was blinded to randomization. A novel definition for adherence using serum 25(OH)D level based on both the absolute change in 25(OH)D over the study period and the subject’s steady-state variation in their 25(OH)D levels were also used to evaluate adherence, following a previously developed method (29).
Measurement of flow mediated dilation
A single ultrasound operator performed the measurements for this study using a Phillips iU22 Ultrasound system with EKG gating and a L9-3 mHz probe in 2D mode. A quality control study was performed on an initial 10 subjects to ensure acceptable correlations between repeated measures before proceeding to the experimental trial. The repeat readings correlated well (r = 0.79, p = 0.007). Subjects fasted for at least 8 hours before the study and were examined in in a quiet, dimly lit, and temperature controlled room. Subjects remained in the recumbent position for 10 minutes prior to the measurement. Baseline measures of brachial artery diameter was made after the 10 minutes of rest. The blood pressure cuff, placed on the ipsilateral forearm, was inflated to 50 mmHg above the patient’s systolic blood pressure for 5 minutes and then released. Endothelium-dependent FMD was measured continuously for 3 minutes after cuff release. Subjects rested for 10 minutes. Then, endothelium-independent dilation was measured 3 minutes after administration of 0.4 mg of sublingual nitroglycerin. Waveform analysis was performed as described by a reviewer blinded to patient treatment or visit number (30, 31) using Vascular Tools Brachial Analyzer for Research version 5.10.8 (Medical Imaging Applications, LLC).
Clinical assessment
Because lupus disease activity could confound the FMD outcome, patients were required to have inactive disease qualified using the SELENA-SLEDAI index (32). Complete metabolic panels (including calcium), complete blood counts, urinalysis, urine protein/creatinine/calcium, serum C3/C4/anti-dsDNA antibodies and total 25(OH)D levels were determined by the accredited MUSC Clinical Laboratory using standard methodology and quality control methods.
Data collection and management
Study data were collected and managed using (Research Electronic Data Capture) REDCap electronic data capture tools hosted at MUSC (33). REDCap is a secure, web-based application designed to support data capture for research studies. De-identified data were exported for analysis using IBM SPSS Statistics version 22.
Statistical analysis
For the primary analysis, patients were divided into 2 groups based on vitamin D status at 16 weeks: repleted or non-repleted. The initial primary endpoint was change in FMD after 16 weeks of treatment. Descriptive statistics were used to characterize the treatment groups with respect to final change in 25(OH)D levels with treatment. A secondary endpoint was added to compare changes in 25(OH)D levels between patients that did and did not have positive changes in FMD. For the primary and secondary endpoints, comparisons between groups were conducted using t-tests, as data were distributed normally by the Kolmogorov-Smirnov normality test. The effect size for change in FMD with vitamin D repletion was calculated using the Cohen’s d calculation (34) and a pooled standard deviation (35). Determination of associations between 25(OH)D repletion and increase in FMD was performed using Fisher exact testing due to the low numbers of patients in each group.
Results
Patient population
Due to restrictive inclusion criteria, recruitment fell below the desired 32 subjects pre-specified by the power analysis. Seventy-two SLE patients were identified by ICD-9 code and 25(OH)D level for potential recruitment into the trial. Of these, 56 failed further pre-screening based on disease activity, medication use, and known cardiovascular disease. Of the 16 who underwent a screening visit, 7 patients failed to meet criteria for enrollment. Nine patients met inclusion and exclusion criteria and were randomized to treatment (n=6) or control (n=3). The study population characteristics are reported in Table I. Subjects were divided into those achieving 25(OH)D levels ≥ 32 ng/ml (repleted) or those with 25(OH)D levels < 32 ng/ml (not repleted) at Week 16. This pharmacologic definition of groups corresponded exactly with treatment groups, as all of the low dose D3 patients failed to replete. The average pill adherence in both groups by pill count was 90 ± 4%. As expected, those who received the high dose of D3 had a significantly larger increases in serum 25(OH)D levels. Those in the repleted group were significantly older.
Table I.
Characteristics of study population by repletion status
| Not Repleted (n=3) * | Repleted (n=6) * | p value | |
|---|---|---|---|
| age | 34 ± 4 | 49 ± 3 | 0.02 |
| % female | 100 | 100 | 1 |
| % African-American | 100 | 83 | 0.67 |
| BMI | 28 ± 3 | 30 ± 3 | 0.56 |
| Waist/Hip ratio | 0.78 ± 0.01 | 0.78 ± 0.09 | 0.97 |
| 25(OH)D baseline | 14 ± 2 | 15 ± 1 | 0.76 |
| change in 25(OH)D | 10 ± 4 | 33 ± 3 | 0.005 |
| change in systolic blood pressure | 0.3 ± 3 | 10 ± 22 | 0.51 |
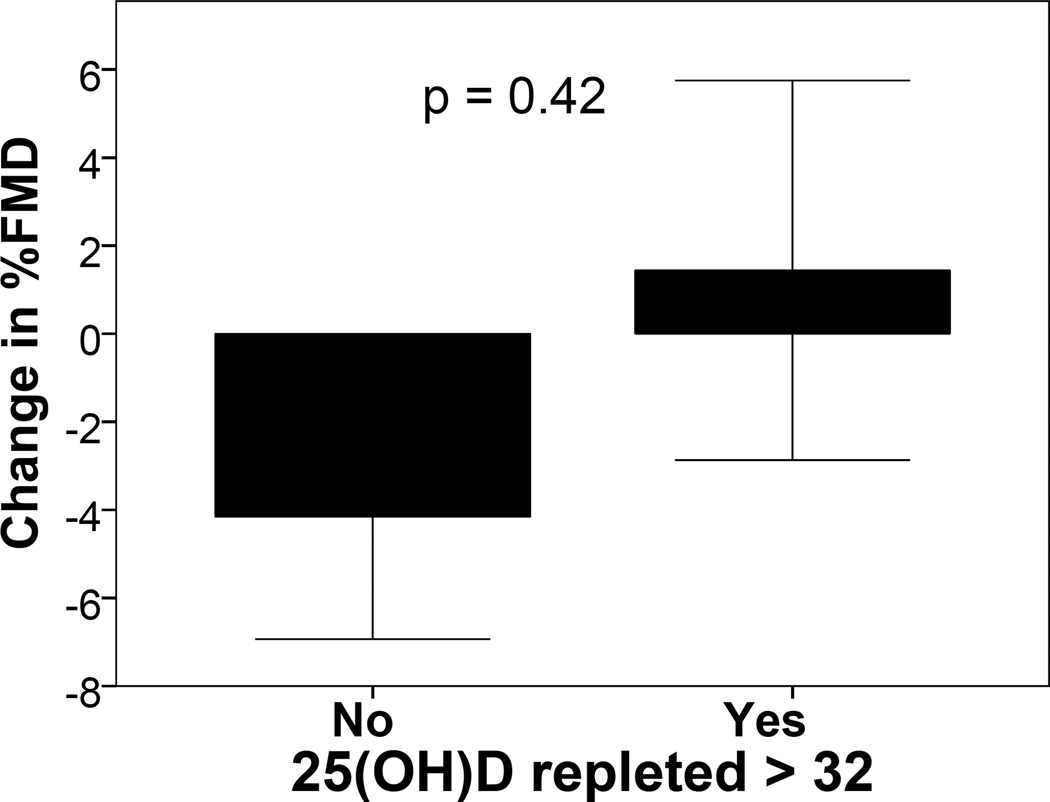
| Change in % FMD | −4.2 ± 2.7 | 1.4 ± 4.3 | 0.42 |
mean ± standard error or percent
Primary endpoint: Calculation of effect size for change in FMD with 25(OH)D repletion
The initial primary endpoint of differences in change in FMD between treatment and control groups from the baseline to the final visit was not achieved. The correlation between the final 25(OH)D level and change in FMD was 0.44 (p = 0.24), providing a trend toward an association between change in vitamin D stores and this surrogate of eNOS function in the vasculature. While those in the repleted group tended to have greater reductions in FMD (Figure 1), the relative 5.6% difference was not statistically significant. The new primary outcome of determining effect size for change in FMD with 25(OH)D repletion was achieved. The Cohen’s d effect size was 0.68, with an effect-size r of 0.32. Based on this effect size, a sample size analysis was performed to determine the size of a subsequent trial to test efficacy. To detect significant differences between 2 groups, assuming 1) normally distributed data, 2) the same effect size, 3) a significance level of 0.05, and 4) a power of 0.8, 35 patients in each group would be required.
Figure 1. Change in %FMD by treatment group.
Vitamin D deficient SLE patients were randomized to doses of D3 that did and did not replete 25(OH)D levels. FMD was measured before and after 16 weeks of treatment. Results are reported as change in %FMD by treatment group.
Secondary endpoint: Change in 25(OH)D levels in patients with and without positive changes in FMD
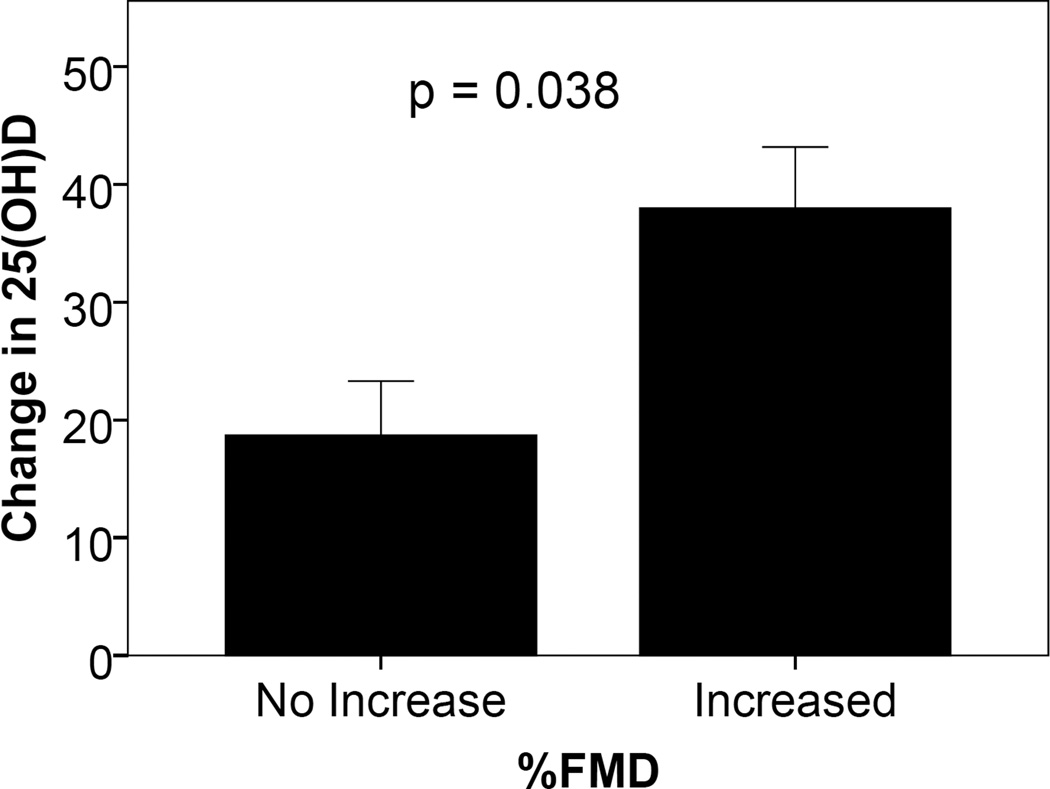
Subjects were divided into 2 groups based on whether there was a positive or negative change in FMD from baseline to after 16 weeks of D3 therapy. All patients in the control group failed to have an increase in FMD at the end of treatment, while 3 of 6 in the treatment group had a significant increase FMD (Table II). By Fisher’s exact test, the 1-sided significance of this association was 0.24. The secondary endpoint of change in 25(OH)D levels between the 2 groups was achieved. Those who achieved increases in FMD had significantly greater changes in 25(OH)D levels from baseline to week 16 (Figure 2) and greater week 16 25(OH)D levels (52 ± 4 vs. 34 ± 5 ng/ml, p = 0.44). This suggests that levels close to those considered low normal (32 ng/ml) may not be adequate in all patients to achieve increases in FMD.
Table II.
Vitamin D repletion status by increase in FMD
| FMD increased | Vitamin D repleted | ||
| No | Yes | ||
| Yes | 0 | 3 | |
| No | 3 | 3 | |
Figure 2. Change in 25(OH)D levels in SLE patients with and without increase in % FMD with D3 therapy.
SLE patients randomized to take low or adequate D3 doses daily for 16 weeks had FMD measured at baseline and 16 weeks. Results are reported as change in 25(OH)D levels over 16 weeks in those who had increases or no increases in FMD during the study period.
Discussion
This pilot study was designed to determine the effect of vitamin D repletion on a clinical measure of endothelial function in vitamin D deficient patients with SLE. Due to low enrollment, there was inadequate power to meet this endpoint. However, from the resulting data, one can estimate a relatively medium effect size of 0.32. Even with this small number of patients, there was a trend toward significant increases in FMD among those treated with 5,000 IU D3 daily for 16 weeks compared to controls. The relative difference in FMD (5.6%) between the two groups was potentially clinically significant, as a 1% change in FMD by the distal cuff measure used in this study is associated with 9% reduction in risk for cardiovascular events (36). These data suggest a number needed to treat for improving FMD of 2, as 50% of those in the high dose and none of those in the low dose group had increases in FMD. However, this estimation is based on a very small number of patients. The 25(OH)D levels seen in patients who had increases in FMD was higher than that necessary to suppress parathyroid hormone (37). This opens the possibility that higher target levels may be required to achieve improvements in FMD in future studies. The revised endpoint of the pilot study was achieved and indicated that 35 subjects in each group would be required to detect significant differences between groups in a similarly designed study.
The concept of restoring endothelial function by repleting vitamin D stores in patients with vitamin D deficiency is not new. Sugden et al demonstrated significant improvements in FMD with vitamin D repletion in diabetics (38). The vitamin D receptor can be detected in endothelial cells, and 25(OH)D is hydroxylated to 1,25(OH)D in several tissues including the endothelium (39, 40). 1,25(OH)D is a steroid hormone that acts in a vitamin D receptor-dependent and –independent fashion. At concentrations present in vivo, increases expression of the eNOS gene and NO production in endothelial cells can be seen. This increase occurs in the presence of phosphorylation of eNOS, possibly through signaling through the vitamin D receptor (16).
The causes of endothelial dysfunction in SLE are likely to be heterogeneous. Several investigators have isolated factors in lupus serum that could induce endothelial cell dysfunction. Among these include oxidized LDL, type I interferons, pro-inflammatory HDL (4), toll-like receptor (TLR) 7 and 9 activation by RNA and DNA containing immune complexes (41), calprotectin (42) (a TLR4 agonist (43)), agiopoeitin-2 (44), Fc receptor activation from immune complexes (45), complement activation (4), anti-endothelial cell antibodies (4) and anti-phospholipid antibodies (46). Targeted interventions for these factors have yet to be proven to restore endothelial function in SLE patients. However, therapies targeting dysfunctional eNOS activity have the potential to be effective in SLE despite heterogeneous extracellular causes of eNOS dysfunction.
The trend toward positive results in this study are seemingly in contrast to a study performed by Kiani et al. In that study, vitamin D deficiency did not predict progression of coronary calcium or carotid intima media thickness (IMT) over 2 years (47). However, the endpoints chosen were long-term consequences of endothelial dysfunction. Detection of differences in these measures between treatment groups is difficult in such a small number of patients. For instance, Spence and colleagues estimate that 300 patients would be required to see a significant change in IMT with therapy over 2 years (48). Others have observed lack of association in coronary calcium score progression and statin use in non-lupus patients, while measures of plaque volume can detect differences between statin and non-statin treatment groups in 50 patients per group in a trial spanning less than 6 months (48). Finally, the above study (47) was not a randomized controlled trial to test the effect of repletion of vitamin D in SLE patients, opening the possibility of unintended confounding factors influencing the outcomes. FMD is a good a surrogate precursor of atherosclerosis and cardiovascular events (49) for proof-of-concept studies. It is possible that our initial measures of 25(OH)D are confounded by the season in which levels were determined. In our previously published study of SLE patients, seasonal variation did not contribute to models predictive of atherosclerosis (13). In this larger study, levels from patients sampled in months with days longer than nights were no different than those sampled in the remaining months (25(OH)D 21 ± 2 vs 20 ± 22 ng/ml, p = 0.7). The current study was designed to supplement 25(OH)D above 32 ng/ml in young adults based on published studies indicating that parathyroid hormone is suppressed above this level. In our previously published study (13), all patients with hyperparathyroidism had levels < 31 ng/ml (data not shown), suggesting that this was a reasonable treatment target in this mostly African-American lupus population. However, the level that is optimal to restore endothelial function may differ from that which suppresses parathyroid hormone.
This is the first study to test the effect of vitamin D repletion on FMD in patients with SLE. Whether vitamin D repletion in SLE patients will improve cardiovascular or renal outcomes is not known. However, a logical initial trial design would be to test whether this intervention improves FMD. This study informs the design of such a trial. The results of this study suggest that future trials should consider multiple treat-to-target 25(OH)D goals (> 32 and > 50 ng/ml, for instance) in the treatment arms rather than fixed dosing of D3. An a priori adaptive trial design could, while maintaining the blind, allow for treat-to-target dose escalation and/or shift randomization into the treatment group most likely to achieve target levels of 25(OH)D, thus reducing the number of patients and length of time required to perform the study.
Acknowledgements
This study would not have been possible without the coordination efforts of Abigail Powell and Lori Ueberroth, repeated FMD measures by Marge Cappuccio, and measurement of study endpoints by Jon Donohue. Special thanks go to Amy Wahlquist, who assisted with study design before funding but who was not available to participate in authoring the manuscript.
Source of Funding
This project was supported by a grant from the Arthritis Foundation. This material is the result of work supported with resources and the use of facilities at the Ralph H. Johnson VA Medical Center, NIH/NCRR MUSC-SCTR Grant number UL1 RR029882, NIH/NIAMS Grant number K23 AR052364, and the MUSC General and Clinical Research Center (M01RR001070).
Abbreviations
- 25(OH)D
25(OH) vitamin D
- D3
vitamin D3
- DNA
deoxyribonucleic acid
- eNOS
endothelial nitric oxide synthase
- Fc
fragment, crystalizable
- FMD
flow-mediated dilation
- IU
international units
- LDL
low density lipoprotein
- NO
nitric oxide
- REDCap
research electronic data capture
- RNA
ribonucleic acid
- SELEDA-SLEDAI
Safety of Estrogens in Lupus Erythematosus National Assessment-SLE Disease Activity Index
- SLE
systemic lupus erythematosus
- SLEDAI
SLE Disease Activity Index
- TLR
toll-like receptor
Footnotes
Conflicts of Interest
None of the authors have financial conflicts of interest to declare.
References
- 1.El-Magadmi M, Bodill H, Ahmad Y, et al. Systemic lupus erythematosus: an independent risk factor for endothelial dysfunction in women. Circulation. 2004;110:399–404. doi: 10.1161/01.CIR.0000136807.78534.50. [DOI] [PubMed] [Google Scholar]
- 2.Urowitz MB, Bookman AA, Koehler BE, et al. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med. 1976;60:221–225. doi: 10.1016/0002-9343(76)90431-9. [DOI] [PubMed] [Google Scholar]
- 3.Turner E, Dishy V, Chung CP, et al. Endothelial function in systemic lupus erythematosus: relationship to disease activity, cardiovascular risk factors, corticosteroid therapy, and coronary calcification. Vasc Health Risk Manag. 2005;1:357–360. doi: 10.2147/vhrm.2005.1.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahlenberg JM, Kaplan MJ. The interplay of inflammation and cardiovascular disease in systemic lupus erythematosus. Arthritis Res Ther. 2011;13:203–212. doi: 10.1186/ar3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 6.Berendji-Grun D, Kolb-Bachofen V, Kroncke KD. Nitric oxide inhibits endothelial IL-1[beta]-induced ICAM-1 gene expression at the transcriptional level decreasing Sp1 and AP-1 activity. Mol Med. 2001;7:748–754. [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SK, Kim JH, Yang WS, et al. Exogenous nitric oxide inhibits VCAM-1 expression in human peritoneal mesothelial cells. Role of cyclic GMP and NF-κB. Nephron. 2002;90:447–454. doi: 10.1159/000054733. [DOI] [PubMed] [Google Scholar]
- 8.Kelleher ZT, Matsumoto A, Stamler JS, et al. NOS2 regulation of NF-kappaB by S-nitrosylation of p65. J Biol Chem. 2007;282:30667–30672. doi: 10.1074/jbc.M705929200. [DOI] [PubMed] [Google Scholar]
- 9.Marshall HE, Stamler JS. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry. 2001;40:1688–1693. doi: 10.1021/bi002239y. [DOI] [PubMed] [Google Scholar]
- 10.Al Gadban MM, German J, Truman J-P, et al. Lack of nitric oxide synthases increases lipoprotein immune complex deposition in the aorta and elevates plasma sphingolipid levels in lupus. Cell Immunol. 2012;276:42–51. doi: 10.1016/j.cellimm.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamen DL, Cooper GS, Bouali H, et al. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev. 2006;5:114–117. doi: 10.1016/j.autrev.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Toloza SM, Cole DE, Gladman DD, et al. Vitamin D insufficiency in a large female SLE cohort. Lupus. 19:13–19. doi: 10.1177/0961203309345775. [DOI] [PubMed] [Google Scholar]
- 13.Ravenell RL, Kamen DL, Spence JD, et al. Premature atherosclerosis is associated with hypovitaminosis D and angiotensin-converting enzyme inhibitor non-use in lupus patients. Am J Med Sci. 2012;344:268–273. doi: 10.1097/MAJ.0b013e31823fa7d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds JA, Haque S, Berry JL, et al. 25-Hydroxyvitamin D deficiency is associated with increased aortic stiffness in patients with systemic lupus erythematosus. Rheumatology (Oxf) 2012;51:544–551. doi: 10.1093/rheumatology/ker352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lertratanakul A, Wu P, Dyer A, et al. 25-hydroxyvitamin D and cardiovascular disease in patients with systemic lupus erythematosus: data from a large international inception cohort. Arthritis Care Res (Hoboken) 2014;66:1167–1176. doi: 10.1002/acr.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molinari C, Uberti F, Grossini E, et al. 1alpha,25-dihydroxycholecalciferol induces nitric oxide production in cultured endothelial cells. Cell Physiol Biochem. 2011;27:661–668. doi: 10.1159/000330075. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Miguel P, Valdivielso JM, Medrano-Andres D, et al. The active form of vitamin D, calcitriol, induces a complex dual upregulation of endothelin and nitric oxide in cultured endothelial cells. Am J Physiol Endocrinol Metab. 2014;307:E1085–E1096. doi: 10.1152/ajpendo.00156.2014. [DOI] [PubMed] [Google Scholar]
- 18.Andrukhova O, Slavic S, Zeitz U, et al. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol Endocrinol. 2014;28:53–64. doi: 10.1210/me.2013-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaul PW. Endothelial nitric oxide synthase, caveolae and the development of atherosclerosis. J Physiol. 2003;547:21–33. doi: 10.1113/jphysiol.2002.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarcin O, Yavuz DG, Ozben B, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–4030. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 21.Dong Y, Stallmann-Jorgensen IS, Pollock NK, et al. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab. 2010;95:4584–4591. doi: 10.1210/jc.2010-0606. [DOI] [PubMed] [Google Scholar]
- 22.Rubinstein LV, Korn EL, Freidlin B, et al. Design issues of randomized phase II trials and a proposal for phase II screening trials. Journal of Clinical Oncology. 2005;23:7199–7206. doi: 10.1200/JCO.2005.01.149. [DOI] [PubMed] [Google Scholar]
- 23.Gilkeson G, James J, Kamen D, et al. The United States to Africa lupus prevalence gradient revisited. Lupus. 2011;20:1095–1103. doi: 10.1177/0961203311404915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamen DL, Meyer AK, Parker TM, et al. Repletion of vitamin D deficiency is safe and well- tolerated among African Americans (AAs) with SLE. Lupus. 2010;19 [Google Scholar]
- 25.Hollis BW. Circulating 25-Hydroxyvitamin D Levels Indicative of Vitamin D Sufficiency: Implications for Establishing a New Effective Dietary Intake Recommendation for Vitamin D. J Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 26.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40 doi: 10.1002/art.1780400928. 1725-. [DOI] [PubMed] [Google Scholar]
- 27.Wright SA, O'Prey FM, McHenry MT, et al. A randomised interventional trial of omega-3-polyunsaturated fatty acids on endothelial function and disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2008;67:841–848. doi: 10.1136/ard.2007.077156. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira GA, Navarro TP, Telles RW, et al. Atorvastatin therapy improves endothelial-dependent vasodilation in patients with systemic lupus erythematosus: an 8 weeks controlled trial. Rheumatology. 2007;46:1560–1565. doi: 10.1093/rheumatology/kem186. [DOI] [PubMed] [Google Scholar]
- 29.Appelgren KE, Nietert PJ, Hulsey TC, et al. Analyzing adherence to prenatal supplement: does pill count measure up? Int J Endocrinol. 2010;2010:631971. doi: 10.1155/2010/631971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peretz A, Leotta D, Sullivan J, et al. Flow mediated dilation of the brachial artery: an investigation of methods requiring further standardization. BMC cardiovascular disorders. 2007;7:11. doi: 10.1186/1471-2261-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 32.Thanou A, Chakravarty E, James JA, et al. How should lupus flares be measured? Deconstruction of the Safety of Estrogen in Lupus Erythematosus National Assessment–Systemic Lupus Erythematosus Disease Activity Index flare index. Rheumatology. 2014 doi: 10.1093/rheumatology/keu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, N.J.: L. Erlbaum Associates; 1988. [Google Scholar]
- 35.Rosnow RL, Rosenthal R. Computing contrasts, effect sizes, and counternulls on other people's published data: General procedures for research consumers. Physiol Methods. 1996;14:331–340. [Google Scholar]
- 36.Green DJ, Jones H, Thijssen D, et al. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57:363–369. doi: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]
- 37.Hollis BW, Wagner CL, Drezner MK, et al. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: An important tool to define adequate nutritional vitamin D status. J Steroid Biochem Mol Biol. 2007;103:631–634. doi: 10.1016/j.jsbmb.2006.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugden JA, Davies JI, Witham MD, et al. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–325. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 39.Zehnder D, Bland R, Chana RS, et al. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13:621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 40.Merke J, Milde P, Lewicka S, et al. Identification and regulation of 1,25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. The Journal of clinical investigation. 1989;83:1903–1915. doi: 10.1172/JCI114097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirou KA, Lee C, George S, et al. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 42.Yu Y, Su K. Neutrophil Extracellular Traps and Systemic Lupus Erythematosus. J Clin Cell Immunol. 2013;4 doi: 10.4172/2155-9899.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim F, Pham M, Luttrell I, et al. Toll-Like Receptor-4 Mediates Vascular Inflammation and Insulin Resistance in Diet-Induced Obesity. Circ Res. 2007;100:1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 44.El-Banawy HS, Gaber EW, Maharem DA, et al. Angiopoietin-2, endothelial dysfunction and renal involvement in patients with systemic lupus erythematosus. J Nephrol. 2012;25:541–550. doi: 10.5301/jn.5000030. [DOI] [PubMed] [Google Scholar]
- 45.Mineo C, Gormley AK, Yuhanna IS, et al. FcgammaRIIB mediates C-reactive protein inhibition of endothelial NO synthase. Circ Res. 2005;97:1124–1131. doi: 10.1161/01.RES.0000194323.77203.fe. [DOI] [PubMed] [Google Scholar]
- 46.Belizna C, Lartigue A, Favre J, et al. Antiphospholipid antibodies induce vascular functional changes in mice: a mechanism of vascular lesions in antiphospholipid syndrome? Lupus. 2008;17:185–194. doi: 10.1177/0961203307086931. [DOI] [PubMed] [Google Scholar]
- 47.Kiani AN, Fang H, Magder LS, et al. Vitamin D deficiency does not predict progression of coronary artery calcium, carotid intima-media thickness or high-sensitivity C-reactive protein in systemic lupus erythematosus. Rheumatology (Oxf) 2013;52:2071–2076. doi: 10.1093/rheumatology/ket271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spence JD. Measurement of Carotid Plaque Burden. JAMA Neurol. 2015 doi: 10.1001/jamaneurol.2014.3002. [DOI] [PubMed] [Google Scholar]
- 49.Shechter M, Shechter A, Koren-Morag N, et al. Usefulness of brachial artery flow-mediated dilation to predict long-term cardiovascular events in subjects without heart disease. Am J Cardiol. 2014;113:162–167. doi: 10.1016/j.amjcard.2013.08.051. [DOI] [PubMed] [Google Scholar]