Abstract
The selection of appropriate responses is a complex endeavor requiring the integration of many different sources of information in fronto-striatal-thalamic circuits. An often neglected but relevant piece of information is provided by proprioceptive inputs about the current position of our limbs. This study examines the importance of striatal and thalamic GABA levels in these processes using GABA-edited magnetic resonance spectroscopy (GABAMRS) and a Simon task featuring proprioception-induced interference in healthy subjects. As a possible model of deficits in the processing of proprioceptive information, we also included Parkinson's disease (PD) patients in this study.
The results show that proprioceptive information about unusual postures complicates response selection processes in controls, but not in PD patients. The well-known deficits of PD patients in processing proprioceptive information can turn into a benefit when altered proprioceptive information would normally complicate response selection processes. Striatal and thalamic GABA levels play dissociable roles in the modulation of response selection processes by proprioceptive information: Striatal GABA levels seem to be important for the general speed of responding, most likely because striatal GABA promotes response selection. In contrast, the modulation of response conflict by proprioceptive information is closely related to thalamic GABA concentrations with higher concentration being related to a smaller response conflict effect. The most likely explanation for this finding is that the thalamus is involved in the integration of sensorimotor, attentional, and cognitive information for the purpose of response formation. Yet, this effect in the thalamus vanishes when controls and PD patients were analyzed separately.
Keywords: striatum, thalamus, GABA, proprioception, response selection, sensorimotor integration, Parkinson's disease
1. Introduction
Fronto-striatal-thalamic circuits are of tremendous importance for response selection and sensorimotor integration processes (Bolam et al., 2000; Middleton and Strick, 2000; Reig and Silberberg, 2014). One of the reasons why response selection processes are quite complex is that the representation of most of our actions and goals comprises several aspects that include different forms of information (Stock et al., 2013). In this context, proprioceptive information about the position / posture of our limbs is an often-neglected aspect that has nevertheless been demonstrated to be of importance (Stock and Beste, 2014; Stock et al., 2013). It has been shown that proprioceptive information on an unusual posture / limb position increases the difficulty to select an appropriate response, especially when there is a conflict to mapping a stimulus onto the appropriate response (Leuthold, 2011; Stock and Beste, 2014; Stock et al., 2013; Wascher et al., 2001). Such conflict effects can, for example, be induced in the Simon Task (e.g. Keye et al., 2013). There, responses are faster and less error-prone in case the task-irrelevant stimulus location corresponds to the location of the (correctly) responding effector, whereas responses are slowed down in case the locations of the stimulus and the responding effector mismatch, thus inducing a conflict (= Simon effect) (e.g. Keye et al., 2013). The size of the Simon effect reflects the extra demands and time required to suppress the interference caused by the incorrect response activation produced in non-corresponding trials that are absent in corresponding trials (Ridderinkhof, 2002; Wylie et al., 2010a). When proprioceptive information is altered (i.e., hand positions are varied) these correspondence effects are also varied (Stock et al., 2013): When an unusual hand position (i.e. crossed hands) is induced, correspondence effects are increased as compared to usual hand positions (i.e. parallel hands), suggesting that altered proprioceptive information complicates and hence slows down response selection processes (Stock et al., 2013).
For response selection processes, it has been suggested that the striatal GABAergic system plays an important role in the selection of appropriate responses. At the striatal level, the GABAergic neurotransmission is considered of importance, because it is assumed to constitute a winner-takes-all (WTA) network that is implemented via medium spiny neurons (MSN) (e.g. Bar-Gad et al., 2003; Bolam et al., 2000; Plenz, 2003). It has been shown that a high integrity of the WTA network leads to fast response selection and execution (e.g. Beste and Saft, 2015; Beste et al., 2012; Willemssen et al., 2011) and it has furthermore already been shown that high striatal GABA levels increase efficiency in response selection (Yildiz et al., 2014). Currently, it is however unknown if the fronto-striatal-thalamic GABAergic system also plays a role in modulatory effects of proprioceptive information on response selection processes. If so, it is likely that this is the case especially in response selection processes that are conflicting. In the current study, we use GABA-edited magnetic resonance spectroscopy (MRS) (Mullins et al., 2014) to investigate this question.
Aside from striatal structures, the thalamus has been suggested to play an important role in the processing of proprioceptive information (Lalonde and Strazielle, 2007; Müller et al., 2013). Moreover, thalamic structures are important for attentional orienting and feature-integration functions (e.g. Kim, 2014; Ruhl and Dicke, 2012; Salmi et al., 2007; Schneider, 2011; Yang and Mayer, 2014). These functions are of importance since congruency/correspondence effects in the Simon task (see above) not only depend on response selection. Instead, it also depends on attentional orienting processes because different stimuli signaling for distinct responses have to be integrated with information on the spatial position of these stimuli (for review: Hommel, 2011). Consequently, functional imaging evidence suggests that thalamic processing is important for performance in the Simon task (Rubia et al., 2011). We will therefore also investigate the role of the thalamic GABAergic system for the above-mentioned modulations of sensorimotor integration processes by proprioceptive information using GABA-MRS.
One possible means to deepen insights into the role of proprioceptive information for sensorimotor integration processes (as induced in the Simon task) and the relevance of the fronto-striatal-thalamic GABAergic system is to investigate the effects of Parkinson's disease (PD). The reason for this is that PD patients are well-known to have an increased threshold for the processing of proprioceptive information (Conte et al., 2013). PD seems particularly useful in this context since the cognitive deficits observed in this disease strongly depend on fronto-striatal-thalamic circuits (e.g. Kehagia et al., 2013). Moreover, response selection in the Simon task has been shown to be altered in PD (Fielding et al., 2005; Plessow et al., 2014; Praamstra and Plat, 2001; van Wouwe et al., 2014; Wylie et al., 2012, 2010b), but no study has yet examined the effects of proprioceptive information. In the current study, we therefore examine PD patients as a possible model of altered proprioceptive information processing thresholds. We hypothesize that because the threshold for proprioceptive information is increased, modulations of proprioceptive information should have smaller effects on sensorimotor integration processes in PD than in controls. This would imply that a deficit associated with PD can also be advantageous in some situations.
2. Materials and Methods
2.1 Sample
Nineteen subjects with mild-to-moderate PD (mean age ± standard deviation: 63.68 ± 9.12 y, 10 male, no dementia, UPDRS-III score off medication: 33.34 ± 10.9) and eighteen healthy controls (mean age ± standard deviation: 59.63 ± 10.24 y, 11 male, UPDRS-III score: 5.43 ± 3.36) were recruited for the study. Three PD patients had never used any Parkinson's medication whereas the remaining patients were withheld from taking Parkinson's medication for at least 12 hours before participating in the study. Subjects with a previous history of neurological disorder, dementia, severe rest tremor, claustrophobia, and those taking GABA-ergic drugs were excluded from the study. The range of disease duration of the PD subjects was between 0.75 and 11 years post-diagnosis. Written informed consent approved by the Indiana University Institutional Review Board was obtained from all subjects prior to participation.
2.2 Task
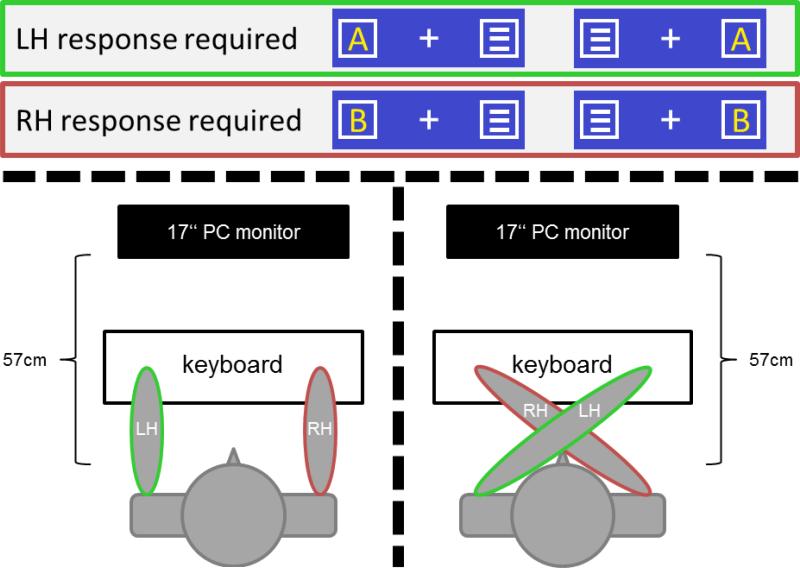
The experimental paradigm is a modified Simon Task, which is identical to a previous study by our group examining the role of proprioceptive information for sensorimotor integration processes (Stock et al., 2013). The experimental setup is shown in Figure 1. For the presentation of stimuli as well as for the recording of the responses (reaction times (RTs) and correctness), Presentation (version 14.9. by Neurobehavioral Systems, Inc.) was used. Responses were collected using a standard computer keyboard. Response buttons were the left and right “Ctrl” keys.
Figure 1.
Experimental setup of the Simon Task. The target stimuli (letters) could be located in either of the boxes as illustrated in the top rows. Letter A required a reaction of the left hand (respective box and limbs edged green) while letter B required a reaction of the right hand (respective box and limbs edged red). The parallel hands condition is shown in the bottom left part of the figure while the crossed hand condition is shown on the bottom right side.
A white fixation cross was continuously displayed in the center of the screen on dark blue background color. Two white frame boxes were laterally presented at the same vertical level as the fixation cross. The distance of the inner border of the two lateralized boxes (one right and one left) from the fixation cross was 1.1 degrees visual angle. Like the fixation cross, the two boxes remained on the screen throughout the experiment. Each trial began with the presentation of a yellow capital letter (A or B) as a target stimulus within one of the two boxes. The opposing box always contained a noise stimulus (three horizontal white bars). Stimuli were approximately 0.5° wide and 0.6° high. These stimuli were presented simultaneously for 200 ms, after which the empty boxes remained on the screen. The study participants (patients and controls) were asked to respond as fast and accurately as possible with the index finger of their left hand, when the letter “A” was presented. When the letter “B” was presented they were required to respond with the index finger of their right hand. These responses were required to be carried out regardless of the target stimulus position on the screen (i.e. in the box left or right of the fixation cross). All trials in which the target stimulus and the correct response button were located in the same hemifield (i.e. on the same side of the body) were classified as spatially correspondent. Hence, all trials in which the stimulus and the button were located in opposing hemifields were classified as spatially non-correspondent.
The first button press after the target onset ended the trial. When the response did not occur within the first 500 ms after the onset of the trial, a speed-up sign („Faster!”) was presented above the stimuli until the end of the trial. If no response was given, the trial automatically ended 1700 ms after its onset and was coded as a „miss”. The trials were separated by response-stimulus intervals (RSIs) during which the fixation cross and the two boxes remained on the screen. The duration of the RSIs varied randomly and ranged between 2000 and 2500 ms. The experiment was made up of eight blocks, each consisting of 100 trials. To vary proprioceptive information, the position of the left and right hand were either parallel to each other (uneven blocks), or the hands were crossed (even blocks) (refer Figure 1). All four conditions (as defined via the spatial stimulus-response (S-R) correspondence of stimulus and response site and hand positions) occurred equally often, resulting in 25 trials per condition and block. The order of the trials was pseudo-randomized. In uneven blocks (blocks 1, 3, 5, and 7), the subjects were instructed to place their arms onto the response panels in parallel so that the left index finger was located on the left response button and the right index finger was located on the right response button. For the even blocks (blocks 2, 4, 6, and 8) the subjects were instructed to cross their arms (with the left arm being on top of the right arm) so that the left index finger was placed on the right response button and vice versa. This setup was identical for controls and PD patients.
2.3 MRS data acquisition and processing
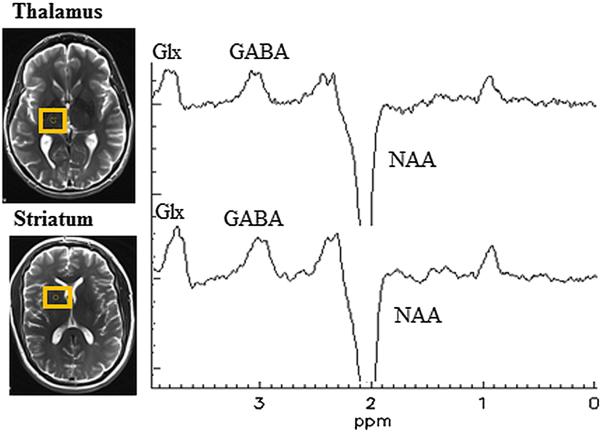
MRI scans were conducted on a whole body 3-T Siemens Magnetom Tim Trio MR scanner (Siemens Healthcare, Erlangen, Germany) using a standard head coil, within 60-90 minutes of performing the Simon test. Fast T2-weighted images (TR/TE= 6000/127 ms) in all three planes were acquired for planning the 25 mm × 30 mm × 25 mm MRS volumes of interest (VOIs) centered on the right thalamus and right striatum (figure 2). High resolution 3D T1-weighted Magnetization Prepared - Rapid Gradient Echo (MP-RAGE) images were also obtained for brain tissue segmentation into white matter (WM), grey matter (GM) and cerebrospinal fluid (CSF). The right hemisphere was chosen in order to be consistent with our earlier studies on manganism (Dydak et al., 2011; Long et al., 2014). Higher order shimming was performed either manually or using FASTMAP (Gruetter, 1993) to achieve a spectral linewidth of < 25 Hz. MEGA-PRESS edited GABA spectra (Mullins et al., 2014; Mescher et al., 1998) were obtained using TR/TE = 2000/68 ms, 256 averages, edit pulse BW = 44 Hz, and edit ON/OFF pulses at 1.9/7.5 ppm. Reference spectra without water suppression were obtained for phase, frequency and eddy current correction. Post-processing and quantification of GABA spectra was done in LCModel (v 6.2-0R) which fits spectra using a linear combination of basis sets (Provencher, 1993). Appropriate basis sets were generated using density matrix simulations and published values of chemical shifts and coupling constants (Kaiser et al., 2008). The LCModel parameter “dkntmn” which defines the flexibility for fitting a spline function to the baseline was set to 0.15. Brain tissue segmentation was performed using SPM8 and voxel co-registration was done using an in-house tool written in MATLAB (v R2013a) to obtain CSF-corrected GABA values referenced to tissue water as described in Chowdhury (Chowdhury et al., 2015). These were then integrated with the behavioural data obtained from the modified Simon task.
Figure 2.
Illustration of the placement of the volumes of interests in the thalamus and striatum including representative examples of the MEGA-PRESS edited GABA spectrum from each brain region. The top part of the figure shows the thalamic volume of interest, the bottom part the striatal volume of interest.
2.4 Statistics
Task performance was analyzed in mixed effects ANOVAs. In these ANOVAs, “correspondence” (correspondent vs. non-correspondent), as well as “hand position” (parallel vs. crossed) were used as within-subject factor. The factor “group” (PD vs. controls) was used as between-subject factor. Greenhouse-Geisser correction was applied and post-hoc tests were bonferroni-corrected, whenever necessary. For all descriptive statistics, the mean and standard error of the mean (SEM) are given. MRS data was integrated with the behavioral data by means of correlation and regression analyses (Quetscher et al., 2014; Yildiz et al., 2014).
3. Results
3.1 Spectral quality of GABA scans
In the thalamus, slightly better shims (linewidth) and signal to noise ratio (SNR) were obtained in the controls than in PD subjects. In the striatum, no significant differences between controls and PD patients were obtained, so no overall difference in data quality was observed between the groups. This is shown in Table 1. It is shown (see section 3.2) that differences observed in thalamus did not modulate the pattern of results as regards the role of GABA for task performance.
Table 1.
Comparison of spectral quality between groups: Mean (standard deviation) of spectral signal to noise ratio (SNR) and linewidth, and Cramér Rao Lower Bounds (%CRLB) of the GABA fits for GABA-edited spectra from thalamus and striatum.
| Thalamus | Striatum | |||||
|---|---|---|---|---|---|---|
| PD | Control | Sig. | PD | Control | Sig. | |
| SNR | 18.64 (4.7) | 23.06 (5.4) | .029 | 17.91 (2.8) | 18.71 (4.1) | .249 |
| Linewidth (Hz) | 21.35 (4.1) | 18.14 (3.2) | 0.17 | 23.91 (2.8) | 24.09 (2.2) | .861 |
| %CRLB of GABA fit | 12.44 (2.1) | 12.87 (1.8) | .533 | 10.81 (1.9) | 12.14 (3.2) | .579 |
3.2 Reaction time data
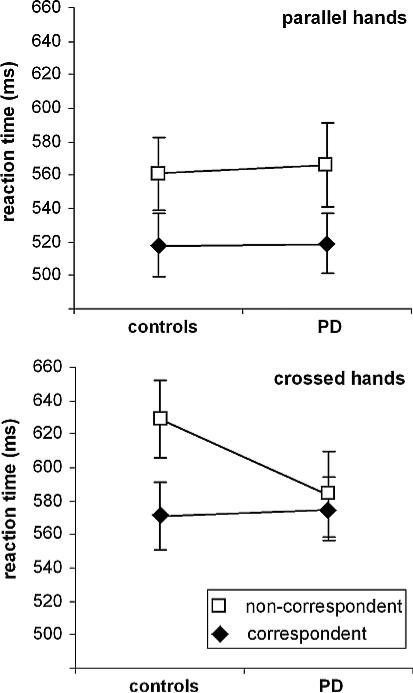
There was a significant main effect of „correspondence” (F(1,32) = 92.59; p < .001; η2 = .743) showing that RTs were longer in non-correspondent (588 ms ± 15) than in correspondent trials (546 ms ± 14). The main effect of “hand position” (F(1,32) = 58.82; p < .001; η2 = .648) showed that RTs were longer in the crossed hands condition (593 ms ± 16) than in the parallel hands condition (541 ms ± 13). Interestingly, there was an interaction of “hand position × correspondence × group” (F(1,32) = 8.15; p = .007; η2 = .203). For this interaction a power analysis revealed a 98% power for our sample size, showing that our study is sufficiently powered. All other main effects or interactions were not significant (all F < 1.6; p > .2). The interaction “hand position × correspondence × group” is shown in Figure 3.
Figure 3.
Reaction time (RT) effects obtained in the Simon task. The top of the figure shows RTs (means ± SEM) in the parallel hands condition for the controls and PD patients. The bottom of the figure shows RTs (means ± SEM) in the crossed hands condition (i.e., unusual proprioceptive information) for the controls and PD patients. White squares show RTs in the non-correspondent condition, black diamonds RTs in the correspondent condition.
Figure 3 suggests that there were no group differences between correspondent and non-correspondent trials in the parallel hands condition. Opposed to this, there seems to be a difference between groups in the crossed hands condition. In comparison to the control group, the PD group performed better in the non-correspondent condition, while there were no group differences in the correspondent condition. This is underlined in the statistical analysis: Calculating the S-R correspondence effect (i.e., non-correspondent minus correspondent RT) revealed that there was no group difference in the correspondence effect in the parallel hands condition (PD: 46 ms ± 7; controls: 42 ms ± 5; t32 = −0.49; p = .312). However, in the crossed hands condition, the correspondence effect was smaller in the PD group (22 ms ± 7) than in the control group (57 ms ± 7) (t32 = 2.67; p = .006). Importantly, there was no group difference in RTs on correspondent trials in the crossed hands condition (t32 = 0.18; p > .4), showing that differences in the correspondence effect were caused by differences in the non-correspondent condition. Compared to the parallel hands condition, the correspondence effect in the crossed-hands condition was larger in controls (t18 = 1.93; p = .035), but not different in PD patients (t18 = −0.95; p > .15).
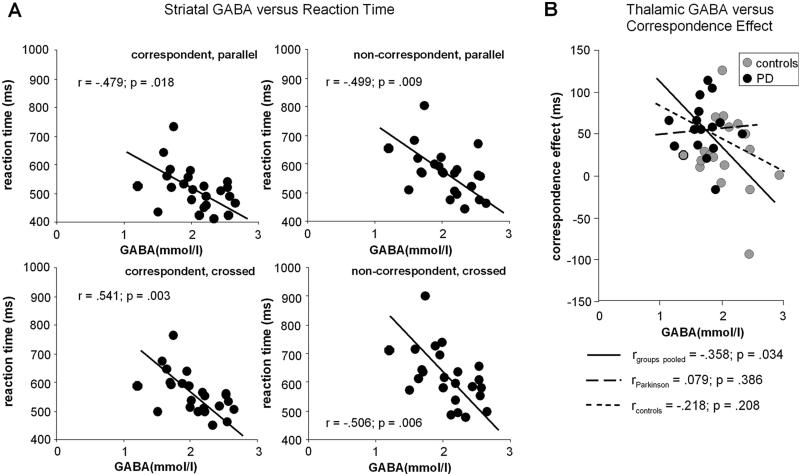
With regard to the importance of the striatal and thalamic GABAergic neural transmission, it could be shown that striatal GABA levels were correlated with RTs in the entire cohort (i.e. including both controls and PD patients) in each of the four experimental conditions (all r > −.479; R2 = .22; p < .018, Bonferroni-corrected). Corresponding scatter plots are shown in figure 4.
Figure 4.
(A) Scatterplots of all subjects denoting the correlation between RTs in the four different Simon Task conditions with striatal GABA concentrations (mmol/l). All four plots show a substantial negative correlation. (B) Scatterplot denoting the negative correlation of the correspondence effect in the crossed hands condition with thalamic GABA concentrations (mmol/l). The correspondence effect is calculated by subtracting RT in the correspondent condition from the non-correspondent condition. Some data points were missing due to spectra rejected for poor quality or unavailability of Simon data.
Higher striatal GABA concentrations were related to shorter RTs in each condition, explaining at least 22% of the observed variance. However, there was no effect of the striatal GABAergic system on correspondence effects (all r < .152; p > .2) in the entire cohort. Opposed to this, the thalamic GABAergic concentrations were correlated with the correspondence effect in the crossed hands condition in the entire cohort (r = −.358; R2 = .12; p < .034, Bonferroni-corrected) (figure 4). Yet, thalamic GABAergic concentrations were not correlated with general RTs in the four different experimental conditions in the entire cohort (all r < .091; p > .3). There was only a trend for a correlation with the correspondence effect in the parallel hands condition in the entire cohort (r = −.275; R2 = .12; p < .058, Bonferroni-corrected). The results therefore suggest dissociable roles of the striatal and thalamic GABAergic system for correspondence effects and general speed (RT) effects in the four different conditions.
In a regression model, we also took the factor “group” into account for the correlation between thalamic GABA levels and the correspondence effect in the crossed hands condition. Using GABA levels and “group” as predictors, this regression analysis showed an overall significant regression model (F(1,32) = 5.90; p = .021). However, only the factor group was a significant predictor (β = −.475; t = −2.51) while GABA level was no longer significant (β = −.202; t = −1.06). Importantly, the thalamic GABA level differed between PDs and controls and was higher in PDs (2.06 ± 0.09) than in controls (1.73 ± 0.09) (t32 = 2.72; p = .005), which explains why GABA level was no longer significant when accounting for the factor group. For the striatal GABA level, there was no difference between PDs and controls (t32 = 0.87; p > .3). Therefore, calculating regression analyses using striatal GABA level and groups as predictors for the speed of responding in the four different experimental conditions, revealed that the GABA level was constantly a significant predictor in each regression model (all β > −.554; t = −2.95; p < .008), while “group” was constantly not predictive (all β < −.02; t = −0.55; p > .5). It may be argued that the obtained correlations with GABA concentrations may be more due to the GM volume content in the voxel examined. However, when adding GM volume content in the voxel as an additional predictor to the regression models, all regression models did not change and it is shown that GM volume was not a significant predictor in any model (β < −.015; t = 0.49; p > .6). Similarly, MRS quality parameters (i.e., SNR, linewidth, GABA %CRLB) did not modulate the pattern of results when added as additional predictors to the regression models for the thalamic and striatal region (all β < −.11; t < −0.8; p > .3). This shows that even though SNR and linewidth were different between the groups (at least for the thalamic VOI), this did not affect the pattern of results.
3.3 Accuracy data
For the rate of correct responses, the main effect of “correspondence” (F(1,32) = 40.27; p < .001; η2 = .557) revealed more correct responses in the correspondent (94.4 % ± 2.1) than in the non-correspondent condition (88.1 % ± 2.1). The main effect of “hand position” (F(1,32) = 4.16; p = .05; η2 = .115) revealed more correct responses in the parallel (93.2 % ± 0.9) than in the crossed hands condition (89.2 % ± 2.7). No other main or interaction effects were significant (all F < 1.6; p > .2). Together with the reaction time data, the accuracy data shows that there was no speed-accuracy trade-off. There were also no correlations with striatal or thalamic GABA levels (all r < .150; p > .3).
4. Discussion
In the current study, we investigated the role of striatal and thalamic GABA concentrations for complex sensorimotor integration processes using a modified Simon paradigm. We examined healthy subjects and patients with Parkinson's disease. The Parkinson disease group served as a possible “model” of altered proprioceptive information processing to examine whether the role of striatal and thalamic GABA for sensorimotor integration processes is different when processing of proprioceptive information is changed. To examine the role of GABA for these processes, MR spectroscopy data was integrated with behavioral data on sensorimotor integration processes.
A major finding of this study is reflected in the group-dependent interaction of hand position (proprioceptive information) and the strength of the correspondence effect. While the unusual proprioceptive information (crossed hands condition) lead to an increase of the correspondence effect in the control group, the degree of the correspondence was not differentially modulated by proprioception in PD patients. This suggests that proprioceptive information is not properly taken into account by PD patients when performing the task. This is well in line with the study's’ hypothesis and the literature, suggesting that PD patients have an increased threshold for position sensitivity of their limbs (Conte et al., 2013) and often seem to fail to prioritize the processing of proprioceptive and sensorimotor information (Bloem et al., 2006; Heremans et al., 2013a; Lafargue et al., 2008; Maurer et al., 2003). It seems that in PD patients, sensorimotor integration processes are not aggravated in the crossed hands condition because proprioceptive information may be sub-threshold or not prioritized to the same extend as in the control group. In this sense, the known deficit of PD patients in processing proprioceptive information (Conte et al., 2013) can also turn into a benefit when altered proprioceptive information would exacerbate sensorimotor integration processes and, subsequently, response selection. Interestingly, thalamic GABA levels were elevated in the PD group, as compared to the control group. It is possible that higher thalamic GABA levels reflect the neurobiological correlate of the increased threshold for proprioceptive information in PD patients reported in literature (Conte et al., 2013). If this is the case, there should be systematic relationships between thalamic GABA levels and task performance. In this regard, the analyses integrating performance data (RTs on correspondence effects) with neurobiochemical data are of interest and suggest that the striatal and the thalamic GABA level play dissociable roles for sensorimotor integration as examined in this study:
Striatal GABA levels were generally related to response times, with higher GABA levels associated with faster responses. This effect was found regardless of whether the stimulus-response mapping was hampered by a crossed hands position, by an non-correspondent stimulus-response mapping or by a combination thereof. Given that GABA inhibits neurotransmission, this may be a counterintuitive result at first glance. However, response processing at a striatal level is considered to take place in a winner-takes-all (WTA) network that is constituted by GABAergic medium spiny neurons (MSN) (Bar-Gad et al., 2003; Bolam et al., 2000; Plenz, 2003). The efficiency of the WTA mechanism strongly depends on the integrity of this network: The higher the integrity, the faster response selection and execution (Beste and Saft, 2015; Beste et al., 2012, p. 201; Willemssen et al., 2011). Higher striatal GABA levels, as measured using MRS, likely increase the efficiency of striatal response selection (Yildiz et al., 2014) which is maybe the reason why higher striatal GABA levels were related to faster responses in this study.
With regard to the above line of arguments, it may seem at odds that the S-R correspondence effect was not affected by striatal GABA levels, but by thalamic GABA levels. Higher thalamic GABA levels were related to a smaller correspondence effect when proprioceptive information is altered (crossed hands condition). Yet, thalamic GABA levels did not affect the general speed of responding. It is already known that PD patients suffer from abnormal sensorimotor integration and reduced processing of sensorimotor information that partly depend on processing changes in the thalamus (Bloem et al., 2006; Patel et al., 2014). This matches previous findings suggesting that the thalamus is involved in the monitoring and coordination of movements and actions (Ku et al., 2014; Sommer and Wurtz, 2002) as well as in our ability to overcome task-irrelevant inferences (Law and Smith, 2012) in this context. It could furthermore be demonstrated that fine motor performance is influenced by thalamic GABA levels (Long et al., 2014). Against this background, our findings suggest that thalamic GABA levels modulate the processing of information important for response selection. Yet, correspondence effects in the Simon task not only depend on response selection, but also on attentional orienting processes as the Simon task requires different stimuli signaling for distinct responses to be integrated with the spatial position of these stimuli (Hommel, 2011). For these attentional orienting and feature-integration functions, the thalamus has also been shown to play an important role (Kim, 2014; Ruhl and Dicke, 2012; Salmi et al., 2007; Schneider, 2011; Yang and Mayer, 2014). Moreover, the thalamus has recently been suggested to integrate different streams of cortical, cerebellar and basal ganglia information (Bosch-Bouju et al., 2013) including proprioceptive information (Lalonde and Strazielle, 2007; Müller et al., 2013). As it was the proprioceptive information that was of particular relevance for the differential effects across groups, and it is known that altered thalamic processing underlies changes in proprioceptive information processing in PD (Müller et al., 2013; Patel et al., 2014), it is likely that attentional orienting and the integration aspect rather than the response selection aspect underlie the effects obtained in the current study.
It is, however, important that the factor “group” (PD vs. control) plays an important role for thalamic GABA levels, as the correlation between thalamic GABA levels and the correspondence effect disappeared when the factor group was taken into account. While this may be an effect of the long-term medication history of the patients, it may also reflect PD pathology-related effects known to involve thalamic structures (Patel et al., 2014). It is hence possible that group differences which are also due to the wide-spread structural neuropathology in PD leverage the effects of GABA. On a related point, it cannot be ruled out that the higher thalamic GABA levels in PD may cause this effect when this aspect is modeled in the statistical analysis by adding the “group” factor. Supporting this interpretation, the results show that in cases with no group differences in GABA levels between the groups (as for the striatal ROI), the factor group was not important as predictor for the correspondence effect.
Although the linewidth and SNRs of the spectra were different between the groups in the thalamus, these parameters did not modulate the effects obtained for GABA levels and task performance. They are therefore not a confounding factor in this study. Since no significant group differences were found between GM, WM or CSF in the thalamus or the striatum, and no modulatory effect of GM volume for the effects obtained for GABA concentrations and task performance, it is ruled out that the GM content is a confounder in the results obtained. For GABA quantification, the contribution of macromolecule (MM) was partly accounted for by using LCModel's baseline. However, the GABA value should still be regarded as GABA+MM. A limitation of the study is that a history of depression was not systematically ruled out in all subjects of this study. Since depression is associated with cortical and possibly sub-cortical GABA deficits, it may be a confounder.
In summary, the study shows that unusual proprioceptive information complicates response selection processes in controls, but not in PD. Deficits in processing proprioceptive information can therefore turn into a benefit when altered proprioceptive information would complicate response selection processes. Striatal and thalamic GABA levels play dissociable roles in these modulations of response selection processes by proprioceptive information. Striatal GABA levels seem to be important for the general speed of responding, most likely because striatal GABA promotes response selection. In contrast, the modulation of response conflict by proprioceptive information is closely related to thalamic GABA concentrations, which diminish the degree of response conflict when elevated. The most likely explanation for this finding is the fact that the thalamus is strongly involved in the integration of sensorimotor, attentional, and cognitive information for the purpose of response formation.
Highlights.
A role of striato-thalamic GABA in response selection and proprioception is tested
Striatal GABA levels affect the general speed of responding
Thalamic GABA levels modulate the strength of response conflict
Proprioception does not modulate response selection in Parkinson's Disease
Acknowledgements
This research was supported by NIH/NIEHS R01 ES020529 to U.D., a grant from the Purdue Research Foundation to U.D. and S.D., and by grants from the Deutsche Forschungsgemeinschaft (DFG) BE4045/10-1 and 10-2 to C.B and the Else Kröner-Fresenius-Stiftung to A.K. and C.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- Bar-Gad I, Morris G, Bergman H. Information processing, dimensionality reduction and reinforcement learning in the basal ganglia. Prog. Neurobiol. 2003;71:439–473. doi: 10.1016/j.pneurobio.2003.12.001. doi:10.1016/j.pneurobio.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Beste C, Ness V, Lukas C, Hoffmann R, Stüwe S, Falkenstein M, Saft C. Mechanisms mediating parallel action monitoring in fronto-striatal circuits. NeuroImage. 2012;62:137–146. doi: 10.1016/j.neuroimage.2012.05.019. doi:10.1016/j.neuroimage.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Beste C, Saft C. Action selection in a possible model of striatal medium spiny neuron dysfunction: behavioral and EEG data in a patient with benign hereditary chorea. Brain Struct. Funct. 2015;220:221–228. doi: 10.1007/s00429-013-0649-9. doi:10.1007/s00429-013-0649-9. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Grimbergen YAM, van Dijk JG, Munneke M. The “posture second” strategy: a review of wrong priorities in Parkinson's disease. J. Neurol. Sci. 2006;248:196–204. doi: 10.1016/j.jns.2006.05.010. doi:10.1016/j.jns.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J. Anat. 2000;196(Pt 4):527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch-Bouju C, Hyland BI, Parr-Brownlie LC. Motor thalamus integration of cortical, cerebellar and basal ganglia information: implications for normal and parkinsonian conditions. Front. Comput. Neurosci. 2013;7:163. doi: 10.3389/fncom.2013.00163. doi:10.3389/fncom.2013.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury FA, O'Gorman RL, Nashef L, Elwes RD, Edden RA, Murdoch JB, Barker GJ, Richardson MP. Investigation of glutamine and GABA levels in patients with idiopathic generalized epilepsy using MEGAPRESS. J. Magn. Reson. Imaging JMRI. 2015;41:694–699. doi: 10.1002/jmri.24611. doi:10.1002/jmri.24611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte A, Khan N, Defazio G, Rothwell JC, Berardelli A. Pathophysiology of somatosensory abnormalities in Parkinson disease. Nat. Rev. Neurol. 2013;9:687–697. doi: 10.1038/nrneurol.2013.224. doi:10.1038/nrneurol.2013.224. [DOI] [PubMed] [Google Scholar]
- Dydak U, Jiang Y-M, Long L-L, Zhu H, Chen J, Li W-M, Edden RAE, Hu S, Fu X, Long Z, Mo X-A, Meier D, Harezlak J, Aschner M, Murdoch JB, Zheng W. In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environ. Health Perspect. 2011;119:219–224. doi: 10.1289/ehp.1002192. doi:10.1289/ehp.1002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding J, Georgiou-Karistianis N, Bradshaw J, Millist L, White O. No sequence dependent modulation of the Simon effect in Parkinson's disease. Brain Res. Cogn. Brain Res. 2005;25:251–260. doi: 10.1016/j.cogbrainres.2005.05.015. doi:10.1016/j.cogbrainres.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn. Reson. Med. Off. J. Soc. Magn. Reson. Med. Soc. Magn. Reson. Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- Heremans E, Nieuwboer A, Vercruysse S. Freezing of gait in Parkinson's disease: where are we now? Curr. Neurol. Neurosci. Rep. 2013b;13:350. doi: 10.1007/s11910-013-0350-7. doi:10.1007/s11910-013-0350-7. [DOI] [PubMed] [Google Scholar]
- Hommel B. The Simon effect as tool and heuristic. Acta Psychol. (Amst.) 2011;136:189–202. doi: 10.1016/j.actpsy.2010.04.011. doi:10.1016/j.actpsy.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Kaiser LG, Young K, Meyerhoff DJ, Mueller SG, Matson GB. A detailed analysis of localized J-difference GABA editing: theoretical and experimental study at 4 T. NMR Biomed. 2008;21:22–32. doi: 10.1002/nbm.1150. doi:10.1002/nbm.1150. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. Cognitive impairment in Parkinson's disease: the dual syndrome hypothesis. Neurodegener. Dis. 2013;11:79–92. doi: 10.1159/000341998. doi:10.1159/000341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keye D, Wilhelm O, Oberauer K, Stürmer B. Individual differences in response conflict adaptations. Front. Psychol. 2013;4:947. doi: 10.3389/fpsyg.2013.00947. doi:10.3389/fpsyg.2013.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Involvement of the dorsal and ventral attention networks in oddball stimulus processing: a meta-analysis. Hum. Brain Mapp. 2014;35:2265–2284. doi: 10.1002/hbm.22326. doi:10.1002/hbm.22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku J, Cho YW, Lee YS, Moon H-J, Chang H, Earley CJ, Allen RP. Functional connectivity alternation of the thalamus in restless legs syndrome patients during the asymptomatic period: a resting-state connectivity study using functional magnetic resonance imaging. Sleep Med. 2014;15:289–294. doi: 10.1016/j.sleep.2013.09.030. doi:10.1016/j.sleep.2013.09.030. [DOI] [PubMed] [Google Scholar]
- Lafargue G, D'Amico A, Thobois S, Broussolle E, Sirigu A. The ability to assess muscular force in asymmetrical Parkinson's disease. Cortex J. Devoted Study Nerv. Syst. Behav. 2008;44:82–89. doi: 10.1016/j.cortex.2005.11.001. doi:10.1016/j.cortex.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Strazielle C. Brain regions and genes affecting postural control. Prog. Neurobiol. 2007;81:45–60. doi: 10.1016/j.pneurobio.2006.11.005. doi:10.1016/j.pneurobio.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Law LM, Smith DM. The anterior thalamus is critical for overcoming interference in a context-dependent odor discrimination task. Behav. Neurosci. 2012;126:710–719. doi: 10.1037/a0029698. doi:10.1037/a0029698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuthold H. The Simon effect in cognitive electrophysiology: A short review. Acta Psychol. (Amst.) 2011;136:203–211. doi: 10.1016/j.actpsy.2010.08.001. doi:10.1016/j.actpsy.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Long Z, Li X-R, Xu J, Edden RAE, Qin W-P, Long L-L, Murdoch JB, Zheng W, Jiang Y-M, Dydak U. Thalamic GABA predicts fine motor performance in manganese-exposed smelter workers. PloS One. 2014;9:e88220. doi: 10.1371/journal.pone.0088220. doi:10.1371/journal.pone.0088220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer C, Mergner T, Xie J, Faist M, Pollak P, Lücking CH. Effect of chronic bilateral subthalamic nucleus (STN) stimulation on postural control in Parkinson's disease. Brain J. Neurol. 2003;126:1146–1163. doi: 10.1093/brain/awg100. [DOI] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000;42:183–200. doi: 10.1006/brcg.1999.1099. doi:10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Müller MLTM, Albin RL, Kotagal V, Koeppe RA, Scott PJH, Frey KA, Bohnen NI. Thalamic cholinergic innervation and postural sensory integration function in Parkinson's disease. Brain J. Neurol. 2013;136:3282–3289. doi: 10.1093/brain/awt247. doi:10.1093/brain/awt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins PG, McGonigle DJ, O'Gorman RL, Puts NAJ, Vidyasagar R, Evans CJ, Cardiff Symposium on MRS of GABA. Edden RAE. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. doi:10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N, Jankovic J, Hallett M. Sensory aspects of movement disorders. Lancet Neurol. 2014;13:100–112. doi: 10.1016/S1474-4422(13)70213-8. doi:10.1016/S1474-4422(13)70213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenz D. When inhibition goes incognito: feedback interaction between spiny projection neurons in striatal function. Trends Neurosci. 2003;26:436–443. doi: 10.1016/S0166-2236(03)00196-6. doi:10.1016/S0166-2236(03)00196-6. [DOI] [PubMed] [Google Scholar]
- Plessow F, Fischer R, Volkmann J, Schubert T. Subthalamic deep brain stimulation restores automatic response activation and increases susceptibility to impulsive behavior in patients with Parkinson's disease. Brain Cogn. 2014;87:16–21. doi: 10.1016/j.bandc.2014.02.009. doi:10.1016/j.bandc.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Plat FM. Failed suppression of direct visuomotor activation in Parkinson's disease. J. Cogn. Neurosci. 2001;13:31–43. doi: 10.1162/089892901564153. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. Off. J. Soc. Magn. Reson. Med. Soc. Magn. Reson. Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Quetscher C, Yildiz A, Dharmadhikari S, Glaubitz B, Schmidt-Wilcke T, Dydak U, Beste C. Striatal GABA-MRS predicts response inhibition performance and its cortical electrophysiological correlates. Brain Struct. Funct. 2014 doi: 10.1007/s00429-014-0873-y. doi:10.1007/s00429-014-0873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig R, Silberberg G. Multisensory integration in the mouse striatum. Neuron. 2014;83:1200–1212. doi: 10.1016/j.neuron.2014.07.033. doi:10.1016/j.neuron.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR. Micro- and macro-adjustments of task set: activation and suppression in conflict tasks. Psychol. Res. 2002;66:312–323. doi: 10.1007/s00426-002-0104-7. doi:10.1007/s00426-002-0104-7. [DOI] [PubMed] [Google Scholar]
- Rubia K, Cubillo A, Woolley J, Brammer MJ, Smith A. Disorder-specific dysfunctions in patients with attention-deficit/hyperactivity disorder compared to patients with obsessive-compulsive disorder during interference inhibition and attention allocation. Hum. Brain Mapp. 2011;32:601–611. doi: 10.1002/hbm.21048. doi:10.1002/hbm.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl T, Dicke U. The role of the dorsal thalamus in visual processing and object selection: a case of an attentional system in amphibians. Eur. J. Neurosci. 2012;36:3459–3470. doi: 10.1111/j.1460-9568.2012.08271.x. doi:10.1111/j.1460-9568.2012.08271.x. [DOI] [PubMed] [Google Scholar]
- Salmi J, Rinne T, Degerman A, Salonen O, Alho K. Orienting and maintenance of spatial attention in audition and vision: multimodal and modality-specific brain activations. Brain Struct. Funct. 2007;212:181–194. doi: 10.1007/s00429-007-0152-2. doi:10.1007/s00429-007-0152-2. [DOI] [PubMed] [Google Scholar]
- Schneider KA. Subcortical mechanisms of feature-based attention. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:8643–8653. doi: 10.1523/JNEUROSCI.6274-10.2011. doi:10.1523/JNEUROSCI.6274-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296:1480–1482. doi: 10.1126/science.1069590. doi:10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- Stock A-K, Beste C. Lateralization of spatial information processing in response monitoring. Front. Psychol. 2014;5:22. doi: 10.3389/fpsyg.2014.00022. doi:10.3389/fpsyg.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock A-K, Wascher E, Beste C. Differential effects of motor efference copies and proprioceptive information on response evaluation processes. PloS One. 2013;8:e62335. doi: 10.1371/journal.pone.0062335. doi:10.1371/journal.pone.0062335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wouwe NC, van den Wildenberg WPM, Claassen DO, Kanoff K, Bashore TR, Wylie SA. Speed pressure in conflict situations impedes inhibitory action control in Parkinson's disease. Biol. Psychol. 2014;101:44–60. doi: 10.1016/j.biopsycho.2014.07.002. doi:10.1016/j.biopsycho.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wascher E, Schatz U, Kuder T, Verleger R. Validity and boundary conditions of automatic response activation in the Simon task. J. Exp. Psychol. Hum. Percept. Perform. 2001;27:731–751. doi: 10.1037//0096-1523.27.3.731. [DOI] [PubMed] [Google Scholar]
- Willemssen R, Falkenstein M, Schwarz M, Müller T, Beste C. Effects of aging, Parkinson's disease, and dopaminergic medication on response selection and control. Neurobiol. Aging. 2011;32:327–335. doi: 10.1016/j.neurobiolaging.2009.02.002. doi:10.1016/j.neurobiolaging.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Wylie SA, Claassen DO, Huizenga HM, Schewel KD, Ridderinkhof KR, Bashore TR, van den Wildenberg WPM. Dopamine agonists and the suppression of impulsive motor actions in Parkinson disease. J. Cogn. Neurosci. 2012;24:1709–1724. doi: 10.1162/jocn_a_00241. doi:10.1162/jocn_a_00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, Ridderinkhof KR, Bashore TR, van den Wildenberg WPM. The effect of Parkinson's disease on the dynamics of on-line and proactive cognitive control during action selection. J. Cogn. Neurosci. 2010a;22:2058–2073. doi: 10.1162/jocn.2009.21326. doi:10.1162/jocn.2009.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, Ridderinkhof KR, Elias WJ, Frysinger RC, Bashore TR, Downs KE, van Wouwe NC, van den Wildenberg WPM. Subthalamic nucleus stimulation influences expression and suppression of impulsive behaviour in Parkinson's disease. Brain J. Neurol. 2010b;133:3611–3624. doi: 10.1093/brain/awq239. doi:10.1093/brain/awq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Mayer AR. An event-related FMRI study of exogenous orienting across vision and audition. Hum. Brain Mapp. 2014;35:964–974. doi: 10.1002/hbm.22227. doi:10.1002/hbm.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz A, Quetscher C, Dharmadhikari S, Chmielewski W, Glaubitz B, Schmidt-Wilcke T, Edden R, Dydak U, Beste C. Feeling safe in the plane: Neural mechanisms underlying superior action control in airplane pilot trainees-A combined EEG/MRS study. Hum. Brain Mapp. 2014 doi: 10.1002/hbm.22530. doi:10.1002/hbm.22530. [DOI] [PMC free article] [PubMed] [Google Scholar]