1. Introduction
The maturational stage of the brain at the time of injury plays a key role in the pattern of brain damage in humans, including regional and cell-type specific susceptibility. In this mini-review we will summarize available models of preterm and at-term ischemic brain injury in rodents and in larger species and discuss how maturation stage of the brain at the time of an insult affects the underlying excitotoxic and inflammatory injury “signatures”. We will review pros and cons of animal modeling for advancing the understanding of the mechanisms of hypoxic-ischemic encephalopathy (HIE) and stroke in human infants.
2. Ischemia-related brain damage in preterm and term infants
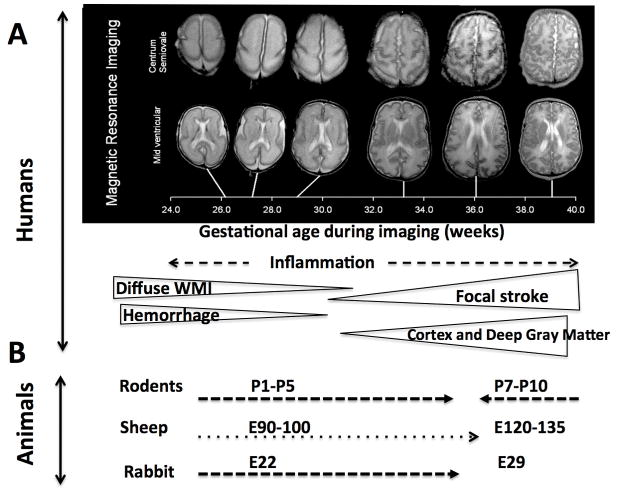
In preterm human babies (23–32 weeks of gestation) periventricular white matter injury (WMI) and its most severe form, periventricular leukomalacia (PVL), as well as intracerebral hemorrhage (ICH) and intraventricular hemorrhage (IVH) are the most common types of ischemia-related injuries 1 (Figure 1 and 2). Vulnerability of oligodendrocyte progenitor cells, loss of subplate neurons and a weak germinal matrix are believed to contribute to the pathology.
Figure 1. Age-dependent preterm and at-term brain injury patterns.
A. MRI-delineated brain maturation during 24–40 weeks of gestation (modified from Kapellou et al, PLoS Med. 2006 2) and the predominant patterns of injury in humans. B. Appropriate age ranges in individual species to mimic ischemia-related preterm and at-term brain pathophysiology.
The pathophysiology of at-term brain damage is multifactorial, with the patterns of ischemic injury differing from those in preterm newborns; injury is no longer diffuse and is manifested focally in gray matter regions, most commonly in the striatum, thalamus and cortex 3 (Figure 1). Infection and inflammation have been shown as the major predisposing and/or modulatory factors in ischemic injury in both preterm and at term infants 4.
Perinatal arterial ischemic stroke, defined as stroke that occurs between 20 weeks of gestation to 28 days postnatal, happens in up to 1 in 2300 live infant births and produces significant morbidity and severe long-term neurological and cognitive deficits including cerebral palsy, epilepsy, neurodevelopmental disabilities, and impaired vision and language function 5.
Increasing evidence suggests that the placenta plays a significant role in HIE and perinatal stroke 6 and that the presence of at least one prothrombotic factor substantially increases the incidence of stroke in term neonates 7.
3. Animal models for ischemia-related preterm and term injury
Numerous models of hypoxia, hypoxia-ischemia (HI), IVH and focal stroke have been developed in rodents and in larger species to mimic the different types of injuries seen in the human infant. Studies discussed in this review focus on ischemic and HI animal models and are summarized in Table 1.
Table 1.
Animal models to study neonatal HI and stroke
| Species | Animal models | Outcomes | Ref |
|---|---|---|---|
| Large animal models | |||
| Macaca nemestrina, near term | UCO | Poor weight gain and cerebellar growth, abnormal brain DTI, behavioral impairment, 43% develop CP. | 8 |
| Fetal sheep, near term | Bilateral CCAO | Shorter HI (<30 min): selective neuronal loss. Longer HI: cortical necrosis. Post-HI EEG suppression related to insult severity and pathology; prevented by hypothermia. | 9, 10 |
| Fetal sheep, midgestation | Bilateral CCAO | Necrosis of subcortical white matter, neuronal loss in thalamus and striatum similar to near term fetus. Little loss of final EEG amplitude. | 11 |
| Fetal sheep, midgestation and near term | UCO | Hippocampal neuronal loss only in near term group. Degree of injury associated with the severity of hypotension during UCO. | 12 |
| Pigs, <24h old | CCAO + hypoxia | Secondary energy failure. Energy metabolism ameliorated by hypothermia (35°C for 12h) at 24h-48h. | 13 |
| Pigs, P9 | Hypotension + hypoxia | ~60% fall in CBF, reduced cerebral O2 uptake, phosphorylated metabolites and pH and increased inorganic phosphate. | 14 |
| Rabbits, 21–22 d gestation | Uterine ischemia | P1 pups: overt posture and tone after ischemia > 37 min, correlates with microgliosis in basal ganglia and thalamus. MRI: WMI in IC. | 15 |
| Rodent models with global hypoxic or excitotoxic component | |||
| Mice @ E8, P0 or P5 | Ibotenate, i.c.v. | PO: laminar neuronal depopulation of layer V–VIa. P5: neuronal loss in all cortical layers, formation of porencephalic cysts. | 16 |
| Pregnant Sprague-Dawley rats, embryonic | Hypoxia E5-E20 | White matter cysts in offspring @ P0–P7, increased lipid peroxidation, WMI and macrophages. | 17 |
| Rodent models with hypoxia-ischemia | |||
| Sprague Dawley rats, P1–P3 | CCAL + hypoxia | Selective vulnerability of late OL progenitors, independent of age. Death of sub-plate neurons, motor deficits, altered thalamocortical connections to somatosensory and visual cortex normal. |
18,19 |
| Sprague-Dawley rats, P7 | CCAL + hypoxia | Unilateral ischemic injury in the cortex, hippocampus, basal ganglia in > 90% of survivors. | 20 |
| Wistar rats, P7 | CCAL + hypoxia | Neutrophils within blood vessels, little infiltration into brain parenchyma after HI. Neutropenia prior to HI reduced brain swelling by 70%. | 21 |
| Wistar rat, P7 | LPS, 4h prior to CCAL + hypoxia | Blocking lymphocyte trafficking reduced brain inflammation, BBB damage, and improved LPS-induced HI brain injury. No effect with pure HI. | 22 |
| C57Bl/6 WT, Tg SOD1, GPx1 over-expressing P7 mice | CCAL + hypoxia | Reduced injury in GPx1-Tg mice but not in SOD1-Tg or GPx1/SOD1. NOS inhibition did not improve outcome in SOD-Tg. | 23 |
| C57BL/6 WT and Gal-3 KO, P9 | CCAL + hypoxia | Increased BBB permeability 2–24h, reduced BBB protein expression. Infarct volume reduction in Gal-3 KO mice. | 24,25 |
| C57BL/6J and TRIF KO mice, P8–9 | Poly I:C, 14h prior to CCAL + hypoxia | Increased infarct volume and WMI, prevented in TRIF KO. Injury linked to inflammatory response & decrease in M2-like microglia. | 26 |
| Focal ischemia rodent models | |||
| Wistar rat, P7 | Permanent MCAO + 1h CCAO | Infarcts in frontoparietal cortex at 3-month recovery. DNA fragmentation from 6–96h. | 27 |
| Sprague Dawley rats, P7 | Transient MCAO, 3h | Severe unilateral perfusion deficits, restoration of CBF upon suture removal. Decreased ADC associated with brain injury at 24h reperfusion. Demonstrated endogenous neuroprotective role of microglial cells after acute injury. | 28,29,30 |
| Sprague Dawley rats, P10 | Transient MCAO, 1.5h | Time resolved cell-type specific increase in HIF-1a and VEGF expression, gliosis. | 31 |
| C57/Bl6 mice, CD36 KO and WT, P9 | Transient MCAO, 1.5h and 3h | Focal ischemia-reperfusion, increased injury and caspase-3 cleavage associated with apoptotic neuronal debris in CD36 KO. Effects independent of NFκB activation. | 32 |
HI, hypoxia-ischemia; CCAO, common carotid artery occlusion; CCAL, common carotid artery ligation; GA, gestational age; UCO, umbilical cord occlusion; CBF, cerebral blood flow; BBB, blood-brain barrier, P, postnatal day; WT, wild type; Tg, transgenic; SOD, Superoxide dismutase; GPx, glutathione peroxidase; MCAO, middle cerebral artery occlusion; ADC, apparent diffusion coefficient; DAP12, DNAX activation protein of 12 kDa; IL, interleukin; TRIF KO mice, C57BL/6J–Ticam1Lps2/J; WMI, white matter injury; CR, callosal radiation; IC, internal capsule; Gal-3, galectin-3.
3.1. Large animal models
A unique model of birth asphyxia has recently been developed in non-human primates by transiently occluding the umbilical cord prior to birth. The animals develop severe asphyxia and die or present with cerebral palsy-like motor abnormalities 8. However, the cost limits the number of study subjects in this model. The most commonly used non-rodent large mammal species to induce HI in the immature brain are sheep, rabbits and pigs, species that have a white/grey matter ratio similar to the human brain.
Sheep are precocial and thus studies are performed during pregnancy to correlate to relevant maturation stages in the human. Cerebral ischemia models in fetal sheep, induced by bilateral transient occlusion of the carotid arteries, were first developed in the near term fetus 9, and, later, during midgestation 11. The chronically instrumented fetal sheep umbilical cord occlusion model is global and allows examination of intrauterine pathophysiology and the contribution of other organs on the brain, without the influence of anesthesia. In these models, the preterm fetuses are more prone to WMI and deep grey matter injury, with an increased vulnerability of cortical grey matter with advancing gestation 12, neuropathology that correlates well with human pathology. On the other hand, fetal models are complicated by maternal/placenta metabolism, which is not present in the human situation of HIE.
Newborn pigs subjected to various combinations of hypoxia and ischemia show changes in metabolism (MR spectroscopy) and CBF similar to that observed in human infants with HIE 14. Generally injury is characterized by neuronal loss in the sensorimotor cortex and basal ganglia and postnatal seizures. The HI models in newborn pigs 13, in parallel with fetal sheep studies 10, have been instrumental in development of therapeutic hypothermia for term infants with HIE.
In rabbits, intrauterine ischemia is induced around 22 days gestation as equivalent to the preterm, and at the end of gestation (at 29 days) to mimic at-term injury. HI in preterm rabbits results in injury mainly in subcortical areas, such as basal ganglia and thalamus 15. Importantly, the preterm rabbit HI model is one of few large animal set-ups, which has demonstrated a correlation between abnormalities on MRI, neuropathology and cerebral palsy-like hypertonic motor deficits in the newborn pups 15.
3.2. Rodent models
Like in humans, in rats and mice much of brain development occurs after birth and, like in humans, individual regions of the rodent brain mature at a different pace, thus making it difficult to adhere to a single postnatal day as a comprehensive representation of brain development in human. Cross-comparisons of gross neuroanatomy, the timing of neurogenesis, synaptogenesis, gliogenesis, maturation and myelination as well as age-dependent molecular and biochemical changes in rodents and humans have demonstrated that the rodent brain at postnatal day 1 (P1)–P5 corresponds to 23–32 weeks of gestation in the humans and is thus suitable for studies of preterm injury, whereas the rodent brain at P7–P10 corresponds to 36–40 weeks of gestation in humans, thus suitable for studying brain injury at term 33 (Figure 1 and Table 1).
Several models of WMI have been developed, including HI in P1–P3 rat, a model that consists of unilateral ligation of the common carotid artery followed by a hypoxic episode 18, 19, ibotenate injection in P5 rats 16 and prolonged low grade gestational hypoxia 17.
The inception of the experimental HI model in P7 rat by Vannucci and colleagues 20 and extension of the HI model to P7-P9 mice has allowed mimicking HIE in the at-term human infant and obtain seminal information about the pathophysiology of the disease, including CBF regulation and energy metabolism 20, 24, inflammation 20,21,23,25, excitotoxic and oxidative injury 23, intracellular injury mechanisms and neuronal death 34 and mechanisms of brain repair 35. HI studies have also demonstrated that genetic background 36 and sex affect mechanisms of neuronal cell death and injury severity 37. A limitation of this model is animal-to-animal variability of injury.
Several models have been established to study the underlying mechanisms of perinatal arterial stroke, including models of permanent middle cerebral artery ligation 27 and suture transient MCA occlusion (tMCAO) in P7-P10 rats 28, 31 and P9 mice 32. Varying the length of tMCAO has allowed induction of injuries of different severity 32. The demonstrated presence of recirculation in the tMCAO model 29 mimics reperfusion, which frequently occurs in arterial stroke in term infants.
4. Excitotoxicity and oxidative injury
Glutamate receptors are prevalent in the developing brain and glutamate-induced excitotoxicity is an important factor that contributes to ischemic injury in both the preterm and at-term brain 38. Maturation-dependent expression of different glutamate receptors have been linked to specific injury patterns. Thus high expression of NMDA and AMPA receptors on neurons in the deep grey matter and cortex are believed to play important roles in at-term brain injury, while AMPA/kainate receptors are more important in the preterm brain. Also, dysregulation of glutamate transporters on oligodendrocytes and microglia likely contribute to excessive extracellular glutamate in the immature brain.
The developing brain is very sensitive to oxidative injury 23. Excitotoxicity and inflammation are major processes that lead to generation of reactive oxidants, which can damage DNA and proteins, both directly and indirectly.
5. Maturation-dependent factors that affect the “inflammatory signatures” in preterm vs at-term ischemic brain damage
5.1. Inflammatory responses
Clinical data have demonstrated that pro-inflammatory cytokines are elevated in CSF of asphyxiated term infants and that the levels of these cytokines reflect the degree of HIE 39. Cytokines are also elevated in newborns with severe IVH and in brains of neonates with PVL (reviewed in 26). There is a robust and continued inflammatory response in the developing brain after HI, including “reactive” microglia and mast cells, as well as infiltration of neutrophils, monocytes and gamma-delta T cells 26. Initially this response is predominantly characterized by production and release of pro-inflammatory cytokines, but over the following days to weeks, an anti-inflammatory/restorative milieu is established.
No single study has compared in detail how the maturation stage of the brain at the time of an insult affects the inflammatory injury “signatures”. However, inflammation is evident in rodents in both preterm HI 40 and at-term 4 injury models, as well as in fetal sheep 41 and fetal rabbits 15. Developmentally regulated inflammatory responses are important determinants of outcome as several reports demonstrate that anti-inflammatory interventions that are beneficial in the immature brain are not so in the adult 24 or vice versa 32. Further, innate immune factors (e.g. stimulation of TLRs), which modify the acute inflammatory response and adaptive immunity, and enhance susceptibility to neonatal HI 26, often have the opposite effect (i.e. pre-conditioning protection) in adult stroke models 42.
There is also growing evidence that the response to immune therapy may be sex-dependent, as was shown for protection of female but not male neonates against HI by an iNOS inhibitor, 2-iminobiotin, and PARP 37.
5.2. Peripheral leukocytes
Neutrophils are one of earliest leukocyte populations to infiltrate and contribute to injury and blood-brain barrier (BBB) disruption in adult transient cerebral ischemia models 43. Unlike in adults, neutrophil infiltration is limited during acute and sub-chronic time points after neonatal HI 21 and focal stroke 29 in rats, likely contributing to better BBB integrity in injured neonates. The exact mechanisms that restrict leukocyte infiltration in the ischemic neonatal brain are not completely understood, and it remains unclear whether the higher resistance of the neonatal BBB to stroke is a cause or a consequence of reduced leukocyte transmigration. However, there is a lack of detailed knowledge on leukocyte cell type specific infiltration in models of neonatal brain injury. Monocyte infiltration is relatively low after acute tMCAO in neonatal rats. On the other hand, on-going studies in neonatal mice show significant monocyte infiltration during the first days after HI injury (Smith et al unpublished). Neither the possibility that peripheral leukocytes contribute to injury without their infiltration nor the possibility that beneficial leukocyte populations enter injured brain via choroid plexus have been sufficiently explored. There is evidence that T-cells infiltrate the neonatal brain during a prolonged period over several months after HI, and a recent study suggests that blocking lymphocyte trafficking is neuroprotective after LPS-sensitized HI injury 22.
5.3. Microglia
Microglia populate the brain before birth and are distinct from peripheral monocytes. Recent data show that microglial cells play key roles in controlling synaptic pruning and the formation of precise synaptic circuits that occurs during the first two weeks of rodent life 44. Improper microglia-neuronal communications during this time and the resultant inappropriate synaptic connections could be the cause of neurodevelopmental disorders.
Specific “hot spots” of amoeboid microglia are found in the developing human brain and are also present in fetal sheep at midgestation in the white matter, a region susceptible to injury. Due to their active-like state during this developmental stage, microglial cells have been suggested to specifically contribute to WMI in the preterm brain. Further, microglia can produce toxic reactive oxidants and injure the immature brain, which has limited antioxidant defenses 23.
However, there is mounting evidence that suggests a protective role for microglia. In the adult, microglial cells can provide neuroprotective effects by producing growth factors although this has so far not been demonstrated in the developing brain. Microglial cells serve as endogenous neuroprotectants in neonatal arterial stroke, as microglial depletion greatly enhances the excitotoxic and inflammatory responses and injury 30. Distinct maturation-dependent microglial phenotypes and “diversity” of the microglial phenotypes at a given time are being increasingly recognized.
The heterogeneity of the microglial pool, the timing of activation and the type of stimulus critically affect an array of microglial effects. A better understanding of these events in the developing brain is likely to benefit the development of novel protective strategies.
5.4. BBB integrity, inflammation and angiogenesis
The presence of a leaky BBB in the immature brain has been commonly assumed, but endothelial tight junctions are already present during early embryonic development 45, specific BBB transporters are present in the brain endothelium during midgestation, and no fenestrations are observed at birth 46. Expression of endothelial BBB proteins undergoes major changes from the embryonic period to adulthood, but susceptibility of the BBB to injury does not decrease linearly with age. In fact, comparison of albumin leakage following acute tMCAO between neonatal and adult rats showed a marked leakage in adults but not in neonates. Distinct expression patterns of the extracellular matrix proteins and tight junction proteins, along with lower than in adults leukocyte infiltration in neonates 29 are the likely contributors to BBB integrity after neonatal stroke. In contrast, in P9 mice subjected to HI, BBB permeability for large and small size tracers is transiently increased during the first 24 hours, changes associated with modified expression of BBB tight junction proteins 25. It is presently unknown if differences in BBB leakage are model- or species-related.
Neurovascular outgrowth (angiogenesis) continues during the first two postnatal weeks in the rat brain 29, but following stroke in P7 rats, only a subtle and delayed angiogenic response is observed in the cortex during 2 weeks after injury 47. The higher resistance of the neonatal BBB to ischemic injury may negatively impact the angiogenic response and ultimate endogenous neurogenesis after stroke, but the relationships between the two processes are still poorly understood.
6. Immune therapies and cell-based therapies to enhance the repair
Currently, hypothermia is used to treat neonatal HIE. However, the therapy is only partially protective and can only be used in term infants. Thus there is a great need to find additional/synergistic therapeutic strategies. A variety of drugs targeting neuroinflammation tested in rodent models of ischemia-related brain injury reported quite variable neuroprotection, depending on the timing and type of intervention and on model and sex of animals used 26. Considering that it is often unknown when injury occurs in human infants, more recently emphasis has shifted to cell therapies or molecules, like growth factors (for example, BDNF and erythropoitin), that can support and enhance the repair 35. While some cell therapies and growth factors have shown promising long-term effects and some are currently in clinical trial (melatonin, erythropoitein), it remains unclear whether cells/agents have direct effects or, which is likely, act via immune-mediated change in brain microenvironment.
7. Pros and cons of different ischemia-related animal models for translation
Large animal cerebral ischemia models would be most suitable in translational research considering that the brains are gyrincephalic and that the white/grey matter ratio is similar to the human brain. Among large non-primate neonatal models, newborn pigs are very appropriate, as their general brain and organ maturation at term are similar to that of humans. Different global models have been developed, where global hypoxia and hypotension often is combined, which results in permanent brain injury, organ failure, post-hypoxic seizures and abnormal neurology, similar to humans on survival. However, practical issues, the high cost of maintenance and long-term neurorehabilitation have dramatically limited the use of larger species over the past decade. New hypoxia models in immature ferrets 46, a species with brain gyrification, may provide a potentially translatable model of preterm WMI.
While parallels are often made between findings in humans and rodents, it is important to realize the limitations and translatability of studies in rodents. Rodents are not gyricephalic species and their physiology, CBF regulation and white/gray matter ratios are vastly different from those in humans. Age-dependent regional vulnerability that may stem from uncoordinated maturation of individual cells in the parenchyma and in the vasculature, should also be considered while interpreting the results from HI or focal stroke studies produced in immature rodents of different postnatal ages.
The role of the systemic effects of neonatal stroke has not been sufficiently addressed in animal models. Stroke is no longer viewed as a disease only of the brain, as it exerts a rapid response in the blood, and in peripheral organs, including liver, heart and spleen, as well as bone marrow 48. Additionally, studying the systemic effects of perinatal stroke are important because injured newborns need intensive care including organ support (ventilation, cardiovascular, renal) and because brain repair may stem from the periphery, but such studies are rather sparse.
8. Conclusion
To summarize, ischemia-related injury to prenatal or early postnatal brain impacts many key neurodevelopmental processes that undergo maturation changes during these time frames, leading to differing structural-functional abnormalities later in life. There is no single animal model that completely recapitulates the complexity of the human condition but utilization of several ischemia-related age-specific models in rodents, and in larger species, and of both sexes, enables the enhanced understanding of brain pathology and development of novel therapies for the immature brain, as was demonstrated for hypothermia.
Supplementary Material
Acknowledgments
Sources of funding
NINDS_NS080015 (ZV), NINDS_NS44025 (ZV), NINDS_NS76726 (ZV), The Leducq Foundation, DSRR_P34404 (CM, ZV), Swedish Research Council 2012-2992 (CM), Swedish Brain Foundation, FO2014-008 (CM).
Footnotes
Disclosures: Dr. Vexler is a consultant for Omniox, Inc.
References
- 1.Volpe JJ. The encephalopathy of prematurity--brain injury and impaired brain development inextricably intertwined. Semin Pediatr Neurol. 2009;16:167–178. doi: 10.1016/j.spen.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapellou O, Counsell SJ, Kennea N, Dyet L, Saeed N, Stark J, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS medicine. 2006;3:e265. doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirton A, Armstrong-Wells J, Chang T, Deveber G, Rivkin MJ, Hernandez M, et al. Symptomatic neonatal arterial ischemic stroke: The international pediatric stroke study. Pediatrics. 2011;128:e1402–1410. doi: 10.1542/peds.2011-1148. [DOI] [PubMed] [Google Scholar]
- 4.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Annals of neurology. 2012;71:444–457. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- 5.Nelson KB. Perinatal ischemic stroke. Stroke; a journal of cerebral circulation. 2007;38:742–745. doi: 10.1161/01.STR.0000247921.97794.5e. [DOI] [PubMed] [Google Scholar]
- 6.Elbers J, Viero S, MacGregor D, DeVeber G, Moore AM. Placental pathology in neonatal stroke. Pediatrics. 2011;127:e722–729. doi: 10.1542/peds.2010-1490. [DOI] [PubMed] [Google Scholar]
- 7.Gunther G, Junker R, Strater R, Schobess R, Kurnik K, Heller C, et al. Symptomatic ischemic stroke in full-term neonates : Role of acquired and genetic prothrombotic risk factors. Stroke; a journal of cerebral circulation. 2000;31:2437–2441. doi: 10.1161/01.str.31.10.2437. [DOI] [PubMed] [Google Scholar]
- 8.Traudt CM, McPherson RJ, Bauer LA, Richards TL, Burbacher TM, McAdams RM, et al. Concurrent erythropoietin and hypothermia treatment improve outcomes in a term nonhuman primate model of perinatal asphyxia. Developmental neuroscience. 2013;35:491–503. doi: 10.1159/000355460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams CE, Gunn AJ, Mallard C, Gluckman PD. Outcome after ischemia in the developing sheep brain: An electroencephalographic and histological study. Annals of neurology. 1992;31:14–21. doi: 10.1002/ana.410310104. [DOI] [PubMed] [Google Scholar]
- 10.Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99:248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy K, Mallard C, Guan J, Marks K, Bennet L, Gunning M, et al. Maturational change in the cortical response to hypoperfusion injury in the fetal sheep. Pediatric research. 1998;43:674–682. doi: 10.1203/00006450-199805000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Mallard EC, Williams CE, Johnston BM, Gluckman PD. Increased vulnerability to neuronal damage after umbilical cord occlusion in fetal sheep with advancing gestation. Am J Obstet Gynecol. 1994;170:206–214. doi: 10.1016/s0002-9378(94)70409-0. [DOI] [PubMed] [Google Scholar]
- 13.Thoresen M, Penrice J, Lorek A, Cady EB, Wylezinska M, Kirkbride V, et al. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatric research. 1995;37:667–670. doi: 10.1203/00006450-199505000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Laptook AR, Hassan A, Peterson J, Corbett RJ, Nunnally RL. Effects of repeated ischemia on cerebral blood flow and brain energy metabolism. NMR Biomed. 1988;1:74–79. doi: 10.1002/nbm.1940010204. [DOI] [PubMed] [Google Scholar]
- 15.Derrick M, Drobyshevsky A, Ji X, Tan S. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke; a journal of cerebral circulation. 2007;38:731–735. doi: 10.1161/01.STR.0000251445.94697.64. [DOI] [PubMed] [Google Scholar]
- 16.Gressens P, Marret S, Evrard P. Developmental spectrum of the excitotoxic cascade induced by ibotenate: A model of hypoxic insults in fetuses and neonates. Neuropathol Appl Neurobiol. 1996;22:498–502. doi: 10.1111/j.1365-2990.1996.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 17.Baud O, Daire JL, Dalmaz Y, Fontaine RH, Krueger RC, Sebag G, et al. Gestational hypoxia induces white matter damage in neonatal rats: A new model of periventricular leukomalacia. Brain Pathol. 2004;14:1–10. doi: 10.1111/j.1750-3639.2004.tb00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheldon RA, Chuai J, Ferriero DM. A rat model for hypoxic-ischemic brain damage in very premature infants. Biology of the neonate. 1996;69:327–341. doi: 10.1159/000244327. [DOI] [PubMed] [Google Scholar]
- 19.Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice JEd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Annals of neurology. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 21.Hudome S, Palmer C, Roberts RL, Mauger D, Housman C, Towfighi J. The role of neutrophils in the production of hypoxic-ischemic brain injury in the neonatal rat. Pediatric research. 1997;41:607–616. doi: 10.1203/00006450-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Yang D, Sun YY, Bhaumik SK, Li Y, Baumann JM, Lin X, et al. Blocking lymphocyte trafficking with fty720 prevents inflammation-sensitized hypoxic-ischemic brain injury in newborns. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:16467–16481. doi: 10.1523/JNEUROSCI.2582-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheldon RA, Jiang X, Francisco C, Christen S, Vexler ZS, Tauber MG, et al. Manipulation of antioxidant pathways in neonatal murine brain. Pediatric research. 2004;56:656–662. doi: 10.1203/01.PDR.0000139413.27864.50. [DOI] [PubMed] [Google Scholar]
- 24.Doverhag C, Hedtjarn M, Poirier F, Mallard C, Hagberg H, Karlsson A, et al. Galectin-3 contributes to neonatal hypoxic-ischemic brain injury. Neurobiol Dis. 2010;38:36–46. doi: 10.1016/j.nbd.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 25.Ek CJ, D’Angelo B, Baburamani AA, Lehner C, Leverin AL, Smith PL, et al. Brain barrier properties and cerebral blood flow in neonatal mice exposed to cerebral hypoxia-ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015;35:818–827. doi: 10.1038/jcbfm.2014.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagberg H, Mallard C, Ferriero D, Vannucci S, Levison S, Vexler Z, et al. The role of inflammation in perinatal brain injury. Nature reviews Neurology. 2015;11:192–208. doi: 10.1038/nrneurol.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renolleau S, Aggoun-Zouaoui D, Ben-Ari Y, Charriaut-Marlangue C. A model of transient unilateral focal ischemia with reperfusion in the p7 neonatal rat: Morphological changes indicative of apoptosis. Stroke; a journal of cerebral circulation. 1998;29:1454–1460. doi: 10.1161/01.str.29.7.1454. discussion 1461. [DOI] [PubMed] [Google Scholar]
- 28.Derugin N, Ferriero DM, Vexler ZS. Neonatal reversible focal cerebral ischemia: A new model. Neurosci Res. 1998;32:349–353. doi: 10.1016/s0168-0102(98)00096-0. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez Lopez D, Faustino J, Daneman R, Zhou L, Lee SY, Derugin N, et al. Blood-brain barrier permeability is increased after acute adult stroke but not neonatal stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:9588–9600. doi: 10.1523/JNEUROSCI.5977-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faustino J, Wang X, Jonhson C, Klibanov A, Derugin N, Wendland M, et al. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:12992–13001. doi: 10.1523/JNEUROSCI.2102-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mu D, Jiang X, Sheldon RA, Fox CK, Hamrick SE, Vexler ZS, et al. Regulation of hypoxia-inducible factor 1alpha and induction of vascular endothelial growth factor in a rat neonatal stroke model. Neurobiol Dis. 2003;14:524–534. doi: 10.1016/j.nbd.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Woo MS, Wang X, Faustino J, Derugin N, Wendland MF, Zhou P, et al. Genetic deletion of cd36 enhances injury after acute neonatal stroke. Annals of neurology. 2012;72:961–970. doi: 10.1002/ana.23727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Progress in neurobiology. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Northington FJ, Chavez-Valdez R, Martin LJ. Neuronal cell death in neonatal hypoxia-ischemia. Annals of neurology. 2011;69:743–758. doi: 10.1002/ana.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Velthoven CT, Gonzalez F, Vexler ZS, Ferriero DM. Stem cells for neonatal stroke- the future is here. Frontiers in cellular neuroscience. 2014;8:207. doi: 10.3389/fncel.2014.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheldon RA, Sedik C, Ferriero DM. Strain-related brain injury in neonatal mice subjected to hypoxia- ischemia. Brain research. 1998;810:114–122. doi: 10.1016/s0006-8993(98)00892-0. [DOI] [PubMed] [Google Scholar]
- 37.Johnston MV, Hagberg H. Sex and the pathogenesis of cerebral palsy. Dev Med Child Neurol. 2007;49:74–78. doi: 10.1017/s0012162207000199.x. [DOI] [PubMed] [Google Scholar]
- 38.Johnston MV. Excitotoxicity in perinatal brain injury. Brain Pathol. 2005;15:234–240. doi: 10.1111/j.1750-3639.2005.tb00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savman K, Blennow M, Gustafson K, Tarkowski E, Hagberg H. Cytokine response in cerebrospinal fluid after birth asphyxia. Pediatric research. 1998;43:746–751. doi: 10.1203/00006450-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Albertsson AM, Bi D, Duan L, Zhang X, Leavenworth JW, Qiao L, et al. The immune response after hypoxia-ischemia in a mouse model of preterm brain injury. Journal of neuroinflammation. 2014;11:153. doi: 10.1186/s12974-014-0153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reddy VM, McElhinney DB, Rajasinghe HA, Rodriguez JL, Hanley FL. Cytokine response to fetal cardiac bypass. J Matern Fetal Investig. 1998;8:46–49. [PubMed] [Google Scholar]
- 42.Stridh L, Mottahedin A, Johansson ME, Valdez RC, Northington F, Wang X, et al. Toll-like receptor-3 activation increases the vulnerability of the neonatal brain to hypoxia-ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:12041–12051. doi: 10.1523/JNEUROSCI.0673-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iadecola C, Anrather J. The immunology of stroke: From mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 45.Kniesel U, Risau W, Wolburg H. Development of blood-brain barrier tight junctions in the rat cortex. Brain Res Dev Brain Res. 1996;96:229–240. doi: 10.1016/0165-3806(96)00117-4. [DOI] [PubMed] [Google Scholar]
- 46.Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res. 2003;314:119–129. doi: 10.1007/s00441-003-0751-z. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez-Lopez D, Faustino J, Derugin N, Vexler ZS. Acute and chronic vascular responses to experimental focal arterial stroke in the neonate rat. Translational Stroke Research. 2013;4:179–188. doi: 10.1007/s12975-012-0214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denes A, Thornton P, Rothwell NJ, Allan SM. Inflammation and brain injury: Acute cerebral ischaemia, peripheral and central inflammation. Brain, behavior, and immunity. 2010;24:708–723. doi: 10.1016/j.bbi.2009.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.