Abstract
Background and Purpose
Mexican Americans (MAs) have an increased risk of stroke and experience worse post-stroke disability than non-Hispanic whites (NHWs), which may translate into worse post-stroke quality of life (QOL). We assessed ethnic differences in post-stroke QOL, as well as potential modification of associations by age, sex, and initial stroke severity.
Methods
Ischemic stroke survivors were identified through the biethnic, population-based Brain Attack Surveillance in Corpus Christi (BASIC) Project. Data were collected from medical records, baseline interviews, and 90-day post-stroke interviews. Post-stroke QOL was measured at approximately 90 days by the validated short-form stroke-specific QOL in 3 domains: overall, physical, and psychosocial (range 0–5; higher scores represent better QOL). Tobit regression was used to model associations between ethnicity and post-stroke QOL scores, adjusted for demographics, clinical characteristics, and pre-stroke cognition and function.
Results
Among 290 eligible stroke survivors (66% MA, 34% NHW, median age=69 years), median scores for overall, physical, and psychosocial post-stroke QOL were 3.3, 3.8 and 2.7, respectively. Overall post-stroke QOL was lower for MAs than NHWs (mean difference = −0.30, 95%CI:−0.59,−0.01) and in the physical domain (mean difference = −0.47, 95%CI:−0.81,−0.14) after multivariable adjustment. No ethnic difference was found in the psychosocial domain. Age modified the associations between ethnicity and post-stroke QOL such that differences were present in older but not younger ages.
Conclusions
Disparities exist in post-stroke QOL for MAs and appear to be driven by differences in older stroke patients. Targeted interventions to improve outcomes among MA stroke survivors are urgently needed.
Keywords: quality of life, stroke, ethnicity, disparities, outcomes research
Introduction
Quality of life (QOL) is a multidimensional construct incorporating individual perception of life circumstances, and its importance has been underscored by both the World Health Organization and Healthy People 2020.1, 2 Mexican Americans (MAs) have an increased risk of stroke compared with non-Hispanic whites (NHWs) and experience worse post-stroke disability even after accounting for demographics, pre-stroke factors and stroke severity.3 This may lead to poorer post-stroke QOL among MAs; however, the impact of disability on post-stroke QOL among MAs may be attenuated by factors more likely to be experienced by MAs, such as higher levels of social support and younger ages at stroke onset, which are associated with better QOL.4–7 Racial disparities exist in post-stroke QOL, with non-white stroke survivors reporting poorer physical QOL as compared to whites, and African Americans experiencing a greater increase in depressive symptoms after stroke than whites, but little is known regarding ethnic disparities in post-stroke QOL.8, 9 Therefore, we assessed ethnic differences in post-stroke QOL among MAs and NHWs in a biethnic population-based stroke study. As ethnic differences in QOL may be more pronounced in subgroups that experience worse long-term outcomes, such as women, older survivors, and those with less severe stroke,4, 8, 10–12 we also explored whether these factors modified the associations between ethnicity and post-stroke QOL.
Methods
Stroke Ascertainment
Ischemic stroke survivors were identified through the ongoing Brain Attack Surveillance in Corpus Christi (BASIC) Project, a population-based stroke surveillance study in Nueces County, Texas. The methods of the BASIC project have been previously described.13 Briefly, stroke cases among patients >45 years of age are obtained through active and passive surveillance. If a patient is identified as a potential stroke case, medical records are systematically reviewed by stroke fellowship trained study physicians blinded to ethnicity and age to validate strokes. Validated ischemic strokes were included using a standard clinical definition; events were limited to an individual’s first event during the study time period.14
Individual-Level Patient Factors
All stroke cases were invited to participate in an in-person baseline interview; a proxy is sought in the event that the case cannot answer a series of orientation questions.15 Approximately 47% of these interviews are conducted in hospital; the remaining interviews occur in the survivor’s home or via telephone (small percentages).10 The primary exposure for this study was self-reported ethnicity, which was obtained from the baseline interview, as were age, sex, educational attainment, marital status, smoking history, and pre-stroke functional and cognitive status. Pre-stroke function was measured within the interview by the modified Rankin Scale (mRS). Pre-stroke cognitive status was measured using the 16-item Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) and completed in-person or over the telephone by an individual who knew the patient well.16 Data collected from the medical record for all patients included the following risk factors and comorbidities: history of stroke/transient ischemic attack (TIA), hypertension, diabetes, coronary artery disease or myocardial infarction (CAD/MI), atrial fibrillation, high cholesterol, smoking status, excessive alcohol use, cancer, chronic obstructive pulmonary disease (COPD), congestive heart failure, Parkinson’s disease, end stage renal disease (ESRD), Alzheimer’s disease or dementia, and epilepsy. Using these variables, a comorbidity index was created, which summed the individual risk factors and comorbidities listed above and ranged from 0 to 15; this comorbidity index has been previously used within this study population.3, 10 Medical record data were collected regarding body mass index (BMI), receipt of tPA, insurance status, and prior residence in a nursing home. Initial NIH Stroke Scale (NIHSS) score was either abstracted from the medical record or calculated from the medical record using a validated method.17
Cases who participate in the baseline interview are asked to complete an in-person outcome interview at approximately 90 days. Whenever possible, these interviews are conducted in-person; however, a small subset is performed over the phone when necessary. In cases where the survivor is unable to complete the interview, a proxy is used. Since May 2010, cases have been asked to answer the twelve item short-form stroke specific quality of life scale (SSQOL). The short-form SSQOL is a standardized tool to measure health-related QOL that has been validated in patients with different types of stroke, as well as within the BASIC study population.18–20 Three summary scores, which served as the primary outcomes, were calculated for each stroke case as the average score within each domain: 1) overall; 2) physical domain; and 3) psychosocial domain. Scores ranged from 0–5; higher scores represent higher QOL. In addition, neurological deficits were measured by the NIHSS administered by a certified study coordinator at the time of the 90-day outcome interview.
Statistical Analysis
Ischemic stroke cases with complete data were included in the analysis. Medians/interquartile ranges (IQR) or frequencies/percentages were calculated for all demographics and risk factors by ethnicity and compared using chi-square and Kruskal-Wallis tests. Tobit regression was used to model unadjusted associations between ethnicity (MA versus NHW) and the 3 post-stroke QOL summary scores. Tobit regression was used to minimize bias due to the QOL scores being restrained by lower and upper bounds.21 Models were then adjusted for the following pre-specified demographic and clinical factors: age, sex, education, insurance status, marital status, residing in a nursing home prior to stroke, pre-stroke mRS, pre-stroke IQCODE, BMI, initial NIHSS, comorbidity index, stroke/TIA history, receipt of tPA, smoking status, presence of hypertension, diabetes, atrial fibrillation, and CAD/MI. Functional forms of continuous covariates were investigated by testing the significance of higher order polynomial terms; age, IQCODE, comorbidity index, and BMI were modeled linearly; initial NIHSS as a quadratic term. To obtain an adjusted Cohen’s standardized effect size, the between ethnic group QOL differences were divided by the overall standard deviation of the QOL measure of interest.22 Separate models were run with interaction terms for ethnicity and age, sex, and initial NIHSS to assess effect modification by these factors. If effect modification was present (p-value<0.10), stratified estimates of the associations were provided.
A post-hoc analysis further explored potential explanations for the observed ethnic differences in post-stroke QOL. First, an age-adjusted ethnic difference of individual questions in the SSQOL was estimated using linear regression models. Second, as neurologic outcome is measuring a different construct from QOL, 90-day post-stroke NIHSS was added to the fully adjusted models to determine if poor outcome explained ethnic differences in QOL. Given that effect modification of the ethnic association by age, post-stroke NIHSS was considered in the final model both as a main effect (modeled quadratically) and as interaction terms with age; estimates of the ethnic associations at the 25th, 50th and 75th percentile of age were evaluated to determine the influence of adjustment for NIHSS.
This study was approved by the Institutional Review Boards (IRB) at the University of Michigan and the local hospitals.
Results
A total of 749 MA and NHW patients had an ischemic stroke from May 2010 – June 2012. Among these patients, 493 (70.1%) agreed to be interviewed. Although participation did not differ by stroke severity (p=0.95), MAs were more likely to participate at baseline (p=0.08). Fifty-eight baseline interviews were completed by a proxy respondent; this did not differ by ethnicity (p=0.80).
Sixty-four participants (13%) were excluded due to mortality prior to completion of the 90-day outcome interview, 49 patients could not be located and 30 patients refused the outcome interview. Among the 350 (81.6%) survivors that completed the outcome interview, participation did not differ by ethnicity (p=0.46); however, those with more severe strokes were more likely to be lost to follow up (p=0.002). Sixty participants were missing data for IQCODE, BMI, education, or SSQOL, resulting in a total of 290 eligible participants (all other variable data were complete); 191 (66%) were MA and 99 (34%) were NHW. Overall, MAs were younger and had more comorbidities than NHWs (Table 1).
Table 1.
Baseline Characteristics by Ethnicity, BASIC Project, May 2010 – June 2012 (n = 290)
| N (%) or Median (Q1–Q3) | |||
|---|---|---|---|
| NHW (n = 99) | MA (n = 191) | p-value | |
| Age (years) | 72 (61–82) | 66 (58–77) | 0.02 |
| Female | 51 (51) | 104 (54) | 0.63 |
| Marital Status | |||
| Married / Living together | 52 (52) | 103 (54) | 0.82 |
| Single | 4 (4) | 7 (4) | 0.87 |
| Widowed | 19 (19) | 51 (27) | 0.16 |
| Divorced / Separated | 24 (24) | 30 (16) | 0.08 |
| Education | |||
| Less than high school | 11 (11) | 99 (52) | <0.0001 |
| High school | 31 (31) | 49 (26) | |
| Vocational / Some College | 29 (29) | 28 (15) | |
| College or more | 28 (28) | 15 (8) | |
| Insured | 92 (93) | 171 (90) | 0.34 |
| Residence in Nursing home | 1 (1) | 3 (2) | 0.7 |
| mRS | |||
| 0–1 | 48 (48) | 85 (45) | 0.77 |
| 2–3 | 43 (43) | 87 (46) | |
| 4+ | 8 (8) | 19 (10) | |
| Treated with tPA | 16 (16) | 17 (9) | 0.06 |
| Atrial Fibrillation | 20 (20) | 13 (7) | 0.0007 |
| Coronary Artery Disease | 32 (32) | 57 (30) | 0.66 |
| Diabetes Mellitus | 26 (26) | 110 (58) | <0.0001 |
| Hypertension | 76 (77) | 165 (86) | 0.04 |
| History of Stroke or TIA | 27 (27) | 62 (32) | 0.36 |
| Current / Former Smoker | 48 (48) | 48 (25) | 0.0003 |
| Initial NIHSS | 4 (2–9) | 4 (2–8) | 0.11 |
| IQCODE | 50 (48–56) | 49 (48–54) | 0.12 |
| Comorbidity Index | 4 (2–5) | 3 (2–4) | 0.05 |
| BMI | 26 (24–32) | 29 (26–34) | 0.0007 |
mRS: Modified Rankin Scale; TIA: Transient Ischemic Attack; NIHSS: National Institutes of Health Stroke Scale; IQCODE: Informant Questionnaire for Cognitive Decline in the Elderly; BMI: Body Mass Index
Quality of life Overall Summary Score
The median overall post-stroke QOL score was 3.0 for MAs (IQR=1.9) and 3.4 (IQR=1.5) for NHWs (scores range from 0–5; higher scores represent higher quality of life). The unadjusted model indicated that MAs experienced lower overall post-stroke QOL as compared to NHWs 90 days post-stroke (mean difference=−0.30 comparing MAs with NHWs, 95% CI: −0.57, −0.03). This ethnic difference persisted after multivariable adjustment (mean difference=−0.30, 95%CI: −0.59, −0.01). This translated into a Cohen’s standardized effect size of 0.27 (considered “small” (0.20) to “medium” (0.50)).22 Lower overall post-stroke QOL was associated with higher pre-stroke mRS, IQCODE, and NIHSS (higher scores represent greater impairment), as well as a history of stroke/TIA, and treatment with tPA (Table 2).
Table 2.
Multivariable Associations between Ethnicity and 90 Day Post-Stroke Quality Of Life (QOL) Summary Scores in the BASIC Project, May 2010 – June 2012 (n = 290)*
| Overall QOL |
95% CI | Physical QOL |
95% CI | Psychosocial QOL |
95% CI | |
|---|---|---|---|---|---|---|
| MA (vs NHW) | −0.30 | −0.59, −0.01 | −0.47 | −0.81, −0.14 | −0.25 | −0.61, 0.12 |
| Age (years) | −0.01 | −0.25, 0.22 | −0.29 | −0.56, −0.02 | 0.26 | −0.04, 0.56 |
| Female (vs male) | −0.05 | −0.29, 0.19 | −0.12 | −0.4, 0.16 | −0.02 | −0.32, 0.29 |
| Single (vs married) | 0.12 | −0.49, 0.73 | 0.23 | −0.47, 0.92 | 0.03 | −0.74, 0.81 |
| Widowed (vs married) | −0.04 | −0.35, 0.26 | 0.07 | −0.28, 0.42 | −0.14 | −0.53, 0.24 |
| Divorced/Separated (vs married) | −0.06 | −0.36, 0.25 | −0.03 | −0.39, 0.32 | −0.08 | −0.47, 0.31 |
| Hig school education (vs no high school) | 0.06 | −0.06, 0.19 | 0.04 | −0.3, 0.39 | 0.06 | −0.32, 0.45 |
| Vocational / Some College (vs no high school) | 0.29 | −0.05, 0.63 | 0.29 | −0.11, 0.68 | 0.37 | −0.07, 0.8 |
| College or more (vs no high school) | 0.23 | −0.14, 0.61 | 0.42 | −0.01, 0.86 | 0.12 | −0.35, 0.6 |
| Insured | 0.00 | −0.41, 0.41 | 0.29 | −0.18, 0.76 | −0.25 | −0.77, 0.26 |
| Residence in Nursing Home | 0.15 | −0.85, 1.16 | 0.42 | −0.72, 1.56 | −0.27 | −1.63, 1.09 |
| mRS 2–3 (vs 0–1) | −0.32 | −0.57, −0.07 | −0.39 | −0.67, −0.11 | −0.31 | −0.62, 0 |
| mRS 4–5 (vs 0–1) | −0.51 | −0.97, −0.04 | −1.14 | −1.68, −0.61 | 0.006 | −0.58, 0.59 |
| IQCODE | −0.12 | −0.22, −0.01 | −0.1 | −0.22, 0.03 | −0.14 | −0.28, −0.01 |
| Comorbidity Index | −0.22 | −0.56, 0.12 | −0.32 | −0.72, 0.07 | −0.15 | −0.58, 0.29 |
| NIHSS | −0.62 | −0.92, −0.32 | −0.65 | −0.99, −0.31 | −0.68 | −1.06, −0.3 |
| NIHSS Squared | 0.00 | 0, 0 | 0.002 | 0, 0 | −0.002 | 0, 0 |
| BMI | −0.02 | −0.16, 0.12 | 0.08 | −0.08, 0.25 | −0.11 | −0.29, 0.07 |
| History of Stroke / TIA | −0.29 | −0.57, −0.02 | −0.25 | −0.57, 0.06 | −0.39 | −0.74, −0.04 |
| Treated with tPA | 0.37 | −0.01, 0.75 | 0.37 | −0.07, 0.81 | 0.41 | −0.06, 0.9 |
| Diabetes Mellitus | 0.02 | −0.26, 0.3 | 0.03 | −0.29, 0.35 | 0.01 | −0.34, 0.37 |
| Hypertension | 0.09 | −0.27, 0.44 | 0.04 | −0.38, 0.45 | 0.16 | −0.29, 0.61 |
| Coronary Artery Disease | 0.06 | −0.24, 0.35 | 0.12 | −0.22, 0.46 | 0.01 | −0.37, 0.39 |
| Atrial Fibrillation | −0.03 | −0.43, 0.38 | −0.13 | −0.6, 0.33 | −0.04 | −0.55, 0.47 |
| Current/former smoker | −0.06 | −0.35, 0.23 | −0.05 | −0.38, 0.28 | −0.1 | −0.47, 0.26 |
Estimated using Tobit regression models
Quality of life Physical Summary Score
The median physical post-stroke QOL score was 3.8 (IQR=2.2) for MAs and 4.2 (IQR=1.7) for NHWs. There was an ethnic difference in physical post-stroke QOL scores when comparing MAs to NHWs (mean difference=−0.43, 95%CI: −0.77, −0.09) that persisted when adjusted for potential confounders (mean difference=−0.47, 95%CI: −0.81, −0.14). This also translated into a Cohen’s standardized effect size considered small to medium (0.36). A lower physical post-stroke QOL score was associated with higher pre-stroke mRS and NIHSS and higher age. In addition, having completed at least college was associated with a higher physical post-stroke QOL as compared to those with less than a high school education.
Quality of life Psychosocial Summary Score
The median psychosocial post-stroke QOL score was 2.7 (IQR=2.2) for MAs and 3.0 (IQR=2.0) for NHWs. The unadjusted association between ethnicity and psychosocial post-stroke QOL indicated that MAs experienced lower psychosocial post-stroke QOL as compared to NHWs (mean difference=−0.32, 95%CI: −0.66, 0.03); however, this difference was not statistically significant before or after multivariable adjustment (mean difference=−0.25, 95%CI: −0.61, 0.12). Lower psychosocial post-stroke QOL score was associated with higher IQCODE and NIHSS and with a history of stroke/TIA.
Effect Modification
Age modified the associations between ethnicity and the three post-stroke QOL summary scores. No ethnic differences were present at younger ages; however, the association between ethnicity and post-stroke QOL became stronger as age increased for all 3 QOL domains (Figure 1). There was some evidence that sex modified the associations between ethnicity and the QOL measures, with ethnic differences in post-stroke QOL being stronger among women; however, this effect modification did not reach statistical significance (Figure 2). Initial NIHSS did not modify the relationships between ethnicity and the three QOL summary scores (p-values > 0.50 for all QOL measures).
Figure 1.
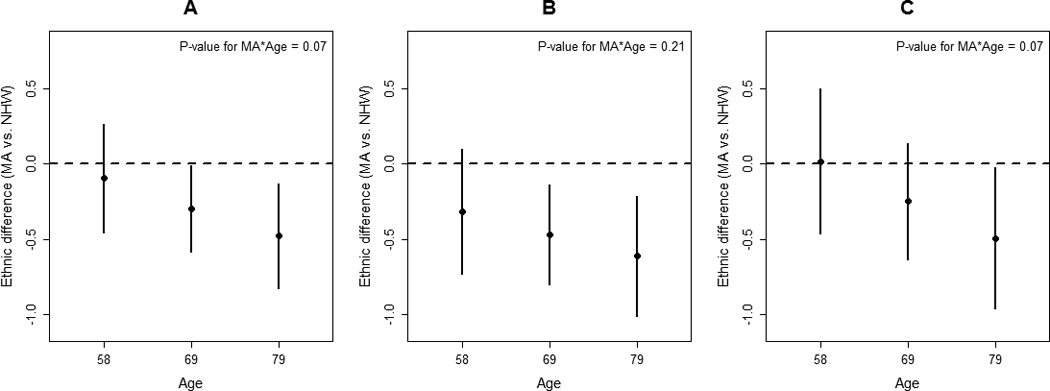
Ethnic differences in the average quality of life (QOL) score (Mexican American vs non-Hispanic White) for (A) Post-stroke Overall QOL, (B) Post-stroke Physical QOL, and (C) Post-stroke Psychosocial QOL at the 25th, 50th and 75th percentile of age distribution (adjusted for demographic, clinical, and pre-stroke characteristics).
Figure 2.
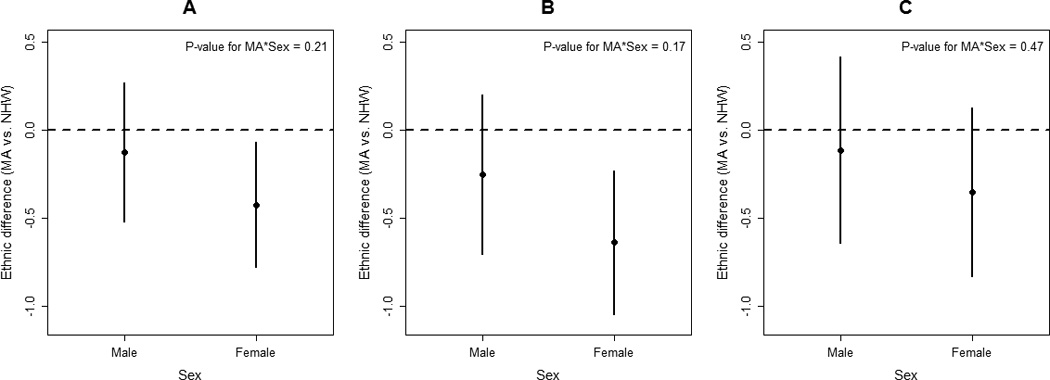
Ethnic differences in the average quality of life (QOL) score (Mexican American vs non-Hispanic White) in (A) Post-stroke Overall QOL, (B) Post-stroke Physical QOL, and (C) Post-stroke Psychosocial QOL across sexes (adjusted for demographic, clinical, and pre-stroke characteristics).
Post-hoc Analysis
MAs consistently reported poorer post-stroke QOL across almost all individual items in the SSQOL (Table 3). However, the majority of the significant ethnic differences were with respect to the physical QOL questions. After the addition of post-stroke NIHSS to the final multivariable model, the association between ethnicity and total QOL was attenuated and borderline significant, and ethnic differences were no longer significant at older ages (Figure I, please see http://stroke.ahajournals.org).
Table 3.
Age-Adjusted Ethnic differences in specific SSQOL items, BASIC Project, May 2010 – June 2012 (n = 290)*
| Item | Mean Difference (MA vs NHW) |
95% Confidence Interval |
|---|---|---|
| Psychosocial Quality of Life | ||
| I felt I was a burden to my family. | −0.49 | −0.90, −0.07 |
| My physical condition interfered with my social life. | −0.25 | −0.67, 0.18 |
| I was too tired to do what I wanted to do. | −0.27 | −0.67, 0.13 |
| I was discouraged about my future | −0.44 | −0.85, −0.02 |
| My personality has changed | −0.25 | −0.66, 0.15 |
| I had trouble remembering things | 0.26 | −0.11, 0.66 |
| Physical Quality of Life | ||
| Did you have to repeat yourself so others could understand you? | −0.3 | −0.65, 0.04 |
| Did you have to stop and rest more than you would like when walking or using the wheelchair? | −0.29 | −0.63, 0.06 |
| Did you have trouble buttoning buttons? | −0.41 | −0.79, −0.03 |
| Did you have trouble seeing the television well enough to enjoy a show? | −0.49 | −0.78, 0.20 |
| Did you have trouble doing daily work around the house? | −0.69 | −1.10, −0.27 |
| Did you need help taking a bath or shower? | −0.72 | −1.12, −0.31 |
Estimated using linear regression models; SSQOL: Short form stroke-specific quality of life
Discussion
In our population of stroke survivors in Nueces County, Texas, MAs experienced worse post-stroke QOL compared to NHWs, both overall and in physical QOL; these differences translated into a small to medium standardized effect size.22 Ethnic differences in post-stroke QOL became stronger as age increased. Our findings suggest that the disparate burden of stroke in MAs extends to QOL. As we accounted for pre-stroke factors, and other studies have demonstrated higher QOL in MAs as compared to NHWs in the general population, these differences likely reflect disparities in post-stroke outcomes.23 Interventions focused among older stroke survivors may improve post-stroke QOL and decrease disparities in the MA population.
Limited data exist on the post-stroke QOL of Hispanics. One study indicated post-stroke QOL differences between Hispanics of predominantly Puerto Rican and Dominican descent and NHWs only existed among those with less severe strokes.12 Our results are consistent in identifying ethnic differences in post-stroke QOL; however, we found ethnic differences across all levels of stroke severity.12 This suggests that MAs may face different challenges post-stroke, or that the NHWs in this population have better stroke outcomes than in previous studies. However, the stroke outcomes in the BASIC NHW population are comparable to those within the Framingham population; therefore, the latter seems unlikely.10
Post-hoc analysis revealed that poor functional and neurologic outcomes are likely driving the ethnic differences in QOL. This is consistent with recent data from the BASIC project that MAs experienced worse functional, neurological and cognitive post-stroke outcomes as compared to NHWs.3 In this community, MAs live in more socioeconomically disadvantaged neighborhoods than NHWs,24 which could contribute to differences in post-stoke outcomes through access to rehabilitation or other health services. These differences could lead to less improvement in functional outcomes over time, although there are little available data to inform this hypothesis.25
The lack of ethnic differences in psychosocial post-stroke QOL despite differences in physical post-stroke QOL may be reflective of cultural differences between the two ethnic groups, such as informal caregiving, familism, and spirituality.26 Hispanics are, in general, more likely to receive informal caregiving, which may also be true for stroke survivors.26, 27 MAs also have higher levels of perceived familial support than NHWs.6 In addition, MAs report more pre-stroke spirituality than NHWs, which may affect psychosocial aspects of the survivor’s life.28 These social support structures may attenuate the effect of stroke on psychosocial QOL among MAs, even despite worse cognitive, neurological and functional outcomes.3 However, it is interesting to note that the average scores for psychosocial post-stroke QOL were lower among both MAs and NHWs as compared to the overall and physical post-stroke QOL scores. This suggests room for improvement in psychosocial post-stroke QOL across both ethnic groups.
Limitations exist to this study. Loss to follow-up is a concern as the likelihood of completing the 90-day post-stroke interview was associated with stroke severity; however, loss to follow-up did not differ by ethnicity. The mRS and IQCODE may be subject to recall bias, as they were administered in reference to the pre-stroke period.16 In addition, the comorbidity index used in this analysis has not been validated. Although we did not have information on pre-stroke QOL, we measured and adjusted for numerous pre-stroke constructs that likely reflect QOL, such as the pre-stroke cognition, function, and comorbidity level. If any potential factors included in our models are located on the causal pathway between ethnicity and post-stroke QOL, the models may be overadjusted. Finally, this study explored ethnic differences in post-stroke QOL within a single community and may not be generalizable to the US as a whole. However, the sociodemographic characteristics of Nueces County are reflective of the changing demographics of the United States.
In conclusion, disparities exist in post-stroke QOL for MAs. Targeted interventions to improve outcomes in the growing population of MA stroke survivors are urgently needed, particularly among older stroke survivors.
Supplementary Material
Acknowledgments
Sources of Funding
This research was funded by the National Institute on Minority Health and Health Disparities (P60MD002249), and the National Institutes of Health/National Institute of Neurological Disorders and Stroke (R01NS038916).
Footnotes
Disclosures
None.
References
- 1.WHOQOL Group. The world health organization quality of life assessment (whoqol): Position paper from the world health organization. Social science & medicine. 1995;41:1403–1409. doi: 10.1016/0277-9536(95)00112-k. [DOI] [PubMed] [Google Scholar]
- 2.HealthyPeople 2020: Health-related quality of life & well-being. Office of Disease Prevention and Health Promotion web site. [Accessed July 14, 2014]; http://www.healthypeople.gov/2020/topics-objectives/topic/health-related-quality-of-life-well-being.
- 3.Lisabeth LD, Sanchez BN, Baek J, Skolarus LE, Smith MA, Garcia N, et al. Neurological, functional, and cognitive stroke outcomes in mexican americans. Stroke. 2014;45:1096–1101. doi: 10.1161/STROKEAHA.113.003912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sturm JW, Donnan GA, Dewey HM, Macdonell RA, Gilligan AK, Srikanth V, et al. Quality of life after stroke: The north east melbourne stroke incidence study (nemesis) Stroke. 2004;35:2340–2345. doi: 10.1161/01.STR.0000141977.18520.3b. [DOI] [PubMed] [Google Scholar]
- 5.King RB. Quality of life after stroke. Stroke. 1996;27:1467–1472. doi: 10.1161/01.str.27.9.1467. [DOI] [PubMed] [Google Scholar]
- 6.Sabogal F, Marín G, Otero-Sabogal R, Marín BV, Perez-Stable EJ. Hispanic familism and acculturation: What changes and what doesn't? Hispanic Journal of Behavioral Sciences. 1987;9:397–412. [Google Scholar]
- 7.Morgenstern LB, Smith MA, Lisabeth LD, Risser JM, Uchino K, Garcia N, et al. Excess stroke in mexican americans compared with non-hispanic whites the brain attack surveillance in corpus christi project. American journal of epidemiology. 2004;160:376–383. doi: 10.1093/aje/kwh225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichols-Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S. Factors influencing stroke survivors' quality of life during subacute recovery. Stroke. 2005;36:1480–1484. doi: 10.1161/01.STR.0000170706.13595.4f. [DOI] [PubMed] [Google Scholar]
- 9.Haley WE, Roth DL, Kissela B, Perkins M, Howard G. Quality of life after stroke: A prospective longitudinal study. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2011;20:799–806. doi: 10.1007/s11136-010-9810-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lisabeth LD, Reeves MJ, Baek J, Skolarus LE, Brown DL, Zahuranec DB, et al. Factors influencing sex differences in poststroke functional outcome. Stroke. 2015;46:860–863. doi: 10.1161/STROKEAHA.114.007985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gargano JW, Reeves MJ. Sex differences in stroke recovery and stroke-specific quality of life: Results from a statewide stroke registry. Stroke. 2007;38:2541–2548. doi: 10.1161/STROKEAHA.107.485482. [DOI] [PubMed] [Google Scholar]
- 12.Dhamoon MS, Moon YP, Paik MC, Boden-Albala B, Rundek T, Sacco RL, et al. Quality of life declines after first ischemic stroke. The northern manhattan study. Neurology. 2010;75:328–334. doi: 10.1212/WNL.0b013e3181ea9f03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgenstern LB, Smith MA, Sanchez BN, Brown DL, Zahuranec DB, Garcia N, et al. Persistent ischemic stroke disparities despite declining incidence in mexican americans. Ann Neurol. 2013;74:778–785. doi: 10.1002/ana.23972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asplund K, Tuomilehto J, Stegmayr B, Wester PO, Tunstall-Pedoe H. Diagnostic criteria and quality control of the registration of stroke events in the monica project. Acta medica Scandinavica. Supplementum. 1988;728:26–39. doi: 10.1111/j.0954-6820.1988.tb05550.x. [DOI] [PubMed] [Google Scholar]
- 15.Smith MA, Risser JM, Moye LA, Garcia N, Akiwumi O, Uchino K, et al. Designing multi-ethnic stroke studies: The brain attack surveillance in corpus christi (basic) project. Ethn Dis. 2004;14:520–526. [PubMed] [Google Scholar]
- 16.Jorm AF. The informant questionnaire on cognitive decline in the elderly (iqcode): A review. International psychogeriatrics / IPA. 2004;16:275–293. doi: 10.1017/s1041610204000390. [DOI] [PubMed] [Google Scholar]
- 17.Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the nih stroke scale. Stroke. 2000;31:858–862. doi: 10.1161/01.str.31.4.858. [DOI] [PubMed] [Google Scholar]
- 18.Williams LS, Weinberger M, Harris LE, Clark DO, Biller J. Development of a stroke-specific quality of life scale. Stroke. 1999;30:1362–1369. doi: 10.1161/01.str.30.7.1362. [DOI] [PubMed] [Google Scholar]
- 19.Kerber KA, Brown DL, Skolarus LE, Morgenstern LB, Smith MA, Garcia NM, et al. Validation of the 12-item stroke-specific quality of life scale in a biethnic stroke population. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2013;22:1270–1272. doi: 10.1016/j.jstrokecerebrovasdis.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Post MW, Boosman H, van Zandvoort MM, Passier PE, Rinkel GJ, Visser-Meily JM. Development and validation of a short version of the stroke specific quality of life scale. Journal of Neurology, Neurosurgery & Psychiatry. 2011;82:283–286. doi: 10.1136/jnnp.2009.196394. [DOI] [PubMed] [Google Scholar]
- 21.Tobin J. Estimation of relationships for limited dependent variables. Econometrica: journal of the Econometric Society. 1958;26:24–36. [Google Scholar]
- 22.Cohen J. Statistical power analysis for the behavioral sciences. 2nd Ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. The analysis of variance and covariance; pp. 273–403. [Google Scholar]
- 23.Farley T, Galves A, Dickinson LM, Perez Mde J. Stress, coping, and health: A comparison of mexican immigrants, mexican-americans, and non-hispanic whites. Journal of immigrant health. 2005;7:213–220. doi: 10.1007/s10903-005-3678-5. [DOI] [PubMed] [Google Scholar]
- 24.Lisabeth LD, Diez Roux AV, Escobar JD, Smith MA, Morgenstern LB. Neighborhood environment and risk of ischemic stroke: The brain attack surveillance in corpus christi (basic) project. Am J Epidemiol. 2007;165:279–287. doi: 10.1093/aje/kwk005. [DOI] [PubMed] [Google Scholar]
- 25.Smith MA, Risser JM, Lisabeth LD, Moyé LA, Morgenstern LB. Access to care, acculturation, and risk factors for stroke in mexican americans the brain attack surveillance in corpus christi (basic) project. Stroke. 2003;34:2671–2675. doi: 10.1161/01.STR.0000096459.62826.1F. [DOI] [PubMed] [Google Scholar]
- 26.Weiss CO, Gonzalez HM, Kabeto MU, Langa KM. Differences in amount of informal care received by non-hispanic whites and latinos in a nationally representative sample of older americans. Journal of the American Geriatrics Society. 2005;53:146–151. doi: 10.1111/j.1532-5415.2005.53027.x. [DOI] [PubMed] [Google Scholar]
- 27.Kemper P. The use of formal and informal home care by the disabled elderly. Health services research. 1992;27:421–451. [PMC free article] [PubMed] [Google Scholar]
- 28.Skolarus LE, Lisabeth LD, Sánchez BN, Smith MA, Garcia NM, Risser JM, et al. The prevalence of spirituality, optimism, depression, and fatalism in a bi-ethnic stroke population. Journal of religion and health. 2012;51:1293–1305. doi: 10.1007/s10943-010-9438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


