Abstract
Background and Purpose
Leptin is a major adipokine that regulates weight balance and energy homeostasis. There is inconsistent evidence linking circulating leptin levels to risk of stroke. We tested the hypothesis that leptin levels are associated with risk of incident stroke in an elderly community-based sample.
Methods
Serum leptin levels were assayed in 757 stroke-free individuals (mean age 79 years, 62% women) from the Framingham Original cohort at the 22nd examination cycle (1990–1994). Incidence of all-stroke and ischemic stroke were prospectively ascertained.
Results
During a mean follow-up of 10 years, 119 individuals developed stroke (99 ischemic stroke). In multivariable Cox regression models, log-leptin levels were not associated with incidence of all-stroke or ischemic stroke (hazard ratios[HR] per standard deviation(SD) increment in log-leptin 0.9 [0.73–1.09] and 0.89 [0.72–1.11], respectively). The results were suggestive for potential effect modification by waist-hip ratio(WHR) for the association between leptin and stroke (P=0.03). Adjusting for age, sex and established stroke risk factors, analysis stratified by WHR quartiles revealed a lower incidence of first-ever all-stroke and ischemic stroke associated with higher leptin levels among only subjects in the top WHR quartile (HR, 0.64 [0.43, 0.95] versus 0.98 [0.77, 1.25], for incident all-stroke and 0.61 [0.39, 0.95] versus 0.96 [0.74, 1.26] for ischemic stroke).
Conclusions
Leptin levels were not directly related to risk of incident stroke overall but there was an inverse association with stroke in the top WHR quartile. Further investigations are required to confirm these findings and explore possible mechanisms for the observed association.
Keywords: Leptin, Adipokines, Risk, Stroke, Obesity
Introduction
Leptin is an adipokine hormone secreted by adipocytes, and has structural and functional similarities with proinflammatory cytokines. It plays a key role in neuroendocrine function and metabolic processes (1). Leptin demonstrates a direct association with body mass. It is higher in obese individuals and lower in persons of normal weight. Leptin inhibits appetite and increases energy expenditure (2). However, central leptin resistance occurs in obese individuals resulting in a hyperleptinemic state (3,4). There is evidence suggesting a potential role of leptin in glucose regulation, insulin sensitivity, hematopoiesis, fatty acid catabolism, angiogenesis, and vascular and endothelial function (5–8). Leptin also induces proinflammatory cytokines such as interleukin-6 (IL6), (9) promotes platelet aggregation and may have a role in arterial thrombosis associated with obesity (10).
Higher circulating leptin levels have been associated with an increased prevalence of various vascular risk factors including insulin resistance, diabetes, hypertriglyceridemia and hypertension, and with lower levels of high-density lipoprotein cholesterol (HDL-C) (11–14). However, the association of leptin with vascular diseases is controversial. Whereas some studies suggest that higher circulating leptin levels increase risk of vascular disease including myocardial infarction and stroke, (15–20) other investigations have reported no such association (21–26), or have reported a protective role for leptin in determining overall mortality and risk of coronary disease (27–29).
Moreover, although several longitudinal studies have examined the association of circulating leptin levels with incident coronary heart disease, there are limited prospective data on their association with the risk of stroke (30). Accordingly, we longitudinally evaluated the association of baseline circulating levels of leptin with the risk of first ever any and ischemic stroke in the Framingham Heart Study to determine to what extent leptin may be an independent risk marker for first-ever stroke.
Materials and Methods
Study sample
The design and details of the Framingham Heart Study cohort have been published elsewhere (31). A total of 5209 participants were included in this study in 1948 to identify cardiovascular disease (CVD) risk factors. Participants are seen in the Heart Study research clinic every 2 years. Visits include laboratory testing, anthropometric measurements, as well as a standardized medical history and physical examination by a study physician. The 1166 participants who were alive at the time of the 22nd biennial examination cycle (between 1992 and 1994) were eligible for the present investigation. Leptin levels were measured in 796 participants. In our study sample, participants who did not have leptin levels measured were significantly older than those with leptin measured. However, after adjustment for age, participants without and with leptin levels did not differ with respect to their baseline clinical or biochemical characteristics. We excluded 39 participants for prevalent stroke. After the exclusions, 757 participants (mean age, 79 years; 470 women) were included in this analysis.
The study protocol was approved by the Institutional Review Board of the Boston University Medical Center and all participants provided written informed consent.
Outcome
Our protocols for stroke surveillance and diagnosis have been published elsewhere (32). Stroke was defined as an acute onset focal neurological deficit of vascular etiology, persisting more than 24 hours. Ischemic stroke was diagnosed if a focal neurological deficit was documented and the imaging showed no hemorrhage, the imaging showed an ischemic infarct that correlated with the clinical deficit, or an ischemic infarct was documented at autopsy.
Leptin Assay
A commercial radioimmunoassay (Linco Research, Inc., St. Louis, MO) was used to determine leptin concentrations from non-fasting plasma samples. The inter-assay coefficient of variation ranged from 3.0–6.2%. The lower sensitivity limit of the assay was 0.5 ng/ml.
Statistical Analysis
Serum leptin levels had a right skewed distribution in each sex and mean values differed between the sexes. Therefore, leptin levels were logarithmically transformed and standardized within each sex (mean=0, standard deviation (SD)=1). Cox regression models were used to relate baseline sex-standardized log-leptin levels to the incidence of stroke after confirming that the assumption of proportionality of hazards was met. Initial analyses were adjusted for age- and sex alone (Model A). Model B was additionally adjusted for Framingham Stroke Risk Profile (FSRP) that includes age, sex, systolic blood pressure, antihypertensive therapy, smoking status, diabetes mellitus and cardiovascular disease. In Model C, we further adjusted for waist-hip ratio (WHR). We chose to include WHR in our model as an index of body fat content because it is more strongly correlated with plasma leptin levels and risk of stroke than body mass index (33). We performed an exploratory analyses to evaluate whether age, sex, or WHR affected relationships of plasma leptin levels with stroke, with a Bonferroni-corrected, 2-tailed α level of 0.033 (3 effect modifiers × 1 exposure = 3 exploratory comparisons; with an alpha level of 0.10 for tests of interaction) and then stratified analyses when significant. Covariates were selected based on biologic interest and associations with exposures. For a sharp focus, we selected sex, age and adiposity as the main determinants of plasma leptin levels. A systematic review of studies published before September 2014 with over 1 year’s follow-up was conducted using a Pubmed search and all published data relating circulating leptin levels to the specific end-point of stroke were abstracted. Odds ratios (OR) were shown with 95% confidence intervals (CI), and 2-tailed probability estimates were used. Statistical analyses were performed using SAS, version 9.3. A p-value <0.05 was considered statistically significant.
Results
A total of 757 individuals were followed for up to 10 years, in which time 119 developed stroke, including 99 individuals with ischemic stroke. Baseline characteristics of our study sample are displayed in Table 1. Log-leptin levels showed no association with incident any or ischemic stroke in any of our models (Table 2). In a meta-analysis of prior studies, the combined risk ratio across all studies was 1.09 (95% CI: 0.87 to 1.37) in the adjusted analyses (Figure 1).
Table 1.
Baseline Characteristics of entire study sample, Framingham Heart Study.
| N | 757 |
|---|---|
| Age, mean±SD | 79±5 |
|
| |
| Female, n(%) | 470 (62) |
|
| |
| Systolic Blood Pressure, mean±SD | 143±21 |
|
| |
| Body Mass Index, mean±SD | 26.78±4.68 |
|
| |
| Waist-Hip Ratio, mean±SD | 0.93±0.09 |
| Q1, mean±SD | 0.83±0.07 |
| Q2, mean±SD | 0.90±0.05 |
| Q3, mean±SD | 0.95±0.04 |
| Q4, mean±SD | 1.02±0.04 |
|
| |
| Current Smoking, n(%) | 62 (8) |
|
| |
| Diabetes Mellitus, n(%) | 86 (12) |
|
| |
| Anti-hypertensive, n(%) | 363 (49) |
|
| |
| Prevalent CVD, n(%) | 241 (32) |
|
| |
| Leptin, Median (Q1, Q3) | 12.75(6.90, 22.35) |
|
| |
| Incident Stroke, n(%) | 119 (16) |
|
| |
| Ischemic Stroke, n(%) | 99 (13) |
CVD: Cardiovascular disease; IGF1: Insulin like growth factor1; N: Number; SD: Standard deviation; Q: Quartile
Table 2.
| HR [CI] (p-value) | ||
|---|---|---|
| Stroke | IS | |
| Model 1 | 0.94 [0.78, 1.13] (0.49) | 0.96 [0.78, 1.17] (0.65) |
| Model 2 | 0.90 [0.73, 1.09] (0.27) | 0.89 [0.72, 1.11] (0.28) |
| Model 3 | 0.93 [0.71, 1.21] (0.57) | 0.93 [0.69, 1.23] (0.59) |
Sex-standardized natural logarithm of leptin;
Using an indicator of top sex-specific quartile.
HR: Hazard ratio; IS: Ischemic stroke;
CI, confidence interval; HR, hazard ratio.
Model1: adjusted for age and sex; Model2: Model 1 plus systolic blood pressure, smoking, diabetes mellitus, Anti-hypertensive treatment, prevalent cardiovascular disease; Model3: Model 2 plus waist-hip ratio.
Figure 1.
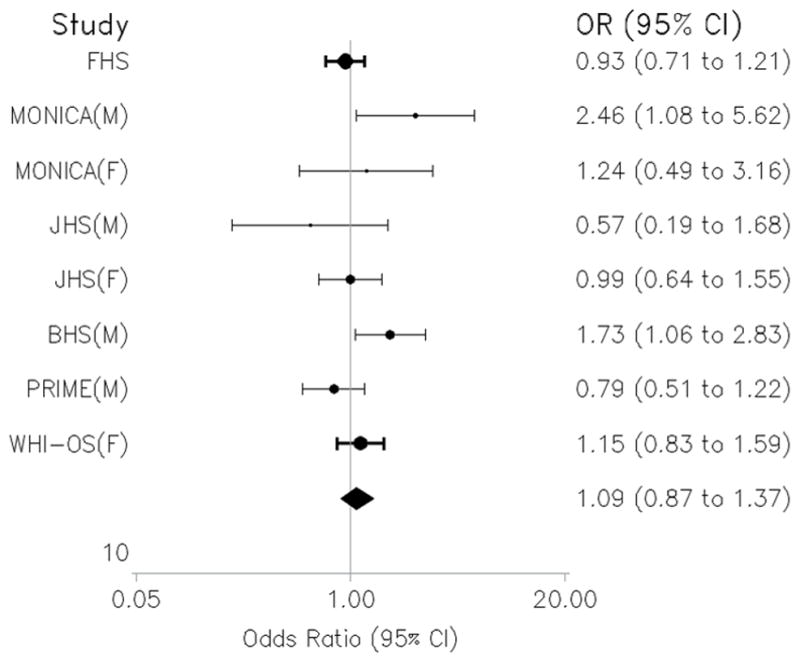
Random effects meta-analysis of prospective studies of circulating leptin levels and Stroke. M: Male; F: Female; FHS: Framingham Heart Study; JHS: Jackson Heart Study; BHS: British Heart Study; WHI-OS: Women Health Initiative – Observational Study.
Estimates from our multiplicative models were suggestive for an interaction by WHR (interaction term P=0.031). Therefore, we evaluated this possibility by comparing stratum-specific estimates that provides a more robust assessment of both biologic and statistical interaction. Analyses stratified by WHR revealed an inverse relationship between plasma leptin levels and risk of incident any (Hazard ratio, HR=0.64 (95%CI: 0.45–0.92), P=0.01) and ischemic stroke (HR=0.65 (95%CI: 0.44–0.95), P=0.02) among subjects in the top WHR quartile, but not in others (HR=1.02 (95%CI: 0.82–1.28), P=0.84 and HR=1.03 (95%CI: 0.80–1.32), P=0.82 for incident any and ischemic stroke, respectively) (Table 3). Theses associations remained unchanged after adjustment for traditional stroke risk factors.
Table 3.
Stratified analysis by WHR for the association between leptin* and incident any and ischemic stroke (IS) **
| Top Quartile of WHR | Bottom 3 quartiles of WHR | |||||||
|---|---|---|---|---|---|---|---|---|
| Stroke | IS | Stroke | IS | |||||
| Model | n | HR [CI] (p-value) | n | HR [CI] (p-value) | n | HR [CI] (p-value) | n | HR [CI] (p-value) |
| Model 1 | 31/189 | 0.64 [0.45, 0.92] (0.01) | 27/189 | 0.65 [0.44, 0.95] (0.02) | 87/564 | 1.02 [0.82, 1.28] (0.84) | 71/564 | 1.03 [0.80, 1.32] (0.82) |
| Model 2 | 31/189 | 0.64 [0.43, 0.95] (0.02) | 27/189 | 0.61 [0.39, 0.95] (0.02) | 87/564 | 0.98 [0.77, 1.25] (0.89) | 71/564 | 0.96 [0.74, 1.26] (0.78) |
Sex-standardized natural logarithm of leptin;
Using an indicator of top sex-specific quartile.
HR: Hazard ratio; CI: Confidence interval; WHR: Weight-to-hip ratio;
Model 1: adjusted for age and sex; Model2: Model 1 plus systolic blood pressure, smoking, diabetes mellitus, Anti-hypertensive treatment, prevalent cardiovascular disease.
Discussion
In this study of older individuals, higher baseline leptin levels were not prospectively associated with total or ischemic stroke incidence in multivariable adjusted models. However, our analysis stratified by WHR was suggestive for a potential protective role for leptin against risk of incident all-stroke and incident ischemic stroke among persons in the top WHR quartile.
Although high circulating leptin levels have been shown to be correlated with established vascular risk factors such as obesity, hypertension, hyperlipidemia and diabetes and an inflammatory state, few prospective studies have investigated the association of serum leptin with incidence of stroke, and prior cross-sectional and case-control studies had inconsistent findings (16,18,34). Recent studies have suggested a leptin surge as an acute response to stress or CVD events (35); therefore, retrospective and cross sectional study designs may not be optimal to study the association. For example, whereas high leptin was reported as a significant risk factor for prevalent stroke in an initial cross-sectional report from the Jackson Heart study (JHS) (18), it was not prospectively associated with incident coronary heart disease or incident stroke during 6 years of follow up in a subsequent report from the same cohort (26). Furthermore, high leptin levels may not be casually linked to vascular risk and may merely reflect a state of hypothalamic leptin resistance in obesity and the co-occurrence of multiple vascular risk factors with obesity.
Our observation of the lack of an overall association between circulating leptin and risk of stroke is in line with 3 recent prospective studies (22,25,26). In a recent prospective nested case-control study among healthy men within the PRIME study, increased plasma leptin was not shown to be associated with an increased 10-year risk of ischemic stroke (25). Similarly, results from a prospective nested case–control study within the Women’s Health Initiative Observational Study cohort with 972 stroke cases suggested no association between serum leptin levels and risk of development of ischemic stroke among postmenopausal women independently of established stroke risk factors (22). On the other hand, in a longitudinal follow-up of the British Regional Heart Study participants with 192 prospectively ascertained cases of stroke, increased leptin was associated with a higher risk of the stroke in older men while obese subjects showed the lowest stroke risk (36). One previous study from the same population observed no association between leptin levels and risk of coronary heart disease, suggesting that this cohort may not be representative of other population samples (37). In our meta-analysis of prior prospective studies of leptin levels and stroke, we did not find any significant association between leptin levels and risk of stroke. None of the previous prospective studies have examined the association of leptin with risk of stroke in different strata of WHR. Our observation of an inverse association between leptin levels and risk of stroke for subjects in the top WHR quartile is intriguing. Recent studies have suggested that leptin can explain the lower rate of cardiovascular events and mortality in the obese (the obesity paradox) (38, 39). Hyperleptinemia generated in the course of diet induced obesity may protect non-adipose tissues from lipotoxicity and oxidative damage (40). Also, leptin is significantly correlated with serum nitric oxide (NO) metabolite concentrations and plays an important role in NO regulation and production in humans (41). Thus, low leptin levels in states of relative leptin resistance, such as obesity may increase risk of vascular events.
Inconsistent findings of the association between leptin and vascular events may be explained by the threshold levels of various leptin-mediated physiological effects in different conditions (42). Recent studies have supported the concept of selective leptin resistance in human and animal models, with a retained sensitivity to leptin in peripheral and vascular tissue in hyperleptinemic states through differential post-receptor signaling feedback loops or brain site-specific mechanisms (4,43,44). In a recent study, leptin was reported to mediate an increase in blood pressure in diet induced obese rodents (45). However, in another study in diet induced obese mice, leptin did not increase blood pressure despite increasing sympathetic activity suggesting that leptin is implicated in other vascular counter-regulatory pathways against hypertension. (46). These concepts suggest the wide array of pathways, beyond blood pressure regulation, by which leptin may differentially modulate the risk of stroke in obese individuals and call for further research, stratified by weight and insulin resistance, to elucidate a possible clinically significant role of leptin resistance in obese individuals.
The prospective ascertainment of first ever stroke in a community-based sample and comprehensive assessment of covariates comprising the Framingham stroke risk profile strengthen our study. However, these results should be interpreted with caution since our data were based on an observational study and cannot yield causal relationship. Also, the study is representative of an elderly population of white individuals that limits the generalizability of our findings to other age-groups or ethnicities. Leptin concentrations were measured from non-fasting plasma samples. However, mean postprandial leptin levels have been shown not to alter from fasting leptin levels after either the low-fat or the high-fat diet (47). Leptin levels were determined in a single measurement, which may not capture a diurnal variation in leptin levels and may result in a misclassification of exposure that may have biased our results toward the null, but this does not invalidate our finding in the obese. Moreover, leptin levels have been consistently shown to be relatively stable over time in an individual (48,49).
Summary
Our findings indicate that serum leptin levels are inversely associated with risk of stroke among subjects within the top WHR quartiles. Additional population studies stratified by weight and experimental studies on the possible protective effects of leptin, particularly in obese individuals, are required to confirm our findings.
Acknowledgments
Sources of Funding
Supported by the Framingham Heart Study’s National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) contract (N01 HC25195 and N01 HHSN-268201500001) and by grants from the National Institute of Neurological Disorders and Stroke (NINDS) (R01 NS017950), the National Institute on Aging (R01 AG16495, AG 033040, AG008122, AG033193, AG031287 and K23 AG038444), and the American Heart Association (11CRP4930020). We also thank the Framingham study team, especially the Neurology team, both investigators and staff, for their contributions to data collection over the past four decades. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart Lung and Blood Institute, the National Institute on Aging or the National Institutes of Health.
Footnotes
Disclosures
Dr. Roubenoff is a full time employee of Novartis Institutes for Biomedical Research. Dr. Shoamanesh is supported by the Marta and Owen Boris Chair in Stroke Research and Care.
References
- 1.Rondinone CM. Adipocyte-derived hormones, cytokines, and mediators. Endocrine. 2006;29:81–90. doi: 10.1385/endo:29:1:81. [DOI] [PubMed] [Google Scholar]
- 2.Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998;1:445–450. doi: 10.1038/2164. [DOI] [PubMed] [Google Scholar]
- 3.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, et al. Diet-Induced Obesity Causes Severe but Reversible Leptin Resistance in Arcuate Melanocortin Neurons. Cell Metab. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52:1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anagnostoulis S, Karayiannakis AJ, Lambropoulou M, Efthimiadou A, Polychronidis A, Simopoulos C. Human leptin induces angiogenesis in vivo. Cytokine. 2008;42:353–357. doi: 10.1016/j.cyto.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 6.O’Rourke L, Gronning LM, Yeaman SJ, Shepherd PR. Glucose-dependent regulation of cholesterol ester metabolism in macrophages by insulin and leptin. J Biol Chem. 2002;277:42557–42562. doi: 10.1074/jbc.M202151200. [DOI] [PubMed] [Google Scholar]
- 7.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 8.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 9.Sommer G, Kralisch S, Stangl V, Vietzke A, Köhler U, Stepan H, et al. Secretory products from human adipocytes stimulate proinflammatory cytokine secretion from human endothelial cells. J Cell Biochem. 2009;106:729–737. doi: 10.1002/jcb.22068. [DOI] [PubMed] [Google Scholar]
- 10.Dellas C, Schäfer K, Rohm I, Lankeit M, Ellrott T, Faustin V, et al. Absence of leptin resistance in platelets from morbidly obese individuals may contribute to the increased thrombosis risk in obesity. Thromb Haemost. 2008;100:1123–1129. [PubMed] [Google Scholar]
- 11.Alikaşifoğlu A, Gönç N, Özön ZA, Sen Y, Kandemir N. The relationship between serum adiponectin, tumor necrosis factor-alpha, leptin levels and insulin sensitivity in childhood and adolescent obesity: adiponectin is a marker of metabolic syndrome. J Clin Res Pediatr Endocrinol. 2009;1:233–239. doi: 10.4274/jcrpe.v1i5.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cleland SJ, Sattar N, Petrie JR, Forouhi NG, Elliott HL, Connell JM. Endothelial dysfunction as a possible link between C-reactive protein levels and cardiovascular disease. Clin Sci (Lond) 2000;98:531–535. [PubMed] [Google Scholar]
- 13.Do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011;57:918–926. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzali G, Di Francesco V, Zoico E, Fantin F, Zamboni G, Benati C, et al. Interrelations between fat distribution, muscle lipid content, adipocytokines, and insulin resistance: effect of moderate weight loss in older women. Am J Clin Nutr. 2006;84:1193–1199. doi: 10.1093/ajcn/84.5.1193. [DOI] [PubMed] [Google Scholar]
- 15.Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104:3052–3056. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 16.Söderberg S, Stegmayr B, Stenlund H, Sjöström L-G, Agren A, Johansson L, et al. Leptin, but not adiponectin, predicts stroke in males. J Intern Med. 2004;256:128–136. doi: 10.1111/j.1365-2796.2004.01351.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim BJ, Lee S-H, Ryu W-S, Kim CK, Yoon B-W. Adipocytokines and ischemic stroke: differential associations between stroke subtypes. J Neurol Sci. 2012;312:117–122. doi: 10.1016/j.jns.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Butler KR, Buxbaum SG, Sung JH, Campbell BW, Taylor HA. Leptinemia and its association with stroke and coronary heart disease in the Jackson Heart Study. Clin Endocrinol (Oxf) 2010;72:32–37. doi: 10.1111/j.1365-2265.2009.03627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sierra-Johnson J, Romero-Corral A, Lopez-Jimenez F, Gami AS, Sert Kuniyoshi FH, Wolk R, et al. Relation of increased leptin concentrations to history of myocardial infarction and stroke in the United States population. Am J Cardiol. 2007;100:234–239. doi: 10.1016/j.amjcard.2007.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Söderberg S, Stegmayr B, Ahlbeck-Glader C, Slunga-Birgander L, Ahrén B, Olsson T. High leptin levels are associated with stroke. Cerebrovasc Dis. 2003;15:63–69. doi: 10.1159/000067128. [DOI] [PubMed] [Google Scholar]
- 21.Brennan AM, Li TY, Kelesidis I, Gavrila A, Hu FB, Mantzoros CS. Circulating leptin levels are not associated with cardiovascular morbidity and mortality in women with diabetes: a prospective cohort study. Diabetologia. 2007;50:1178–1185. doi: 10.1007/s00125-007-0635-y. [DOI] [PubMed] [Google Scholar]
- 22.Rajpathak SN, Kaplan RC, Wassertheil-Smoller S, Cushman M, Rohan TE, McGinn AP, et al. Resistin, but not adiponectin and leptin, is associated with the risk of ischemic stroke among postmenopausal women: results from the Women’s Health Initiative. Stroke. 2011;42:1813–1820. doi: 10.1161/STROKEAHA.110.607853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welsh P, Murray HM, Buckley BM, de Craen AJM, Ford I, Jukema JW, et al. Leptin predicts diabetes but not cardiovascular disease: results from a large prospective study in an elderly population. Diabetes Care. 2009;32:308–310. doi: 10.2337/dc08-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stott DJ, Welsh P, Rumley A, Robertson M, Ford I, Sattar N, et al. Adipocytokines and risk of stroke in older people: a nested case-control study. Int J Epidemiol. 2009;38:253–261. doi: 10.1093/ije/dyn215. [DOI] [PubMed] [Google Scholar]
- 25.Prugger C, Luc G, Haas B, Arveiler D, Machez E, Ferrieres J, et al. Adipocytokines and the risk of ischemic stroke: the PRIME Study. Ann Neurol. 2012;71:478–486. doi: 10.1002/ana.22669. [DOI] [PubMed] [Google Scholar]
- 26.Bidulescu A, Musani SK. Associations of adiponectin and leptin with incident coronary heart disease and ischemic stroke in African Americans: the Jackson Heart Study. Front Public Health. 2013;1:16. doi: 10.3389/fpubh.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scholze A, Rattensperger D, Zidek W, Tepel M. Low serum leptin predicts mortality in patients with chronic kidney disease stage 5. Obesity (Silver Spring) 2007;15:1617–1622. doi: 10.1038/oby.2007.191. [DOI] [PubMed] [Google Scholar]
- 28.Smith CCT, Mocanu MM, Davidson SM, Wynne AM, Simpkin JC, Yellon DM. Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br J Pharmacol. 2006;149:5–13. doi: 10.1038/sj.bjp.0706834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ku IA, Farzaneh-Far R, Vittinghoff E, Zhang MH, Na B, Whooley MA. Association of low leptin with cardiovascular events and mortality in patients with stable coronary artery disease: the Heart and Soul Study. Atherosclerosis. 2011;217:503–508. doi: 10.1016/j.atherosclerosis.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savopoulos C, Michalakis K, Apostolopoulou M, Miras A, Hatzitolios A. Adipokines and stroke: a review of the literature. Maturitas. 2011;70:322–327. doi: 10.1016/j.maturitas.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 33.Peltz G, Sanderson M, Pérez A, Sexton K, Ochoa Casares D, Fadden MK. Serum leptin concentration, adiposity, and body fat distribution in Mexican-Americans. Arch Med Res. 2007;38:563–570. doi: 10.1016/j.arcmed.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Romero-Corral A, Sierra-Johnson J, Lopez-Jimenez F, Thomas RJ, Singh P, Hoffmann M, et al. Relationships between leptin and C-reactive protein with cardiovascular disease in the adult general population. Nat Clin Pract Cardiovasc Med. 2008;5:418–425. doi: 10.1038/ncpcardio1218. [DOI] [PubMed] [Google Scholar]
- 35.Meisel SR, Ellis M, Pariente C, Pauzner H, Liebowitz M, David D, et al. Serum leptin levels increase following acute myocardial infarction. Cardiology. 2001;95:206–211. doi: 10.1159/000047373. [DOI] [PubMed] [Google Scholar]
- 36.Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Adiposity, adipokines, and risk of incident stroke in older men. Stroke. 2013;44:3–8. doi: 10.1161/STROKEAHA.112.670331. [DOI] [PubMed] [Google Scholar]
- 37.Sattar N, Wannamethee G, Sarwar N, Chernova J, Lawlor DA, Kelly A, et al. Leptin and coronary heart disease: prospective study and systematic review. J Am Coll Cardiol. 2009;53:167–175. doi: 10.1016/j.jacc.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 38.Wolk R, Berger P, Lennon RJ, Brilakis ES, Johnson BD, Somers VK. Plasma leptin and prognosis in patients with established coronary atherosclerosis. J Am Coll Cardiol. 2004;44:1819–1824. doi: 10.1016/j.jacc.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 39.Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Papacosta O, Sattar N. The obesity paradox in men with coronary heart disease and heart failure: the role of muscle mass and leptin. Int J Cardiol. 2014;171:49–55. doi: 10.1016/j.ijcard.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Unger RH. Hyperleptinemia: protecting the heart from lipid overload. Hypertension. 2005;45:1031–1034. doi: 10.1161/01.HYP.0000165683.09053.02. [DOI] [PubMed] [Google Scholar]
- 41.Tsuda K, Nishio I. Leptin and nitric oxide production in normotensive and hypertensive men. Obes Res. 2004;12:1223–1237. doi: 10.1038/oby.2004.154. [DOI] [PubMed] [Google Scholar]
- 42.Rosenbaum M, Leibel RL. Leptin: a molecule integrating somatic energy stores, energy expenditure and fertility. Trends Endocrinol Metab. 1998;9:117–124. doi: 10.1016/s1043-2760(98)00028-9. [DOI] [PubMed] [Google Scholar]
- 43.Correia MLG, Haynes WG, Rahmouni K, Morgan DA, Sivitz WI, Mark AL. The concept of selective leptin resistance: evidence from agouti yellow obese mice. Diabetes. 2002;51:439–442. doi: 10.2337/diabetes.51.2.439. [DOI] [PubMed] [Google Scholar]
- 44.Münzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 45.Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, et al. Leptin mediates the increase in blood pressure associated with obesity. Cell. 2014;159:1404–1416. doi: 10.1016/j.cell.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belin de Chantemèle EJ, Mintz JD, Rainey WE, Stepp DW. Impact of leptin-mediated sympatho-activation on cardiovascular function in obese mice. Hypertension. 2011;58:271–279. doi: 10.1161/HYPERTENSIONAHA.110.168427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weigle DS, Duell PB, Connor WE, Steiner RA, Soules MR, Kuijper JL. Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels. J Clin Endocrinol Metab. 1997;82:561–565. doi: 10.1210/jcem.82.2.3757. [DOI] [PubMed] [Google Scholar]
- 48.Linkov F, Gu Y, Arslan AA, Liu M, Shore RE, Velikokhatnaya L, et al. Reliability of tumor markers, chemokines, and metastasis-related molecules in serum. Eur Cytokine Netw. 2009;20:21–26. doi: 10.1684/ecn.2009.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee S-A, Kallianpur A, Xiang Y-B, Wen W, Cai Q, Liu D, et al. Intra-individual variation of plasma adipokine levels and utility of single measurement of these biomarkers in population-based studies. Cancer Epidemiol Biomarkers Prev. 2007;16:2464–2470. doi: 10.1158/1055-9965.EPI-07-0374. [DOI] [PubMed] [Google Scholar]


