Abstract
Background and Purpose
We recently observed early white matter injury after experimental subarachnoid hemorrhage (SAH) but the underlying mechanisms are uncertain. This study investigated the potential role of matrix metalloproteinase (MMP)-9 in blood-brain barrier (BBB) disruption and consequent white matter injury.
Methods
SAH was induced by endovascular perforation in adult male mice. The following three experiments were devised: (1) mice underwent MRI at 24 h after SAH and were euthanized to determine BBB disruption and MMP-9 activation in white matter; (2) to investigate the role of MMP-9 in BBB disruption, lesion volumes on MRI were compared between wild-type (WT) and MMP-9-knockout (MMP-9−/−) mice at 24 h after SAH; (3) WT and MMP-9−/− mice underwent MRI at 1 and 8 days after SAH to detect time-dependent changes in brain injury. Brains were used to investigate myelin integrity in white matter.
Results
In WT mice with SAH, white matter showed BBB disruption (albumin leakage) and T2-hyperintensity on MRI. MMP-9 activity was elevated at 24 h after SAH. MMP-9−/− mice had less white matter T2-hyperintensity after SAH than WT mice. At 8 days after SAH, WT mice had decreased myelin integrity and MMP-9−/− mice developed less white matter injury.
Conclusions
SAH causes BBB disruption and consequent injury in white matter. MMP-9 plays an important role in those pathologies and could be a therapeutic target for SAH-induced white matter injury.
Keywords: blood-brain barrier, matrix metalloproteinase-9, subarachnoid hemorrhage, white matter injury
Introduction
Subarachnoid hemorrhage (SAH) is a devastating cerebrovascular disorder with particularly high mortality and morbidity rates1. Many physiological derangements, such as elevated intracranial pressure and decreased cerebral blood flow, are immediately induced by SAH and these events initiate inflammation and oxidative stress which result in blood-brain barrier (BBB) disruption, brain edema as well as neuronal injury 2. Acute brain edema is recognized as a major consequence of SAH, and is identified as an independent risk factor for poor clinical outcome 3. As yet, the few available SAH therapies focus on preventing aneurysmal rebleeding and prophylaxis for vasospasm, and there are no effective treatments available against early brain injury and consequent edema formation 4.
Matrix metalloproteinases (MMPs), particularly MMP-9, is well known as a key factor for the development of vasogenic edema after various brain injuries including SAH 5. Thus, MMP-9 is upregulated and activated after cerebrovascular disorders in both clinical and experimental settings 6, and MMP-9 deletion ameliorates brain edema and leads to better neurological recovery in SAH mice 7.
Recently, we observed early white matter injury after experimental SAH 8. However, the detailed mechanisms involved in such injury remain uncertain. We hypothesized that MMP-9 causes acute BBB disruption in white matter after SAH resulting in consequent white matter injury. In the present study, we investigated this hypothesis using a mouse model of experimental SAH.
Methods
All animal protocols were approved by the University of Michigan Committee on the Use and Care of Animals. Adult male C57BL/6 mice (Charles River Laboratories; Portage, MI) weighing 22 to 30 g and male MMP-9-knockout (MMP-9−/−) mice with C57BL/6 background (University of Michigan Breeding Core) were housed at a controlled temperature under a 12-hour light-dark cycle. Food and water were available to all animals ad libitum.
Experimental design
This study included the following three experiments. Throughout the current study, we used forty-two WT and twenty-seven MMP-9−/− mice. Experiment 1 were conducted in WT mice, experiment 2 and 3 were conducted in WT and MMP-9−/− mice.
Experiment 1
To determine the extent of BBB disruption and MMP-9 activation/expression in white matter after SAH, wild-type (WT) mice were divided into sham and SAH groups. Mice underwent magnetic resonance imaging (MRI) at 24 hours after SAH and were then euthanized. Brains were used for immunohistochemistry, transmission electron microscopy and gelatin zymography.
Experiment 2
To investigate the role of MMP-9 in white matter injury, WT and MMP-9−/− mice underwent endovascular perforation. They underwent MRI 24 hours after SAH induction. Severity of SAH, neurological conditions including mortality, and lesion volumes detected by MRI in each group were compared. We used 28 WT (n=11 for sham, and n=17 for SAH) and 13 MMP-9−/− mice in experiment 1 and 2.
Experiment 3
To investigate time-dependent changes in white matter, MRI was performed 1 and 8 days after SAH induction in WT and MMP-9−/− mice. Mice without any surgery were set as normal control. Animals were euthanized at day 8 and the brains were used for immunohistochemistry. Mortality, neurological score and body weight during the observation period were also recorded. In experiment 3, we used 14 WT and 14 MMP-9−/− mice (n=10 for SAH and n=4 for normal control, for each).
Mouse SAH model
SAH was induced by endovascular perforation method as previously described 8. Details of surgical procedures are presented in the online-only Data Supplement.
Magnetic resonance imaging technique and measurement of lesion volume
MRI was performed using a 7.0-T Varian MR scanner (Varian Inc, Palo Alto, CA) with acquisition of T2 fast spin-echo and T2* gradient-echo sequences using a field of view of 20×20 mm, matrix of 256×256 mm, and 25 coronal slices (0.5 mm thick). The volume of T2-hyperintensity in the white matter and ventricular volume were measured as previously described 8, 9.
Evaluation of SAH severity and neurological scores
The extent of SAH was assessed using a modified grading system 10, 11, and the neurological scores were evaluated by a blinded observer at determined time points in each experiment as previously described 12. Details for the evaluation methods are in the online-only Data Supplement.
Transmission electron microscopy
Transmission electron microscopy was performed as previously described 13 (see details in online-only Data Supplement).
Gelatin zymography for measurement of MMP activity
Activities of MMP-9 and -2 were analyzed by gelatin zymography using commercially available kit (Cosmo Bio, Tokyo, Japan) as previously described 14. Details are available in the online-only Data Supplement.
Immunohistochemistry and histochemistry
Immunohistochemistry and histochemistry were performed on 10-μm-thick coronal sections15 (see online-only Data Supplement).
Immunofluorescence
Double-label immunofluoresence was performed as described previously 15(see online-only Data Supplement).
Quantification of immunostaining
All analyses were performed by blinded investigator according to previous report 16 (see online-only Data Supplement).
Statistics
Data are expressed as mean ± SD and analyzed using JMP 7 software (SAS Institute Inc, Cary, NC). Statistical differences among the groups were analyzed using 1-way ANOVA, repeated-measures ANOVA, Spearman rank correlation test, and Log-rank test. A Bonferroni correction was used for multiple comparisons. P<0.05 was considered statistically significant.
Results
SAH caused BBB disruption in white matter
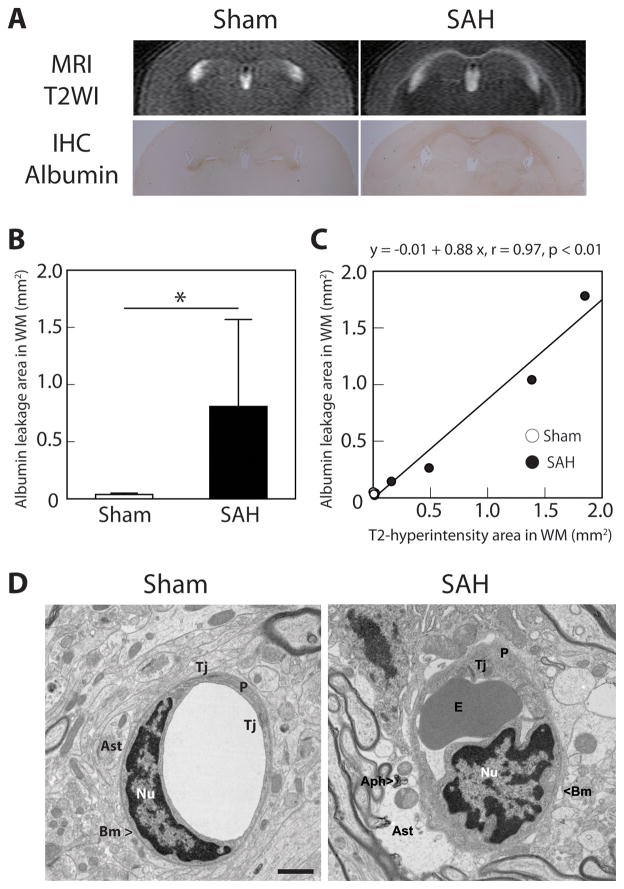
Mice that underwent endovascular perforation (SAH) but not a sham operation developed white matter T2-hyperintensity at 24 hours. SAH also induced albumin leakage (BBB disruption) along the white matter. The area of albumin leakage was significantly larger in SAH-than in sham-operated mice (0.81 ± 0.76 vs. 0.04 ± 0.01 mm2; P<0.05; n=4, for each; Figure 1B). There was a good correlation between T2-hyperintensity and albumin leakage (r=0.97; P<0.01; Figure 1C).
Figure 1.
Subarachnoid hemorrhage (SAH) causes blood-brain barrier disruption at 24 hours after endovascular perforation. (A) Coronal T2-weighted imaging (T2WI) on MRI demonstrated hyperintensity along the white matter (WM) in SAH mice, and this was consistent with albumin leakage demonstrated by immunohistochemistry (IHC). (B) The area of WM albumin leakage was significantly larger after SAH than in sham-operated mice. (C) The area of albumin leakage on IHC correlated with the area of T2-hyperintensity on MRI. Values are mean±SD; *P<0.05; n=4 for each. (D) Transmission electron microscopy demonstrated WM microvascular ultrastructural abnormalities after SAH: swollen astrocyte endfeet with autophagosome, tight junction detachment, erythrocytes trapped in capillary lumen, and irregularity of basement membrane. Aph – autophagosome, Ast – astrocyte, BM – basement membrane, E – erythrocyte, Nu – nucleus of endothelium, P – pericyte, Tj – tight junction. Scale bar = 1μm.
An electron microscopic examination showed ultrastructural abnormalities in white matter microvessels after SAH (Figure 1D). Abnormalities included swollen astrocyte endfeet with autophagosomes, tight junction detachment, erythrocytes in capillary lumen (after perfusion), and basement membrane irregularities. Degenerating axons, indicating acute white matter injury, were also observed in after SAH (data not shown). These results strongly suggest that SAH causes BBB disruption in white matter, as well as acute axonal injury.
MMP-9 activity in white matter was elevated after SAH
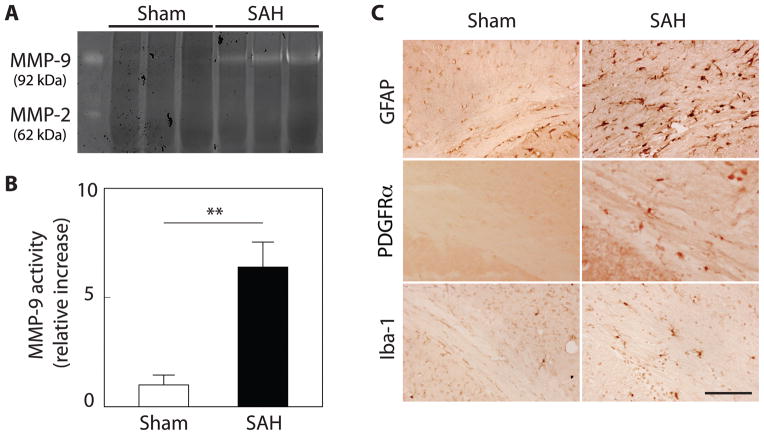
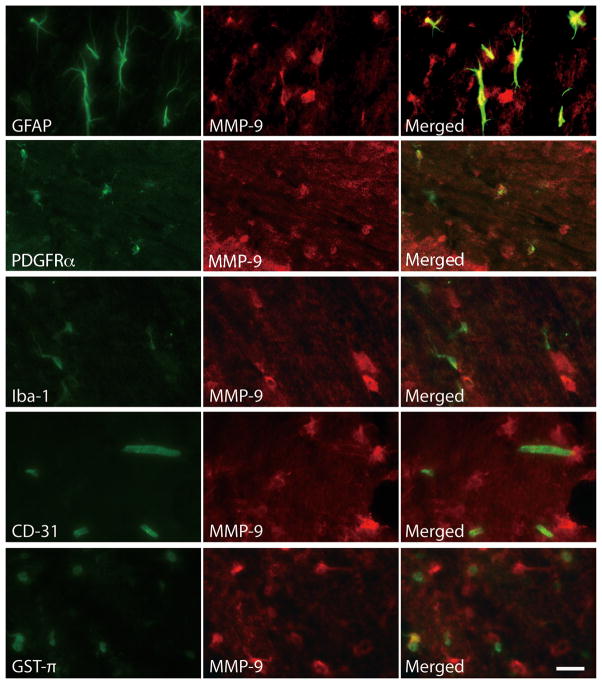
Gelatin zymography revealed that MMP-9, but not MMP-2, activity was strongly elevated in the white matter at 24 hours after SAH (P<0.01, vs. sham; n=3, for each; Figure 2A, B). In immunohistochemistry, GFAP, PDGFRα, and Iba-1 expression were prominently observed in the white matter after SAH (Figure 2C). Immunofluorescent double labeling showed that MMP-9 positive cells in white matter were mainly astrocytes (GFAP positive) and oligodendrocyte precursor cells (PDGFRα positive), but rarely microglia (Iba-1 positive), endothelial cells (CD-31 positive), or mature oligodendrocytes (GST-π positive; Figure 3).
Figure 2.
SAH induces MMP-9 activation and activates astrocytes, oligodendrocyte precursors and microglia in white matter. (A) Representative gelatin zymography demonstrates that activity of MMP-9, but not MMP-2, increases in SAH compared to sham mice at 24 h after SAH induction. (B) Quantification of MMP-9 activity on gelatin zymography. **P < 0.01; Values are mean ± SD; n=3 for each. (C) Representative 3,3 diaminobenzidine (DAB) staining of glial fibrillary acid protein (GFAP), PDGFRα, and Iba-1 in the corpus callosum in sham and SAH mice at 24 hours (Scale bar = 100μm).
Figure 3.
Immunofluorescent double labeling for MMP-9 with glial fibrillary acid protein (GFAP; astrocyte marker), PDGFRα (oligodendrocyte precursor marker), Iba-1 (microglial marker), CD-31 (endothelial marker) or GST-π (mature oligodendrocyte marker). MMP-9 expressing cells were mainly astrocytes and oligodendrocyte precursors, and not microglia, endothelial cells or mature oligodendrocytes. (Scale bar = 20μm).
MMP-9 deletion ameliorated early white matter injury
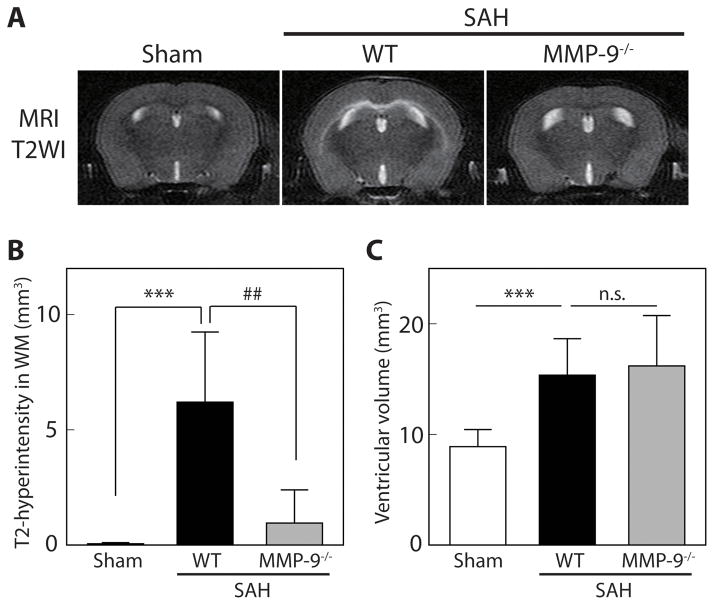
WT and MMP-9−/− mice were examined at 24 hours after SAH induction. Mortality rates were 24% (4/17) and 23% (3/13) in WT and in MMP-9−/− mice, respectively (P=0.98). No sham animal died (n=11). One WT mouse that developed a large hemispheric infarction after SAH was excluded from further investigation. The SAH severity score (degree of bleeding) was equivalent in WT and MMP-9−/− animals (9.0 ± 3.3 and 9.1 ± 4.0, respectively; P=0.95). Neurological scores at 24 hours after SAH were also similar (WT 13.1 ± 3.0, MMP-9−/− mice 13.8 ± 3.7, P=0.55). However, while all WT mice developed T2-hyperintensity in white matter at 24 hours after SAH (as also shown in our previous study 8), this was much less evident in MMP-9−/− mice (Figure 4A). Thus, the volume of white matter T2-hyperintensity in WT mice with SAH was 6.2 ± 3.0 vs. 0.06 ± 0.05 mm3 in WT sham (P<0.001; n=12 and 11, respectively), while it was only 1.0 ± 1.4 mm3 in MMP-9−/− mice with SAH (P<0.01; vs. WT with SAH; n=10 MMP-9−/− mice with SAH; Figure 4B). Ventricular volume was significantly greater in WT with SAH mice than in sham mice (15.4 ± 3.3 and 8.9 ± 1.6 mm3, respectively; P<0.001). Ventricular volume after SAH in MMP-9−/− mice (16.2±4.6 mm3) was similar to that in WT mice with SAH (P=0.56; Figure 4C).
Figure 4.
MMP-9 deletion ameliorated BBB disruption in white matter (WM) after SAH. (A) Representative coronal T2 images of wild-type (WT) sham, WT with SAH, and MMP-9 knockout (MMP-9−/−) with SAH at 24 hours. (B) The volume of T2-hyperintensity in the WM, and (C) ventricular volume at 24 hours after endovascular perforation or sham procedure. Values are mean ± SD; ***P < 0.001 vs. sham, and ##P < 0.01 vs. WT with SAH; n.s. not significant; n=10 to 12.
Time course of MRI findings and neurological conditions following SAH
The time course of neurological deficits, body weight and MRI changes after SAH was examined in another set of ten WT and ten MMP-9−/− mice which underwent endovascular perforation. Mortality rates at 8 days after SAH were 20% (2/10) in each group, and the survivors were used for the following investigation. The initial reduction in neurological score (14.4 ± 2.7 in MMP-9−/− vs. 13.6 ± 1.9 in WT) and loss of body weight (10.0 ± 4.0% in MMP-9−/− vs. 11.1 ± 3.5% in WT) were very similar in both genotypes. By 8 days, there were tendencies for MMP-9−/− mice to have a better recovery in neurological deficits (17.1 ± 1.6 vs. 15.6 ± 1.6 in WT) and body weight (2.5 ± 4.5 and 7.1 ± 3.5% loss in MMP-9−/− and WT mice, respectively). However, these did not reach statistical significance (repeated measures ANOVA; P=0.25 and P=0.10, respectively).
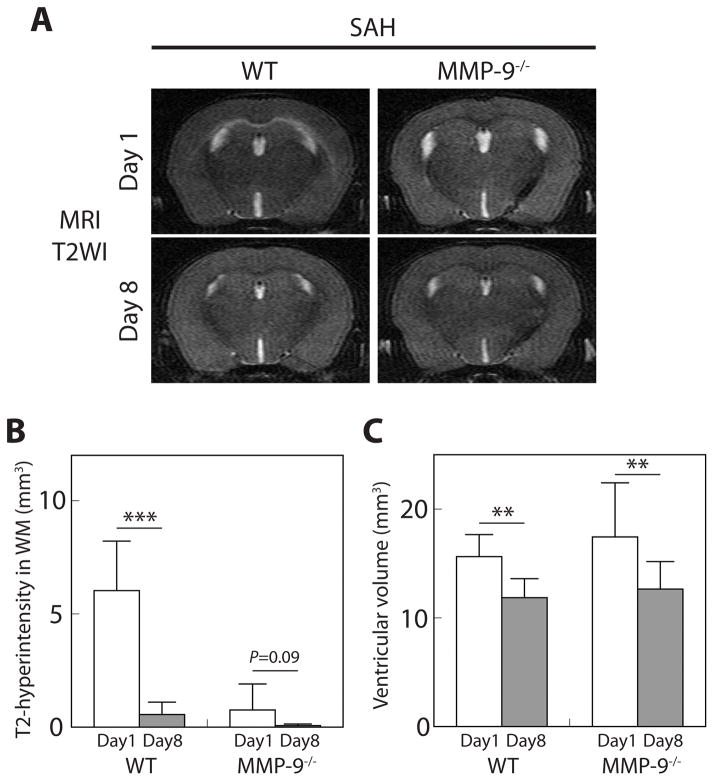
Compared to 1 day, by 8 days after SAH, T2-hyperintensity in the white matter was dramatically reduced, particularly in WT mice (Figure 5A). The volumes of T2-hyperintensity in WT mice were 6.0 ± 2.2 and 0.6 ± 0.5 mm3, and those in MMP-9−/− mice were 0.8 ± 1.1 and 0.07 ± 0.07 mm3, at 1 and 8 days after SAH, respectively (P<0.001, and P=0.09, respectively; Figure 5B). No marked albumin leakage was detected in the white matter at day 8 after SAH in either WT mice or MMP-9−/− mice. Also, ventricular volumes at 8 days were smaller than at 1 day after SAH in both WT and MMP-9−/− mice. Ventricular volumes in WT mice were 15.6 ± 2.0 and 11.8 ± 1.7 mm3, and those in MMP-9−/− mice were 17.4 ± 5.0 and 12.6 ± 2.5 mm3, at 1 and 8 days after SAH, respectively (P<0.01 for each; Figure 5C).
Figure 5.
White matter (WM) T2-hyperintensity and ventricular dilatation improved at 8 days after subarachnoid hemorrhage (SAH). (A) Repeat coronal T2-weighted imaging (T2WI) in wild-type (WT) and MMP-9−/− mice at 1 and 8 days after SAH. Quantification of (B) the volume of WM T2-hyperintensity and (C) ventricular volume after SAH. Values are mean ± SD; ***P < 0.001, and **P < 0.01; n=8 for each.
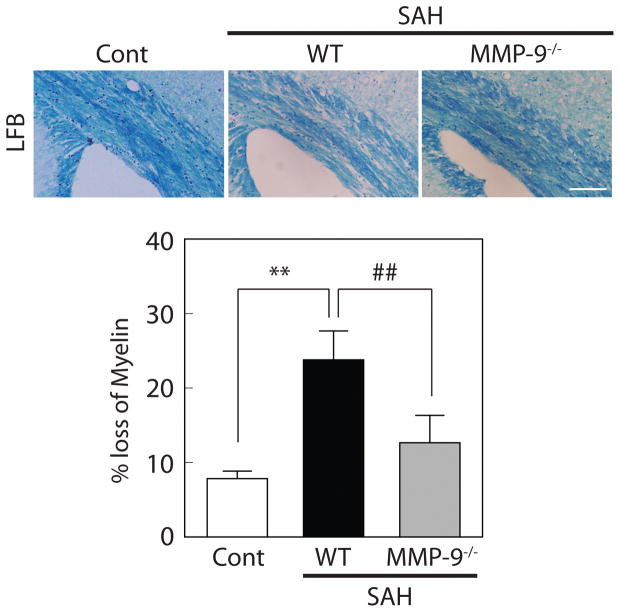
MMP-9 deletion attenuated white matter damage after SAH
White matter injury in WT and MMP-9−/− mice eight days after SAH was examined using Luxol fast blue (LFB) staining (Figure 6). Mice without any surgery (n=4, for WT and MMP-9−/− mice) were set as normal control. Estimated LFB integrity (% loss of myelin) was equivalent in WT and MMP-9−/− mice without any surgery (data not shown). As expected, decreased myelin integrity was observed in WT mice with SAH compared to controls (24 ± 4 vs. 8 ± 1% myelin loss, respectively; P<0.01). This loss was ameliorated in MMP9−/− mice (13 ± 4% myelin loss; P<0.01 vs. WT SAH; n=4 for each; Figure 6).
Figure 6.
MMP-9 deletion ameliorated white matter injury after SAH. Representative luxol fast blue (LFB) staining in wild-type (WT) control, WT with SAH, and MMP-9 knockout (MMP-9−/−) mice with SAH at day 8. Values are mean ± SD; **P < 0.01 vs WT control, and ##P < 0.01 vs WT with SAH; n=4 for each. Scale bar = 100μm.
Discussion
The present study contains three major findings: (1) experimental SAH induced by endovascular perforation caused acute BBB disruption in white matter; (2) MMP-9 activity in white matter was increased after SAH and the main sources of MMP-9 were reactive astrocytes and oligodendrocyte precursors; (3) MMP-9 deletion ameliorated BBB disruption and white matter injury.
Brain edema and BBB disruption are major components of early brain injury after SAH and brain edema is an independent risk factor of poor clinical prognosis after SAH3. While we recently reported that SAH causes acute white matter injury 8, the underlying mechanisms are still uncertain. In animal models of other central nervous system injuries, such as trauma17 and multiple sclerosis18, or chronic ischemic stroke19, BBB disruption occurs in the early stage of disease and contributes to progression of white matter dysfunction at later time points. It is possible that similar mechanisms are involved in white matter pathology after SAH. However, to our best knowledge, there is no previous study that investigated acute BBB disruption in white matter after SAH. In the present study, T2-hyperintensity was more prominent in white matter than in other cerebral regions 24 hours after SAH, and this was consistent with albumin leakage detected by immunostaining. In addition, transmission electron microscopy showed ultrastructural abnormalities in white matter microvessels, including swollen astrocyte endfeet with autophagosomes, tight junction detachment, erythrocyte trapping within vessel lumens, and basement membrane irregularities. These results clearly demonstrate that BBB disruption occurs acutely in white matter following SAH.
The MMP family, especially MMP-9, are well accepted as key neurovascular proteases that can induce BBB damage and cause edema, hemorrhage and neuronal death20, 21. Increased MMP-9 activity has been reported at 24 hours after SAH22, 23. That MMP-9 contributes to early brain injury with MMP-9−/− mice developing less brain edema after SAH 7. In this study, we confirmed that white matter MMP-9 activity was significantly increased at 24 hours after SAH induction. Activated astrocytes, microglia and, as with our previous report8, oligodendrocyte precursors were prominently observed in white matter after SAH. The main MMP-9 sources were activated astrocytes and oligodendrocyte precursors, and not activated microglia, endothelial cells or mature oligodendrocytes.
Oxidative injury caused by excessive hemoglobin and iron, a major hemoglobin degradation product, significantly contributes to brain injury after hemorrhagic stroke10, 24. It was reported that astrocytes play an important role in maintaining BBB integrity25. Also, MMP-9 can be activated in reactive astrocytes induced by oxidative stress resulting in BBB disruption after intracerebral hemorrhage or traumatic brain injury26, 27. Our current results suggest similar mechanisms are involved in astrocytic induction of MMP-9 in white matter after SAH leading to BBB disruption. On the other hand, it is well known that the components of white matter, such as axons and oligodendrocytes are highly sensitive to oxidative stress and, therefore, white matter is vulnerable to damage in various neurological disorders28. As shown in the current study and our previous results8, acute white matter injury including axonal degeneration and demyelination occurs at 24 hours after SAH. Oligodendrocyte precursors are considered to maintain homeostasis and mediate long-term repair in white matter after injury 29. They rapidly proliferate, migrate and fill in the damaged lesion in response to demyelinating signals to differentiate into mature oligodendrocytes and restore myelin sheaths30, 31. Recently, it was reported that oligodendrocyte precursors can rapidly respond to chronic hypoperfusion-induced white matter injury and secrete MMP-9 that leads to BBB disruption32. These findings are comparable to our current results. Taken together, oligodendrocyte precursors are considered another player that mediates acute BBB disruption in white matter after SAH. In addition, studies have suggested that other mechanisms such as inflammation and excitotoxicity may also contribute to early white matter injury following SAH.2
We previously reported that lipocalin-2 (LCN-2) plays an important role in SAH-induced acute white matter injury8. LCN-2 has the potential to preserve MMP-9 activity by preventing its degradation33, and there is a close relationship between LCN-2 expression and MMP-9 activity in a mouse model of breast cancer34. Similarly, our unpublished data confirmed that the activity of MMP-9 in the white matter after SAH is significantly lower in LCN-2 knockout mice than in WT mice. The effect of LCN-2 on MMP-9 related pathogenesis, such as BBB disruption after SAH shown in the current study, needs further investigation.
Our current results demonstrated that MMP-9 deletion effectively attenuated acute BBB disruption and consequent white matter injury. However, some previous studies indicate that MMP-9 plays a beneficial role in promoting neurovascular remodeling in the delayed stage of stroke recovery21, 35. In adult white matter, it was also reported that MMP-9 promotes white matter remodeling through accelerating angiogenesis in the recovery stage after injury36, although MMP-9 plays a deleterious role in myelin damage and BBB disruption during acute injury37. Because of these dual-edged actions of MMPs, the use of a broad-spectrum MMP inhibitor during the first few hours after cerebral ischemia reduced infarction, but delayed use of the same inhibitor worsened outcome38. In this study, we used MMP-9−/− mice because specific MMP-9 inhibitors are currently unavailable. Future studies should determine the optimal timing of MMP-9 inhibition against SAH-induced white matter injury.
In conclusion, SAH results in rapid activation of astrocytes and oligodendrocyte precursors in white matter. They secrete MMP-9 leading to acute BBB disruption, axonal damage and/or demyelination. MMP-9 deletion effectively reduced acute BBB disruption and attenuated consequent white matter injury. Hence, MMP-9 could be a therapeutic target for SAH-induced white matter injury.
Supplementary Material
Acknowledgments
Sources of Funding
Supported by NIH grants NS-073595, NS-079157, NS-084049 and NS-091545, and Japan Heart Foundation / Bayer Yakuhin Research Grant Abroad.
Footnotes
Disclosures
None.
References
- 1.Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: A meta-analysis. Lancet neurology. 2009;8:635–642. doi: 10.1016/S1474-4422(09)70126-7. [DOI] [PubMed] [Google Scholar]
- 2.Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res. 2013;4:432–446. doi: 10.1007/s12975-013-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claassen J, Carhuapoma JR, Kreiter KT, Du EY, Connolly ES, Mayer SA. Global cerebral edema after subarachnoid hemorrhage: Frequency, predictors, and impact on outcome. Stroke. 2002;33:1225–1232. doi: 10.1161/01.str.0000015624.29071.1f. [DOI] [PubMed] [Google Scholar]
- 4.Kooijman E, Nijboer CH, van Velthoven CT, Kavelaars A, Kesecioglu J, Heijnen CJ. The rodent endovascular puncture model of subarachnoid hemorrhage: Mechanisms of brain damage and therapeutic strategies. Journal of neuroinflammation. 2014;11:2. doi: 10.1186/1742-2094-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg GA. Matrix metalloproteinases in brain injury. Journal of neurotrauma. 1995;12:833–842. doi: 10.1089/neu.1995.12.833. [DOI] [PubMed] [Google Scholar]
- 6.Ning M, Furie KL, Koroshetz WJ, Lee H, Barron M, Lederer M, et al. Association between tpa therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology. 2006;66:1550–1555. doi: 10.1212/01.wnl.0000216133.98416.b4. [DOI] [PubMed] [Google Scholar]
- 7.Feiler S, Plesnila N, Thal SC, Zausinger S, Scholler K. Contribution of matrix metalloproteinase-9 to cerebral edema and functional outcome following experimental subarachnoid hemorrhage. Cerebrovascular diseases. 2011;32:289–295. doi: 10.1159/000328248. [DOI] [PubMed] [Google Scholar]
- 8.Egashira Y, Hua Y, Keep RF, Xi G. Acute white matter injury after experimental subarachnoid hemorrhage: Potential role of lipocalin 2. Stroke. 2014;45:2141–2143. doi: 10.1161/STROKEAHA.114.005307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okubo S, Strahle J, Keep RF, Hua Y, Xi G. Subarachnoid hemorrhage-induced hydrocephalus in rats. Stroke. 2013;44:547–550. doi: 10.1161/STROKEAHA.112.662312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JY, Keep RF, He Y, Sagher O, Hua Y, Xi G. Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. Journal of cerebral blood flow and metabolism. 2010;30:1793–1803. doi: 10.1038/jcbfm.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. Journal of neuroscience methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S, Ma Q, Krafft PR, Chen Y, Tang J, Zhang J, et al. P2x7 receptor antagonism inhibits p38 mitogen-activated protein kinase activation and ameliorates neuronal apoptosis after subarachnoid hemorrhage in rats. Critical care medicine. 2013;41:e466–474. doi: 10.1097/CCM.0b013e31829a8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu S, Xi G, Jin H, He Y, Keep RF, Hua Y. Thrombin-induced autophagy: A potential role in intracerebral hemorrhage. Brain research. 2011;1424:60–66. doi: 10.1016/j.brainres.2011.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egashira Y, Suzuki Y, Azuma Y, Takagi T, Mishiro K, Sugitani S, et al. The growth factor progranulin attenuates neuronal injury induced by cerebral ischemia-reperfusion through the suppression of neutrophil recruitment. Journal of neuroinflammation. 2013;10:105. doi: 10.1186/1742-2094-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin H, Xi G, Keep RF, Wu J, Hua Y. Darpp-32 to quantify intracerebral hemorrhage-induced neuronal death in basal ganglia. Translational Stroke Research. 2013;4:130–134. doi: 10.1007/s12975-012-0232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flygt J, Djupsjo A, Lenne F, Marklund N. Myelin loss and oligodendrocyte pathology in white matter tracts following traumatic brain injury in the rat. The European journal of neuroscience. 2013;38:2153–2165. doi: 10.1111/ejn.12179. [DOI] [PubMed] [Google Scholar]
- 17.Glushakova OY, Johnson D, Hayes RL. Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood-brain barrier disruption, and progressive white matter damage. Journal of neurotrauma. 2014;31:1180–1193. doi: 10.1089/neu.2013.3080. [DOI] [PubMed] [Google Scholar]
- 18.Maggi P, Macri SM, Gaitan MI, Leibovitch E, Wholer JE, Knight HL, et al. The formation of inflammatory demyelinated lesions in cerebral white matter. Annals of neurology. 2014;76:594–608. doi: 10.1002/ana.24242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamoto N, Pham LD, Maki T, Liang AC, Arai K. A radical scavenger edaravone inhibits matrix metalloproteinase-9 upregulation and blood-brain barrier breakdown in a mouse model of prolonged cerebral hypoperfusion. Neuroscience letters. 2014;573:40–45. doi: 10.1016/j.neulet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for mmps and timps in cerebral ischemia. Glia. 2005;50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- 21.Lo EH. A new penumbra: Transitioning from injury into repair after stroke. Nature medicine. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 22.Sehba FA, Mostafa G, Knopman J, Friedrich V, Jr, Bederson JB. Acute alterations in microvascular basal lamina after subarachnoid hemorrhage. Journal of neurosurgery. 2004;101:633–640. doi: 10.3171/jns.2004.101.4.0633. [DOI] [PubMed] [Google Scholar]
- 23.Guo Z, Sun X, He Z, Jiang Y, Zhang X. Role of matrix metalloproteinase-9 in apoptosis of hippocampal neurons in rats during early brain injury after subarachnoid hemorrhage. Neurological sciences. 2010;31:143–149. doi: 10.1007/s10072-009-0192-x. [DOI] [PubMed] [Google Scholar]
- 24.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet neurology. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 25.Laird MD, Vender JR, Dhandapani KM. Opposing roles for reactive astrocytes following traumatic brain injury. Neuro-Signals. 2008;16:154–164. doi: 10.1159/000111560. [DOI] [PubMed] [Google Scholar]
- 26.Tejima E, Zhao BQ, Tsuji K, Rosell A, van Leyen K, Gonzalez RG, et al. Astrocytic induction of matrix metalloproteinase-9 and edema in brain hemorrhage. Journal of cerebral blood flow and metabolism. 2007;27:460–468. doi: 10.1038/sj.jcbfm.9600354. [DOI] [PubMed] [Google Scholar]
- 27.Ralay Ranaivo H, Hodge JN, Choi N, Wainwright MS. Albumin induces upregulation of matrix metalloproteinase-9 in astrocytes via mapk and reactive oxygen species-dependent pathways. Journal of neuroinflammation. 2012;9:68. doi: 10.1186/1742-2094-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. Journal of cerebral blood flow and metabolism. 2003;23:263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- 29.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (ng2 cells): Multifunctional cells with lineage plasticity. Nature reviews Neuroscience. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 30.Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult cns. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 31.Redwine JM, Armstrong RC. In vivo proliferation of oligodendrocyte progenitors expressing pdgfalphar during early remyelination. Journal of neurobiology. 1998;37:413–428. doi: 10.1002/(sici)1097-4695(19981115)37:3<413::aid-neu7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 32.Seo JH, Miyamoto N, Hayakawa K, Pham LD, Maki T, Ayata C, et al. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. The Journal of clinical investigation. 2013;123:782–786. doi: 10.1172/JCI65863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (mmp) activity is a complex of gelatinase b/mmp-9 and neutrophil gelatinase-associated lipocalin (ngal). Modulation of mmp-9 activity by ngal. The Journal of biological chemistry. 2001;276:37258–37265. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 34.Leng X, Ding T, Lin H, Wang Y, Hu L, Hu J, et al. Inhibition of lipocalin 2 impairs breast tumorigenesis and metastasis. Cancer research. 2009;69:8579–8584. doi: 10.1158/0008-5472.CAN-09-1934. [DOI] [PubMed] [Google Scholar]
- 35.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nature medicine. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 36.Pham LD, Hayakawa K, Seo JH, Nguyen MN, Som AT, Lee BJ, et al. Crosstalk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia. 2012;60:875–881. doi: 10.1002/glia.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kieseier BC, Seifert T, Giovannoni G, Hartung HP. Matrix metalloproteinases in inflammatory demyelination: Targets for treatment. Neurology. 1999;53:20–25. doi: 10.1212/wnl.53.1.20. [DOI] [PubMed] [Google Scholar]
- 38.Zhao BQ, Tejima E, Lo EH. Neurovascular proteases in brain injury, hemorrhage and remodeling after stroke. Stroke. 2007;38:748–752. doi: 10.1161/01.STR.0000253500.32979.d1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.