Introduction
Currently, diagnosis of intracranial large vessel pathologies is based on luminal appearance using angiographic studies either non-invasively with MR or CT, or invasively with catheter angiography. However, exciting new methodologies are in development that allow imaging beyond the lumen, to characterize the disease process of the vessel wall1, 2. Inflammation has been implicated in large artery cerebrovascular lesions, having a role in the progression of symptomatic intracranial atherosclerosis3, growth and rupture of brain aneurysms4, delayed cerebral ischemia following subarachnoid hemorrhage5, and rupture risk of brain arteriovenous malformations6. Imaging the spatial distribution, severity and temporal changes in cerebrovascular inflammation may offer insight into the clinical diagnosis and treatment decisions in the near future. Substantial challenges remain for the development of high resolution, specific and sensitive techniques to quantify patterns of inflammation in large intracranial arteries. This review serves to highlight developments in vascular wall imaging and molecular imaging techniques to better elucidate the predictive value of vascular inflammation in the progression of cerebrovascular disease.
Methods
We conducted searches using PubMed and Google Scholar for relevant articles published since 1980 in English. We used search words such as Cerebral Arteries or Cerebrovasculature, Inflammation, or Imaging combined with subject headings search words representing different pathological conditions or imaging modalities such as MRI, Molecular Imaging, Positron Emission Tomography, Ultrasound, Vessel Wall Imaging, Intracranial Atherosclerosis, Intracranial Aneurysm, Angitis, Vasculitis, Moyamoya Disease, Giant Cell Arteritis, or Ultrasmall Superparamagnetic Iron Oxide. The first search was performed in January 2015 and the last in May 2015. Articles were screened by title and abstract, and if relevant the article was reviewed in its entirety. Due to restrictions in Stroke on the publication of Topical Reviews, final discretion of selected references was determined by the authors.
Vessel-Wall Imaging
Black blood MRI is the workhorse of vessel wall examinations in vivo. Suppression of signals from the blood is fundamental to generate contrast between the lumen and the vessel wall. Adequate wall visualization is further dependent on careful compensation for residual blood signals arising from slow and laminar flow. Additional sensitivity for detection of intracranial arterial wall irregularities related to inflammatory processes is provided by gadolinium contrast-enhanced T1-weighted MRI. Selective gadolinium uptake in diseased arterial wall segments - in part due to increased density of intracranial arterial vasa vasorum (Figure a, b) - has been shown to be associated with the progression of cerebrovascular diseases such as intracranial atherosclerosis or brain aneurysms7. Initial examinations at 1.5T revealed age-related increases in intracranial arterial wall enhancement that could result from neovascularity seen with atherosclerotic disease,8 while at 3T patterns of focal eccentric wall enhancement in atherosclerotic disease patients could be distinguished from smooth and concentric enhancement patterns in patients with inflammatory diseases such as vasculitis and arteritis.9
However, translation of pulse sequence techniques that were developed for vessel wall imaging of the extracranial carotid artery to the intracranial circulation is not straightforward. Intracranial arterial wall imaging is particularly challenging because of anatomical variability, tortuous geometry, and much smaller vessel diameters and wall thicknesses in branches distal to the circle of Willis.10 These factors heighten the requirements for spatial resolution, especially since thick-slice anisotropic acquisition geometries can lead to wall thickness measurement errors in curved vessels.11 Moreover, wall thickness measurements in healthy and diseased vessels and detection of focal abnormalities are hampered by insufficient contrast to discriminate the vessel wall from surrounding brain parenchyma and cerebrospinal fluid (CSF).10, 12, 13 Since smaller voxels and CSF-suppression come at the cost of signal-to-noise ratio, many studies employ fast spin-echo techniques at 3T and higher field strengths.10 In order to further limit acquisition times in clinical settings, brain coverage is often reduced, thus necessitating precise targeting of the vessel of interest.10 As a result, most studies are limited to a small subset of the intracranial vasculature and focus mainly on the larger arteries comprising the circle of Willis.10
Several techniques have been proposed to overcome the limitations specific to imaging of the intracranial arterial wall. Since the effectiveness of double inversion recovery for blood suppression is reduced in three-dimensional imaging,14 novel techniques such as motion-sensitized driven-equilibrium (MSDE) magnetization preparation15 and non-selective delay alternating with nutation for tailored excitation (DANTE) pulse trains14 have been optimized for high-resolution black blood MRI. In combination with a contrast-enhanced three-dimensional fast spin-echo sequence, improved blood suppression with MSDE enabled visualization of wall enhancement in saccular, mostly ruptured aneurysms,16 arguably related to wall inflammation.17
Black blood MRI with additional CSF-suppression was achieved at 7T with inversion recovery magnetization preparation and a fast spin echo sequence,12 which was further improved to enable whole brain coverage.18 Similarly, a carefully optimized DANTE pre-pulse was described for improved suppression of signals from blood while jointly nulling cerebrospinal fluid signal, which could be used in combination with a proton density-weighted fast spin echo technique.13 Still, even with suppression of CSF, similar signal intensities in surrounding brain parenchyma reportedly precluded complete arterial wall visualization in about 30% of the intracranial arterial segments.13 In a multi-contrast vessel wall imaging approach, these techniques can be implemented alongside T1-weighted acquisitions in proton density- and T2-weighted sequences,13, 18 which could provide valuable information to discriminate inflammatory diseases from other intracranial vasculopathies.19
Vessel Wall Imaging in Intracranial Atherosclerosis
Despite efforts to suppress potentially misleading signals from laminar blood flow and improve visibility of the vessel wall by reducing perivascular CSF signals, a standard of reference to quantify the sensitivity and specificity of measurements in healthy and diseased vessel walls is currently not available. Recently, studies have correlated high resolution MRI findings with intracranial atherosclerotic disease (ICAD)20, 21. Turan et al20 collected the postmortem ICAD specimens 4 days after 3T MRI examination and processed the specimens for histology. Different image signal characteristics were found to be consistent with various atherosclerotic components, namely, lipid and loose matrix, fibrous tissue, and calcium. Similar findings were noted in van der Kolk’s study21 that employed vessel wall imaging at 7T to successfully distinguished different plaque components. These studies provided valuable insight into the ICAD pathology. However, the characterization of ICAD was limited to postmortem evaluation since intracranial atherosclerotic plaques are not removed from living patients during clinical interventions.22
Currently, there is no standard protocol for MRI of ICAD, nor is there a gold standard phantom to compare MRI pulse sequences and imaging platforms. A small batch manufacturing technique was developed to create a hydrogel plaque phantom that provided a platform for establishing a uniform imaging method for diagnosis of ICAD. The phantom was scanned on different 3T systems and preliminary data showed that variations in signal intensity between the fibrous cap, lipid core and vessel wall were observed on the T2 images.23 Thus, additional work is needed for standardization of ICAD MR imaging techniques to allow for multicenter studies.
Imaging of Cerebral Vasculitis
Primary angitis of the central nervous system (PACNS) shares with ICAD, Moyamoya disease and reversible cerebral vasoconstriction syndrome (RCVS) the same pattern on 3D time-of-flight MRA: focal arterial stenoses of medium-sized intracranial arteries24, 25. The accurate positive diagnosis is often difficult to reach even with digital subtraction angiography and cerebral/meningeal biopsy24. 3T contrast-enhanced high resolution MR acquisitions may be reliable to distinguish these diseases26–28. Indeed, some authors have described an absence of vessel wall enhancement on high-resolution MRI in RCVS as compared to PACNS27. Different patterns of wall thickening have been reported, such as a diffuse and uniform wall thickening in RCVS whereas a focal thickening with concentric (70%) or eccentric (30%) vessel wall enhancement in vasculitis was more frequently observed29. Mossa-Basha et al27 have shown that a high resolution MR protocol combining pre- and post-contrast T1 and T2 sequences, the sensitivity in differentiating ICAD from other vasculopathies could increase over 96%. In young adults (≤ 55 y) with unilateral M1 segment stenosis, 3T high resolution MRI protocol including T1 pre- and post-contrast and proton density acquisitions perpendicular to the M1 segment, may help to distinguish ICAD (eccentric wall thickening and enhancement) from vasculitis (circumferential wall thickening and enhancement), intracranial dissection (dissecting flap, pseudolumen and mural hematoma) or from Moyamoya disease (concentric wall enhancement without wall thickening and fine meshwork of basal collateral vessels)30, 31. High-resolution fat-saturated T1-weighted MRI (3T) before and after IV gadolinium administration may also help to reach the positive diagnosis of giant cell arteritis (GCA), in which arterial stenoses may be absent32. Indeed, wall enhancement of the extracranial arteries (mainly the superficial temporal and occipital arteries) is depicted on high-resolution fat-saturated T1-weighted MRI with a sensitivity of 0.80 and a specificity of 0.80. In addition, intradural arteries enhancement (mainly the ICA) is observed in more than half cases in GCA patients. It should also be mentioned that an animal study on a mouse model of Kawasaki disease has demonstrated the advantage of MPO-Gd as it increased the vessel wall intensity about 2.5 fold higher than regular gadolinium contrast-enhanced high-resolution T1-weighted MRI at 7T33. However, vessel wall enhancement should be interpreted carefully, especially in the pediatric population (< 18 y), since normal intracranial parietal enhancement is frequently seen in both cavernous and petrous ICA, as well as in M1 segment on high resolution fat-saturated contrast-enhanced MRI34. High-field (7T) 3D time-of-flight MRA has shown its benefits in detecting small size vessels like thalamoperforating arteries35. By providing higher spatial resolution, these 7T MRA exams would be able to depict stenoses missed by regular 3T MRA on small vessels. Recent studies have also shown the potential of 7T magnetization preparation inversion recovery (MPIR) turbo spin-echo MR sequence to improve detection of vessel wall abnormalities36. However, one should keep in mind that 7T MR acquisitions’ quality may be significantly impaired by a higher sensitivity to artifacts, especially close to the skull base and the paranasal sinuses37. Additionally, long scanning time in 7T MR sequences may discourage its use in daily clinical practice (> 10 min)38.
Role of Intravascular Imaging
Although our review is mostly focused on non-invasive vessel wall imaging techniques, advances in catheter based technology for the minimally invasive treatment of cerebrovascular disease is also enabling the development of high-resolution intra-vascular imaging technology39–41. Albeit invasive, with resolution approaching microscopy, these technologies have secured an important role in characterization of lesions in the peripheral and coronary circulations to support endovascular treatments. The cerebrovascular system presents significant challenges for intravascular imaging, with fragile, thin-walled vessels that have a tortuous path. Dedicated ongoing research to optimize optical coherence intravascular imaging systems specifically for brain arteries presents an exciting paradigm not only for accurate diagnosis, but to guide ideal endovascular treatments.
Molecular Imaging of Cerebrovascular Inflammation
Neuroinflammation Imaging in Stroke
Major pathophysiology caused by vascular dysfunction and morphological manifestation of damage to the brain can be investigated using an emerging hybrid imaging technique which combines positron emission tomography (PET) and MRI. This technique enables regional quantification of physiological parameters and assesses the distribution of molecular markers in the brain while providing an excellent view of the anatomy in ischemic brain. Cerebral ischemia induces hypoxia and the resultant neuronal injury leads to the activation of the inflammatory cascade, in which migratory microglial cells initially play the central role. These cells (10% of the total brain cells) are activated and exhibit phagocytic activity in response to neuronal dysfunction due to cell stress and apoptosis after an ischemic stroke. The upregulation of mitochondrial translocator protein 18 kDa (TSPO18) in microglia has been explored as a potential biomarker for imaging of microglial activation in neuroinflammation42. In humans, the appearance of activated microglia occurs early in the ischemic core, whereas at later time points activated cells appear on the periphery of brain infarct. Both PET and MRI use molecular imaging probes and sensors to visualize inflammation. The post-ischemic PET imaging using11C-labeled PK11195 TSPO18 ligand (based on isoquinoline carboxamide) enabled time-dependent imaging of neuroinflammation that showed binding along the outer border of ischemic lesions as well as in regions distal to the primary lesion43. After several days the areas populated by activated microglia proximal to the infarct colocalize with an increased FDG uptake. Findings from PET studies suggest neuroinflammation around the infarct correlates with negative outcomes. More recently a number of longer half-life 18F-labeled ligands of TSPO18 were reported (e.g. dimethylpyrazolopyrimidine 18F-DPA 714)44 and imidazopyridines 18F-PBR102 and 18F-PBR11145) that, in direct comparative imaging experiments in animals, showed better imaging characteristics than PK11195. Innate immune system cells, i.e. neutrophils and monocyte/macrophages originating from brain blood supply are also actively engaged in the neuroinflammatory cascade and neutrophil infiltration correlates positively with ischemic damage. It has been reported that myeloperoxidase (MPO), a specific enzymatic marker of terminal differentiation of neutrophils, was consistently found peaking early at 3 days post-infarct and widely distributed in ischemic tissues, correlating with the size of the lesions46. Neutrophil-derived ectopic MPO activity in the ischemia is responsible for enzymatic chlorination and nitrosylation of proteins with potential deleterious effect on brain cells. MR Imaging of MPO enzymatic activity after an intravenous injection of a small molecular weight paramagnetic MPO substrate DTPA(Gd) bis-hydroxyindolamide (bis-5HT-DTPAGd), or MPO-Gd47 enabled detection of inflammation on the periphery of infracted zone in animal models. The infiltration of leukocytes into the ischemic brain areas results in oxidative damage and resultant neuronal degeneration. PET is capable of providing quantitative assessment of such areas by delineating microenvironments that experienced oxidative stress. Copper-64 or -62 radioisotope labeled diacetyl-bis(N4-methylthiosemicarbazone, 64Cu-ATSM) is one of the most promising candidates for imaging oxidative stress in the brain using PET, which can be used in combination with 18FDG imaging48, 49. In addition, 62Cu-ATSM – assisted PET in the brain allows for initial evaluation of cerebral blood flow (CBF), and the regional uptake at 20 min (delayed phase) reflects over-reduction of ATSM that serves as an imaging signature of oxidative stress displayed as a result of mitochondrial dysfunction48.
Imaging of Endothelial Activation in Stroke
The expression of endothelial leukocyte adhesion molecules P- and E-selectin is transcriptionally activated in brain ischemia50 supposedly by pro-inflammatory cytokines. It has been reported that inducible E-selectin expression can be visualized in vivo using antibody-targeted MR iron-oxide (IO) based agents51.The IO-assisted MRI approach has been applied to imaging of early (several hours) or late (24 hours) endothelial activation in stroke using the sialyl LewisX-decorated iron oxide nanoparticles that serve as multivalent ligands of endothelial selectins52. In endothelin-induced stroke, these targeted nanoparticles resulted in hypointense signals on T2*-weighted MR images at 3 hours after the injury but only 24 hours after transient artery occlusion in mice. Paramagnetic GdDTPA derivatives functionalized with a sialyl LewisX mimetics rather than superparamagnetic IO can potentially serve as alternatives to selectin-targeted IO in stroke imaging53. The importance of MR imaging of activated endothelial lining of brain blood vessels is in that it allows the delineation of the area of potentially salvageable penumbra, in contrast to diffusion-weighted MRI which detects primarily the smaller infarct area.
Molecular Imaging of Intracranial Aneurysms
The pathophysiology of symptomatic unruptured intracranial aneurysms (IA) resembles ruptured aneurysms, generally showing significant endothelial cell damage, structural changes of the wall and inflammatory cell infiltration54, 55. Early reports that investigated inflammation-induced antigens (VCAM-1, C3b) expression in aneurysmal tissues established also the elevated presence of CD68 and CD3+ cells in unruptured IA (versus normal basilar arteries) pointing to an existing link between IA progression and inflammation56. Both hemodynamic stress and inflammation promote vascular smooth muscle cell death and activate their migration resulting in thin and dilated areas of the cerebral vasculature57. Pro-inflammatory cytokines (TNF-alpha, INF-gamma, IL-6) secreted by brain resident mast cells, recruited neutrophils, and macrophages potentially up-regulate the expression of adhesion molecules in endothelium resulting in leukocyte recruitment essential to the pathogenesis of vascular inflammation58. Network-based gene expression analysis showed major histocompatibility complex class II gene overexpression in IA pointing to the definite role of professional antigen-presenting and/or INF-gamma activated cells in IA formation59. Another important pathway that may be altered due to genetic factors is endothelin signaling via type A endothelin receptors. These receptors are situated on smooth muscle cells and their activation results in vasoconstriction60. Therefore, the abundant evidence already gathered by several independent research groups suggests that imaging of molecules associated with local inflammation would greatly assist in determining active remodeling and progression of IA to rupture55. Such imaging can be accomplished either by molecular, or cell imaging, i.e. the former aimed at imaging molecular target expression and distribution in vivo, while the latter designed to track myeloid cells of interest. Molecular imaging of cerebral arteries is challenging due to their specific morphology, i.e. thin walls (0.06–0.25 mm61, 62) located in the intradural space and surrounded by cerebrospinal fluid and widened peri-vascular spaces resulting in a compartment that could non-specifically trap imaging probes.
MR Imaging of Myeloperoxidase in Aneurysms
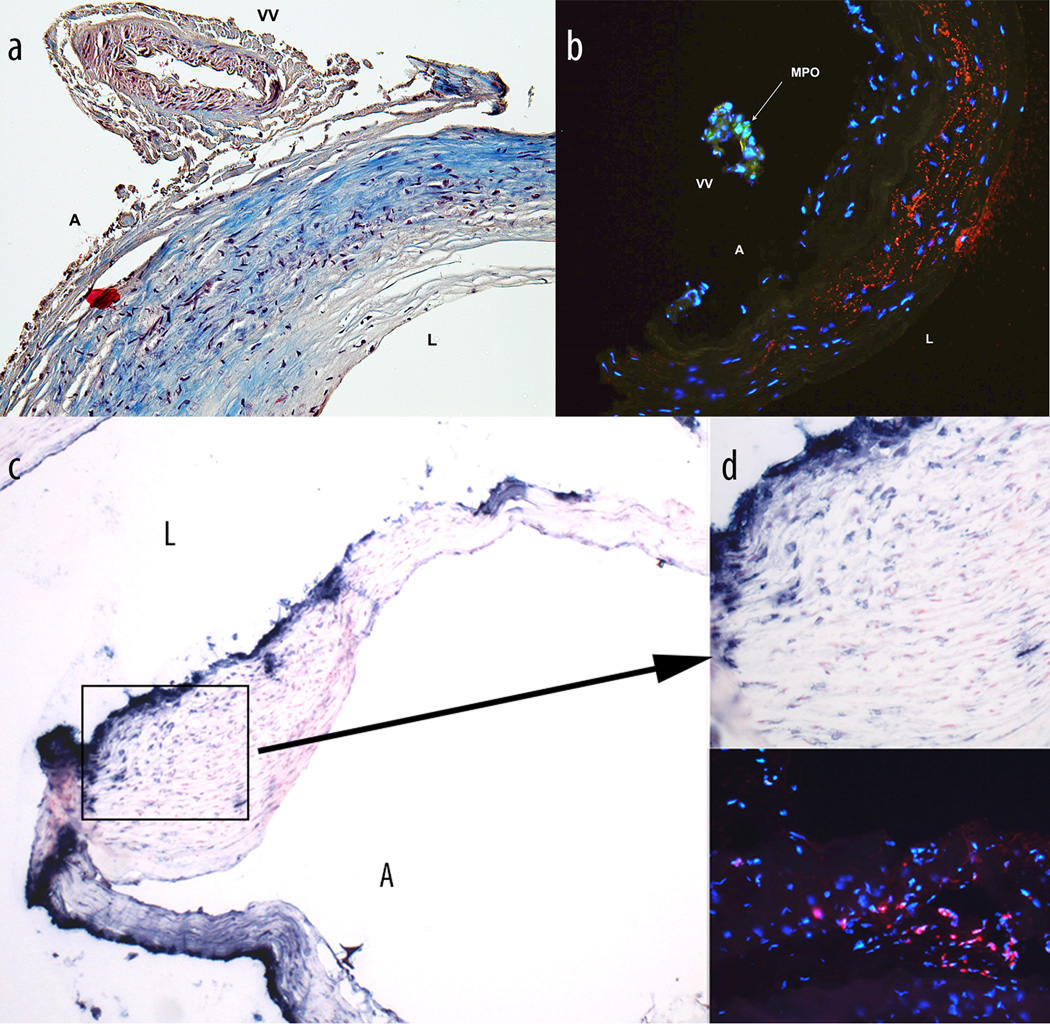
Recently, we noticed a correlation between neutrophil MPO presence in human surgically excised unruptured aneurysms and rupture risk predicted by the PHASES score model63 suggesting the importance of MPO detection in aneurysmal wall as a predictor of instability (Figure 1). We have observed in human aneurysm specimens both adventitial vascularization as a pathway for neutrophilic MPO infiltration (Figure 1a and b) as well as mural presence of MPO in the absence of vasa vasorum (Figure 1c and d). We have previously explored the use of MPO-specific MR signal enhancement for imaging inflammation in an animal model of saccular aneurysm64. Non-invasive imaging of myeloperoxidase activity in blood vessel was performed by using high-resolution MRI combined with IV administration of bis-5HT-DTPAGd MPO substrate. To induce local intramural inflammation of the vessel wall in a model aneurysm, we used image-guided intramural injection of the E.coli lipopolysaccharide solution into the vessel and compared these animals to non-injected controls. The procedure leads to infiltration of MPO positive neutrophils and MPO-negative macrophages at the site of injection. After injecting bis-5HT-DTPAGd we observed a visible and lasting T1-weighted MR signal enhancement in LPS-injected aneurysms. Future clinical translation of this MRI technique requires an optimization of the pulse sequence enabling a specific imaging of vascular wall in aneurysms that have non-linear blood flow. We optimized an MSDE prepared turbo spin echo MRI approach for the detection of an MPO-specific imaging probe in experimental aneurysm models and further applied it for imaging realistic in vivo rabbit aneurysm models. Blood signal within the lumen of the aneurysm was sufficiently suppressed in combination with fat-saturation and inversion-pulse specific for aneurysm wall imaging. The optimized imaging protocol in the rabbit model of saccular aneurysms revealed a significant increase in the change of signal-to-noise ratio from pre-contrast to post-contrast imaging in the inflamed aneurysms as compared to naïve aneurysms and the adjacent carotid artery (p<0.0001)65.
Figure 1.
(a) and (b): Histology sections of an unruptured, 9mm pericallosal artery aneurysm resected following surgical clipping. This aneurysm in a 71 year-old female was surveilled for a period of 10 years and underwent treatment due to imaging evidence of growth. Structure of the wall of the aneurysm (a, Maisson trichrome stain, 10×) shows a large vessel (VV – vasa vasorum) coursing on the adventitial surface (A). Frozen, non-consecutive section of the same aneurysm (b, 10x) shows myeloperoxidase (anti-human MPO mAb staining, green fluorescence) in the vasculature of the aneurysm wall demonstrating an “outside-in” pathway for migration of inflammatory cells. (b): Blue fluorescence - nuclei (DAPI), red fluorescence – endothelial (anti-human CD31 mAb), L – vessel lumen. (c) and (d): Sections of an unruptured, 7 mm, irregular middle cerebral artery bifurcation aneurysm resected from a 54 year-old female (c, myeloperoxidase stain, 4×) with dense staining for myeloperoxidase presence (d, top panel, 20×) and activity (d, bottom panel, 20×) in the layers of smooth muscle cells indicating an “inside-out” pathway for sustained local inflammation. (d, bottom panel): Blue fluorescence – nuclei (DAPI), red fluorescence – myeloperoxidase activity staining using Cy3-(5-hydroxytryptamide) substrate.
Ultrasmall Superparamagnetic Iron Oxide Contrast in MR Imaging of Aneurysmal Inflammation
Despite early concerns about the safety of using Ultra Small Super-Paramagnetic Iron Oxide (USPIO) particles, MR imaging with these contrast agents has continued to gain acceptance as a feasible means of detection and risk assessment of aneurysms and other vascular pathologies. The power of this technique lies in high level of USPIO uptake into inflammatory cells (monocytes, macrophages, and microglia), which have now been firmly established as both structural components and biological mediators of lesion progression in vascular disease66. Uptake of USPIOs in lesions induces localized magnetic susceptibility which primarily shorten T2 and T2* relaxation times, and thus are detected as hypointense areas in corresponding MR images. The localization of USPIOs in infiltrated macrophages associated with carotid plaques has been verified repeatedly through histology, and a clear connection between amount of inflammatory cell burden and disease severity and outcome has been established67, 68. However, while the majority of animal studies show USPIO-laden macrophage accumulation several days following insult68–70 there is growing evidence showing early (within hours) accumulation of particles not associated with inflammatory cells,71–73 which may reflect other sources of USPIO accumulation such as the extravasation of free USPIOs. In some cases this pattern overlaps that of increased vascular permeability as detected by Gd-DTPA suggesting USPIOs may cross the leaky vascular wall via passive diffusion,74, 75 however in other cases a mismatch between the two contrast mechanisms may indicate entrapment or non-passive transport into tissue75–77. In some animal models the time course of USPIO contrast enhancement follows more closely with the appearance and remission of clinical signs than other sources of MR contrast68. Trials of USPIO enhanced detection of human aneurysms have yielded promising results. In patients imaged 72 hours after USPIO administration, 7 out of 9 aneurysms (78%) showed USPIO mediated contrast in the lesion wall and moreover, aneurysm tissue harvested from these patients stained positive for macrophages (CD68+) and iron stain78. In a follow up study, USPIO induced signal changes in aneurysm wall of five patients could be partly ameliorated after patients were given a regimen of daily aspirin79. Related larger clinical studies are now underway80 which should provide general validation of USPIO-mediated MR contrast imaging for rupture risk assessment of human aneurysms.
Summary
Imaging inflammation in large intracranial artery pathology may play an important role in diagnosis of and risk stratification for a variety of cerebrovascular diseases. Looking beyond the lumen has already generated widespread excitement in the stroke community, and the potential to unveil molecular processes in the vessel wall is a natural evolution to develop a more comprehensive understanding of the pathogenesis of diseases such as ICAD and brain aneurysms.
Supplementary Material
Acknowledgments
Matthew J Gounis: Research Grants / Fee-for-service Research (money paid to institution) – Blockade Medical, CereVasc LLC, Codman Neurovascular, Cook Medical, eV3 Neurovascular/ Covidien, InNeuroCo Inc, Neuronal Protection System LLC, Omniox Inc, NIH, Philips Healthcare, Spineology Inc, Silk Road, Stryker Neurovascular. Consultation / Fee-per-hour (money paid to individual) – Stryker Neurovascular. Investment/ Stocks - InNeuroCo Inc.
Ajit S Puri: Research Grants / Fee-for-service Research (money paid to institution) – eV3 Neurovascular/Covidien, Stryker Neurovascular. Consultation / Fee-per-hour (money paid to individual) – eV3 Neurovascular, Codman Neurovascular
Alexei A. Bogdanov: NIH
Footnotes
Disclosures:
Kajo van der Marel: None
Miklos Marosfoi: None
Mary L. Mazzanti: None
Ju-Yu Chueh: None
Literature Cited
- 1.Swartz RH, Bhuta SS, Farb RI, Agid R, Willinsky RA, Terbrugge KG, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology. 2009;72:627–634. doi: 10.1212/01.wnl.0000342470.69739.b3. [DOI] [PubMed] [Google Scholar]
- 2.Krings T, Mandell DM, Kiehl TR, Geibprasert S, Tymianski M, Alvarez H, et al. Intracranial aneurysms: from vessel wall pathology to therapeutic approach. Nat Rev Neurol. 2011;7:547–559. doi: 10.1038/nrneurol.2011.136. [DOI] [PubMed] [Google Scholar]
- 3.Arenillas JF, Alvarez-Sabin J, Molina CA, Chacon P, Fernandez-Cadenas I, Ribo M, et al. Progression of symptomatic intracranial large artery atherosclerosis is associated with a proinflammatory state and impaired fibrinolysis. Stroke. 2008;39:1456–1463. doi: 10.1161/STROKEAHA.107.498600. [DOI] [PubMed] [Google Scholar]
- 4.Tulamo R, Frosen J, Hernesniemi J, Niemela M. Inflammatory changes in the aneurysm wall: a review. J Neurointerv Surg. 2010;2:120–130. doi: 10.1136/jnis.2009.002055. [DOI] [PubMed] [Google Scholar]
- 5.Carr KR, Zuckerman SL, Mocco J. Inflammation, cerebral vasospasm, and evolving theories of delayed cerebral ischemia. Neurol Res Int. 2013;2013:506584. doi: 10.1155/2013/506584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Zhu W, Bollen AW, Lawton MT, Barbaro NM, Dowd CF, et al. Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery. 2008;62:1340–1349. doi: 10.1227/01.neu.0000333306.64683.b5. discussion 1349–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Portanova A, Hakakian N, Mikulis DJ, Virmani R, Abdalla WM, Wasserman BA. Intracranial vasa vasorum: insights and implications for imaging. Radiology. 2013;267:667–679. doi: 10.1148/radiol.13112310. [DOI] [PubMed] [Google Scholar]
- 8.Aoki S, Shirouzu I, Sasaki Y, Okubo T, Hayashi N, Machida T, et al. Enhancement of the intracranial arterial wall at MR imaging: relationship to cerebral atherosclerosis. Radiology. 1995;194:477–481. doi: 10.1148/radiology.194.2.7824729. [DOI] [PubMed] [Google Scholar]
- 9.Swartz RH, Bhuta SS, Farb RI, Agid R, Willinsky RA, Terbrugge KG, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology. 2009;72:627–634. doi: 10.1212/01.wnl.0000342470.69739.b3. [DOI] [PubMed] [Google Scholar]
- 10.Dieleman N, van der Kolk AG, Zwanenburg JJM, Harteveld AA, Biessels GJ, Luijten PR, et al. Imaging intracranial vessel wall pathology with magnetic resonance imaging: current prospects and future directions. Circulation. 2014;130:192–201. doi: 10.1161/CIRCULATIONAHA.113.006919. [DOI] [PubMed] [Google Scholar]
- 11.Antiga L, Wasserman BA, Steinman DA. On the overestimation of early wall thickening at the carotid bulb by black blood MRI, with implications for coronary and vulnerable plaque imaging. Magn Reson Med. 2008;60:1020–1028. doi: 10.1002/mrm.21758. [DOI] [PubMed] [Google Scholar]
- 12.van der Kolk AG, Zwanenburg JJM, Brundel M, Biessels G-J, Visser F, Luijten PR, et al. Intracranial vessel wall imaging at 7.0-T MRI. Stroke. 2011;42:2478–2484. doi: 10.1161/STROKEAHA.111.620443. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Helle M, Zhou Z, Börnert P, Hatsukami TS, Yuan C. Joint blood and cerebrospinal fluid suppression for intracranial vessel wall MRI. [Accessed June 25, 2015];Magn Reson Med. 2015 doi: 10.1002/mrm.25667. [pulished online ahead of print March 13, 2015]. http://onlinelibrary.wiley.com/doi/10.1002/mrm.25667/abstract;jsessionid=EA7E2D1E3526799BE2EFA64111558333.f03t02. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Miller KL, Jezzard P. DANTE-prepared pulse trains: a novel approach to motion-sensitized and motion-suppressed quantitative magnetic resonance imaging. Magn Reson Med. 2012;68:1423–1438. doi: 10.1002/mrm.24142. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Yarnykh VL, Hatsukami T, Chu B, Balu N, Yuan C. Improved suppression of plaque-mimicking artifacts in black-blood carotid atherosclerosis imaging using a multislice motion-sensitized driven-equilibrium (MSDE) turbo spin-echo (TSE) sequence. Magn Reson Med. 2007;58:973–981. doi: 10.1002/mrm.21385. [DOI] [PubMed] [Google Scholar]
- 16.Nagahata S, Nagahata M, Obara M, Kondo R, Minagawa N, Sato S, et al. Wall enhancement of the intracranial aneurysms revealed by magnetic resonance vessel wall imaging using three-dimensional turbo spin-echo sequence with motion-sensitized driven-equilibrium: a sign of ruptured aneurysm? [Accessed June 25, 2015];Clin Neuroradiol. 2014 doi: 10.1007/s00062-014-0353-z. [published online ahead of print October 21, 2014]. http://link.springer.com/article/10.1007%2Fs00062-014-0353-z. [DOI] [PubMed] [Google Scholar]
- 17.Edjlali M, Gentric J-C, Régent-Rodriguez C, Trystram D, Hassen WB, Lion S, et al. Does aneurysmal wall enhancement on vessel wall MRI help to distinguish stable from unstable intracranial aneurysms? Stroke. 2014;45:3704–3706. doi: 10.1161/STROKEAHA.114.006626. [DOI] [PubMed] [Google Scholar]
- 18.van der Kolk AG, Hendrikse J, Brundel M, Biessels GJ, Smit EJ, Visser F, et al. Multi-sequence whole-brain intracranial vessel wall imaging at 7.0 tesla. Eur Radiol. 2013;23:2996–3004. doi: 10.1007/s00330-013-2905-z. [DOI] [PubMed] [Google Scholar]
- 19.Mossa-Basha M, Hwang WD, De Havenon A, Hippe D, Balu N, Becker KJ, et al. Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes. Stroke. 2015;46:1567–1573. doi: 10.1161/STROKEAHA.115.009037. [DOI] [PubMed] [Google Scholar]
- 20.Turan TN, Rumboldt Z, Granholm AC, Columbo L, Welsh CT, Lopes-Virella MF, et al. Intracranial atherosclerosis: correlation between in-vivo 3T high resolution MRI and pathology. Atherosclerosis. 2014;237:460–463. doi: 10.1016/j.atherosclerosis.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Kolk AG, Zwanenburg JJ, Denswil NP, Vink A, Spliet WG, Daemen MJ, et al. Imaging the Intracranial Atherosclerotic Vessel Wall Using 7T MRI: Initial Comparison with Histopathology. AJNR: Am J Neuroradiol. 2015;36:694–701. doi: 10.3174/ajnr.A4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li ML, Xu WH, Song L, Feng F, You H, Ni J, et al. Atherosclerosis of middle cerebral artery: evaluation with high-resolution MR imaging at 3T. Atherosclerosis. 2009;204:447–452. doi: 10.1016/j.atherosclerosis.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Chueh JY, Turan TN, Brown TR, LeMatty T, Mao H, Brooks OW, et al. Preparation of a hydrogel phantom of human atherosclerotic plaque for medical simulation and imaging. Paper presented at: Summer Biomechanics, Bioengineering and Biotransport Conference; June 17, 2015; Snowbird, Utah. [Google Scholar]
- 24.Hajj-Ali RA, Singhal AB, Benseler S, Molloy E, Calabrese LH. Primary angiitis of the CNS. Lancet Neurol. 2011;10:561–572. doi: 10.1016/S1474-4422(11)70081-3. [DOI] [PubMed] [Google Scholar]
- 25.Singhal AB. Diagnostic challenges in RCVS, PACNS, and other cerebral arteriopathies. Cephalalgia. 2011;31:1067–1070. doi: 10.1177/0333102411410084. [DOI] [PubMed] [Google Scholar]
- 26.Pfefferkorn T, Linn J, Habs M, Opherk C, Cyran C, Ottomeyer C, et al. Black blood MRI in suspected large artery primary angiitis of the central nervous system. J Neuroimaging. 2013;23:379–383. doi: 10.1111/j.1552-6569.2012.00743.x. [DOI] [PubMed] [Google Scholar]
- 27.Mossa-Basha M, Hwang WD, De Havenon A, Hippe D, Balu N, Becker KJ, et al. Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes. Stroke. 2015;46:1567–1573. doi: 10.1161/STROKEAHA.115.009037. [DOI] [PubMed] [Google Scholar]
- 28.Kim YJ, Lee DH, Kwon JY, Kang DW, Suh DC, Kim JS, et al. High resolution MRI difference between moyamoya disease and intracranial atherosclerosis. Eur J Neurol. 2013;20:1311–1318. doi: 10.1111/ene.12202. [DOI] [PubMed] [Google Scholar]
- 29.Obusez EC, Hui F, Hajj-Ali RA, Cerejo R, Calabrese LH, Hammad T, et al. High-resolution MRI vessel wall imaging: spatial and temporal patterns of reversible cerebral vasoconstriction syndrome and central nervous system vasculitis. AJNR Am J Neuroradiol. 2014;35:1527–1532. doi: 10.3174/ajnr.A3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahn SH, Lee J, Kim YJ, Kwon SU, Lee D, Jung SC, et al. Isolated MCA disease in patients without significant atherosclerotic risk factors: a high-resolution magnetic resonance imaging study. Stroke. 2015;46:697–703. doi: 10.1161/STROKEAHA.114.008181. [DOI] [PubMed] [Google Scholar]
- 31.Ryoo S, Cha J, Kim SJ, Choi JW, Ki CS, Kim KH, et al. High-resolution magnetic resonance wall imaging findings of Moyamoya disease. Stroke. 2014;45:2457–2460. doi: 10.1161/STROKEAHA.114.004761. [DOI] [PubMed] [Google Scholar]
- 32.Siemonsen S, Brekenfeld C, Holst B, Kaufmann-Buehler AK, Fiehler J, Bley TA. 3T MRI reveals extra- and intracranial involvement in giant cell arteritis. AJNR Am J Neuroradiol. 2015;36:91–97. doi: 10.3174/ajnr.A4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su HS, Nahrendorf M, Panizzi P, Breckwoldt MO, Rodriguez E, Iwamoto Y, et al. Vasculitis: molecular imaging by targeting the inflammatory enzyme myeloperoxidase. Radiology. 2012;262:181–190. doi: 10.1148/radiol.11110040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mineyko A, Kirton A, Ng D, Wei XC. Normal intracranial periarterial enhancement on pediatric brain MR imaging. Neuroradiology. 2013;55:1161–1169. doi: 10.1007/s00234-013-1206-1. [DOI] [PubMed] [Google Scholar]
- 35.Conijn MM, Hendrikse J, Zwanenburg JJ, Takahara T, Geerlings MI, Mali WP, et al. Perforating arteries originating from the posterior communicating artery: a 7.0-Tesla MRI study. Eur Radiol. 2009;19:2986–2992. doi: 10.1007/s00330-009-1485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Kolk AG, Hendrikse J, Brundel M, Biessels GJ, Smit EJ, Visser F, et al. Multi-sequence whole-brain intracranial vessel wall imaging at 7.0 tesla. Eur Radiol. 2013;23:2996–3004. doi: 10.1007/s00330-013-2905-z. [DOI] [PubMed] [Google Scholar]
- 37.Jeon JS, Kim JE. The present and future roles of 7Tesla MRI in cerebrovascular diseases. Neurosurgery. 2013;73:N20–N22. doi: 10.1227/01.neu.0000432625.34095.6b. [DOI] [PubMed] [Google Scholar]
- 38.van der Kolk AG, Zwanenburg JJ, Brundel M, Biessels GJ, Visser F, Luijten PR, et al. Intracranial vessel wall imaging at 7.0-T MRI. Stroke. 2011;42:2478–2484. doi: 10.1161/STROKEAHA.111.620443. [DOI] [PubMed] [Google Scholar]
- 39.Lopes DK, Johnson AK. Evaluation of cerebral artery perforators and the pipeline embolization device using optical coherence tomography. J Neurointerv Surg. 2012;4:291–294. doi: 10.1136/neurintsurg-2011-010102. [DOI] [PubMed] [Google Scholar]
- 40.Kan P, Mokin M, Abla AA, Eller JL, Dumont TM, Levy EI, et al. Utility of intravascular ultrasound in intracranial and extracranial neurointerventions: experience at University at Buffalo Neurosurgery-Millard Fillmore Gates Circle Hospital. Neurosurg Focus. 2012;32:E6. doi: 10.3171/2011.10.FOCUS11242. [DOI] [PubMed] [Google Scholar]
- 41.McVeigh PZ, Sacho R, Weersink RA, Pereira VM, Kucharczyk W, Seibel EJ, et al. High-resolution angioscopic imaging during endovascular neurosurgery. Neurosurgery. 2014;75:171–180. doi: 10.1227/NEU.0000000000000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heiss WD. Radionuclide imaging in ischemic stroke. J Nucl Med. 2014;55:1831–1841. doi: 10.2967/jnumed.114.145003. [DOI] [PubMed] [Google Scholar]
- 43.Gerhard A, Schwarz J, Myers R, Wise R, Banati RB. Evolution of microglial activation in patients after ischemic stroke: a [11C](R)-PK11195 PET study. Neuroimage. 2005;24:591–595. doi: 10.1016/j.neuroimage.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 44.Boutin H, Prenant C, Maroy R, Galea J, Greenhalgh AD, Smigova A, et al. [18F]DPA-714: direct comparison with [11C]PK11195 in a model of cerebral ischemia in rats. PLoS One. 2013;8:e56441. doi: 10.1371/journal.pone.0056441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Callaghan PD, Wimberley CA, Rahardjo GL, Berghofer PJ, Pham TQ, Jackson T, et al. Comparison of in vivo binding properties of the 18-kDa translocator protein (TSPO) ligands [(18)F]PBR102 and [(18)F]PBR111 in a model of excitotoxin-induced neuroinflammation. Eur J Nucl Med Mol Imaging. 2015;42:138–151. doi: 10.1007/s00259-014-2895-3. [DOI] [PubMed] [Google Scholar]
- 46.Breckwoldt MO, Chen JW, Stangenberg L, Aikawa E, Rodriguez E, Qiu S, et al. Tracking the inflammatory response in stroke in vivo by sensing the enzyme myeloperoxidase. Proc Natl Acad Sci U S A. 2008;105:18584–18589. doi: 10.1073/pnas.0803945105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen JW, Pham W, Weissleder R, Bogdanov A., Jr Human myeloperoxidase: a potential target for molecular MR imaging in atherosclerosis. Magn Reson Med. 2004;52:1021–1028. doi: 10.1002/mrm.20270. [DOI] [PubMed] [Google Scholar]
- 48.Ikawa M, Okazawa H, Arakawa K, Kudo T, Kimura H, Fujibayashi Y, et al. PET imaging of redox and energy states in stroke-like episodes of MELAS. Mitochondrion. 2009;9:144–148. doi: 10.1016/j.mito.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 49.Okazawa H, Ikawa M, Tsujikawa T, Kiyono Y, Yoneda M. Brain imaging for oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Q J Nucl Med Mol Imaging. 2014;58:387–397. [PubMed] [Google Scholar]
- 50.Haring HP, Berg EL, Tsurushita N, Tagaya M, del Zoppo GJ. E-selectin appears in nonischemic tissue during experimental focal cerebral ischemia. Stroke. 1996;27:1386–1391. doi: 10.1161/01.str.27.8.1386. [DOI] [PubMed] [Google Scholar]
- 51.Kang HW, Torres D, Wald L, Weissleder R, Bogdanov AA., Jr Targeted imaging of human endothelial-specific marker in a model of adoptive cell transfer. Lab Invest. 2006;86:599–609. doi: 10.1038/labinvest.3700421. [DOI] [PubMed] [Google Scholar]
- 52.van Kasteren SI, Campbell SJ, Serres S, Anthony DC, Sibson NR, Davis BG. Glyconanoparticles allow pre-symptomatic in vivo imaging of brain disease. Proc Natl Acad Sci U S A. 2009;106:18–23. doi: 10.1073/pnas.0806787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin AY, Tuor UI, Rushforth D, Kaur J, Muller RN, Petterson JL, et al. Reduced blood brain barrier breakdown in P-selectin deficient mice following transient ischemic stroke: a future therapeutic target for treatment of stroke. BMC Neurosci. 2010;11:12. doi: 10.1186/1471-2202-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kataoka K, Taneda M, Asai T, Kinoshita A, Ito M, Kuroda R. Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke. 1999;30:1396–1401. doi: 10.1161/01.str.30.7.1396. [DOI] [PubMed] [Google Scholar]
- 55.Frosen J, Piippo A, Paetau A, Kangasniemi M, Niemela M, Hernesniemi J, et al. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004;35:2287–2293. doi: 10.1161/01.STR.0000140636.30204.da. [DOI] [PubMed] [Google Scholar]
- 56.Chyatte D, Bruno G, Desai S, Todor DR. Inflammation and intracranial aneurysms. Neurosurgery. 1999;45:1137–1146. doi: 10.1097/00006123-199911000-00024. [DOI] [PubMed] [Google Scholar]
- 57.Penn DL, Witte SR, Komotar RJ, Sander Connolly E., Jr The role of vascular remodeling and inflammation in the pathogenesis of intracranial aneurysms. J Clin Neurosci. 2014;21:28–32. doi: 10.1016/j.jocn.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J, Alcaide P, Liu L, Sun J, He A, Luscinskas FW, et al. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PLoS One. 2011;6:e14525. doi: 10.1371/journal.pone.0014525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krischek B, Kasuya H, Tajima A, Akagawa H, Sasaki T, Yoneyama T, et al. Network-based gene expression analysis of intracranial aneurysm tissue reveals role of antigen presenting cells. Neuroscience. 2008;154:1398–1407. doi: 10.1016/j.neuroscience.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 60.Yasuno K, Bakircioglu M, Low SK, Bilguvar K, Gaal E, Ruigrok YM, et al. Common variant near the endothelin receptor type A (EDNRA) gene is associated with intracranial aneurysm risk. Proc Natl Acad Sci U S A. 2011;108:19707–19712. doi: 10.1073/pnas.1117137108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walmsley JG, Campling MR, Chertkow HM. Interrelationships among wall structure, smooth muscle orientation, and contraction in human major cerebral arteries. Stroke. 1983;14:781–790. doi: 10.1161/01.str.14.5.781. [DOI] [PubMed] [Google Scholar]
- 62.Monson KL, Goldsmith W, Barbaro NM, Manley GT. Significance of source and size in the mechanical response of human cerebral blood vessels. J Biomech. 2005;38:737–744. doi: 10.1016/j.jbiomech.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Gounis MJ, Vedantham S, Weaver JP, Puri AS, Brooks CS, Wakhloo AK, et al. Myeloperoxidase in human intracranial aneurysms: preliminary evidence. Stroke. 2014;45:1474–1477. doi: 10.1161/STROKEAHA.114.004956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeLeo MJ, 3rd, Gounis MJ, Hong B, Ford JC, Wakhloo AK, Bogdanov AA., Jr Carotid artery brain aneurysm model: in vivo molecular enzyme-specific MR imaging of active inflammation in a pilot study. Radiology. 2009;252:696–703. doi: 10.1148/radiol.2523081426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gounis MJ, van der Bom IM, Wakhloo AK, Zheng S, Chueh JY, Kuhn AL, et al. MR Imaging of Myeloperoxidase Activity in a Model of the Inflamed Aneurysm Wall. AJNR Am J Neuroradiol. 2015;36:146–152. doi: 10.3174/ajnr.A4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trivedi RA, Mallawarachi C, U-King-Im JM, Graves MJ, Horsley J, Goddard MJ, et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol. 2006;26:1601–1606. doi: 10.1161/01.ATV.0000222920.59760.df. [DOI] [PubMed] [Google Scholar]
- 67.Tang T, Howarth SP, Miller SR, Trivedi R, Graves MJ, King-Im JU, et al. Assessment of inflammatory burden contralateral to the symptomatic carotid stenosis using high-resolution ultrasmall, superparamagnetic iron oxide-enhanced MRI. Stroke. 2006;37:2266–2270. doi: 10.1161/01.STR.0000236063.47539.99. [DOI] [PubMed] [Google Scholar]
- 68.Floris S, Blezer EL, Schreibelt G, Dopp E, van der Pol SM, Schadee-Eestermans IL, et al. Blood-brain barrier permeability and monocyte infiltration in experimental allergic encephalomyelitis: a quantitative MRI study. Brain. 2004;127:616–627. doi: 10.1093/brain/awh068. [DOI] [PubMed] [Google Scholar]
- 69.Dousset V, Delalande C, Ballarino L, Quesson B, Seilhan D, Coussemacq M, et al. In vivo macrophage activity imaging in the central nervous system detected by magnetic resonance. Magn Reson Med. 1999;41:329–333. doi: 10.1002/(sici)1522-2594(199902)41:2<329::aid-mrm17>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 70.Saleh A, Schroeter M, Ringelstein A, Hartung HP, Siebler M, Modder U, et al. Iron oxide particle-enhanced MRI suggests variability of brain inflammation at early stages after ischemic stroke. Stroke. 2007;38:2733–2737. doi: 10.1161/STROKEAHA.107.481788. [DOI] [PubMed] [Google Scholar]
- 71.Kleinschnitz C, Bendszus M, Frank M, Solymosi L, Toyka KV, Stoll G. In vivo monitoring of macrophage infiltration in experimental ischemic brain lesions by magnetic resonance imaging. J Cereb Blood Flow Metab. 2003;23:1356–1361. doi: 10.1097/01.WCB.0000090505.76664.DB. [DOI] [PubMed] [Google Scholar]
- 72.Oude Engberink RD, Blezer EL, Hoff EI, van der Pol SM, van der Toorn A, Dijkhuizen RM, et al. MRI of monocyte infiltration in an animal model of neuroinflammation using SPIO-labeled monocytes or free USPIO. J Cereb Blood Flow Metab. 2008;28:841–851. doi: 10.1038/sj.jcbfm.9600580. [DOI] [PubMed] [Google Scholar]
- 73.Farr TD, Seehafer JU, Nelles M, Hoehn M. Challenges towards MR imaging of the peripheral inflammatory response in the subacute and chronic stages of transient focal ischemia. NMR Biomed. 2011;24:35–45. doi: 10.1002/nbm.1553. [DOI] [PubMed] [Google Scholar]
- 74.Oude Engberink RD, Blezer EL, Dijkstra CD, van der Pol SM, van der Toorn A, de Vries HE. Dynamics and fate of USPIO in the central nervous system in experimental autoimmune encephalomyelitis. NMR Biomed. 2010;23:1087–1096. doi: 10.1002/nbm.1536. [DOI] [PubMed] [Google Scholar]
- 75.Desestret V, Brisset JC, Moucharrafie S, Devillard E, Nataf S, Honnorat J, et al. Early-stage investigations of ultrasmall superparamagnetic iron oxide-induced signal change after permanent middle cerebral artery occlusion in mice. Stroke. 2009;40:1834–1841. doi: 10.1161/STROKEAHA.108.531269. [DOI] [PubMed] [Google Scholar]
- 76.Kleinschnitz C, Schutz A, Nolte I, Horn T, Frank M, Solymosi L, et al. In vivo detection of developing vessel occlusion in photothrombotic ischemic brain lesions in the rat by iron particle enhanced MRI. J Cereb Blood Flow Metab. 2005;25:1548–1555. doi: 10.1038/sj.jcbfm.9600151. [DOI] [PubMed] [Google Scholar]
- 77.Wiart M, Davoust N, Pialat JB, Desestret V, Moucharrafie S, Cho TH, et al. MRI monitoring of neuroinflammation in mouse focal ischemia. Stroke. 2007;38:131–137. doi: 10.1161/01.STR.0000252159.05702.00. [DOI] [PubMed] [Google Scholar]
- 78.Hasan D, Chalouhi N, Jabbour P, Dumont AS, Kung DK, Magnotta VA, et al. Early change in ferumoxytol-enhanced magnetic resonance imaging signal suggests unstable human cerebral aneurysm: a pilot study. Stroke. 2012;43:3258–3265. doi: 10.1161/STROKEAHA.112.673400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hasan DM, Chalouhi N, Jabbour P, Magnotta VA, Kung DK, Young WL. Imaging aspirin effect on macrophages in the wall of human cerebral aneurysms using ferumoxytol-enhanced MRI: preliminary results. J Neuroradiol. 2013;40:187–191. doi: 10.1016/j.neurad.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McBride OM, Berry C, Burns P, Chalmers RT, Doyle B, Forsythe R, et al. MRI using ultrasmall superparamagnetic particles of iron oxide in patients under surveillance for abdominal aortic aneurysms to predict rupture or surgical repair: MRI for abdominal aortic aneurysms to predict rupture or surgery-the MA(3)RS study. Open Heart. 2015;2:e000190. doi: 10.1136/openhrt-2014-000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.