Abstract
Providing a stable physical connection between the nucleus and the cytoskeleton is essential for a wide range of cellular functions and could also participate in mechanosensing by transmitting intra- and extracellular mechanical stimuli via the cytoskeleton to the nucleus. Nesprins and SUN proteins, located at the nuclear envelope, form the LINC (Linker of Nucleus and Cytoskeleton) complex that connects the nucleus to the cytoskeleton; underlying nuclear lamins contribute to anchoring LINC complex components at the nuclear envelope. Disruption of the LINC complex or loss of lamins can result in disturbed perinuclear actin and intermediate filament networks and causes severe functional defects, including impaired nuclear positioning, cell polarization, and cell motility. Recent studies have identified the LINC complex as the major force transmitting element at the nuclear envelope and suggest that many of the aforementioned defects can be attributed to disturbed force transmission between the nucleus and the cytoskeleton. Thus, mutations in nesprins, SUN proteins or lamins, which have been linked to muscular dystrophies and cardiomyopathies, may weaken or completely eliminate LINC complex function at the nuclear envelope and result in impaired intracellular force transmission, thereby disrupting critical cellular functions.
Keywords: Nucleus, nesprins, SUN proteins, lamins, cell mechanics, forces
Introduction
The importance of efficient force transmission between cells and their surroundings has long been recognized and studied in the context of cell adhesion, migration, and cell-cell and cell-matrix interactions. Many of the molecular players, such as integrins, cadherins, or members of the dystroglycan complex, are now well characterized. In contrast, even though it is evident that extracellular forces are transmitted across the cytoskeleton to the nucleus [1], the molecular details involved in coupling the nucleus to the cytoskeleton have only recently begun to emerge. The current model suggests that nesprins, a family of spectrin-repeat proteins located at the nuclear envelope, can simultaneously bind to cytoskeletal filaments and interact across the luminal space with the inner nuclear membrane proteins SUN1 and SUN2. This physical link between the cytoskeleton and the nucleus is referred to as the LINC (Linker of Nucleoskeleton and Cytoskeleton) complex [2, 3]. SUN proteins can in turn bind to lamins, chromatin, and other nuclear envelope proteins [4], further extending the physical connection to the nuclear interior [5]. Nonetheless, despite the many recent advances, it remains unclear if/how other nuclear envelope proteins can modulate the interaction of LINC complex proteins or independently contribute to nucleo-cytoskeletal coupling.
To date, at least four nesprin genes (nesprin-1 through 4) have been identified. As a result of multiple initiation and splicing sites, nesprins are expressed in multiple isoforms that vary in length, domain composition, and expression patterns. Nesprins can be located at the inner or outer nuclear membrane, likely in the form of dimers or other complexes. The largest (>800 kDa) isoforms of nesprin-1 and nesprin-2 contain an N-terminal actin binding domain that can interact with cytoplasmic actin filaments [6, 7], while nesprin-3 isoforms (~100 kDa) bind to plectin proteins, which crosslink intermediate filaments [8]. Nesprin-4, only expressed in secretory epithelial cells, is a kinesin-binding protein that connects the nucleus to microtubules [9]. The C-terminal domain of all four nesprins consists of a highly conserved KASH domain [10] that can bind to the C-terminal luminal domain of SUN proteins and is required for the localization of nesprins at the nuclear envelope [2, 4, 11, 12]. SUN1 and SUN2 are retained at the inner nuclear membrane by the interaction of their N-terminal domain with lamins, chromatin and other nuclear membrane proteins and the loss of lamins A/C results in increased mobility of nesprins and SUN proteins at the nuclear envelope [4, 13, 14].
Loss of nesprins, SUN proteins, and lamins from the nuclear envelope results in defects in nuclear positioning, cell polarization, and migration
Many of the initial insights into nucleo-cytoskeletal coupling have come from studies in Caenorhabditis elegans and Drosophila melanogaster. In C. elegans, the nesprin-1/2 ortholog ANC-1 is critical for actin-dependent nuclear positioning in muscle cells during development [15], and mutations in the SUN1 ortholog UNC-84 similarly result in defective nuclear migration and anchoring [16]. Recent independent studies in mouse and human fibroblasts expressing dominant negative LINC complex components or lacking specific nesprin or SUN proteins have reported defects in centrosome attachment to the nucleus [17]; in scratch-wound assays, the initial cell polarization towards the wound, which consists of rearward nuclear movement and positioning of the centrosome towards the leading edge, is often absent, and the subsequent migration of cells into the wound is impaired [13, 18-22]. For example, knockdown of nesprin-1 prevents the reorientation of endothelial cells in response to cyclic strain and causes a decrease in endothelial cell migration in a scratch-wound assay [22]. In fibroblasts, nesprin-2-giant is required for the rearward nuclear movement that precedes polarization and migration [23]. Similar results can be seen when disrupting the LINC complex with dominant negative constructs and plating cells on micropatterned substrates that induce cell polarization (Fig. 1). In addition to nesprin and SUN proteins, impaired nuclear polarization and migration has been demonstrated in lamin A/C–deficient fibroblasts [19]. In genetically engineered mice, mutations in the LINC complex proteins or lamins have been shown to cause abnormal nuclear positioning of synaptic nuclei at the neuromuscular junction in skeletal muscle [24-27].
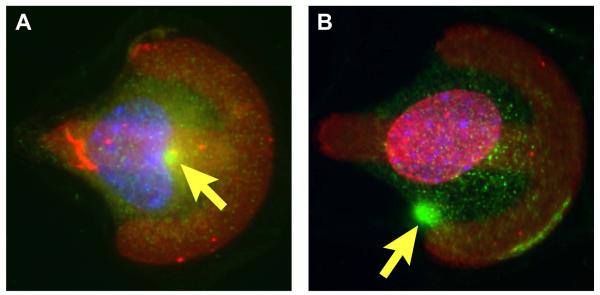
Figure 1.
Impaired cell polarization in mouse embryonic fibroblasts stably expressing dominant negative nesprin constructs. Cells were plated on fibronectin-micropatterned substrates (red) and fluorescently stained for γ-tubulin (green) as centrosomal marker and Hoechst 33342 nuclear stain (blue). (A) Control fibroblast expressing mCherry only polarize towards the bowed front, characterized by a rearward nuclear position and a forward facing centrosome position (arrow). (B) Cells expressing a dominant negative nesprin mCherry fusion construct fail to polarize properly. This figure was originally published in [29]: Lombardi et al. The Interaction between Nesprins and Sun Proteins at the Nuclear Envelope Is Critical for Force Transmission between the Nucleus and Cytoskeleton. J Biol Chem. 2011. 286: 26743-26753 © the American Society for Biochemistry and Molecular Biology.
Nucleo-cytoskeletal coupling is crucial for intracellular force transmission
How can mutations in nuclear envelope proteins result in such dramatic defects in cellular organization and function? Force transmission from the cytoskeleton to the nuclear interior is essential for many cellular processes (Fig. 2). In migrating cells, movement of the nucleus is closely coordinated with the dynamic remodeling of the cytoskeleton, resulting in the appearance of the nucleus to remain stationary relative to the moving cell; during interkinetic nuclear migration, the nucleus periodically moves between the basal and apical side of the neuron; in muscle cells, synaptic nuclei are moved towards the cell periphery and anchored at neuro-muscular junctions [3]. To shift the nucleus within the cell or, in the case of migrating cells, along with the cell, cytoskeletal forces must pull or push on the nucleus [28]; even to retain the nucleus in a fixed position within the cell, the nucleus must be tightly anchored to the surrounding cytoskeleton to prevent random movement. In a seminal study, Luxton and colleagues [23] identified the interaction of SUN proteins and nesprins as critical for intracellular force transmission in mammalian cells by demonstrating that the rearward nuclear movement during the initial cell polarization in a scratch wound assay is driven by the retrograde flow of actin cables that engage the nucleus via nesprin-2 and SUN2, creating structures referred to as transmembrane actin-associated nuclear (TAN) lines, and that knockdown of either nesprin-2 or SUN-2 is sufficient to abrogate nuclear movement. In addition to actin-mediated forces, microtubule-associated motors such as dynein or kinesin can also propel the nucleus within the cell. A nuclear envelope-spanning complex of kinesin-1, nesprin-2, SUN proteins and lamins is required for cell polarization in a scratch-wound assay [17], and ectopic expression of nesprin-4, which interacts with kinesin-1, can result in dramatic cellular rearrangement and separation between the nucleus and the centrosome [9]. Further support for the importance of the LINC complex in intracellular force transmission comes from a recent study in which precisely controlled strain was applied to adherent fibroblasts either locally by displacing a microneedle inserted into the cytoskeleton in close proximity to the nucleus, or globally by stretching the elastic substrate on which the cells were cultured [29]. Intracellular force transmission can then be inferred from the induced cytoskeletal and nuclear displacements (Fig. 3) and compared between cells in which the LINC complex had been disrupted by expression of dominant negative nesprin (or SUN constructs) and mock controls. In these experiments, LINC complex disruption resulted in impaired propagation of intracellular forces between the cytoskeleton and the nucleus, evidenced by reduced nuclear displacements in cells subjected to internal or external mechanical strain [29]. Taken together, these studies indicate that the LINC complex is critical for efficient force transmission between the nucleus and the cytoskeleton.
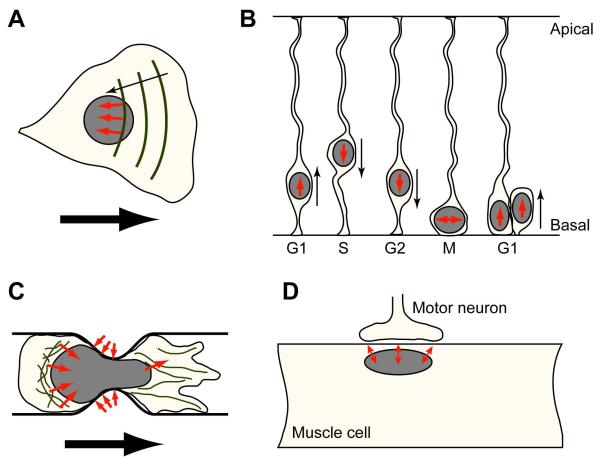
Figure 2.
Examples of nucleo-cytoskeletal force transmission and relevance in cellular function. Forces acting on the nucleus are depicted as small black arrows (A) Rearward nuclear movement in polarizing cells via retrograde flow of actin cables coupled to the nucleus trough nesprin-2/SUN2 [23]. (B) Interkinetic nuclear migration in neurons. Microtubule-associated motors move the nucleus during the cell cycle. Cell cycle phases are indicated as G1, S, G2, and M. (C) Nuclear movement in a migrating cell passing through a narrow constriction. (D) Nuclear anchoring at neuromuscular junctions via lamins, nesprins, and SUN proteins.
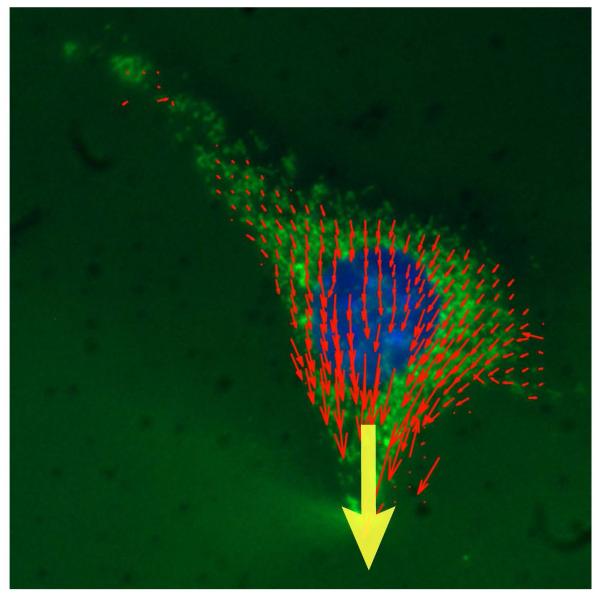
Figure 3.
Representative displacement vector map of induced cytoskeletal deformations in a microneedle manipulation assay. Precisely controlled localized strain was applied with a microneedle near the nucleus towards the cell periphery (yellow arrow). Final cytoskeletal displacements (red vectors) were computed by tracking fluorescently labeled mitochondria (Mitotracker Green) during force application. The fibroblast nucleus was labeled with Hoechst 33342 nuclear stain (blue). Shown are the final displacements at the end of the localized strain application. Vector length shown at 2× magnification for clarity.
Disruption of nucleo-cytoskeletal coupling alters perinuclear cytoskeletal organization
Cytoskeletal components remodel in response to applied forces, and this dynamic feedback with intra- and extracellular forces has an important impact on cellular morphology. For example, in cells subjected to fluid shear stress, actin stress fibers align with the flow direction within 12 hours [30]. Since cytoskeletal components, such as actin, vimentin, and microtubules, are directly associated with the LINC complex, it is not surprising that disruption of the LINC complex can result in altered cytoskeletal organization by eliminating anchoring sites at the nuclear envelope and reducing force transmission between the cytoskeleton and the nucleus. Cultured fibroblasts expressing dominant negative forms of nesprin and SUN proteins have discontinuous and fragmented actin stress fibers in the perinuclear area and also disturbed organization of the perinuclear vimentin filament systems, characterized by a looser and more irregular network surrounding the nucleus [13, 18, 20-22, 29]. The reduced density of the perinuclear cytoskeleton could in turn contribute further to the reduced force transmission between the nucleus and the cytoskeleton. Of note, lamin A/C–deficient cells display similar defects in perinuclear cytoskeletal organization [18, 31] and have reduced cytoskeletal stiffness compared to wild-type controls [19, 32], suggesting that lamins may play a similar role in nucleo-cytoskeletal coupling and cytoskeletal organization as nesprins and SUN proteins, possibly by anchoring LINC complex proteins at the nuclear envelope.
Defective nucleo-cytoskeletal coupling may impair activation of mechanosensitive genes
Cells respond to mechanical stimulation with activation of specific signaling pathways and expression of mechanosensitive genes, a process referred to as mechanotransduction. The nucleus itself has often been proposed as a cellular mechanosensor [5]; for example, nuclear deformations could cause conformational changes in chromatin structure and organization that modulate accessibility of transcription factors or transcriptional processes [5]. Initial support for the existence of nuclear mechanosensing came from findings in lamin A/C–deficient and emerin–deficient mouse embryo fibroblasts that have altered nuclear mechanics and impaired expression of the mechanosensitive genes Iex-1 and Egr-1 in response to cyclic strain application [32, 33]. However, since lamins A and C can also directly modulate signaling pathways by binding to transcriptional regulars such as c-Fos and Erk1/2 [34], it remains unclear whether the observed mechanotransduction defects result from impaired nucleo-cytoskeletal coupling or from the loss of biochemical interactions between transcriptional regulators and specific nuclear envelope proteins. Recent experiments in which nucleo-cytoskeletal coupling was disrupted with dominant negative nesprin constructs without affecting lamin A/C or emerin levels suggest the latter, as the mechanically induced expression of Iex-1 and Egr-1 was indistinguishable from control cells, in spite of dramatically reduced nuclear deformations in the LINC complex disrupted cells [29]. One likely explanation is that mechanotransduction signals triggered at focal adhesions, the plasma membrane, or the peripheral cytoskeleton are sufficient to initiate cellular mechanotransduction pathways [30]. Nonetheless, disturbed nuclear and/or cytoskeletal organization resulting from LINC complex disruption could have additional, possibly modulating effects on specific transcriptional pathways. In one recent example, C2C12 cells expressing dominant negative nesprin and SUN protein constructs showed enhanced activation of NF-κB signaling, regardless whether the stimulation was mechanical (cyclic strain) or chemical (TNF-α)[35].
Defective nucleo-cytoskeletal coupling and force transmission in human diseases
Muscular dystrophies and congenital dilated cardiomyopathies are often caused by mutations in cytoskeletal proteins that disrupt cellular force transmission [36, 37]. For example, Duchenne muscular dystrophy, the most common form of muscular dystrophy, is caused by mutations in the dystrophin gene, which disturbs connections between the cytoskeleton and the extracellular matrix and renders the plasma membrane more fragile [37]. Recently, nesprin-1 and -2 and SUN proteins joined lamins A/C, emerin, and torsin A in the growing group of nuclear envelope proteins that can cause muscular dystrophies and dilated cardiomyopathy when mutated [20, 38-41]. Of note, all of these nuclear envelope proteins are either part of the LINC complex or directly interact with LINC complex proteins. For most of these genes, it has already been demonstrated that functional loss causes impaired nucleo-cytoskeletal coupling and defects in intracellular force transmission, cell polarization, and migration. Future experiments should be aimed at investigating whether the disease-causing mutations elicit similar phenotypes as complete loss of protein or the dominant negative constructs used in the current studies. This is particularly important in light of the fact that the nesprin mutations identified in muscular dystrophy patients to date lie outside the KASH domain. For lamins A and C, work by the Gundersen group revealed that lamin mutants associated with muscular dystrophy, but not those linked to lipodystrophy, abolished retrograde nuclear movement in a scratch wound assay [42], suggesting that loss of nucleo-cytoskeletal coupling is particularly relevant to muscular laminopathies.
Nonetheless, the consequences of defects in nucleo-cytoskeletal coupling are not limited to muscle cells. Mutations in the gene encoding lamins A and C are notorious for causing a plethora of human diseases that include Emery-Dreifuss muscular dystrophy and limb-girdle muscular dystrophy, but also Hutchinson-Gilford progeria syndrome, familial partial lipodystrophy, and Charcot-Marie-Tooth disease [43]; mutations in nesprin-1 also cause Meckel-Gruber syndrome and autosomal recessive cerebellar ataxia with neurological defects in the cerebellum [44, 45]; Duplication of the lamin B1 gene has been linked to autosomal dominant leukodystrophy, a neurological disorder [46], and mouse embryo fibroblasts lacking full length lamin B1 have defective nucleo-cytoskeletal coupling, causing the nuclei of these cells to undergo spontaneous sustained rotations [47]. In mice, loss of lamin B2 results in severe defects in neuronal migration in the cerebral cortex [48], and mice lacking either nesprin-2 or Sun1 and Sun2 have mislocalized photoreceptor nuclei in the retina [49], further illustrating the importance of sufficient nucleo-cytoskeletal coupling in neuronal development and intracellular nuclear migration.
Conclusions and perspectives
The LINC complex and LINC complex associated proteins provide an essential connection between the cytoskeleton and the nucleus critical for intracellular force transmission. Functional loss—either either by mutations, dominant negative constructs, or genetic manipulation—of these proteins can result in defective cytoskeletal organization, impaired nuclear positioning and anchoring, and loss of cell polarization and reduced motility. In recent years, a rapidly growing number of mutations in nuclear envelope proteins have been identified as the cause of at least fifteen human diseases; impaired nucleo-cytoskeletal coupling and disturbed intracellular force transmission are likely to contribute to the disease mechanism, particularly in muscle and neuronal cells. Despite many recent advances, we still have an incomplete understanding of the various functions of the diverse nuclear envelope proteins and their complex interactions. For example, just in the case of nesprins, numerous isoforms exist, and yet even the functional differences between nesprin-1 and -2 have not been fully addressed. Similarly, considering how promiscuous the interactions between KASH and SUN domain proteins appear, little is known about the mechanism by which interactions between nesprins, SUN proteins, and other nuclear envelope proteins are regulated to control nucleo-cytoskeletal coupling in cells. Future research, combining clever genetic and experimental approaches to probe nucleo-cytoskeletal coupling in vitro and in vivo, will be required to address these questions.
Acknowledgements
We apologize to all authors whose work could not be cited due to space constraints. This work was supported by National Institutes of Health (NIH) awards [R01 NS059348 and R01 HL082792] and the Brigham and Women’s Hospital Cardiovascular Leadership Group Award.
Abbreviations
- LINC
Linker of Nucleus and Cytoskeleton
References
- 1.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94(3):849–54. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172(1):41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol. 2010;26:421–44. doi: 10.1146/annurev-cellbio-100109-104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haque F, Lloyd DJ, Smallwood DT, Dent CL, Shanahan CM, Fry AM, Trembath RC, Shackleton S. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol Cell Biol. 2006;26(10):3738–51. doi: 10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10(1):75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 6.Padmakumar VC, Abraham S, Braune S, Noegel AA, Tunggal B, Karakesisoglou I, Korenbaum E. Enaptin, a giant actin-binding protein, is an element of the nuclear membrane and the actin cytoskeleton. Exp Cell Res. 2004;295(2):330–9. doi: 10.1016/j.yexcr.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Zhen YY, Libotte T, Munck M, Noegel AA, Korenbaum E. NUANCE, a giant protein connecting the nucleus and actin cytoskeleton. J Cell Sci. 2002;115(Pt 15):3207–22. doi: 10.1242/jcs.115.15.3207. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, van den Bout I, Raymond K, Sonnenberg A. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J Cell Biol. 2005;171(5):799–810. doi: 10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roux KJ, Crisp ML, Liu Q, Kim D, Kozlov S, Stewart CL, Burke B. Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc Natl Acad Sci U S A. 2009;106(7):2194–9. doi: 10.1073/pnas.0808602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Ragnauth CD, Skepper JN, Worth NF, Warren DT, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprin-2 is a multi-isomeric protein that binds lamin and emerin at the nuclear envelope and forms a subcellular network in skeletal muscle. J Cell Sci. 2005;118(Pt 4):673–87. doi: 10.1242/jcs.01642. [DOI] [PubMed] [Google Scholar]
- 11.Padmakumar VC, Libotte T, Lu W, Zaim H, Abraham S, Noegel AA, Gotzmann J, Foisner R, Karakesisoglou I. The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J Cell Sci. 2005;118(Pt 15):3419–30. doi: 10.1242/jcs.02471. [DOI] [PubMed] [Google Scholar]
- 12.Ketema M, Wilhelmsen K, Kuikman I, Janssen H, Hodzic D, Sonnenberg A. Requirements for the localization of nesprin-3 at the nuclear envelope and its interaction with plectin. J Cell Sci. 2007;120(Pt 19):3384–94. doi: 10.1242/jcs.014191. [DOI] [PubMed] [Google Scholar]
- 13.Stewart-Hutchinson PJ, Hale CM, Wirtz D, Hodzic D. Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness. Exp Cell Res. 2008;314(8):1892–905. doi: 10.1016/j.yexcr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostlund C, Folker ES, Choi JC, Gomes ER, Gundersen GG, Worman HJ. Dynamics and molecular interactions of linker of nucleoskeleton and cytoskeleton (LINC) complex proteins. J Cell Sci. 2009;122(Pt 22):4099–108. doi: 10.1242/jcs.057075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starr DA, Han M. Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science. 2002;298(5592):406–9. doi: 10.1126/science.1075119. [DOI] [PubMed] [Google Scholar]
- 16.Malone CJ, Fixsen WD, Horvitz HR, Han M. UNC-84 localizes to the nuclear envelope and is required for nuclear migration and anchoring during C. elegans development. Development. 1999;126(14):3171–81. doi: 10.1242/dev.126.14.3171. [DOI] [PubMed] [Google Scholar]
- 17.Schneider M, Lu W, Neumann S, Brachner A, Gotzmann J, Noegel AA, Karakesisoglou I. Molecular mechanisms of centrosome and cytoskeleton anchorage at the nuclear envelope. Cell Mol Life Sci. doi: 10.1007/s00018-010-0535-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hale CM, Shrestha AL, Khatau SB, Stewart-Hutchinson PJ, Hernandez L, Stewart CL, Hodzic D, Wirtz D. Dysfunctional connections between the nucleus and the actin and microtubule networks in laminopathic models. Biophys J. 2008;95(11):5462–75. doi: 10.1529/biophysj.108.139428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JS, Hale CM, Panorchan P, Khatau SB, George JP, Tseng Y, Stewart CL, Hodzic D, Wirtz D. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys J. 2007;93(7):2542–52. doi: 10.1529/biophysj.106.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, Ragnauth CD, Yi Q, Mellad JA, Warren DT, Wheeler MA, Ellis JA, Skepper JN, Vorgerd M, Schlotter-Weigel B, Weissberg PL, Roberts RG, Wehnert M, Shanahan CM. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet. 2007;16(23):2816–33. doi: 10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]
- 21.Schneider M, Lu W, Neumann S, Brachner A, Gotzmann J, Noegel AA, Karakesisoglou I. Molecular mechanisms of centrosome and cytoskeleton anchorage at the nuclear envelope. Cell Mol Life Sci. 2010 doi: 10.1007/s00018-010-0535-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chancellor TJ, Lee J, Thodeti CK, Lele T. Actomyosin tension exerted on the nucleus through nesprin-1 connections influences endothelial cell adhesion, migration, and cyclic strain-induced reorientation. Biophys J. 2010;99(1):115–23. doi: 10.1016/j.bpj.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luxton GW, Gomes ER, Folker ES, Vintinner E, Gundersen GG. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science. 2010;329(5994):956–9. doi: 10.1126/science.1189072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grady RM, Starr DA, Ackerman GL, Sanes JR, Han M. Syne proteins anchor muscle nuclei at the neuromuscular junction. Proc Natl Acad Sci U S A. 2005;102(12):4359–64. doi: 10.1073/pnas.0500711102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Xu R, Zhu B, Yang X, Ding X, Duan S, Xu T, Zhuang Y, Han M. Syne-1 and Syne-2 play crucial roles in myonuclear anchorage and motor neuron innervation. Development. 2007;134(5):901–8. doi: 10.1242/dev.02783. [DOI] [PubMed] [Google Scholar]
- 26.Lei K, Zhang X, Ding X, Guo X, Chen M, Zhu B, Xu T, Zhuang Y, Xu R, Han M. SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proc Natl Acad Sci U S A. 2009;106(25):10207–12. doi: 10.1073/pnas.0812037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mejat A, Decostre V, Li J, Renou L, Kesari A, Hantai D, Stewart CL, Xiao X, Hoffman E, Bonne G, Misteli T. Lamin A/C-mediated neuromuscular junction defects in Emery-Dreifuss muscular dystrophy. J Cell Biol. 2009;184(1):31–44. doi: 10.1083/jcb.200811035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedl P, Wolf K, Lammerding J. Nuclear mechanics during cell migration. Curr Opin Cell Biol. 2011;23(1):55–64. doi: 10.1016/j.ceb.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. The Interaction between Nesprins and Sun Proteins at the Nuclear Envelope Is Critical for Force Transmission between the Nucleus and Cytoskeleton. J Biol Chem. 2011;286(30):26743–53. doi: 10.1074/jbc.M111.233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10(1):63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broers JL, Peeters EA, Kuijpers HJ, Endert J, Bouten CV, Oomens CW, Baaijens FP, Ramaekers FC. Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum Mol Genet. 2004;13(21):2567–80. doi: 10.1093/hmg/ddh295. [DOI] [PubMed] [Google Scholar]
- 32.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin A/C Deficiency Causes Defective Nuclear Mechanics and Mechanotransduction. J Clin Invest. 2004;113(3):370–8. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lammerding J, Hsiao J, Schulze PC, Kozlov S, Stewart CL, Lee RT. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol. 2005;170(5):781–91. doi: 10.1083/jcb.200502148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez JM, Navarro-Puche A, Casar B, Crespo P, Andres V. Fast regulation of AP-1 activity through interaction of lamin A/C, ERK1/2, and c-Fos at the nuclear envelope. J Cell Biol. 2008;183(4):653–66. doi: 10.1083/jcb.200805049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brosig M, Ferralli J, Gelman L, Chiquet M, Chiquet-Ehrismann R. Interfering with the connection between the nucleus and the cytoskeleton affects nuclear rotation, mechanotransduction and myogenesis. Int J Biochem Cell Biol. 2010;42(10):1717–28. doi: 10.1016/j.biocel.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Roux KJ, Burke B. Nuclear envelope defects in muscular dystrophy. Biochim Biophys Acta. 2006 doi: 10.1016/j.bbadis.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Emery AE. The muscular dystrophies. Lancet. 2002;359(9307):687–95. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 38.Bione S, Maestrini E, Rivella S, Mancini M, Regis S, Romeo G, Toniolo D. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet. 1994;8(4):323–7. doi: 10.1038/ng1294-323. [DOI] [PubMed] [Google Scholar]
- 39.Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L, Muntoni F, Greenberg CR, Gary F, Urtizberea JA, Duboc D, Fardeau M, Toniolo D, Schwartz K. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet. 1999;21(3):285–8. doi: 10.1038/6799. [DOI] [PubMed] [Google Scholar]
- 40.Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M, Atherton J, Vidaillet HJ, Jr., Spudich S, De Girolami U, Seidman JG, Seidman C, Muntoni F, Muehle G, Johnson W, McDonough B. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med. 1999;341(23):1715–24. doi: 10.1056/NEJM199912023412302. [DOI] [PubMed] [Google Scholar]
- 41.Slominsky PA, Markova ED, Shadrina MI, Illarioshkin SN, Miklina NI, Limborska SA, Ivanova-Smolenskaya IA. A common 3-bp deletion in the DYT1 gene in Russian families with early-onset torsion dystonia. Hum Mutat. 1999;14(3):269. doi: 10.1002/(SICI)1098-1004(1999)14:3<269::AID-HUMU12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 42.Folker ES, Ostlund C, Luxton GW, Worman HJ, Gundersen GG. Lamin A variants that cause striated muscle disease are defective in anchoring transmembrane actin-associated nuclear lines for nuclear movement. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1000824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Worman HJ, Fong LG, Muchir A, Young SG. Laminopathies and the long strange trip from basic cell biology to therapy. J Clin Invest. 2009;119(7):1825–36. doi: 10.1172/JCI37679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dawe HR, Adams M, Wheway G, Szymanska K, Logan CV, Noegel AA, Gull K, Johnson CA. Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton. J Cell Sci. 2009;122(Pt 15):2716–26. doi: 10.1242/jcs.043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dupre N, Bouchard JP, Gros-Louis F, Rouleau GA. [Mutations in SYNE-1 lead to a newly discovered form of autosomal recessive cerebellar ataxia] Med Sci (Paris) 2007;23(3):261–2. doi: 10.1051/medsci/2007233261. [DOI] [PubMed] [Google Scholar]
- 46.Padiath QS, Saigoh K, Schiffmann R, Asahara H, Yamada T, Koeppen A, Hogan K, Ptacek LJ, Fu YH. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat Genet. 2006;38(10):1114–23. doi: 10.1038/ng1872. [DOI] [PubMed] [Google Scholar]
- 47.Ji JY, Lee RT, Vergnes L, Fong LG, Stewart CL, Reue K, Young SG, Zhang Q, Shanahan CM, Lammerding J. Cell nuclei spin in the absence of lamin b1. J Biol Chem. 2007;282(27):20015–26. doi: 10.1074/jbc.M611094200. [DOI] [PubMed] [Google Scholar]
- 48.Coffinier C, Chang SY, Nobumori C, Tu Y, Farber EA, Toth JI, Fong LG, Young SG. Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc Natl Acad Sci U S A. 2010;107(11):5076–81. doi: 10.1073/pnas.0908790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu J, Lei K, Zhou M, Craft CM, Xu G, Xu T, Zhuang Y, Xu R, Han M. KASH protein Syne-2/Nesprin-2 and SUN proteins SUN1/2 mediate nuclear migration during mammalian retinal development. Hum Mol Genet. 2011;20(6):1061–73. doi: 10.1093/hmg/ddq549. [DOI] [PMC free article] [PubMed] [Google Scholar]