Abstract
Understanding how transcriptional regulators are themselves controlled is important in attaining a complete picture of the intracellular effects that follow signaling cascades during early development and cell-restricted differentiation. We have addressed this issue by focusing on the regulation of EKLF/KLF1, a zinc finger transcription factor that plays a necessary role in the global regulation of erythroid gene expression. Using biochemical affinity purification, we have identified the DEK oncoprotein as a critical factor that interacts with an essential upstream enhancer element of the EKLF promoter and exerts a positive effect on EKLF levels. This element also binds a core set of erythroid transcription factors, suggesting that DEK is part of a tissue-restricted enhanceosome that contains BMP4-dependent and -independent components. Together with local enrichment of properly coded histones and an open chromatin domain, optimal transcriptional activation of the EKLF locus can be established.
INTRODUCTION
Studies of the erythroid lineage have led to the successful characterization of intracellular regulators that act as transcription factors to generate red-cell-specific expression (1). However, in many cases it remains unresolved how these factors are themselves regulated. Based on this notion, we have been studying the regulation of erythroid Krüppel-like factor (EKLF or KLF1 [2]), a zinc finger hematopoietic transcription factor that plays a global role in activation of genes critical for genetic control within the erythroid lineage (3–5). It performs this function by binding to its cognate DNA 5′CCMCRCCCN3′ element and recruiting chromatin-remodeling proteins and histone modifiers.
Regulation of EKLF itself is of interest because of a number of functional properties and expression characteristics. EKLF expression remains tissue specific throughout early development and in the adult. Its onset in the yolk sac is strictly limited to the mesodermal, primitive erythroid cells that populate the blood islands at the early headfold stage (embryonic day 7.5 [E7.5]), switching by E9.5 to definitive cells within the hepatic primordia and then to the red pulp of the adult spleen and the bone marrow (6). During definitive hematopoietic differentiation, EKLF is expressed at low levels in multipotent progenitors (MPP) and retains an expression pattern restricted to the common myeloid progenitor (CMP) and megakaryocyte erythroid progenitor (MEP) prior to eventual segregation to erythroid progeny (7–9).
We have demonstrated that a 950-bp region adjacent to the EKLF start site of transcription, encompassing two critical erythroid hypersensitive sites (EHS1 and -2) and the proximal promoter (10), contains all the information needed for developmentally regulated, blood-cell-specific expression of a linked reporter in vivo in mice (11). The tissue specificity and enhancer properties of hypersensitive sites in this region are also seen in human erythroid cells (12). The EHS1 enhancer element contains a very highly conserved (7 species) cluster of Smad, Gata, and E box elements that are critical for optimal promoter function (10, 13–15) and have been shown to bind their cognate proteins (15–18). Removal of the endogenous 49-bp EHS1 enhancer decreases KLF1 levels by 50-fold in mice (19).
Recruitment of transcription factors to enhancers can be dependent on and regulated by growth factor signals. In this context, BMP4 has been shown to play a necessary role in the induction of EKLF transcription, and the BMPR/Smad pathway is critical (20). The importance of the Gata and Smad elements in EHS1 and the proximal promoter has been verified by mutational analyses using a 950-bp KLF1 promoter/green fluorescent protein (GFP) transgene that faithfully recapitulates the onset of endogenous KLF1 expression during mouse embryoid body (EB) differentiation (15). Chromatin immunoprecipitation (ChIP) of GATA proteins reveals a switch from GATA2 to GATA1 in Gata site occupancy (21, 22) when comparing early and late times of EB differentiation (15). In addition, the use of a doxycycline-inducible small hairpin RNA (shRNA) line directed against Smad5 verified its critical importance for KLF1 expression. Together with the promoter analyses, these data led to the proposal of a two-tiered mechanism for transcriptional regulation of KLF1, with GATA2 and SMAD5 proteins initially generating low transcript levels, followed by upregulation of KLF1 expression after GATA1 protein is produced (15).
The slow kinetics of BMP4 induction of EKLF (20) raised the possibility that other factors are corequired prior to EKLF onset. Therefore, we focused on isolating proteins that interact with the particularly strong enhancer element located within EHS1. Using a biochemical affinity purification approach, we have identified one of these factors as the DEK oncoprotein (23). DEK was originally identified as a fusion with the CAN (now called Nup214 [24]) protein in a subset of patients with acute myeloid leukemia (AML) who carry a t(6;9)(p23;q34) translocation (25). We now show that DEK plays a critical role within the protein complex at EHS1 and enables optimal levels of EKLF transcription.
MATERIALS AND METHODS
Cell culture.
Murine erythroleukemia (MEL) line 745A, 32DEpo1, and 293T cells were grown as previously described (10, 26).
Purification of EHS1-binding proteins.
Competitive gel shifts were performed with oligonucleotide 2 (oligo2), oligo3, and oligo4 (10) to help us determine which one to select for purification of EHS1-binding proteins. Use of competitive gel shifts continued during our determination of the optimal purification procedure and in the subsequent follow-through (e.g., see Fig. 2C). M280 magnetic Dynabeads coupled with streptavidin (Life Technologies) were incubated with biotinylated oligonucleotides to create the oligonucleotide Dynabeads using buffers, washes, and conditions as recommended by the manufacturer's protocol. oligo1 and oligo2 sequences have been described previously (10). The 32DEpo1 cell lysate (prepared as described in reference 10) was added, and incubation was continued for 2 to 3 h at room temperature with rotation. A magnetic apparatus was used for all washes. The beads were sequentially washed in 0.2 M NaCl (which removes most of the nonspecific binding proteins) and 0.5 M NaCl before elution in 1 M NaCl. Eluted protein was dialyzed and concentrated by acetone precipitation prior to SDS-PAGE. Proteins were visualized by staining with colloidal blue, and bands of interest were excised. Mass spectrometry analyses of the gel slices were performed by the Rockefeller University Protein and DNA Technology Center using the Sonar MS/MS search engine coupled with statistical scoring methods (27). DEK was significantly enriched in two separate analyses.
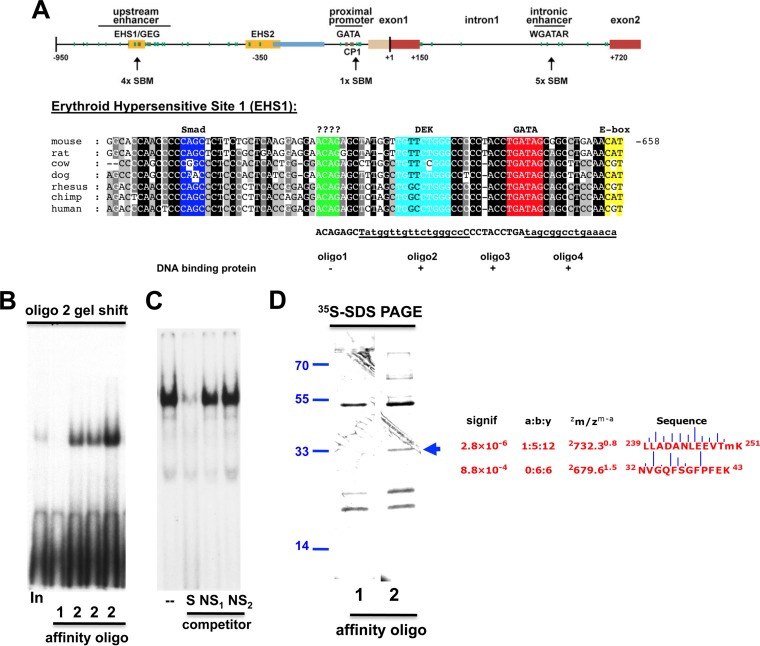
FIG 2.
oligo2 binding proteins within EKLF EHS1. (A) (Top) Schematic (15) of conserved Eklf cis-regulatory elements (upstream enhancer and proximal promoter) together with erythroid hypersensitive sites (EHS1 and EHS2), the Gata-E box-Gata site (GEG) at EHS1, the Gata and Cp1 sites at the proximal promoter, and potential Smad binding motifs (SBM). (Bottom) Detail of EKLF upstream enhancer (EHS1) region. Blocks of conserved sequence homology between seven mammalian species are color coded for their transcription factor binding sites (if known); locations of oligo1 through -4 sequences as identified by reference 10 within EHS1 are as indicated, along with evidence of their in vitro ability to bind proteins in cell extracts. (B) Enrichment of oligo2 binding protein after affinity chromatography with an oligo1 or oligo2 (3 samples) probe as indicated was monitored by a gel shift assay with radioactively labeled oligonucleotide 2. Input is nonenriched 32DEpo1 cell extract. (C) Competitive gel shifts were performed during the purification to assess specificity of binding by extracts to oligonucleotide 2. Specific (S) or nonspecific (NS) competitor DNA was included at a 100-fold excess as indicated. (D) DNA binding proteins that interact with oligo2 were monitored by SDS-PAGE analysis of [35S]methionine-labeled proteins after oligo1 or oligo2 affinity purification. Purified material derived from ∼5 × 106 cell equivalents is shown. The arrow marks the protein unique to the oligonucleotide 2 preparation that is the focus of this study, along with the mass spectrometry data (Sonar MS/MS analysis: a:b:y ratio is of the fragmentation ions; zm/zm−a is the chargemass/chargemeasured−calculated mass; the vertical bar between amino acid pairs indicates the ion intensity within the peptide fragment [27]) and peptide sequences used in its identification; molecular masses are indicated in kilodaltons on the left.
Electrophoretic mobility shift assay.
EHS1 DNA oligonucleotides were fill-in labeled with [32P]dCTP, incubated with purified His-tagged human recombinant DEK protein (pET-28a vector [28]) or with 32DEpo1 extracts, and analyzed on 5% or 8% native polyacrylamide gels as described previously (10, 26, 29). We also performed purified DEK DNA binding reactions in 20 mM HEPES (pH 7.9), 60 mM KCl, 3% Ficoll, 0.5 mM MgCl2, 0.06% Nonidet P-40, 1 mM dithiothreitol, and 20 ng of double-stranded poly(dI-dC). Mouse oligo1 and oligo2 sequences were as described previously (10); human oligo2 (5′-AGCTTCTAGCTGGCCTGGGCCC) and scrambled (5′-AGCTGCGCTGCTCGTACGTTAG) were also used (the top strand only is shown; the EKLF sequence is underlined). The DEK10 sequence was 5′AGCTTATGGTAATTATAGACCC (the italicized bases were changes from the mouse wild type).
shRNA knockdowns.
A 21-nucleotide (nt) stretch within the mouse DEK mRNA sequence was identified according to the RNA interference (RNAi) target design rules put forth by reference 30 (bp 392 to 412 of mouse DEK mRNA, NCBI accession no. NM_025900) and incorporated into a double-stranded DNA 70-mer to encode an anti-DEK small hairpin RNA (shRNA) according to the design rules described in reference 31: 5′-AGATCCCCCTGCTTTACAACAGGCCGGTTCAAGAGACCGGCCTGTTGTAAAGCAGTTTTTGGAAAAGCTT. This 70-mer was cloned into the pSUPER vector via its BglII and HindIII sites and verified by sequencing. The resulting plasmid, the empty vector (carrying a neomycin resistance gene), or a GFP-expressing control plasmid (carrying a neomycin resistance gene) was transfected into the murine erythroid leukemia cell line MEL 745A and selected for G418 resistance. Stable clones were expanded and monitored for DEK and EKLF protein expression levels by immunoblotting (mouse monoclonal anti-DEK antibody [BD Transduction Laboratories]) of cell lysates. This yielded “sh1” as used in Fig. 4.
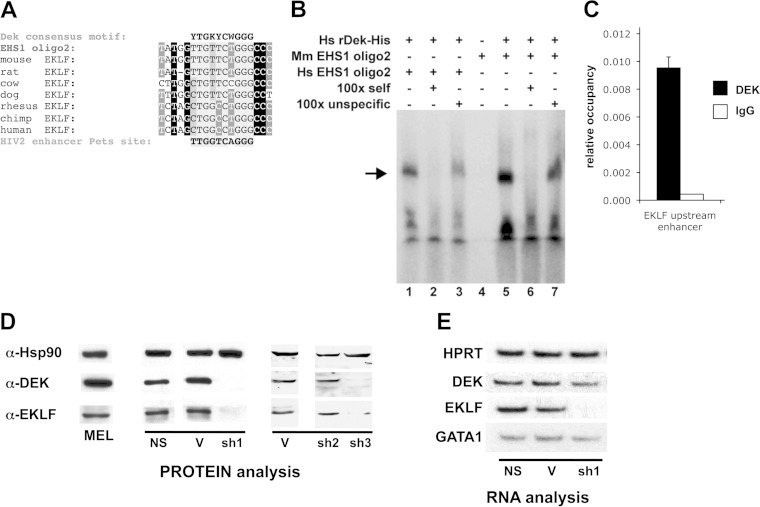
FIG 4.
DEK is critical for EKLF expression. (A) DEK consensus motif. Multispecies EKLF EHS1 oligo2 shares significant similarity with the peri-ets (pets) site bound by DEK in the HIV-2 enhancer. (B) DEK protein interacts with EHS1 in a sequence-specific manner in vitro. DEK binding to oligonucleotide 2 was monitored by gel shift analysis of purified recombinant DEK protein with labeled human (Hs) (lanes 1 to 3) or murine (Mm) (lanes 4 to 7) oligonucleotide 2. Incubations were neat (lanes 1 and 5) or included self (lanes 2 and 6) or scrambled (lanes 3 and 7) cold oligonucleotide 2 at a 100-fold excess. The arrow indicates the band of interest. (C) Quantitative analysis of chromatin immunoprecipitation of DEK protein at the EKLF EHS1 (upstream enhancer) region. Isotype IgG was used as a negative control. Results are the averages of triplicates from two experiments. (D) MEL cells were stably transfected (sh1) or infected (sh2 and sh3) with DEK shRNA-expressing constructs and their appropriate empty vector controls (V) or a nonspecific gene (NS). Untransfected MEL cells (745A) served as an additional control. Results of Western blotting of extracts probed with the indicated antibodies are shown; Hsp90 was used as a loading control. (E) RNA isolated from the same experiment as in panel D was semiquantitatively analyzed for expression of DEK, EKLF, and Gata1 expression; hypoxanthine phosphoribosyltransferase served as a control. Note that sh1 must be exerting its effect by translational repression (114), as DEK RNA levels are not affected in spite of effective protein knockdown.
Additionally, “verified” lentiviral vectors containing shRNAs targeting mouse DEK cloned in pLKO.1 (32) were obtained from Sigma (Mission shRNA Library). Lentiviral supernatants were produced in Phoenix cells by transient transfection with Fugene 6 (Roche). Two rounds of transduction of 25 × 104 murine erythroleukemic cells were carried out as described previously (33), followed by selection in 1 μg/ml puromycin (Sigma). After 4 days, samples were prepared for mRNA and protein analysis. This yielded “sh2” and “sh3” as used in Fig. 4. The sequence for sh3 is 5′-CCGGCGAACTCGTGAAGAGGATCTTCTCGAGAAGATCCTCTTCACGAGTTCGTTTTTG. For control experiments, cells were infected with either empty viral vector or vector expressing a scrambled shRNA.
mRNA levels were detected via semiquantitative or quantitative reverse transcription-PCR (RT-PCR) as previously described (20). DEK mRNA was detected using 5′-AAAGGAACGGAACAGTTCTGG (forward) and 5′ TTGTGACTTCTTCCAAGTTAGC (reverse) primers.
Chromatin analysis.
Chromatin immunoprecipitation analyses were performed as described previously (15, 34) using anti-DEK (rabbit antibody; kind gift from F. Kappes [35]), anti-Tal1 (Santa Cruz; sc-12984X), or anti-P300 (Santa Cruz; sc-585X). Quantitative DNA analyses focused on EKLF EHS1 using 5′-AAGGAGGAACAGAGCTATGGTTGT (forward) and 5′-CAGGCATTATCAGACACACCAGAT (reverse) primers. The specificity of the rabbit anti-DEK antibody has been tested by showing that shRNA knockdown of DEK expression in HEK293 cells leads to loss of signal on a Western blot (36).
Cell and in vivo analyses.
Embryonic stem (ES) cell differentiation into embryoid bodies followed established protocols (15, 37, 38). The ES cell line containing the 950-bp EKLF promoter directly upstream of GFP (Peklf-GFP) is as described previously (15); a variant line containing a deletion of the oligo2 site (Peklf-1xΔDek-GFP) was established after transfection/selection as previously described (15). The parental line is engineered to enable insertion of the entire reporter unidirectionally and in single copy into the same homing site, thus eliminating position effect variegation (39). Postimplantation embryos dissected from pregnant female CD1 or ICR mice (40, 41) were staged according to morphological landmarks (42). Total cellular RNA was isolated as described previously (43). Procurement and yolk sac isolation from CRE/loxP matings were performed as described previously (44). Quantitative analyses of RNA (45) used primers previously described (15).
Cell manipulations.
Transfections, extractions, coprecipitation, and Western blot analyses were performed as described previously (33, 45, 46) using pFLag-Smad5 and pHA-DEK.
Gene editing of MEL cells.
The CRISPR (clustered regularly interspaced short palindromic repeat) design tool at http://tools.genome-engineering.org (47) was used to identify the proper guide sequence with the fewest predicted off-target sites that was closest to the target DEK site to be edited. This web tool enabled selection of the sequence used in Fig. 6, which was cloned into the pX330 vector (48). In addition, a 150-nt (single-strand) donor DNA strand was synthesized (IDT), containing changes in 5 bases of the DEK target sequence that altered the most conserved residues. MEL cells were cotransfected with the pXM330/DEK plasmid, the donor polynucleotide, and a red fluorescent protein (RFP)-expressing plasmid using the Neon transfection system (Life Technologies) set at 1,450 V and 3 pulses with a bandwidth of 10 ms. RFP-expressing cells were single cell sorted with a FACSAria II (Becton Dickinson) into 96-well dishes for growth. Genomic DNA from these clonal populations was isolated and directly sequenced across the region of interest. Because nonhomologous end joining is more efficient than homologous recombination, most of the clones contained deletions in one or both alleles at the DEK binding site even though we had included donor DNA; we selected one clone with an identical deletion in both alleles (indel8) and one with a perfectly recombined target (DEK10) for analysis.
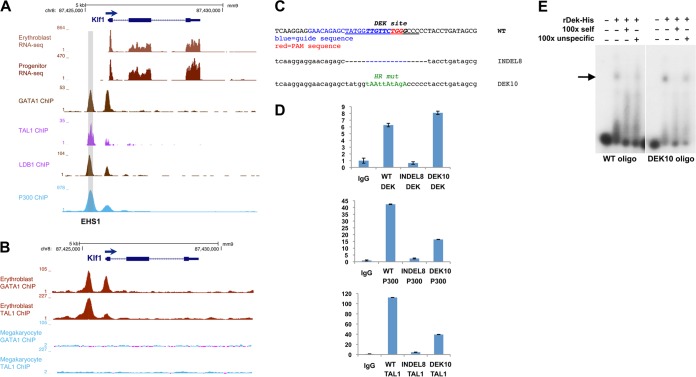
FIG 6.
Critical importance of the oligonucleotide 2 region for erythroid core complex formation. (A) EKLF/Klf1 genome browser data (49, 52–54) showing EKLF transcript levels and location as they relate to Gata1, Tal1, Ldb1, and P300 binding to EHS1 (gray shading). (B) EKLF/Klf1 genome browser data (49, 50) showing Gata1 and Tal1 binding in erythroblasts and megakaryocytes. (C) CRISPR-Cas9 transfection of MEL cells was used for genomic editing. The top panel shows the guide sequence (blue) along with the PAM sequence (red). The DEK sequence is in bold, and the oligonucleotide 2 region is underlined. The bottom panel shows the deletion or substitution identified after genomic sequence analysis of two selected clones, indel8 and DEK10. (D) ChIP analysis of DEK (top), P300 (middle), and Tal1 (bottom) protein binding to EHS1 in wild-type (WT), indel8, or DEK10 MEL cells as indicated. (E) DEK binding to wild-type oligonucleotide 2 (left) or oligonucleotide containing the DEK10 altered sequence (right) was monitored by gel shift analysis with purified recombinant DEK protein. Samples also contained self or scrambled cold oligonucleotide 2 at a 100-fold excess as indicated. The arrow indicates the band of interest.
Data sets analyzed.
Databases used as part of this study include histone modification (49–51), transcription factor (49, 50, 52–54), and erythroid expression (55) sources and are indicated within the relevant figure legends.
RESULTS
The conserved EKLF upstream enhancer element is the site of epigenetic modulation.
We have shown by cotransfection and in vivo analyses that the small 950-bp region adjacent to the EKLF transcription start site is sufficient for tissue-restricted expression (10, 11) and wished to address whether the epigenetic profile of this region supports its importance. Database perusal of modified histone H3 interactions within the critical 950-bp region of the EKLF promoter/enhancer during hematopoiesis (51, 56) shows that levels of the H3K4me1 active enhancer mark increase exactly in parallel with activation of EKLF expression during hematopoiesis (7), that is, low within the hematopoietic repopulating cells (long-term [LT] and short-term [ST] hematopoietic stem cells [HSC]) and in myeloid (CMP and MPP) progenitors but high in MEPs and erythroblasts (Fig. 1A). High levels of the active H3K4me2 and H3K27ac marks are also evident in this region of the EKLF promoter in erythroblasts (57, 58), as is a wider H3K4me3 mark (59) within the body of the gene (Fig. 1A).
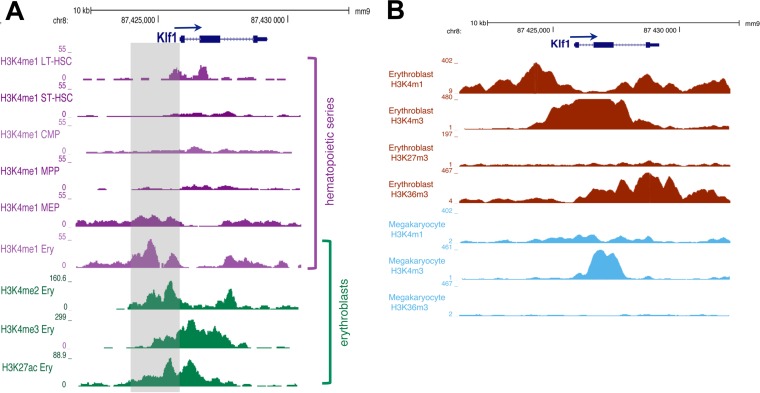
FIG 1.
Database histone H3 modifications associated with the EKLF (Klf1) transcription unit. (A) Histone H3K4me1 association data during hematopoietic differentiation, along with those of H3K4me2, H3K4me3, and H3K27ac from erythroblasts, are compared (iChIP database from reference 51). The shaded area is the adjacent enhancer/promoter region of mouse EKLF that is the focus of the present study. (B) Histone H3K4me1, H3K4me3, H3K27me3, and H3K36me3 association data from the ENCODE/PSU analysis (49, 50) are compared between erythroblasts and megakaryocytes.
Other erythroid database analyses (49, 50) support this pattern and additionally show that the active H3K36me3 mark is preferentially associated with the 3′ end of the gene and that the repressive H3K27me3 mark is not present (Fig. 1B). Although EKLF levels are high in the bipotent MEP, its expression in MEP progeny changes such that it is transcribed at 80-fold-greater levels in erythroid than in megakaryocyte cells (7). Active H3 histone marks at the EKLF gene show a smaller breadth of H3K4me3 and a virtual absence of H3K4me1 and H3K36me3 marks in megakaryocytes compared to erythroblasts (Fig. 1B). Together, these data demonstrate that the EKLF gene, along with its proximal control region, is dynamically altered in activated histone occupancy, consistent with changes in its expression pattern during hematopoiesis.
Isolation of proteins that bind the core fragment in EHS1.
Within the evolutionarily conserved 950-bp region adjacent to the EKLF transcription initiation site, our in vitro analysis of the 49-bp EHS1 region (−715 to −666) (10) found unique DNA binding proteins when oligonucleotides spanning this region were used in gel shift assays with extracts from 32DEpo1 erythroid cells. We had originally used these cells because of their high transfection efficiency in reporter assays; as a result, we retained the same cells for the biochemical purification of binding proteins. We focused on the 18-bp oligo2 binding activity (Fig. 2A) because its DNA/protein complex was more resistant to dissociation than that formed with oligo3, there was not any detectable complex formed with oligo1, and oligo3/oligo4 span two Gata factor sites already known to bind the Gata1-Scl/Tal1 complex (14).
In our approach, double-stranded oligonucleotide 2 (or oligonucleotide 1 as a negative control) is 5′ biotinylated at one end and attached to a streptavidin/magnetic bead column, which provides the solid-state matrix to which the protein(s) is to be bound (60, 61). This column was sequentially washed in 0.2 M NaCl (which removes most of the nonspecific binding proteins) and 0.5 M NaCl before elution in 1 M NaCl, which contains almost all of the specific DNA binding responsible for the oligonucleotide 2 probe shift in the crude extract. Elution is efficient, as active DNA binding material is recovered from the oligonucleotide 2 column but not from the oligonucleotide 1 column (Fig. 2B and C), and a second elution does not recover any additional activity (not shown). Based on DNA binding activity and protein estimates, this step represents an ∼5,000-fold purification.
We performed two analyses to ascertain whether any proteins had been sufficiently enriched for subsequent analysis by this procedure. First, we prepared extracts from [35S]methionine-labeled cells and performed the affinity purification. Analysis of labeled proteins indicates that two proteins (molecular masses of ∼60 and 33 kDa) are uniquely present in the oligonucleotide 2-purified fraction (Fig. 2D), demonstrating that we can well resolve the proteins that are specific to oligonucleotide 2. Second, we scaled up the protocol and monitored a portion of the eluted material on a higher-percentage, silver-stained SDS gel. This higher-resolution analysis confirmed that the two proteins seen previously are uniquely present in the oligonucleotide 2-derived material compared to that from oligonucleotide 1 (not shown).
We purified protein from >10 liters of 32DEpo1 cells, performed matrix-assisted laser desorption ionization (MALDI-TOF) and tandem mass spectrometric analysis, and focused on the material that migrated at ∼35 kDa and that is uniquely present in the oligonucleotide 2-derived preparation. This preparation (but not the negative control) yields the expected gel shift with a labeled oligonucleotide 2 probe. Using the mass spectrometry data, software tools enabled appropriate database searches and determination of which subset of peptides gives the specified ion fragments (62). Of particular interest are two peptides that match the mammalian DEK oncoprotein with high significance (Fig. 2D).
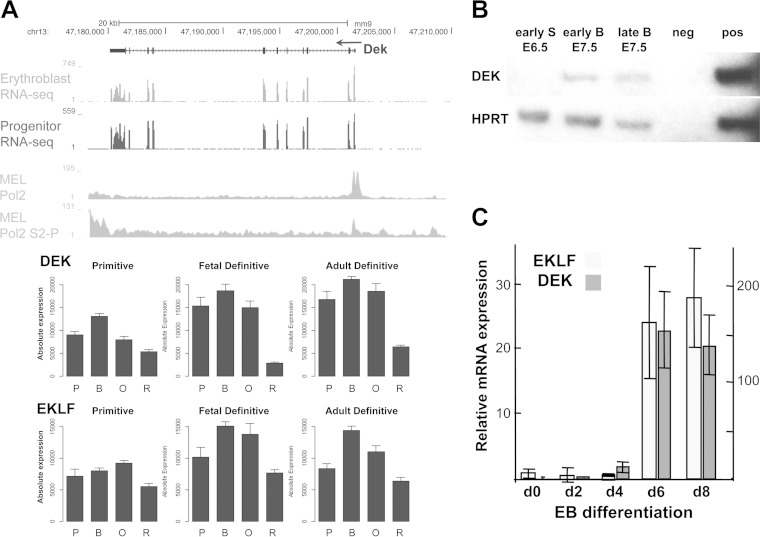
DEK is a protein of particular relevance to the present studies as it is predicted to be involved in the induction of mesoderm during early embryonic development (63), and it plays a role in a subset of myeloid leukemia as a translocation protein fusion product with Nup214 (25). Database inspection shows that all DEK exons are strongly expressed in primitive and definitive erythroid cells at all stages, including erythroid progenitors and the proerythroblast, with an expression pattern that parallels that of EKLF; although it dips in the reticulocyte, this remains at a relatively high level (Fig. 3A). In the gastrulating embryo, DEK expression increases dramatically between E6.5 and early E7.5 (Fig. 3B), just prior to or coincident with the onset of EKLF at E7.5 (6), at the time when the blood islands of the yolk sac are first apparent. DEK onset is also coincident with that of EKLF during EB differentiation at a time when hematopoiesis initiates (Fig. 3C). As a result, DEK is appropriately present to exert an effect on EKLF expression, both in the erythroid cell and during early development and differentiation.
FIG 3.
Erythroid and developmental expression of DEK. (A) (Top) DEK RNA expression in mouse erythroid progenitors and erythroblasts and RNA polymerase II density (total and elongation phospho-Ser2 forms) from the genome browser (50, 52). (Bottom) Data from the Erythron database (55) show expression levels of DEK during mouse primitive and definitive (fetal liver or bone marrow) differentiation. P, proerythroblasts; B, basophilic erythroblasts; O, orthochromatiphilic erythroblasts; R, reticulocytes. (B) Semiquantitative RT-PCR analysis of DEK expression was performed with RNA from staged whole embryos at E6.5 (S, streak) and early or late E7.5 (B, bud) as indicated; water was used as a negative (neg) control; 32DEpo1 RNA was used as a positive (pos) control. Results are representative of two experiments. (C) Levels of EKLF (left scale) or DEK (right scale) were measured in RNA from EBs harvested at the indicated day of differentiation using quantitative RT-PCR and normalized to glyceraldehyde-3-phosphate dehydrogenase. Values are presented relative to day 0, which is set to 1. Results are the averages of triplicates from a single experiment that is representative of two experiments.
DEK binding to its target site is important for optimal EKLF expression.
Sequence inspection indicates that oligonucleotide 2, although not absolutely conserved across its entire length, contains a core region that is identical across KLF1 promoters from seven vertebrate species (Fig. 4A). In addition, this core region matches nicely (only one mismatch) with the HIV-2 enhancer peri-ets site (pets site) bound specifically by DEK in vitro (28) (Fig. 4A). We used in vitro DNA binding assays to test whether recombinant DEK protein can bind oligonucleotide 2 sequences derived from mouse or human EKLF genomic DNA. These sequences are not identical (5/18 bp are different), but as shown in Fig. 4B, each interacts specifically with DEK in vitro as monitored by gel mobility shift experiments. We addressed by chromatin immunoprecipitation (ChIP) whether DEK is bound to the EKLF EHS1 in vivo in erythroid cells; Fig. 4C shows that it does.
A prediction from these analyses is that manipulating DEK expression should have a direct effect on EKLF levels. To test this idea, DEK mRNA was targeted for degradation via RNAi. Using two different vectors (pSUPER [31] and pLKO.1 [32]) with either transfection/selection- or infection/selection-based approaches, we monitored the effects of stably expressing three DEK shRNAs in MEL (line 745A) cells (shDEK lines). Expression analyses from two of the three shDEK lines (but not the empty vector or the nonspecific control lines) show a strong decrease of expression of EKLF protein, which correlates with the loss of DEK protein (Fig. 4D). EKLF but not Gata1 RNA levels are decreased (Fig. 4E).
Collectively, these studies demonstrate that DEK is required for EKLF expression and directly interacts in vitro and in vivo with its cognate site in the EKLF EHS1 enhancer.
Relevance of BMP4 pathway to transcription factor expression.
Studies of the EKLF promoter have implicated the BMPR/BMP/Smad pathway in its regulation (15, 18, 20, 64). This conclusion was supported by the demonstration that BMP4 was necessary and sufficient to induce EKLF during serum-free ES cell differentiation and that interference with the pathway by dominant negative BMPR1B or constitutive Smad6 expression prevented EKLF expression even during serum-containing ES cell differentiation (20). At the same time, the Smad complex has been shown to play a direct role in EKLF induction via Smad5 (15, 18). However, it takes 2 to 3 days for BMP4 to induce EKLF in differentiating EBs (20). The delayed nature of this effect led us to postulate the presence of a required protein that either is synthesized independently or is codependent on BMP4 induction.
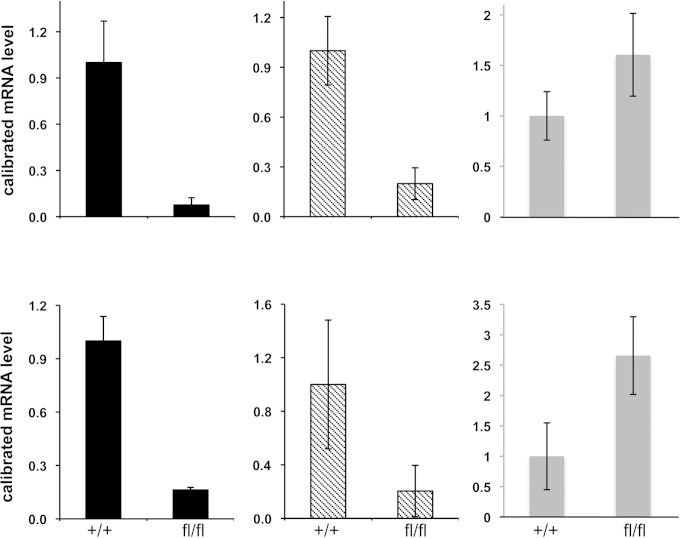
To examine whether DEK might fit into such a scheme, we used the Flk1+/CRE (65) and the floxed BMPR1A (Alk3) and Smad4 (44) mouse lines to address whether DEK and EKLF expression is altered specifically within the Flk1+ population of the developing embryo after conditional ablation. Flk1 is expressed by E7.0 in the extraembryonic and paraxial-lateral embryonic mesoderm (66, 67), and Flk1+ cells are critical for formation of blood vessels and hematopoietic cells (68, 69). Embryos that result from a cross between Flk1+/CRE and floxed Alk3 or Smad4 mice die by E11 (44). RNA was isolated from either E10.5 or E9.5 yolk sacs and analyzed by quantitative RT-PCR for expression of EKLF, Gata1, and DEK. The results (Fig. 5) show that normalized EKLF and Gata1 levels drop by 70 to 90% in the absence of Alk3 or Smad4 in vivo, consistent with our previous studies in differentiating ES cells (20). Of relevance to our test model, DEK levels do not change, or are even slightly elevated, under these ablation conditions, showing that DEK expression in erythroid cells is not regulated by the BMP4 pathway. These data provide in vivo genetic evidence for the requirement of the BMPR/Smad pathway for EKLF and Gata1 expression, independently of DEK expression.
FIG 5.
Effects of yolk sac (YS)-specific ablation of Alk3 or Smad4 on selected targets. EKLF (left), Gata1 (middle), and DEK (right) transcript levels were monitored in yolk sac cells derived from Flk1-Cre (Flk1+/Cre) × Smad4 (wild-type or fl/fl) E9.5 embryos (bottom) or Flk1-Cre (Flk1+/Cre) × Alk3 (wild-type or fl/fl) E10.5 embryos (top). Samples were processed for RNA isolation and analyzed by quantitative RT-PCR. Levels are normalized to glyceraldehyde-3-phosphate dehydrogenase and then set to “1” for wild type. Results are averages from two to three independent biological replicates performed each in triplicate.
Establishment of a multiprotein activation complex at EHS1.
The DEK binding site is located within a conserved Smad-DEK-Gata-E box-Gata-Smad cluster in EHS1 (Fig. 2A) that is critical for EKLF transcription (10, 13–15, 19). We have shown that Gata2 and Gata1 sequentially interact with that site in vivo during the early-to-late transition in developing EBs (15). Several other groups have also shown that Gata1, Gata2, and Smad5 proteins bind the KLF1 promoter in vivo (16–18, 70, 71). Examination of genome browser data shows that Gata1, Tal1, Ldb1, and P300 interact with EHS1 (Fig. 6A), consistent with formation of a “core” erythroid transcription network (56, 72–76), which helps to explain the erythroid cell-specific enhancer properties of EHS1 (10). Its importance is further underscored by analysis of this region in primary megakaryocyte cells that do not express EKLF: these do not show Gata1 or Tal1 protein interaction at EHS1, unlike that of primary erythroblasts analyzed in the same series (Fig. 6B).
To test whether the 18-bp oligonucleotide 2 site plays a role in maintenance of this complex, we used a CRISPR/Cas9 gene-editing approach (47, 48) to modify the endogenous oligonucleotide 2 site in MEL cells. This resulted in a series of indels of various extents, of which one (indel8) contains a biallelic deletion that overlaps oligonucleotide 2 (Fig. 6C). We investigated the status of DEK and two of the core erythroid components in this line and found that the low level of DEK interaction with the oligonucleotide 2-edited EHS1 site was mirrored by a dramatic decrease in occupancy by P300 and Tal1 (Fig. 6D).
The DEK consensus target site within oligonucleotide 2 is fairly loose (Fig. 4A and B), and its DNA recognition properties are unusual (see Discussion), making a directed mutagenesis design less straightforward. Nevertheless, we used CRISPR/Cas9 under homologous recombination conditions to establish a MEL cell line with five substitutions within the most conserved nucleotide residues (DEK10 [Fig. 6C]). Interestingly, although DEK binding is not affected, binding by P300 and Tal1 is decreased by ∼70% (Fig. 6D), suggesting that the core erythroid complex does not remain intact in the midst of this DEK-directed mutation. Direct testing by in vitro analysis supports the lack of effect of the mutated DEK10 site on DEK binding, which maintains specificity, as it is not competed by a scrambled oligonucleotide 2 variant (Fig. 6E).
We next addressed the importance of the oligonucleotide 2 sequence on EKLF expression level in the edited MEL lines, indel8 and DEK10, where we find a small (∼40%) but significant effect on expression, at both the RNA and protein levels (Fig. 7A). This quantitative effect was further supported by analysis of the previously characterized ES cell line containing the 950-bp EKLF promoter linked to a GFP reporter that faithfully reproduces endogenous activation (15). We precisely deleted the oligonucleotide 2 sequence within the promoter and quantified expression levels during embryoid body formation. The data reveal a 2-fold drop in promoter-directed expression in the oligonucleotide 2-deleted cells (Fig. 7B).
FIG 7.
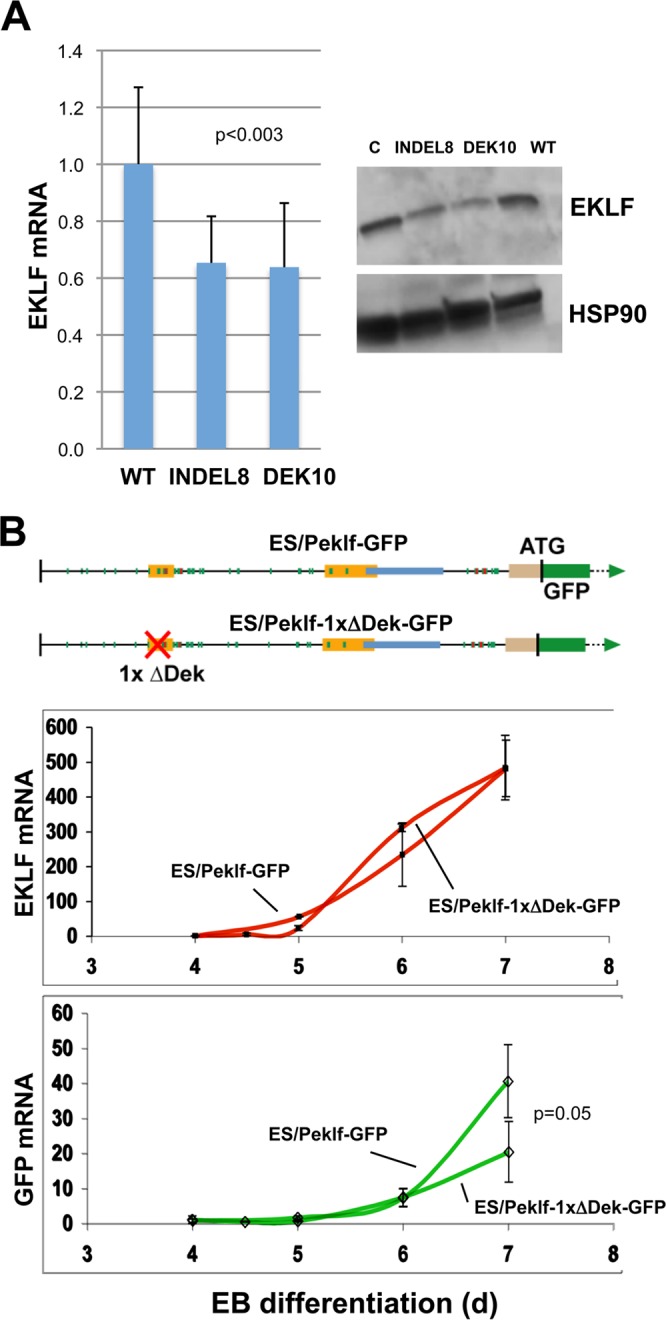
Importance of the oligonucleotide 2 sequence for optimal EKLF expression. (A) Total RNA was isolated or whole-cell extracts were prepared from wild-type (WT), indel8, or DEK10 MEL cells and analyzed for expression of EKLF RNA (quantitative RT-PCR; analysis of 11 analytical replicates each from two experiments) or protein (Western blotting) as indicated. “C” is an additional loading control to balance the EKLF protein signals across the gel. (B) (Top) Schematic of constructs containing the KLF1 promoter or one that has the oligonucleotide 2 sequence removed that were stably integrated into mouse ES cells adjacent to a GFP reporter, yielding two stable ES lines (“Peklf-GFP” and “Peklf-1xΔDek-GFP”). These are single-copy, unidirectional integrations into the same homing site, which thus avoids position effect variegation. Locations of the mapped upstream enhancer, proximal promoter, and intronic enhancer are as indicated. (Middle and bottom) ES cells from each were differentiated to EBs for the indicated number of days, and samples were quantitatively analyzed for expression of endogenous KLF1 (red) or exogenous reporter (green). The data show that the two cell lines differentiated and expressed endogenous KLF1 normally but that the Peklf-1xΔDek-GFP line generated a significantly lower relative level of reporter than did the Peklf-GFP line. Data are from three experiments analyzed in triplicate.
Together, these results demonstrate that the oligonucleotide 2 sequence is vital for formation of the critical core erythroid enhanceosome complex within EHS1 that minimally includes Tal1, P300, and DEK (Fig. 8). Although removal of the 49-bp EHS1 site has a dramatic effect in vivo, removal of the DEK/oligo2 element yields a more nuanced effect on adjacent gene expression and yet remains necessary to establish optimal levels of EKLF RNA and protein expression.
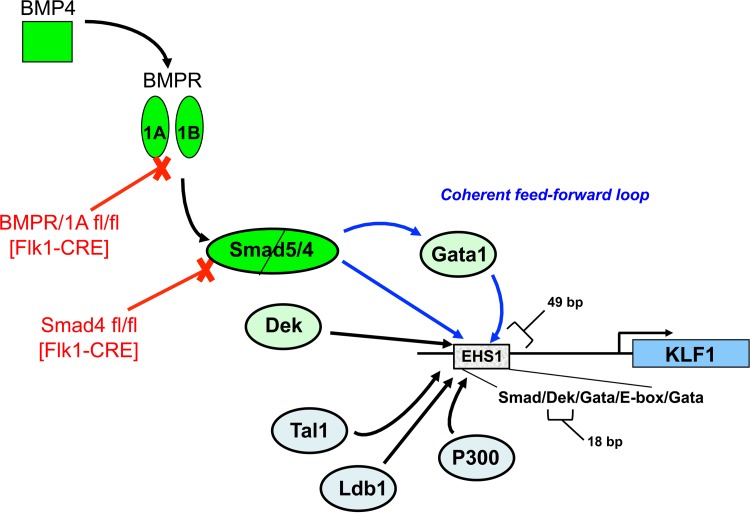
FIG 8.
Extra- and intracellular inputs into regulation of EKLF/KLF1 expression. The BMP4 pathway is linked to erythroid hypersensitive site 1 (EHS1) via Smad5/Smad4, which also induces Gata1, leading to a coherent feed-forward activation loop. Although not shown in the model, Gata2 provides the initial impetus for EKLF and Gata1 expression. The “core” erythroid transcription network that includes Tal1, Ldb1, Gata1 or -2, and P300 is brought into play via the conserved Smad/Dek/Gata/E box/Gata layout at EHS1. Within the 49-bp EHS1 is the 18-bp oligo2 sequence that binds DEK and is important for the full integrity of the complex.
DISCUSSION
We have used a biochemical approach to identify the DEK oncoprotein as a critical factor for EKLF expression via its interaction with the 18-bp oligonucleotide 2 region of the 49-bp EHS1 enhancer element. Removal of the oligonucleotide 2 target site yields a significant disruption of the core erythroid complex at EHS1, and removal of DEK protein (via shRNA constructs) has a dramatic effect on EKLF expression. Our studies suggest that DEK forms a critical part of the core erythroid complex that is formed at EHS1.
Unusual and relevant regulatory properties of DEK.
DEK was first identified as a fusion protein expressed from the t(6;9)(p23;q34) translocation found in a subset of patients with AML (25; see references 23 and 77 to 79 for recent reviews). The DEK gene encodes a 375-amino-acid nuclear protein whose only structural relatedness to known proteins is a well-conserved SAP domain (35, 80, 81), which forms one of its two DNA binding domains (82). DEK plays a role in gene control via its binding of nucleic acids, although the specific nature of these interactions remains complex. For example, while there is evidence that it may play a non-sequence-specific scaffolding function leading to induced alterations of chromatin topology (81, 83, 84), there is also evidence that it recognizes specific target DNA sequences, notably within the HIV-2 enhancer peri-ets sequence (28, 85) and in the class II major histocompatibility complex (MHC) gene promoter at its Y box sequence (86). These target sequences are activation elements and are both homologous to the oligonucleotide 2 sequence of the EKLF promoter (Fig. 4).
The association of DEK with Daxx and histone deacetylase (HDAC) suggests that it plays a role in gene repression (87) and in maintaining heterochromatin integrity via HP1α (36). However, and of most relevance to the present studies, DEK interacts with AP-2α and stimulates transactivation (88) and also interacts with C/EBPα, an important coactivator of myeloid target genes (89), where DEK knockdown disrupts granulocyte differentiation. DEK, accompanied by AP-2α recruitment, becomes enriched at a site proximal to the CD21 promoter upon its transcriptional activation in B lymphocytes (90). DEK plays a critical role in enabling activation of myogenic satellite cells and their exit from quiescence (91) and binds to highly expressed genes within open chromatin in U937 cells (92). Drosophila DEK is found at ecdysone-induced puffs and is a coactivator for the ecdysone receptor (93). Although the levels of hematopoietic stem cells (HSC) and progenitors are slightly but significantly enhanced in the absence of DEK, engraftment of HSC is curtailed (94).
Given the divergence in functions attributed to DEK, no direct connection to the regulation of the EKLF gene was apparent at first. However, the pets site motif that is occupied by DEK in the HIV-2 enhancer is almost identical to the oligo2 sequence of EHS1, which in turn is highly conserved between species. Furthermore, DEK is predicted to play a role in the induction of mesoderm during early embryonic development (63). Together, these properties provided us with a compelling reason to more fully investigate DEK as a functional component of the EKLF promoter, specifically via an interaction with the oligo2 region of EHS1. Based on its expression pattern during erythroid differentiation and development, on the activating role of its target DNA site, and on the negative effects of its knockdown, our findings are most consistent with a stimulatory function for DEK during erythropoiesis, similar to its role in myeloid gene activation.
As summarized in Fig. 8, DEK onset occurs independently of the BMP4 pathway, suggesting that its expression is separate but possibly coordinately required with BMP4 activation of Smad5 to fully induce EKLF. We have previously proposed that Gata2 is required for EKLF's initial activation, which is then activated to higher levels once Gata1 is fully induced by Gata2 (15). An intact Gata1 protein is required for full activation of EKLF, as Gata1s is deficient (95). The requirement of the BMP4 pathway for Gata1 expression suggests a coherent type 1 feed-forward mechanism on EKLF induction between BMP4/Smad5 and Gata1 once Gata1 is induced by Gata2 (96, 97). Based on our earlier studies, this requires high levels of BMP4 (20).
Extended protein/DNA complex interactions.
The oligonucleotide 2 target site is situated within the EKLF EHS1 enhancer region (Fig. 8) that is known to interact with “core” erythroid transcription components that consist of Gata1 or -2, Tal1, Ldb1, and P300 (56, 72–75, 98). Of relevance to the present study, a recent analysis shows that the accuracy of enhancer prediction is improved by including Smad binding to this core set (99). The sequence and protein layout are highly reminiscent of the beta interferon (IFN-β) enhanceosome (100, 101), a conserved 50-bp sequence with overlapping binding sites that associate with a specific set of transcriptional activators. Using DNA- and protein-dependent mechanisms, these cooperate to synergistically activate transcription. CBP/P300 is a strong coactivator that interacts with many of these regulators and stabilizes the complex. Supporting this analogy, global analyses of blood-cell-specific expression demonstrate that motifs with preferential spacing are prevalent in hematopoietic promoters and that they interact with multiple transcription factors (102). The spacing among these motifs is critical for maximal activity (102); for example, the Gata/E box motif pair retains an ∼9-bp spacing, a property also seen within EHS1. Gata2 and Smad4 (the partner of Smad5) form part of a combinatorial interaction network (103), and we also find that DEK and Smad5 can interact after cotransfection (unpublished observations).
Our studies suggest that DEK is a critical non-tissue-specific component that supports formation of a complex structure of core erythroid activators at selected promoters/enhancers that contain Smad/Gata/E box/Gata DNA sequences (73–75, 98). DEK may or may not be needed for the integrity of the other components of the complex; the ChIP data (Fig. 6) suggest that it contributes to it but also that the DNA site plays a significant role in its coherence, such that the DEK10 mutant site is just not optimal for maximum transcription efficiency even though it binds DEK. In addition, other proteins may be involved (e.g., CTCF [104]). It will be of interest to query other erythroid sites that also interact with these core components to determine the extent of overlap with DEK.
Although we have focused our attention on the importance of EHS1, it is essential to remember that the proximal promoter is absolutely required for EKLF expression in cell culture and in vivo (10, 11, 15), and there is evidence that a conserved intron 1 element contributes to full activity as well (15). EHS1 and intron 1 may each provide quantitative contributions to expression that together with the crucial proximal promoter element are needed for full EKLF induction in vivo.
How might DEK function be so critical for EKLF transcriptional activation? DEK properties suggest a number of speculative scenarios. For example, DEK's ability to interact with altered DNA and chromatin structures may come into play within any erythroid “activation hubs” (e.g., see references 105 to 107) that might form at the EKLF genetic region. Given that Scl/Tal1, a protein that is known to play a critical role in the formation of such 3-dimensional structures in erythroid cells via the Ldb complex (108), also binds at EHS1, it is not far-fetched to imagine such a scenario. In addition, DEK interacts with histone H3.3, particularly after its phosphorylation by CK2, and preferentially enriches H3.3 localization to actively transcribed regions (93). Finally, DEK is found associated with splicing complexes (80, 109, 110), where it enables accurate discrimination of potential 3′ splice sites via its interaction with U2AF (110), thus potentially linking primary transcription to effective accumulation of the processed EKLF transcript (discussed in references 111 to 113).
ACKNOWLEDGMENTS
Chunhong Gong, Changwon Park, and Deanna Mohn were involved in different phases of this study. We thank Nithya Gnanapragasam for help with fluorescence-activated cell sorting. We thank David Markovitz and Ferdinand Kappes for DEK plasmids and antibodies.
This work was supported by NIH grants R01 DK48721 and DK46865 to J.J.B., R01 HL55337 to K.C., and R01 HL62248 to M.H.B.
REFERENCES
- 1.Novershtern N, Subramanian A, Lawton LN, Mak RH, Haining WN, McConkey ME, Habib N, Yosef N, Chang CY, Shay T, Frampton GM, Drake AC, Leskov I, Nilsson B, Preffer F, Dombkowski D, Evans JW, Liefeld T, Smutko JS, Chen J, Friedman N, Young RA, Golub TR, Regev A, Ebert BL. 2011. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell 144:296–309. doi: 10.1016/j.cell.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller IJ, Bieker JJ. 1993. A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Krüppel family of nuclear proteins. Mol Cell Biol 13:2776–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siatecka M, Bieker JJ. 2011. The multifunctional role of EKLF/KLF1 during erythropoiesis. Blood 118:2044–2054. doi: 10.1182/blood-2011-03-331371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tallack MR, Perkins AC. 2010. KLF1 directly coordinates almost all aspects of terminal erythroid differentiation. IUBMB Life 62:886–890. doi: 10.1002/iub.404. [DOI] [PubMed] [Google Scholar]
- 5.Yien YY, Bieker JJ. 2013. EKLF/KLF1, a tissue-restricted integrator of transcriptional control, chromatin remodeling, and lineage determination. Mol Cell Biol 33:4–13. doi: 10.1128/MCB.01058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Southwood CM, Downs KM, Bieker JJ. 1996. Erythroid Kruppel-like factor (EKLF) exhibits an early and sequentially localized pattern of expression during mammalian erythroid ontogeny. Dev Dyn 206:248–259. doi:. [DOI] [PubMed] [Google Scholar]
- 7.Frontelo P, Manwani D, Galdass M, Karsunky H, Lohmann F, Gallagher PG, Bieker JJ. 2007. Novel role for EKLF in megakaryocyte lineage commitment. Blood 110:3871–3880. doi: 10.1182/blood-2007-03-082065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui K, Zang C, Roh TY, Schones DE, Childs RW, Peng W, Zhao K. 2009. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell 4:80–93. doi: 10.1016/j.stem.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li B, Ding L, Li W, Story MD, Pace BS. 2012. Characterization of the transcriptome profiles related to globin gene switching during in vitro erythroid maturation. BMC Genomics 13:153. doi: 10.1186/1471-2164-13-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Reitman M, Bieker JJ. 1998. Chromatin structure and transcriptional control elements of the erythroid Kruppel-like factor (EKLF) gene. J Biol Chem 273:25031–25040. doi: 10.1074/jbc.273.39.25031. [DOI] [PubMed] [Google Scholar]
- 11.Xue L, Chen X, Chang Y, Bieker JJ. 2004. Regulatory elements of the EKLF gene that direct erythroid cell-specific expression during mammalian development. Blood 103:4078–4083. doi: 10.1182/blood-2003-09-3231. [DOI] [PubMed] [Google Scholar]
- 12.Xiong Q, Zhang Z, Chang KH, Qu H, Wang H, Qi H, Li Y, Ruan X, Yang Y, Sandstrom R, Sabo PJ, Li Q, Stamatoyannopoulos G, Stamatoyannopoulos JA, Fang X. 2013. Comprehensive characterization of erythroid-specific enhancers in the genomic regions of human Kruppel-like factors. BMC Genomics 14:587. doi: 10.1186/1471-2164-14-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson KP, Crable SC, Lingrel JB. 1998. Multiple proteins binding to a GATA-E box-GATA motif regulate the erythroid Kruppel-like factor (EKLF) gene. J Biol Chem 273:14347–14354. doi: 10.1074/jbc.273.23.14347. [DOI] [PubMed] [Google Scholar]
- 14.Anderson KP, Crable SC, Lingrel JB. 2000. The GATA-E box-GATA motif in the EKLF promoter is required for in vivo expression. Blood 95:1652–1655. [PubMed] [Google Scholar]
- 15.Lohmann F, Bieker JJ. 2008. Activation of Eklf expression during hematopoiesis by Gata2 and Smad5 prior to erythroid commitment. Development 135:2071–2082. doi: 10.1242/dev.018200. [DOI] [PubMed] [Google Scholar]
- 16.Fujiwara T, O'Geen H, Keles S, Blahnik K, Linnemann AK, Kang YA, Choi K, Farnham PJ, Bresnick EH. 2009. Discovering hematopoietic mechanisms through genome-wide analysis of GATA factor chromatin occupancy. Mol Cell 36:667–681. doi: 10.1016/j.molcel.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu M, Riva L, Xie H, Schindler Y, Moran TB, Cheng Y, Yu D, Hardison R, Weiss MJ, Orkin SH, Bernstein BE, Fraenkel E, Cantor AB. 2009. Insights into GATA-1-mediated gene activation versus repression via genome-wide chromatin occupancy analysis. Mol Cell 36:682–695. doi: 10.1016/j.molcel.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perna F, Vu LP, Themeli M, Kriks S, Hoya-Arias R, Khanin R, Hricik T, Mansilla-Soto J, Papapetrou EP, Levine RL, Studer L, Sadelain M, Nimer SD. 2015. The polycomb group protein L3MBTL1 represses a SMAD5-mediated hematopoietic transcriptional program in human pluripotent stem cells. Stem Cell Rep 4:658–669. doi: 10.1016/j.stemcr.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou D, Liu K, Sun CW, Pawlik KM, Townes TM. 2010. KLF1 regulates BCL11A expression and gamma- to beta-globin gene switching. Nat Genet 42:742–744. doi: 10.1038/ng.637. [DOI] [PubMed] [Google Scholar]
- 20.Adelman CA, Chattopadhyay S, Bieker JJ. 2002. The BMP/BMPR/Smad pathway directs expression of the erythroid-specific EKLF and GATA1 transcription factors during embryoid body differentiation in serum-free media. Development 129:539–549. [DOI] [PubMed] [Google Scholar]
- 21.Bresnick EH, Lee HY, Fujiwara T, Johnson KD, Keles S. 2010. GATA switches as developmental drivers. J Biol Chem 285:31087–31093. doi: 10.1074/jbc.R110.159079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dore LC, Chlon TM, Brown CD, White KP, Crispino JD. 2012. Chromatin occupancy analysis reveals genome-wide GATA factor switching during hematopoiesis. Blood 119:3724–3733. doi: 10.1182/blood-2011-09-380634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanden C, Gullberg U. 2015. The DEK oncoprotein and its emerging roles in gene regulation. Leukemia 29:1632–1636. doi: 10.1038/leu.2015.72. [DOI] [PubMed] [Google Scholar]
- 24.Kraemer D, Wozniak RW, Blobel G, Radu A. 1994. The human CAN protein, a putative oncogene product associated with myeloid leukemogenesis, is a nuclear pore complex protein that faces the cytoplasm. Proc Natl Acad Sci U S A 91:1519–1523. doi: 10.1073/pnas.91.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Lindern M, Fornerod M, van Baal S, Jaegle M, de Wit T, Buijs A, Grosveld G. 1992. The translocation (6;9), associated with a specific subtype of acute myeloid leukemia, results in the fusion of two genes, dek and can, and the expression of a chimeric, leukemia-specific dek-can mRNA. Mol Cell Biol 12:1687–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siatecka M, Lohmann F, Bao S, Bieker JJ. 2010. EKLF directly activates the p21WAF1/CIP1 gene by proximal promoter and novel intronic regulatory regions during erythroid differentiation. Mol Cell Biol 30:2811–2822. doi: 10.1128/MCB.01016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Field HI, Fenyo D, Beavis RC. 2002. RADARS, a bioinformatics solution that automates proteome mass spectral analysis, optimises protein identification, and archives data in a relational database. Proteomics 2:36–47. [PubMed] [Google Scholar]
- 28.Fu GK, Grosveld G, Markovitz DM. 1997. DEK, an autoantigen involved in a chromosomal translocation in acute myelogenous leukemia, binds to the HIV-2 enhancer. Proc Natl Acad Sci U S A 94:1811–1815. doi: 10.1073/pnas.94.5.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Bieker JJ. 1996. Erythroid Krüppel-like factor (EKLF) contains a multifunctional transcriptional activation domain important for inter- and intramolecular interactions. EMBO J 15:5888–5896. [PMC free article] [PubMed] [Google Scholar]
- 30.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 31.Brummelkamp TR, Bernards R, Agami R. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 32.Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G, Piqani B, Eisenhaure TM, Luo B, Grenier JK, Carpenter AE, Foo SY, Stewart SA, Stockwell BR, Hacohen N, Hahn WC, Lander ES, Sabatini DM, Root DE. 2006. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 33.Soni S, Pchelintsev N, Adams PD, Bieker JJ. 2014. Transcription factor EKLF (KLF1) recruitment of the histone chaperone HIRA is essential for beta-globin gene expression. Proc Natl Acad Sci U S A 111:13337–13342. doi: 10.1073/pnas.1405422111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Im H, Grass JA, Johnson KD, Kim SI, Boyer ME, Imbalzano AN, Bieker JJ, Bresnick EH. 2005. Chromatin domain activation via GATA-1 utilization of a small subset of dispersed GATA motifs within a broad chromosomal region. Proc Natl Acad Sci U S A 102:17065–17070. doi: 10.1073/pnas.0506164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kappes F, Scholten I, Richter N, Gruss C, Waldmann T. 2004. Functional domains of the ubiquitous chromatin protein DEK. Mol Cell Biol 24:6000–6010. doi: 10.1128/MCB.24.13.6000-6010.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kappes F, Waldmann T, Mathew V, Yu J, Zhang L, Khodadoust MS, Chinnaiyan AM, Luger K, Erhardt S, Schneider R, Markovitz DM. 2011. The DEK oncoprotein is a Su(var) that is essential to heterochromatin integrity. Genes Dev 25:673–678. doi: 10.1101/gad.2036411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi K, Chung YS, Zhang WJ. 2005. Hematopoietic and endothelial development of mouse embryonic stem cells in culture. Methods Mol Med 105:359–368. [DOI] [PubMed] [Google Scholar]
- 38.Manwani D, Galdass M, Bieker JJ. 2007. Altered regulation of beta-like globin genes by a redesigned erythroid transcription factor. Exp Hematol 35:39–47. doi: 10.1016/j.exphem.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kyba M, Perlingeiro RC, Daley GQ. 2002. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell 109:29–37. doi: 10.1016/S0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 40.Belaoussoff M, Farrington SM, Baron MH. 1998. Hematopoietic induction and respecification of A-P identity by visceral endoderm signaling in the mouse embryo. Development 125:5009–5018. [DOI] [PubMed] [Google Scholar]
- 41.Dyer MA, Farrington SM, Mohn D, Munday JR, Baron MH. 2001. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development 128:1717–1730. [DOI] [PubMed] [Google Scholar]
- 42.Downs KM, Davies T. 1993. Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development 118:1255–1266. [DOI] [PubMed] [Google Scholar]
- 43.Baron MH, Mohn D. 2005. Mouse embryonic explant culture system for analysis of hematopoietic and vascular development. Methods Mol Med 105:231–256. [DOI] [PubMed] [Google Scholar]
- 44.Park C, Lavine K, Mishina Y, Deng CX, Ornitz DM, Choi K. 2006. Bone morphogenetic protein receptor 1A signaling is dispensable for hematopoietic development but essential for vessel and atrioventricular endocardial cushion formation. Development 133:3473–3484. doi: 10.1242/dev.02499. [DOI] [PubMed] [Google Scholar]
- 45.Siatecka M, Xue L, Bieker JJ. 2007. Sumoylation of EKLF promotes transcriptional repression and is involved in inhibition of megakaryopoiesis. Mol Cell Biol 27:8547–8560. doi: 10.1128/MCB.00589-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X, Bieker JJ. 2001. Unanticipated repression function linked to erythroid Kruppel-like factor. Mol Cell Biol 21:3118–3125. doi: 10.1128/MCB.21.9.3118-3125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. 2013. Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. 2013. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu W, Cheng Y, Keller CA, Ernst J, Kumar SA, Mishra T, Morrissey C, Dorman CM, Chen KB, Drautz D, Giardine B, Shibata Y, Song L, Pimkin M, Crawford GE, Furey TS, Kellis M, Miller W, Taylor J, Schuster SC, Zhang Y, Chiaromonte F, Blobel GA, Weiss MJ, Hardison RC. 2011. Dynamics of the epigenetic landscape during erythroid differentiation after GATA1 restoration. Genome Res 21:1659–1671. doi: 10.1101/gr.125088.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stamatoyannopoulos JA, Snyder M, Hardison R, Ren B, Gingeras T, Gilbert DM, Groudine M, Bender M, Kaul R, Canfield T, Giste E, Johnson A, Zhang M, Balasundaram G, Byron R, Roach V, Sabo PJ, Sandstrom R, Stehling AS, Thurman RE, Weissman SM, Cayting P, Hariharan M, Lian J, Cheng Y, Landt SG, Ma Z, Wold BJ, Dekker J, Crawford GE, Keller CA, Wu W, Morrissey C, Kumar SA, Mishra T, Jain D, Byrska-Bishop M, Blankenberg D, Lajoie BR, Jain G, Sanyal A, Chen KB, Denas O, Taylor J, Blobel GA, Weiss MJ, Pimkin M, Deng W, Marinov GK, Williams BA, Fisher-Aylor KI, Desalvo G, Kiralusha A, Trout D, Amrhein H, Mortazavi A, Edsall L, McCleary D, Kuan S, Shen Y, Yue F, Ye Z, Davis CA, Zaleski C, Jha S, Xue C, Dobin A, Lin W, Fastuca M, Wang H, Guigo R, Djebali S, Lagarde J, Ryba T, Sasaki T, Malladi VS, Cline MS, Kirkup VM, Learned K, Rosenbloom KR, Kent WJ, Feingold EA, Good PJ, Pazin M, Lowdon RF, Adams LB. 2012. An encyclopedia of mouse DNA elements (Mouse ENCODE). Genome Biol 13:418. doi: 10.1186/gb-2012-13-8-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lara-Astiaso D, Weiner A, Lorenzo-Vivas E, Zaretsky I, Jaitin DA, David E, Keren-Shaul H, Mildner A, Winter D, Jung S, Friedman N, Amit I. 2014. Immunogenetics. Chromatin state dynamics during blood formation. Science 345:943–949. doi: 10.1126/science.1256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pilon AM, Ajay SS, Kumar SA, Steiner LA, Cherukuri PF, Wincovitch S, Anderson SM, Mullikin JC, Gallagher PG, Hardison RC, Margulies EH, Bodine DM. 2011. Genome-wide ChIP-Seq reveals a dramatic shift in the binding of the transcription factor erythroid Kruppel-like factor during erythrocyte differentiation. Blood 118:e139–e148. doi: 10.1182/blood-2011-05-355107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soler E, Andrieu-Soler C, de Boer E, Bryne JC, Thongjuea S, Stadhouders R, Palstra RJ, Stevens M, Kockx C, van Ijcken W, Hou J, Steinhoff C, Rijkers E, Lenhard B, Grosveld F. 2010. The genome-wide dynamics of the binding of Ldb1 complexes during erythroid differentiation. Genes Dev 24:277–289. doi: 10.1101/gad.551810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kassouf MT, Hughes JR, Taylor S, McGowan SJ, Soneji S, Green AL, Vyas P, Porcher C. 2010. Genome-wide identification of TAL1's functional targets: insights into its mechanisms of action in primary erythroid cells. Genome Res 20:1064–1083. doi: 10.1101/gr.104935.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kingsley PD, Greenfest-Allen E, Frame JM, Bushnell TP, Malik J, McGrath KE, Stoeckert CJ, Palis J. 2013. Ontogeny of erythroid gene expression. Blood 121:e5–e13. doi: 10.1182/blood-2012-04-422394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu J, Shao Z, Glass K, Bauer DE, Pinello L, Van Handel B, Hou S, Stamatoyannopoulos JA, Mikkola HK, Yuan GC, Orkin SH. 2012. Combinatorial assembly of developmental stage-specific enhancers controls gene expression programs during human erythropoiesis. Dev Cell 23:796–811. doi: 10.1016/j.devcel.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thurman RE, Rynes E, Humbert R, Vierstra J, Maurano MT, Haugen E, Sheffield NC, Stergachis AB, Wang H, Vernot B, Garg K, John S, Sandstrom R, Bates D, Boatman L, Canfield TK, Diegel M, Dunn D, Ebersol AK, Frum T, Giste E, Johnson AK, Johnson EM, Kutyavin T, Lajoie B, Lee BK, Lee K, London D, Lotakis D, Neph S, Neri F, Nguyen ED, Qu H, Reynolds AP, Roach V, Safi A, Sanchez ME, Sanyal A, Shafer A, Simon JM, Song L, Vong S, Weaver M, Yan Y, Zhang Z, Lenhard B, Tewari M, Dorschner MO, Hansen RS, Navas PA, Stamatoyannopoulos G, Iyer VR, Lieb JD, Sunyaev SR, Akey JM, Sabo PJ, Kaul R, Furey TS, Dekker J, Crawford GE, Stamatoyannopoulos JA. 2012. The accessible chromatin landscape of the human genome. Nature 489:75–82. doi: 10.1038/nature11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.ENCODE Project Consortium 2012. An integrated encyclopedia of DNA elements in the human genome. Nature 489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benayoun BA, Pollina EA, Ucar D, Mahmoudi S, Karra K, Wong ED, Devarajan K, Daugherty AC, Kundaje AB, Mancini E, Hitz BC, Gupta R, Rando TA, Baker JC, Snyder MP, Cherry JM, Brunet A. 2014. H3K4me3 breadth is linked to cell identity and transcriptional consistency. Cell 158:673–688. doi: 10.1016/j.cell.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gabrielsen OS, Hornes E, Korsnes L, Ruet A, Oyen TB. 1989. Magnetic DNA affinity purification of yeast transcription factor tau—a new purification principle for the ultrarapid isolation of near homogeneous factor. Nucleic Acids Res 17:6253–6267. doi: 10.1093/nar/17.15.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gershon PD, Moss B. 1990. Early transcription factor subunits are encoded by vaccinia virus late genes. Proc Natl Acad Sci U S A 87:4401–4405. doi: 10.1073/pnas.87.11.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McLafferty FW, Fridriksson EK, Horn DM, Lewis MA, Zubarev RA. 1999. Biomolecule mass spectrometry. Science 284:1289–1290. doi: 10.1126/science.284.5418.1289. [DOI] [PubMed] [Google Scholar]
- 63.Hollnagel A, Oehlmann V, Heymer J, Ruther U, Nordheim A. 1999. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem 274:19838–19845. doi: 10.1074/jbc.274.28.19838. [DOI] [PubMed] [Google Scholar]
- 64.Kang YJ, Shin JW, Yoon JH, Oh IH, Lee SP, Kim SY, Park SH, Mamura M. 2012. Inhibition of erythropoiesis by Smad6 in human cord blood hematopoietic stem cells. Biochem Biophys Res Commun 423:750–756. doi: 10.1016/j.bbrc.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 65.Motoike T, Markham DW, Rossant J, Sato TN. 2003. Evidence for novel fate of Flk1+ progenitor: contribution to muscle lineage. Genesis 35:153–159. doi: 10.1002/gene.10175. [DOI] [PubMed] [Google Scholar]
- 66.Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. 1993. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development 118:489–498. [DOI] [PubMed] [Google Scholar]
- 67.Dumont DJ, Fong GH, Puri MC, Gradwohl G, Alitalo K, Breitman ML. 1995. Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev Dyn 203:80–92. doi: 10.1002/aja.1002030109. [DOI] [PubMed] [Google Scholar]
- 68.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. 1995. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 69.Lugus JJ, Park C, Ma YD, Choi K. 2009. Both primitive and definitive blood cells are derived from Flk-1+ mesoderm. Blood 113:563–566. doi: 10.1182/blood-2008-06-162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodriguez P, Bonte E, Krijgsveld J, Kolodziej KE, Guyot B, Heck AJ, Vyas P, de Boer E, Grosveld F, Strouboulis J. 2005. GATA-1 forms distinct activating and repressive complexes in erythroid cells. EMBO J 24:2354–2366. doi: 10.1038/sj.emboj.7600702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vakoc CR, Letting DL, Gheldof N, Sawado T, Bender MA, Groudine M, Weiss MJ, Dekker J, Blobel GA. 2005. Proximity among distant regulatory elements at the beta-globin locus requires GATA-1 and FOG-1. Mol Cell 17:453–462. doi: 10.1016/j.molcel.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 72.Li L, Freudenberg J, Cui K, Dale R, Song SH, Dean A, Zhao K, Jothi R, Love PE. 2013. Ldb1-nucleated transcription complexes function as primary mediators of global erythroid gene activation. Blood 121:4575–4585. doi: 10.1182/blood-2013-01-479451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su MY, Steiner LA, Bogardus H, Mishra T, Schulz VP, Hardison RC, Gallagher PG. 2013. Identification of biologically relevant enhancers in human erythroid cells. J Biol Chem 288:8433–8444. doi: 10.1074/jbc.M112.413260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tallack MR, Magor GW, Dartigues B, Sun L, Huang S, Fittock JM, Fry SV, Glazov EA, Bailey TL, Perkins AC. 2012. Novel roles for KLF1 in erythropoiesis revealed by mRNA-seq. Genome Res 22:2385–2398. doi: 10.1101/gr.135707.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wontakal SN, Guo X, Smith C, MacCarthy T, Bresnick EH, Bergman A, Snyder MP, Weissman SM, Zheng D, Skoultchi AI. 2012. A core erythroid transcriptional network is repressed by a master regulator of myelo-lymphoid differentiation. Proc Natl Acad Sci U S A 109:3832–3837. doi: 10.1073/pnas.1121019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rogers H, Wang L, Yu X, Alnaeeli M, Cui K, Zhao K, Bieker JJ, Prchal J, Huang S, Weksler B, Noguchi CT. 2012. T-cell acute leukemia 1 (TAL1) regulation of erythropoietin receptor and association with excessive erythrocytosis. J Biol Chem 287:36720–36731. doi: 10.1074/jbc.M112.378398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Broxmeyer HE, Mor-Vaknin N, Kappes F, Legendre M, Saha AK, Ou X, O'Leary H, Capitano M, Cooper S, Markovitz DM. 2013. Concise review: role of DEK in stem/progenitor cell biology. Stem Cells 31:1447–1453. doi: 10.1002/stem.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Privette Vinnedge LM, Kappes F, Nassar N, Wells SI. 2013. Stacking the DEK: from chromatin topology to cancer stem cells. Cell Cycle 12:51–66. doi: 10.4161/cc.23121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Riveiro-Falkenbach E, Soengas MS. 2010. Control of tumorigenesis and chemoresistance by the DEK oncogene. Clin Cancer Res 16:2932–2938. doi: 10.1158/1078-0432.CCR-09-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sitwala KV, Mor-Vaknin N, Markovitz DM. 2003. Minireview: DEK and gene regulation, oncogenesis and AIDS. Anticancer Res 23:2155–2158. [PubMed] [Google Scholar]
- 81.Waldmann T, Scholten I, Kappes F, Hu HG, Knippers R. 2004. The DEK protein—an abundant and ubiquitous constituent of mammalian chromatin. Gene 343:1–9. doi: 10.1016/j.gene.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 82.Bohm F, Kappes F, Scholten I, Richter N, Matsuo H, Knippers R, Waldmann T. 2005. The SAF-box domain of chromatin protein DEK. Nucleic Acids Res 33:1101–1110. doi: 10.1093/nar/gki258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Waldmann T, Baack M, Richter N, Gruss C. 2003. Structure-specific binding of the proto-oncogene protein DEK to DNA. Nucleic Acids Res 31:7003–7010. doi: 10.1093/nar/gkg864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alexiadis V, Waldmann T, Andersen J, Mann M, Knippers R, Gruss C. 2000. The protein encoded by the proto-oncogene DEK changes the topology of chromatin and reduces the efficiency of DNA replication in a chromatin-specific manner. Genes Dev 14:1308–1312. [PMC free article] [PubMed] [Google Scholar]
- 85.Cleary J, Sitwala KV, Khodadoust MS, Kwok RP, Mor-Vaknin N, Cebrat M, Cole PA, Markovitz DM. 2005. p300/CBP-associated factor drives DEK into interchromatin granule clusters. J Biol Chem 280:31760–31767. doi: 10.1074/jbc.M500884200. [DOI] [PubMed] [Google Scholar]
- 86.Adams BS, Cha HC, Cleary J, Haiying T, Wang H, Sitwala K, Markovitz DM. 2003. DEK binding to class II MHC Y-box sequences is gene- and allele-specific. Arthritis Res Ther 5:R226–233. doi: 10.1186/ar774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hollenbach AD, McPherson CJ, Mientjes EJ, Iyengar R, Grosveld G. 2002. Daxx and histone deacetylase II associate with chromatin through an interaction with core histones and the chromatin-associated protein Dek. J Cell Sci 115:3319–3330. [DOI] [PubMed] [Google Scholar]
- 88.Campillos M, Garcia MA, Valdivieso F, Vazquez J. 2003. Transcriptional activation by AP-2alpha is modulated by the oncogene DEK. Nucleic Acids Res 31:1571–1575. doi: 10.1093/nar/gkg247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koleva RI, Ficarro SB, Radomska HS, Carrasco-Alfonso MJ, Alberta JA, Webber JT, Luckey CJ, Marcucci G, Tenen DG, Marto JA. 2012. C/EBPalpha and DEK coordinately regulate myeloid differentiation. Blood 119:4878–4888. doi: 10.1182/blood-2011-10-383083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hu HG, Illges H, Gruss C, Knippers R. 2005. Distribution of the chromatin protein DEK distinguishes active and inactive CD21/CR2 gene in pre- and mature B lymphocytes. Int Immunol 17:789–796. doi: 10.1093/intimm/dxh261. [DOI] [PubMed] [Google Scholar]
- 91.Cheung TH, Quach NL, Charville GW, Liu L, Park L, Edalati A, Yoo B, Hoang P, Rando TA. 2012. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature 482:524–528. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanden C, Jarvstrat L, Lennartsson A, Brattas PL, Nilsson B, Gullberg U. 2014. The DEK oncoprotein binds to highly and ubiquitously expressed genes with a dual role in their transcriptional regulation. Mol Cancer 13:215. doi: 10.1186/1476-4598-13-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sawatsubashi S, Murata T, Lim J, Fujiki R, Ito S, Suzuki E, Tanabe M, Zhao Y, Kimura S, Fujiyama S, Ueda T, Umetsu D, Ito T, Takeyama K, Kato S. 2010. A histone chaperone, DEK, transcriptionally coactivates a nuclear receptor. Genes Dev 24:159–170. doi: 10.1101/gad.1857410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Broxmeyer HE, Kappes F, Mor-Vaknin N, Legendre M, Kinzfogl J, Cooper S, Hangoc G, Markovitz DM. 2012. DEK regulates hematopoietic stem engraftment and progenitor cell proliferation. Stem Cells Dev 21:1449–1454. doi: 10.1089/scd.2011.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chlon TM, McNulty M, Goldenson B, Rosinski A, Crispino JD. 2015. Global transcriptome and chromatin occupancy analysis reveal the short isoform of GATA1 is deficient for erythroid specification and gene expression. Haematologica 100:575–584. doi: 10.3324/haematol.2014.112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shoval O, Alon U. 2010. SnapShot: network motifs. Cell 143:326–e321. doi: 10.1016/j.cell.2010.09.050. [DOI] [PubMed] [Google Scholar]
- 97.Yosef N, Regev A. 2011. Impulse control: temporal dynamics in gene transcription. Cell 144:886–896. doi: 10.1016/j.cell.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ulirsch JC, Lacy JN, An X, Mohandas N, Mikkelsen TS, Sankaran VG. 2014. Altered chromatin occupancy of master regulators underlies evolutionary divergence in the transcriptional landscape of erythroid differentiation. PLoS Genet 10:e1004890. doi: 10.1371/journal.pgen.1004890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dogan N, Wu W, Morrissey CS, Chen KB, Stonestrom A, Long M, Keller CA, Cheng Y, Jain D, Visel A, Pennacchio LA, Weiss MJ, Blobel GA, Hardison RC. 2015. Occupancy by key transcription factors is a more accurate predictor of enhancer activity than histone modifications or chromatin accessibility. Epigenetics Chromatin 8:16. doi: 10.1186/s13072-015-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ford E, Thanos D. 2010. The transcriptional code of human IFN-beta gene expression. Biochim Biophys Acta 1799:328–336. doi: 10.1016/j.bbagrm.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 101.Panne D. 2008. The enhanceosome. Curr Opin Struct Biol 18:236–242. doi: 10.1016/j.sbi.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 102.Ng FS, Schutte J, Ruau D, Diamanti E, Hannah R, Kinston SJ, Gottgens B. 2014. Constrained transcription factor spacing is prevalent and important for transcriptional control of mouse blood cells. Nucleic Acids Res 42:13513–13524. doi: 10.1093/nar/gku1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ravasi T, Suzuki H, Cannistraci CV, Katayama S, Bajic VB, Tan K, Akalin A, Schmeier S, Kanamori-Katayama M, Bertin N, Carninci P, Daub CO, Forrest AR, Gough J, Grimmond S, Han JH, Hashimoto T, Hide W, Hofmann O, Kamburov A, Kaur M, Kawaji H, Kubosaki A, Lassmann T, van Nimwegen E, MacPherson CR, Ogawa C, Radovanovic A, Schwartz A, Teasdale RD, Tegner J, Lenhard B, Teichmann SA, Arakawa T, Ninomiya N, Murakami K, Tagami M, Fukuda S, Imamura K, Kai C, Ishihara R, Kitazume Y, Kawai J, Hume DA, Ideker T, Hayashizaki Y. 2010. An atlas of combinatorial transcriptional regulation in mouse and man. Cell 140:744–752. doi: 10.1016/j.cell.2010.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hou C, Dale R, Dean A. 2010. Cell type specificity of chromatin organization mediated by CTCF and cohesin. Proc Natl Acad Sci U S A 107:3651–3656. doi: 10.1073/pnas.0912087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Drissen R, Palstra RJ, Gillemans N, Splinter E, Grosveld F, Philipsen S, de Laat W. 2004. The active spatial organization of the beta-globin locus requires the transcription factor EKLF. Genes Dev 18:2485–2490. doi: 10.1101/gad.317004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stadhouders R, Thongjuea S, Andrieu-Soler C, Palstra RJ, Bryne JC, van den Heuvel A, Stevens M, de Boer E, Kockx C, van der Sloot A, van den Hout M, van Ijcken W, Eick D, Lenhard B, Grosveld F, Soler E. 2012. Dynamic long-range chromatin interactions control Myb proto-oncogene transcription during erythroid development. EMBO J 31:986–999. doi: 10.1038/emboj.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou Y, Kurukuti S, Saffrey P, Vukovic M, Michie AM, Strogantsev R, West AG, Vetrie D. 2013. Chromatin looping defines expression of TAL1, its flanking genes, and regulation in T-ALL. Blood 122:4199–4209. doi: 10.1182/blood-2013-02-483875. [DOI] [PubMed] [Google Scholar]
- 108.Song SH, Kim A, Ragoczy T, Bender MA, Groudine M, Dean A. 2010. Multiple functions of Ldb1 required for beta-globin activation during erythroid differentiation. Blood 116:2356–2364. doi: 10.1182/blood-2010-03-272252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McGarvey T, Rosonina E, McCracken S, Li Q, Arnaout R, Mientjes E, Nickerson JA, Awrey D, Greenblatt J, Grosveld G, Blencowe BJ. 2000. The acute myeloid leukemia-associated protein, DEK, forms a splicing-dependent interaction with exon-product complexes. J Cell Biol 150:309–320. doi: 10.1083/jcb.150.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Soares LM, Zanier K, Mackereth C, Sattler M, Valcarcel J. 2006. Intron removal requires proofreading of U2AF/3′ splice site recognition by DEK. Science 312:1961–1965. doi: 10.1126/science.1128659. [DOI] [PubMed] [Google Scholar]
- 111.Schor IE, Gomez Acuna LI, Kornblihtt AR. 2013. Coupling between transcription and alternative splicing. Cancer Treat Res 158:1–24. doi: 10.1007/978-3-642-31659-3_1. [DOI] [PubMed] [Google Scholar]
- 112.Hsin JP, Manley JL. 2012. The RNA polymerase II CTD coordinates transcription and RNA processing. Genes Dev 26:2119–2137. doi: 10.1101/gad.200303.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Darnell JE., Jr 2013. Reflections on the history of pre-mRNA processing and highlights of current knowledge: a unified picture. RNA 19:443–460. doi: 10.1261/rna.038596.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fabian MR, Sonenberg N. 2012. The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC. Nat Struct Mol Biol 19:586–593. doi: 10.1038/nsmb.2296. [DOI] [PubMed] [Google Scholar]