Abstract
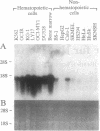
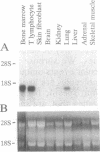
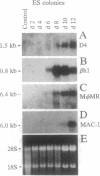
We have identified the mRNA for a human gene, denoted D4, which is expressed at very high levels in hematopoietic cell lines and in normal cells of lymphoid and myeloid origin. The 1.5-kb transcript is absent or detectable only at low levels in nonhematopoietic tissues. D4 encodes a 201-amino acid protein with homology to rhoGDI, an inhibitor of GDP dissociation for the ras-homologous protein rho. D4 might function also as a regulator of guanine nucleotide exchange for small GTP-binding proteins. A homologous transcript of similar size is also preferentially expressed in murine hematopoietic tissues. When totipotent murine embryonic stem cells develop in vitro into hematopoietic cells, the gene is activated with the onset of hematopoiesis. When hematopoietic cell lines are induced to differentiate, the expression of D4 is modulated. Thus, D4 appears to be a developmentally regulated gene. Its preferential expression in hematopoietic cells indicates that D4 likely plays some significant role in the growth and differentiation processes of hematopoietic cells. This significance is underscored by increasing evidence for the involvement of regulators of G proteins in clinical diseases.
Full text
PDF



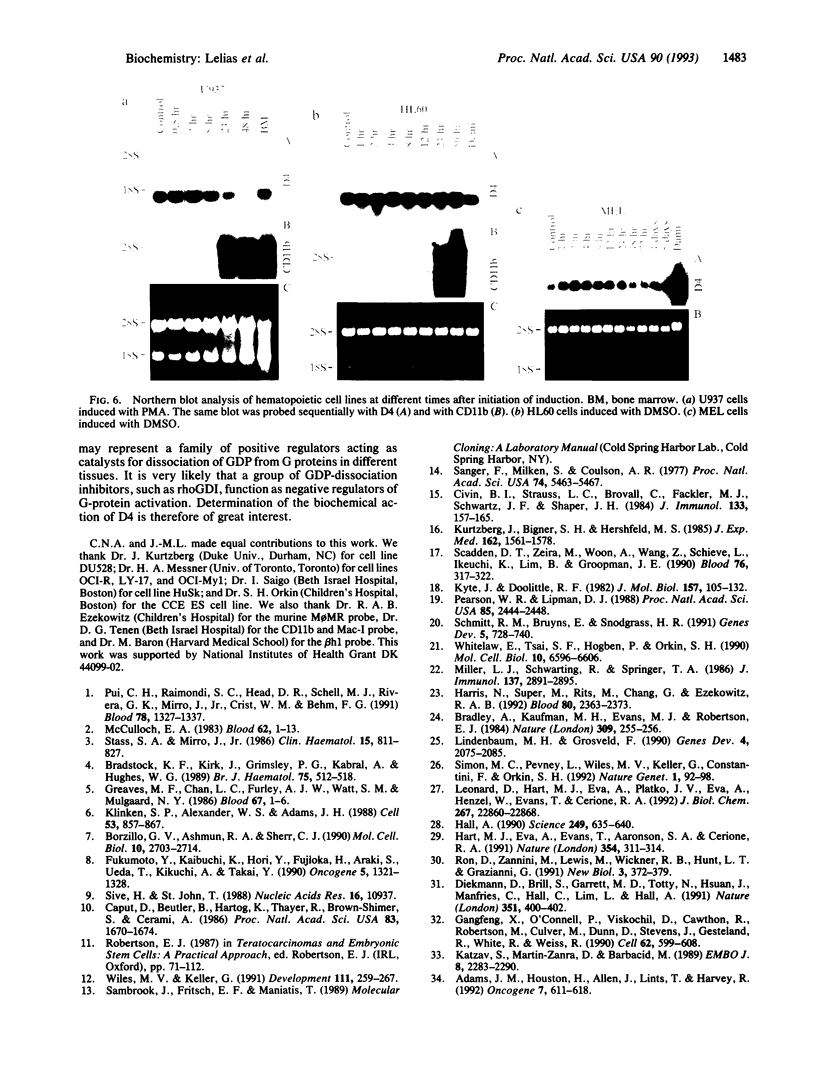
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Houston H., Allen J., Lints T., Harvey R. The hematopoietically expressed vav proto-oncogene shares homology with the dbl GDP-GTP exchange factor, the bcr gene and a yeast gene (CDC24) involved in cytoskeletal organization. Oncogene. 1992 Apr;7(4):611–618. [PubMed] [Google Scholar]
- Borzillo G. V., Ashmun R. A., Sherr C. J. Macrophage lineage switching of murine early pre-B lymphoid cells expressing transduced fms genes. Mol Cell Biol. 1990 Jun;10(6):2703–2714. doi: 10.1128/mcb.10.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley A., Evans M., Kaufman M. H., Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984 May 17;309(5965):255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Bradstock K. F., Kirk J., Grimsley P. G., Kabral A., Hughes W. G. Unusual immunophenotypes in acute leukaemias: incidence and clinical correlations. Br J Haematol. 1989 Aug;72(4):512–518. doi: 10.1111/j.1365-2141.1989.tb04315.x. [DOI] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civin C. I., Strauss L. C., Brovall C., Fackler M. J., Schwartz J. F., Shaper J. H. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984 Jul;133(1):157–165. [PubMed] [Google Scholar]
- Diekmann D., Brill S., Garrett M. D., Totty N., Hsuan J., Monfries C., Hall C., Lim L., Hall A. Bcr encodes a GTPase-activating protein for p21rac. Nature. 1991 May 30;351(6325):400–402. doi: 10.1038/351400a0. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y., Kaibuchi K., Hori Y., Fujioka H., Araki S., Ueda T., Kikuchi A., Takai Y. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene. 1990 Sep;5(9):1321–1328. [PubMed] [Google Scholar]
- Greaves M. F., Chan L. C., Furley A. J., Watt S. M., Molgaard H. V. Lineage promiscuity in hemopoietic differentiation and leukemia. Blood. 1986 Jan;67(1):1–11. [PubMed] [Google Scholar]
- Hall A. The cellular functions of small GTP-binding proteins. Science. 1990 Aug 10;249(4969):635–640. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- Harris N., Super M., Rits M., Chang G., Ezekowitz R. A. Characterization of the murine macrophage mannose receptor: demonstration that the downregulation of receptor expression mediated by interferon-gamma occurs at the level of transcription. Blood. 1992 Nov 1;80(9):2363–2373. [PubMed] [Google Scholar]
- Hart M. J., Eva A., Evans T., Aaronson S. A., Cerione R. A. Catalysis of guanine nucleotide exchange on the CDC42Hs protein by the dbl oncogene product. Nature. 1991 Nov 28;354(6351):311–314. doi: 10.1038/354311a0. [DOI] [PubMed] [Google Scholar]
- Katzav S., Martin-Zanca D., Barbacid M. vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J. 1989 Aug;8(8):2283–2290. doi: 10.1002/j.1460-2075.1989.tb08354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinken S. P., Alexander W. S., Adams J. M. Hemopoietic lineage switch: v-raf oncogene converts Emu-myc transgenic B cells into macrophages. Cell. 1988 Jun 17;53(6):857–867. doi: 10.1016/s0092-8674(88)90309-1. [DOI] [PubMed] [Google Scholar]
- Kurtzberg J., Bigner S. H., Hershfield M. S. Establishment of the DU.528 human lymphohemopoietic stem cell line. J Exp Med. 1985 Nov 1;162(5):1561–1578. doi: 10.1084/jem.162.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Leonard D., Hart M. J., Platko J. V., Eva A., Henzel W., Evans T., Cerione R. A. The identification and characterization of a GDP-dissociation inhibitor (GDI) for the CDC42Hs protein. J Biol Chem. 1992 Nov 15;267(32):22860–22868. [PubMed] [Google Scholar]
- Lindenbaum M. H., Grosveld F. An in vitro globin gene switching model based on differentiated embryonic stem cells. Genes Dev. 1990 Dec;4(12A):2075–2085. doi: 10.1101/gad.4.12a.2075. [DOI] [PubMed] [Google Scholar]
- McCulloch E. A. Stem cells in normal and leukemic hemopoiesis (Henry Stratton Lecture, 1982). Blood. 1983 Jul;62(1):1–13. [PubMed] [Google Scholar]
- Miller L. J., Schwarting R., Springer T. A. Regulated expression of the Mac-1, LFA-1, p150,95 glycoprotein family during leukocyte differentiation. J Immunol. 1986 Nov 1;137(9):2891–2900. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pui C. H., Raimondi S. C., Head D. R., Schell M. J., Rivera G. K., Mirro J., Jr, Crist W. M., Behm F. G. Characterization of childhood acute leukemia with multiple myeloid and lymphoid markers at diagnosis and at relapse. Blood. 1991 Sep 1;78(5):1327–1337. [PubMed] [Google Scholar]
- Ron D., Zannini M., Lewis M., Wickner R. B., Hunt L. T., Graziani G., Tronick S. R., Aaronson S. A., Eva A. A region of proto-dbl essential for its transforming activity shows sequence similarity to a yeast cell cycle gene, CDC24, and the human breakpoint cluster gene, bcr. New Biol. 1991 Apr;3(4):372–379. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden D. T., Zeira M., Woon A., Wang Z., Schieve L., Ikeuchi K., Lim B., Groopman J. E. Human immunodeficiency virus infection of human bone marrow stromal fibroblasts. Blood. 1990 Jul 15;76(2):317–322. [PubMed] [Google Scholar]
- Schmitt R. M., Bruyns E., Snodgrass H. R. Hematopoietic development of embryonic stem cells in vitro: cytokine and receptor gene expression. Genes Dev. 1991 May;5(5):728–740. doi: 10.1101/gad.5.5.728. [DOI] [PubMed] [Google Scholar]
- Simon M. C., Pevny L., Wiles M. V., Keller G., Costantini F., Orkin S. H. Rescue of erythroid development in gene targeted GATA-1- mouse embryonic stem cells. Nat Genet. 1992 May;1(2):92–98. doi: 10.1038/ng0592-92. [DOI] [PubMed] [Google Scholar]
- Sive H. L., St John T. A simple subtractive hybridization technique employing photoactivatable biotin and phenol extraction. Nucleic Acids Res. 1988 Nov 25;16(22):10937–10937. doi: 10.1093/nar/16.22.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stass S. A., Mirro J., Jr Lineage heterogeneity in acute leukaemia: acute mixed-lineage leukaemia and lineage switch. Clin Haematol. 1986 Aug;15(3):811–827. [PubMed] [Google Scholar]
- Whitelaw E., Tsai S. F., Hogben P., Orkin S. H. Regulated expression of globin chains and the erythroid transcription factor GATA-1 during erythropoiesis in the developing mouse. Mol Cell Biol. 1990 Dec;10(12):6596–6606. doi: 10.1128/mcb.10.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles M. V., Keller G. Multiple hematopoietic lineages develop from embryonic stem (ES) cells in culture. Development. 1991 Feb;111(2):259–267. doi: 10.1242/dev.111.2.259. [DOI] [PubMed] [Google Scholar]
- Xu G. F., O'Connell P., Viskochil D., Cawthon R., Robertson M., Culver M., Dunn D., Stevens J., Gesteland R., White R. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990 Aug 10;62(3):599–608. doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]