Abstract
Background:
Periodontal disease is one of the most common and complex disease affecting mankind. Being multifactorial in etiology it encompasses a variety of infectious entities with various unique microbial constellations and immune responses. A bacteriologic cause alone seems insufficient in explaining several clinical features of the periodontal disease. Recent studies suggest that periodontal herpes viruses comprise an important source of triggering periodontal tissue destruction. The following study aims to assess human cytomegalovirus (HCMV), Epstein-Barr virus (EBV-I) interaction with the established periodontopathic bacteriae, Porphyromonas gingivalis (Pg) and Aggregatibacter actinomycetemcomitans (Aa) in pathogenesis of aggressive periodontitis (AgP) using Hotstart polymerase chain reaction (PCR).
Materials and Methods:
A total of 30 subjects, 15 with AgP and 15 healthy controls contributed random subgingival plaque samples. PCR methodology was used to identify the subgingival herpesviruses, Pg, and Aa. Yates corrected Chi-square test was employed to identify a statistical association between herpesviruses and periodontopathic bacteriae.
Results:
Findings suggested that viruses may be pertinent to disease progression. The prevalence of the periodontopathic bacteria Aa was found in 53.33% (P = 0.0168, S) and Pg in 40% (P = 0.2155, NS) of the AgP patients. Herpesviruses, HCMV and EBV-I were found to have a prevalence of 46.67% (P = 0.039, S) and 40% (P = 0.084, NS). The viral and bacterial co-infection was found to be 77.78% (P = 0.0002, S) with Aa and HCMV.
Conclusion:
The present data reveals, viruses may exert periodontopathic effect by causing local immunosupression which may set a stage for the subgingival colonization and multiplication of periodontal bacteriae. Further studies are needed to develop an understanding into the significance of herpesviruses in human periodontitis which, may allow for improved diagnosis, more specific therapy and ultimately disease prevention.
Keywords: Aggressive periodontitis, Aggregatibacter actinomycetemcomitans, Epstein-Barr virus, human cytomegalovirus, polymerase chain reaction, Porphyromonas gingivalis
Introduction
Periodontal disease affects four out of every five adults. This makes periodontal disease one of the most common and complex disease affecting mankind and is the leading cause of tooth loss in adults. Relationship between dental disease and soft deposits on teeth has been suspected since ancient times. Researchers of the late 19th century acknowledged the role of these deposits in the initiation and progression of diseases of teeth and their supporting structures.
Periodontal diseases being multifactorial in etiology encompass a variety of infectious entities with various unique microbial constellations and immune responses. Furthermore, the course of periodontitis can vary considerably between young and adults. The course of the disease in adolescents and young adults is typically aggressive and relatively brief. These observations may suggest that aggressive periodontitis (AgP) in young patients require less infectious agent stimulus to trigger a progressive disease response than the more chronic type of disease.
AgP comprises a group of rare, severe rapidly progressive forms of periodontitis often characterized by an early age of clinical manifestation and the rapid destructive tendency for cases to aggregate in families. The events that initiate AgP have been difficult to delineate because of the complexity of the pathogenic microbiota and pathophysiologic effects of several pro- and anti-inflammatory mediators. A number of bacterial species have been incriminated in the etiopathogenesis of periodontitis, but a bacteriologic cause alone seems insufficient in explaining several clinical features of the disease. Recent studies suggest that periodontal herpes viruses comprise an important source of triggering periodontal tissue destruction.1
The presence of human cytomegalovirus (HCMV) and Epstein-Barr virus (EBV) has been linked to a high occurrence of subgingival Porphyromonas gingivalis (Pg), Aggregatibacter actinomycetemcomitans (Aa) and other bacterial pathogens. Conceivably, herpesviruses rely on co-infection with to produce periodontitis and, conversely bacteria may develop on viruses for the initiation and progression of some types of periodontal diseases. Presumably, herpesviruses residing in inflammatory cells enter gingival tissues with the development of gingivitis. Subsequently, herpesviruses reactivation then diminishes the resistance of periodontal tissues causing local immunosupression, leading to overgrowth of periodontopathic bacteria.2
Polymerase chain reaction (PCR) developed by Mullis in 1985 is one such diagnostic method, which is emerging as an alternative on the basis of its ability to directly and rapidly assess the microorganisms with extreme levels of sensitivity, which would be particularly helpful since some individuals may harbor periodontal pathogens at levels below that detectable by culture or other diagnostic methods.3 Hence, PCR assays have the potential form being an ideal detection method of periodontal microorganisms. It is relatively easy to perform and demonstrates excellent detection limits and little cross-reactivity under optimal conditions.
Hence, the aim of this study was to detect the presence of subgingival herpesviruses (HCMV and EBV-I) along with putative bacterial pathogens (Aa and Pg) in AgP patients using Hotstart PCR so as to evaluate their synergistic role in the pathogenesis of periodontal breakdown.
Materials and Methods
This study was carried out in the Department of Periodontics, PMNM Dental College and Hospital, Bagalkot, Karnataka, India. A total of 30 systemically healthy subjects were selected after obtaining an informed consent. The study was approved by the ethical committee of the institution. They were categorized into two groups; study group consisted of 15 subjects diagnosed as having AgP (generalized AgP [GAgP]/localized AgP [LAgP]) while the control group comprised of 15 subjects.
A diagnosis of AgP was assigned to each of the patient according to the criteria established by 1999 International Workshop for Classification of periodontal disease and conditions.4 In order to receive a diagnosis of AgP, patients were required to: Be systemically healthy, display a severity of disease disproportionate to the amount of local etiological factors, show evidence of rapid attachment loss (Figure 1b,d) and bone loss based on comparisons of available sets of clinical and radiographic records (Figure 1c). Patients were sub-classified according to the distribution of bone loss and number of teeth involved into LAgP or GAgP subgroup. The following criteria were taken into consideration for control groups: No signs of periodontal disease, should not be on any kind of antimicrobial drug for 6 months.
Figure 1.
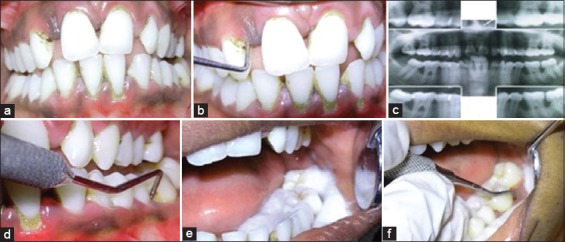
(a) Intraoral view if patient with aggressive periodontitis (b) Periodontal destruction in relation to 13 region (c) Radiographic view (d) Periodontal destruction in relation to 36 region (e, f) isolation and procurement of subgingival samples using sterile curettes.
The exclusion criteria included: Smokers, patients with known systemic diseases like diabetes mellitus or hypertension and other chronic diseases, which may have some influence on the periodontal disease initiation and/or progression. Patients who have undergone oral prophylaxis or any other periodontal therapy or use of antibiotics for any reasons within 6 months prior to the study.
Clinical assessment
Full-mouth periodontal examinations were conducted using a William’s graduated periodontal probe. Probing depth (PD) and clinical attachment level (CAL) were measured at four sites (mesial, facial, distal and lingual/palatal) per tooth for all teeth present, and the measurements were recorded approximately to the nearest whole millimeter. CAL was measured as the distance from the bottom of the pocket to the cementoenamel junction. Total score of all teeth divided by the number of teeth examined gave the average PD and the mean CAL. The indices recorded were Green and Vermillion Oral Hygiene Index-Simplified5 and Millers’s Tooth Mobility Index.6
Microbial sampling
Based on the clinical findings, tooth with the deepest pocket in each quadrant was selected for sampling. After isolating the area with cotton rolls the tooth was then cleaned with a sterile cotton pellet (Figure 1e). For random subgingival plaque collection, a sterile curette tip was introduced through the pocket orifice as far apically as possible and then removed with slight pressure against the tooth in a single vertical stroke (Figure 1f). Pooled subgingival plaque samples from the deepest pocket in each quadrant were taken. The subgingival plaque samples collected were stored at −20°C following the addition of 500 µl of TE buffer (10 mM Tris hydrochloride, 1 mM ethylenediaminetetraacetic acid, pH 7.5). The subgingival samples were processed within 48 h as the organisms are susceptible to atmospheric oxygen. The laboratory investigations were carried out using Hotstart PCR (Figure 2 a,b).
Figure 2.
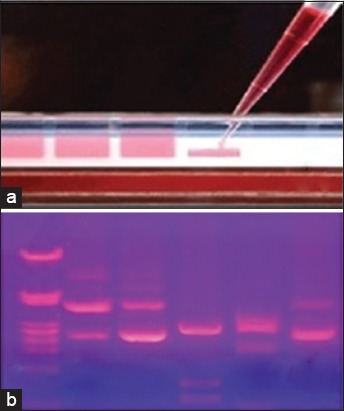
(A) Loading of templates in agarose gel for analysis, (b) templates observed and analyzed under ultraviolet light.
Statistical analysis
The data obtained were statistically analyzed and the data were described as numbers and percentage. Yate’s (corrected) Chi-square test was applied to find out a significant correlation between the two viruses HCMV and EBV, and the two bacteria Pg and Aa. All statistical analysis was performed by means of SPSS version 16 software.
Results
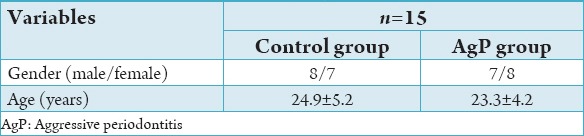
A total of 30 subjects participated in the study. Table 1 shows the demographic data of both the groups. In the study group, of 15 subjects, 8 were males and 7 females, whereas in the control group, 7 males and 8 females. The experimental group depicted a mean age of 24.66 ± 5.53 while the control group showed a mean age of 23.6 ± 4.01.
Table 1.
Population and clinical variables (mean±SD) of control and GAP group.
Prevalence of Aa, Pg, HCMV and EBV-1 according to AgP and control groups is shown in Table 2. The PCR revealed the prevalence of Aa in a total of 8 subjects which accounted for 53.33% of the experimental group, while only 1 subject showed the presence of Aa which accounted for 6.67% of the control group (Graph 1a). The prevalence of Pg was found to be present in total of 6 subjects of the experimental group accounting for 40%, whereas the control group showed a presence of Pg in 2 subjects 13.33% (Graph 1b). The prevalence of HCMV was found to be 46.67% (n = 7) in the experimental group and the same was found to be present in 1 (6.67%) subject (Graph 1c). EBV-I was found to be prevalent in 40% (n = 6) of the subjects of the study samples, whereas it was found to be present in 1 (6.67%) subject of the control group (Graph 1d).
Table 2.
Prevalence of EBV-I according to groups.
Graph 1.
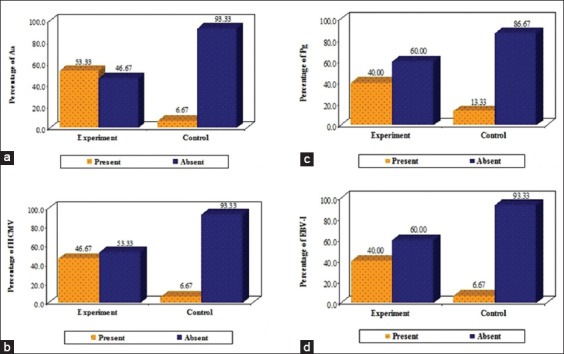
Prevalence of Aggregatibacter actinomycetemcomitans (a), Porphyromonas gingivalis (b), human cytomegalovirus (c) and Epstein-Barr virus-I (d) according to groups.
Results of correlation of Aa with HCMV and EBV-1 is shown in Table 3. When subjects were evaluated using the PCR a synergistic correlation was found between Aa and HCMV accounting to a total of 77.78% (n = 7), which bear a statistical significant value of P = 0.0002 which implies that both the microorganisms acts in a synergistic manner resulting in the periodontal pathogenesis (Graph 2a). When correlating the subjects for the presence of Aa and EBV-I a value of 22.22% (n = 2) was obtained, which did not bear any statistical significance (P = 0.706) (Graph 2b).
Table 3.
Correlation of Aa and Pg with HCMV and EBV-1.
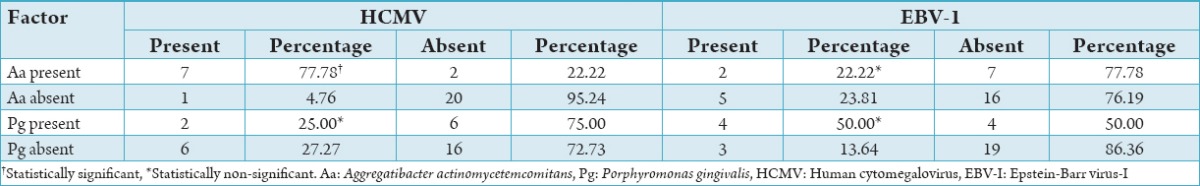
Graph 2.
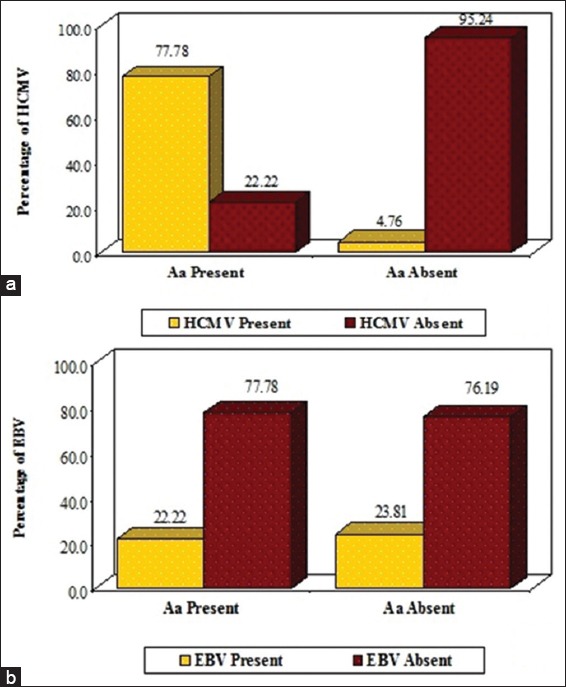
Correlation of Aggregatibacter actinomycetemcomitans with human cytomegalovirus (a) and Epstein-Barr virus-I (b).
Correlation of Pg with HCMV and EBV-1 is shown in Table 3. Presence of Pg in synergistic correlation with HCMV revealed a value of 25% (n = 2), which was not statistically significant (P = 0.732) (Graph 3a). The presence of Pg in correlation with EBV-I revealed a value of 50% (n = 4), which did not state any significant value (P = 0.110) (Graph 3b).
Graph 3.
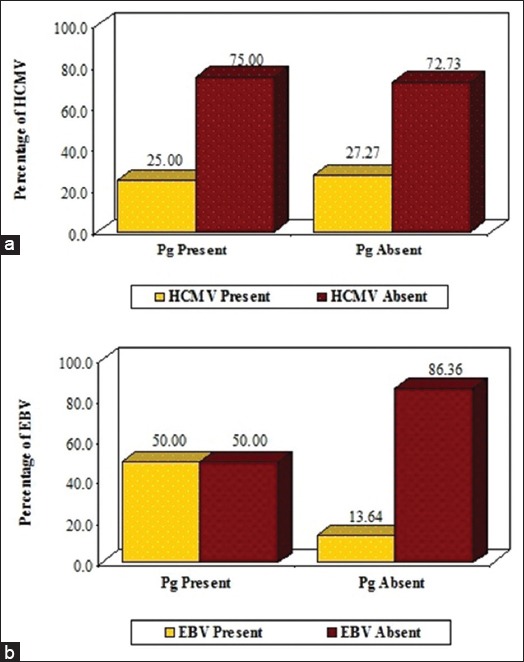
Correlation of Porphyromonas gingivalis with human cytomegalovirus (a) and Epstein-Barr virus-I (b).
Discussion
Periodontitis a disease of multifactorial has overwhelming evidence shows that organisms present in microbial plaque and in the regions of gingival sulcus and pocket or substance derived from them, constitute the primary and possibly the only extrinsic etiologic agent participating in the cause of inflammatory gingival and periodontal disease.
Since early 90s, herpesviruses have emerged as a putative pathogen in various types of periodontitis. In particular, HCMV and EBV-I seem to play important roles in the etiopathogenesis of severe types of periodontitis. Genomes of the two herpesviruses occur at high frequency in progressive periodontitis in all forms.
Detection and quantification of periodontopathic bacterial species is not found useful all the time for identifying subjects at elevated risk of periodontitis and do not consistently predict clinical outcome.7 Further the difficulties encountered on a routine basis regarding the identification of the pathogens by routine laboratory investigations acts as a setback to the clinician in the implementation of appropriate preventive and treatment protocols. On the contrary, PCR has an upper hand as it serves to be highly specific and sensitive diagnostic methodology used to identify the periodontal pathogens that are much below the levels detectable by the standard diagnostic tests.3
This study was an attempt toward identifying the possible presence of the two herpesviruses, HCMV and EBV in a synergistic relationship with the two major periodontopathic bacteriae Aa and Pg as having a contributory role in the etiopathogenesis of AgPs.
In this study, the prevalence of Aa, a small, non-motile, Gram-negative, saccharolytic, capnophilic, round-ended rod was found to be 53.33% with a P = 0.0168 depicting statistical significance. The data were in accordance to the study conducted by Umeda et al.8 where the prevalence was found in a range from 35.7% to 58.3%. Saygun et al.9 found a prevalence of 44.4% in their study on herpesviral – bacterial interrelationships in AgP patients.
Pg another gram-negative anaerobic, non-motile, asaccharolytic rod was found to be prevalent in 40% with a P = 0.2155. According to the work carried out by Kamma et al.10 where the bacteria was detected in 71.9% of the subjects. In another study, the prevalence of Pg was found to be 24% of the samples by PCR.11 Few other studies also revealed a wide range of prevalence from 16.7% to 66% with the variability observed in the sample size, whereas few other reported a still higher prevalence of about 84.2% in the AgPs cases.12-14 All the above-mentioned data points toward the association of Pg with actively progressing lesions associated with increased tendency for alveolar bone loss and attachment level loss. However, we did not notice any significant values to depict the prevalence of Pg.
HCMV and EBV-I, the two of which seem to play important roles in the etiopathogenesis of severe types of periodontitis were found in 46.67% and 40% with a P = 0.0390 (S) and 0.08424 (NS). Studies related to the herpes group of viruses revealed presence of 51.7% (HCMV) and 4.2% (EBV-I). Researchers demonstrated the presence of HCMV in 64.7% and EBV-I in 70.6% of the AgP patients.15 It has been hypothesized that the herpes group of viruses have a synergistic role in the etiopathogenesis of progressive periodontal breakdown along with periodontopathic bacteriae.3 The data obtained from our study pointed out the definitive role of HCMV in contrast to values obtained for EBV-I which did not depict any statistical significance.
Periodontitis has multifactorial infectious agents and interconnected cellular and humoral host immune responses.16-18 However, it has been difficult to unravel the precise role of various pathogens and host responses in the periodontitis. It is not understood why in hosts with comparable levels of risk factors, some periodontal infections result in loss of periodontal attachment and alveolar bone while other infections are limited to inflammation of gingiva with little or no discernible clinical consequences. Detection and quantification of periodontopathic bacterial species are useful for identifying subjects at elevated risk of periodontitis, but do not consistently predict clinical outcome.7 These uncertainties have galvanized efforts to find additional etiologic factors for periodontitis. Recent studies have implicated HCMV and EBV-1 of herpes group in the pathogenesis of human periodontal disease. Periodontal presence of the two herpesviruses is associated with increased occurrence of subgingival Pg and Aa.10 The present study revealed a 77.78% of correlation between Aa and HCMV (P = 0.0002, S). Further a correlation of 22.22% between Aa and EBV (P = 0.7063, NS), 25% between Pg and HCMV (P = 0.732, NS), and 50% between Pg and EBV-I (P = 0.110, NS). The above data was in accordance with study be Kamma et al.10 which revealed a prevalence of EBV-I with Pg to be 20%. It seems clear that periodontal tissue breakdown occurs more frequently and progresses more rapidly in herpesvirus-infected than in herpesvirus-free periodontal sites. Herpesvirus may cause periodontal pathosis as a direct result of virus infection and replication or as a consequence of virally induced impairment of the periodontal immune defense, resulting in heightened virulence of the resident bacterial pathogens. It is assumed that the ability of herpesviruses to express cytopathogenic effects, immune evasion, immunopathogenecity, latency, reactivation from latency and tissue tropism is of relevance for the development of periodontitis.7
Herpesvirus may cause a direct cytopathic effect on fibroblasts, keratinocytes, endothelial cells, inflammatory cells, and possibly bone cells.7 Genco RJ & Slots J.19 found that phagocytic and bactericidal capacities of periodontal neutrophils, cells of key importance in the periodontal defense, were significantly impaired in subjects who carried herpesviruses in oral lymphocytes and epithelial cells as compared to virus-free persons. In addition, herpesvirus infection of the fibroblasts and other key periodontal cells may hamper tissue turnover and repair following regenerative periodontal therapy. Furthermore, herpesvirus infection and damage of the periodontal pocket epithelium may contribute to gingival bleeding, as suggested by a high prevalence of HCMV and EBV DNA in periodontal sites exhibiting bleeding on probing.
Periodontal herpesvirus infection may lead to overgrowth of periodontopathic bacteria. AgPs lesions with active HCMV infection tend to yield elevated Aa count.7
Infectious disease model for the development of periodontitis showed that initially with bacterial infection causing inflammatory cells to enter gingival tissue. In response to antiviral host defenses, herpesviruses have devised a number of elaborate immunosubmassive mechanisms to ensure persistent infections. Herpesviruses can trigger dysregulation of macrophages and lymphocytes for the purpose of down-regulating the antiviral host immune response.7
HCMV can interfere with the immune functions of antigen-presenting monocyte-derived dendritic cells by impairing their maturation, antigen presentation, and allostimulatory capacity.20 HCMV and other herpes viruses have also the ability to inhibit the expression of major histocompatibility complex (MHC) Class I and II on the surface of macrophages to evade cytotoxic T-cells recognition and attempt induction of antiviral immunity and to encode proteins that interfere with the presentation of viral peptide antigens to cytotoxic T-cells.21 The presence of genes that encode proteins that interfere with HCMV antigen presentation helps herpesvirus infected cells to escape CD8+ and CD4+ T-cell immunosurvelliance. Cells that lack MHC Class I and II pathways within macrophages, which markedly impair their principal role in antigen presentation, together with silencing of NK cells, help ensure the permanence of herpesvirus infection. Some herpesviruses gene protect cell from undergoing apoptosis to prolong the lives of infected cells.22,23
Herpesviruses also affect cytokine-chemokine networks. Cytokines and chemokines play important roles in the first line of defense against human herpesvirus infection and also contribute significantly to the regulation of acquired immune responses. By a diverse array of strategies, herpesviruses are able to interfere with the cytokine production or divert potent antiviral cytokine responses.7
All the above mechanisms grounds reactivation of herpesviruses from latency. Herpesviruses with their virulent factors induce localized immune impairment, which may cause an up growth of resident gram-negative anaerobic bacteria. Hence, it is very much evident that herpesvirus relies on co-infection with periodontal bacteria to produce periodontitis and, conversely periodontopathic bacteria may depend on viral presence for the initiation and progression of some types of periodontitis.
Conclusion
Based on the present microbiological findings it can be concluded that herpesvirus may cause direct damage to periodontal tissues or impair the resistance of the periodontium, thereby permitting subgingival overgrowth of pathogenic bacteria. Prolonged periods of latency interspersed with periods of reactivation of herpesvirus infections may in part be responsible for the burst-like episodes of periodontal diseases progression which may account for the rapid periodontal breakdown in some patients showing little dental plaque.
Further studies are needed to investigate the present novel hypothesis of interrelationship between bacteria and viruses in the etiopathogenesis of various forms of periodontal diseases so as to develop prevention and treatment modalities of periodontitis which may focus on controlling the causative viruses.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Mardirossian A, Contreras A, Navazesh M, Nowzari H, Slots J. Herpesviruses 6, 7 and 8 in HIV- and non-HIV-associated periodontitis. J Periodontal Res. 2000;35(5):278–84. doi: 10.1034/j.1600-0765.2000.035005278.x. [DOI] [PubMed] [Google Scholar]
- 2.Contreras A, Zadeh HH, Nowzari H, Slots J. Herpesvirus infection of inflammatory cells in human periodontitis. Oral Microbiol Immunol. 1999;14(4):206–12. doi: 10.1034/j.1399-302x.1999.140402.x. [DOI] [PubMed] [Google Scholar]
- 3.Sanz M, Lau L, Herrera D, Morillo JM, Silva A. Methods of detection of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Tannerella forsythensis in periodontal microbiology, with special emphasis on advanced molecular techniques: A review. J Clin Periodontol. 2004;31(12):1034–47. doi: 10.1111/j.1600-051X.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 4.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4(1):1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 6.Anderegg CR, Metzler DG. Tooth mobility revisited. J Periodontol. 2001;72(7):963–7. doi: 10.1902/jop.2001.72.7.963. [DOI] [PubMed] [Google Scholar]
- 7.Slots J. Herpesviruses in periodontal diseases. Periodontol 2000. 2005;38:33–62. doi: 10.1111/j.1600-0757.2005.00109.x. [DOI] [PubMed] [Google Scholar]
- 8.Umeda M, Contreras A, Chen C, Bakker I, Slots J. The utility of whole saliva to detect the oral presence of periodontopathic bacteria. J Periodontol. 1998;69(7):828–33. doi: 10.1902/jop.1998.69.7.828. [DOI] [PubMed] [Google Scholar]
- 9.Saygun I, Kubar A, Ozdemir A, Yapar M, Slots J. Herpesviral-bacterial interrelationships in aggressive periodontitis. J Periodontal Res. 2004;39(4):207–12. doi: 10.1111/j.1600-0765.2004.00728.x. [DOI] [PubMed] [Google Scholar]
- 10.Kamma JJ, Contreras A, Slots J. Herpes viruses and periodontopathic bacteria in early-onset periodontitis. J Clin Periodontol. 2001;28(9):879–85. doi: 10.1034/j.1600-051x.2001.028009879.x. [DOI] [PubMed] [Google Scholar]
- 11.Riggio MP, Macfarlane TW, Mackenzie D, Lennon A, Smith AJ, Kinane D. Comparison of polymerase chain reaction and culture methods for detection of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in subgingival plaque samples. J Periodontal Res. 1996;31(7):496–501. doi: 10.1111/j.1600-0765.1996.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi Y, Umeda M, Sakamoto M, Benno Y, Huang Y, Ishikawa I. Treponema socranskii, Treponema denticola, and Porphyromonas gingivalis are associated with severity of periodontal tissue destruction. J Periodontol. 2001;72(10):1354–63. doi: 10.1902/jop.2001.72.10.1354. [DOI] [PubMed] [Google Scholar]
- 13.Mullally BH, Dace B, Shelburne CE, Wolff LF, Coulter WA. Prevalence of periodontal pathogens in localized and generalized forms of early-onset periodontitis. J Periodontal Res. 2000;35(4):232–41. doi: 10.1034/j.1600-0765.2000.035004232.x. [DOI] [PubMed] [Google Scholar]
- 14.Avila-Campos MJ, Velasquez-Melendez G. Prevalence of putative periodontopathogens from periodontal patients and healthy subjects in Sao Paulo, SP, Brazil. Rev Inst Med Trop Sao Paulo. 2002;44(1):1–5. doi: 10.1590/s0036-46652002000100001. [DOI] [PubMed] [Google Scholar]
- 15.Yapar M, Saygun I, Ozdemir A, Kubar A, Sahin S. Prevalence of human herpesviruses in patients with aggressive periodontitis. J Periodontol. 2003;74(11):1634–40. doi: 10.1902/jop.2003.74.11.1634. [DOI] [PubMed] [Google Scholar]
- 16.Novak KF, Novak MJ. Aggressive periodontitis. In: Newman MG, Takei HH, Klokkevold PR, Carranza FA, editors. Carranza’s Clinical Periodontology. 10th ed. New Delhi: Elsevier; 2007. pp. 506–12. [Google Scholar]
- 17.Daniel MA, Van Dyke TE. Alterations in phagocyte function and periodontal infection. J Periodontol. 1996;67(10 Suppl):1070–5. doi: 10.1902/jop.1996.67.10s.1070. [DOI] [PubMed] [Google Scholar]
- 18.Gemmell E, Seymour GJ. Modulation of immune responses to periodontal bacteria. Curr Opin Periodontol. 1994:28–38. [PubMed] [Google Scholar]
- 19.Genco RJ, Slots J. Host responses in periodontal diseases. J Dent Res. 1984;63(3):441–51. doi: 10.1177/00220345840630031601. [DOI] [PubMed] [Google Scholar]
- 20.Ongrádi J, Sallay K, Kulcsár G. The decreased antibacterial activity of oral polymorphonuclear leukocytes coincides with the occurrence of virus-carrying oral lymphocytes and epithelial cells. Folia Microbiol (Praha) 1987;32(5):438–47. doi: 10.1007/BF02887577. [DOI] [PubMed] [Google Scholar]
- 21.Bennekov T, Spector D, Langhoff E. Induction of immunity against human cytomegalovirus. Mt Sinai J Med. 2004;71(2):86–93. [PubMed] [Google Scholar]
- 22.Tortorella D, Gewurz BE, Furman MH, Schust DJ, Ploegh HL. Viral subversion of the immune system. Annu Rev Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- 23.Braud VM, Tomasec P, Wilkinson GW. Viral evasion of natural killer cells during human cytomegalovirus infection. Curr Top Microbiol Immunol. 2002;269:117–29. doi: 10.1007/978-3-642-59421-2_8. [DOI] [PubMed] [Google Scholar]


