Abstract
Background:
Both osteoporosis (OP) and periodontitis are chronic inflammatory diseases associated with bone loss mediated by local and systemic factors. The two diseases share common risk factors. Previous studies have suggested that OP in itself is a predisposing factor for periodontal tissue destruction in postmenopausal women. However, only a moderate correlation has been shown between the two conditions. In this study, we compared the severity of periodontal disease in postmenopausal osteoporotic women and postmenopausal women without OP.
Materials and Methods:
The study group consisted of 100 postmenopausal women in the age group of 50-65 years: Group 1 (50 osteoporotic) and Group 2 (50 non-osteoporotic women). Periodontal parameters included sulcus bleeding index, oral hygiene index simplified, probing pocket depth (PPD), and clinical attachment loss (CAL), interproximal alveolar bone loss (ABL), and number of missing teeth. The correlation of periodontal disease status with systemic bone mineral density (BMD) was evaluated by dual-energy X-ray absorptiometry.
Results:
The results indicated that osteoporotic (Group 1) women had a significantly greater PPD, CAL, and ABL when compared with the non-osteoporotic Group 2 (P < 0.0001). There was no significant correlation between BMD and various parameters between the groups.
Conclusions:
Within the limitations of the present study it was noted that postmenopausal OP is associated with an increased incidence and severity of periodontal disease. Educating postmenopausal osteoporotic women regarding the importance of good oral care should be part of their management regime. Hence, it could be inferred a possibility of a probable relationship between OP and periodontal disease, but long-term prospective studies are warranted in the future in order to provide definitive evidence.
Keywords: Bone density, periodontal disease, postmenopausal osteoporosis
Introduction
Osteoporosis (OP) affects more than 20 million people and results in nearly 2 million fractures per year; most of which are in women. OP is a condition that is represented by a decrease in the bone mass and loss of the micro-architecture of the bone scaffolding that result in increased bone fragility and susceptibility to fractures. It is a physiologic, gender, and age-related condition resulting from bone mineral content loss and structural changes in bone.1
OP affects both cortical and trabecular bone; and the rate of bone loss is approximately 2 times greater in women than men. In women, postmenopausal OP (PMO) is a heterogeneous disorder, which begins after natural or surgical menopause and leads to fractures within 5-10 years from the cessation of ovarian function.
A number of risk factors have been identified for OP. These include modifiable risk factors such as smoking, alcohol, low intake of calcium or vitamin D, physical inactivity (lack of exercise), medications (such as glucocorticoids), and certain diseases (such as hyperparathyroidism) and non-modifiable risk factors such as age and gender.2 Even though there is a difference in the pathogenesis of OP a, these diseases have many common risk factors.3 One of the risk factors is an increased prevalence of disease with age, smoking, and the use of medications that interfere with healing. Considering the similarities, several studies have examined the relationship between OP and periodontitis.4
The risk for periodontal disease may increase due to OP. A decreased alveolar bone density, which turns to be more susceptible to resorption by the effect of co-existing or subsequent periodontal infection and inflammation due to OP is an established hypothesis that is borne out from experimental animal models. As per the hypothesis, though OP does not initiate periodontal disease, OP may affect the course of the disease by reducing trabecular bone mass and density even though it does not cause the initiation of periodontal disease. The relation between radiographic measurements of alveolar bone height and skeletal bone mineral density (BMD) in dentate individuals have been evaluated by human studies that reported correlations between bone density and alveolar bone height bone density at the femoral neck or spine and forearm to be moderate. In spite of evidence-based studies suggesting that the risk of periodontal disease is associated with the status of the extracranial skeleton; several cross-sectional studies conducted thus far have been inconclusive.4
Potential role of OP in periodontitis
Based on the literature knowledge concerning the risk factors that affect both periodontal disease and OP, a hypothesis has been proposed regarding the interrelationship.1 Periodontitis results from bacteria that elicit a host inflammatory response, which while being protective, may induce loss of alveolar bone as well as loss of collagenous support of the tooth. OP - A generalized rather than localized disease of bone loss, results in the loss of BMD in the maxilla and mandible and throughout the body as well. Hence, as a result, local reduction of BMD in the jaw may set the stage for the more significant loss of alveolar bone. The presence of bone resorbing factors could be expected to be an attributing factor to cause a greater loss of alveolar crest height than in a non-osteoporotic individual.5 Other risk factors, such as diet, hormone levels smoking, diabetes, that affect the systemic bone loss and these factors may also contribute to periodontitis. Hence, the potential mechanisms that have been proposed are:5
In OP, low BMD in the jaw bones may be associated with low systemic bone density. This low bone density or loss of bone density may lead to increased susceptibility to resorption of alveolar bone in areas of periodontitis
Systemic factors that have a modified local tissue response to periodontal infection may also affect bone remodeling. Individuals with systemic bone loss are known to have increased systemic production of cytokines (interleukin-1 [IL-1], IL-6) that may have effects on bone throughout the body including the bone of the maxilla and mandible. The resulting low density in the jawbones leads to increased alveolar porosity altered the trabecular pattern and more rapid alveolar bone resorption following invasion by periodontal pathogens. Periodontal infections have been shown to increase local cytokine production that in turn, increases local osteoclastic activity resulting in increased bone resorption. Genetic factors that predispose a person to systemic bone loss also influence or predispose a person to periodontal destruction6
The increased gene expression of IL-6 with age may be the reason why both OP and chronic periodontal diseases are age-related
Certain lifestyle factors such as cigarette smoking and suboptimal calcium intake, among others, may put individuals at risk for development of both OP and periodontal disease.7
In the present study, we examined whether PMO is associated with an increased incidence and severity of periodontal disease.
Materials and Methods
The study population consisted of 100 postmenopausal women (age range: 50-65 years). The study protocol was explained to each subject in detail and written informed consent was obtained from those who agreed to participate. Ethical clearances were obtained from the Institution’s Ethics Committee and review boards.
A double-blind, case-control study was designed consisting of 100 postmenopausal women: 50 cases (Group 1, postmenopausal women) with OP for at least 5 years. The information regarding the history of OP was obtained from the hospital records and Group 2 consisted of 50 postmenopausal women without OP (control) based on medical history and dual energy X-ray absorptiometry technique (DEXA).
Those who agreed to participate voluntarily were evaluated for age, socioeconomic status, age at menopause, sulcus bleeding index (SBI), oral hygiene index-simplified (OHI-S), probing depth8 (PD), clinical attachment loss9 (CAL), interproximal alveolar bone loss10 (ABL), and number of missing teeth. DEXA was used to determine the correlation of systemic BMD with periodontal disease status.
Postmenopausal women with a presence of at least 10 natural teeth in each jaw and no history of systemic diseases like hyperparathyroidism that could influence the BMD formed the inclusion criteria for the study.
Individuals on hormone replacement therapy, long-term steroid medication who had experienced early onset menopause, who had a hysterectomy or oophorectomy or had experienced osteoporotic fractures, smokers or individuals with endocrine, and metabolic bone diseases that could affect the results of the study formed the exclusion criteria for this study. Subjects who had undergone any type of periodontal treatment within 1year were also excluded from the study.
All subjects had a routine BMD test (lumbar level) using a DEXA technique. The DEXA technique is considered as the “gold standard” for diagnosing OP.10,11 Based on the BMD test, aided by the medical history, subjects were divided into two groups: Group 1 (50 osteoporotic) and Group 2 (50 non-osteoporotic women).
Assessment of socioeconomic status for individual patients was expressed using “Kuppuswamy’s socio-economic status scale.”12
Statistical analysis
The data were analyzed using the following tests. To investigate the significance between the means of the two populations, Student’s t-test was used. To analyze the categorical data, Chi-square test was used and to investigate whether the difference between the sample correlation coefficient and zero was statistically significant Pearson’s correlation coefficient was used. A P < 0.05 was considered significant.
Results
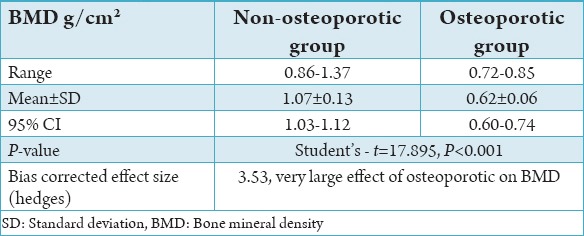
This was a double-blind case-control study consisted of 50 cases and 50 controls to evaluate the correlation between OP and periodontal disease. Relation between age and years after menopause and correlation of the osteoporotic and non-osteoporotic groups with the socio-economic status has been tabulated in Table 1. There was no statistical difference between cases and controls when compared for age, socio-economic status, and years after menopause (Table 1). Comparison of BMD between the controls and test groups is charted in Table 2.
Table 1.
Distribution of age and years after menopause in control and test cases.
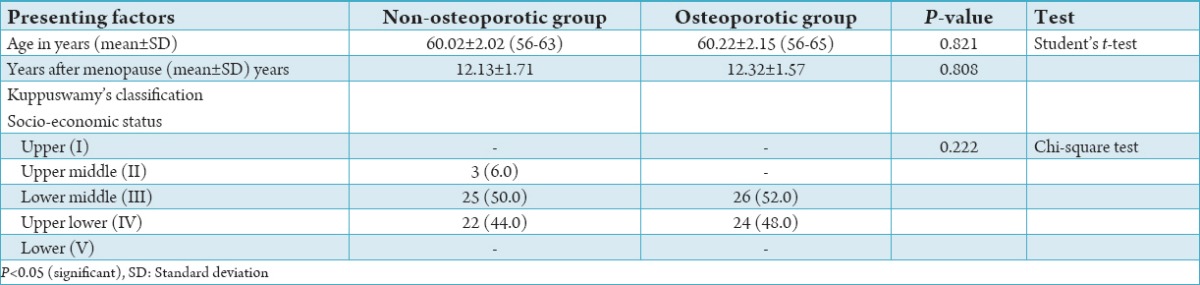
Table 2.
Comparison of BMD between the controls and test cases.
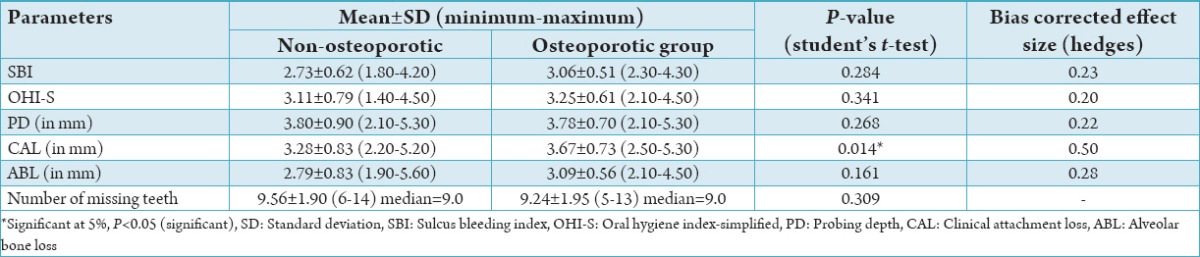
The results indicated no statistical significant difference between parameters such as SBI, OHI-S, PD, ABL, and number of missing teeth between two groups however CAL was significantly higher in the osteoporotic group when compared to the non-osteoporotic group (Table 3).
Table 3.
Comparison of various parameters between the controls and test cases.
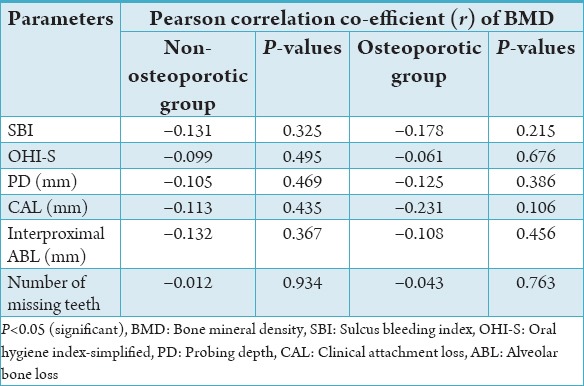
Thus, un-biased selection of subjects was ensured between the groups. Periodontal disease status determined by SBI; and the oral hygiene status was similar in both the groups as measured by OHI-S. OP was associated with periodontal attachment loss to a lesser extent. There was no statistical difference obtained in the PD, interproximal ABL, and number of missing teeth between the groups indicating that the periodontal disease status is similar in both the groups (Table 2). Furthermore, the correlation between the BMD and the various parameters did not reach statistical significance indicating that there was no relationship (Table 4).
Table 4.
Correlation between BMD and various parameters in control and test cases.
Discussion
Researchers have suggested a relationship between periodontal disease (or tooth loss) and the presence of OP in postmenopausal women.13
In the present study, we attempted to determine whether PMO is associated with an increase in the incidence and severity of periodontal disease. The study subjects were dichotomized based on the BMD test, aided by medical history.
Although various techniques are available for assessing OP, the DEXA technique was used in the present study as it uses a very low level of radiation and is non-invasive. In all subjects, the BMD values were lower than the normal reference values suggesting that generalized osteopenia occurred in the control group.7,8,12,14-16
The probing pocket depth was recorded at three sites on the buccal and lingual surfaces of all teeth. The present study showed statistically similar mean PDs in both controls and cases. The correlation between the BMD values and the PD was not statistically significant. These results are consistent with previous studies.17-19 CAL has been used as the most dependent variable or parameter to represent periodontal disease by many authors. In the present study, a statistically significant difference was observed between the groups showing a mild effect of OP. However, the results showed a negative correlation between the BMD and the CAL, which did not attain statistical significance. A negative correlation was seen between attachment level and BMD. These results are consistent with other studies,2,20-24 who found no association.
The present study showed no statistically significant difference in the interproximal ABL between the groups. Earlier studies did not define or quantify the bone loss and employed no statistical analysis but the conclusions drawn were mainly based on the subjective observations. In those studies, a relationship between systemic bone loss and ABL was a common finding. Recent studies employed quantitative measurements of variables of periodontal parameters. The results of the present study showed a negative correlation between the BMD and ABL, which did not attain statistical significance and are consistent similarly with previous studies.10-12 Alternatively, one study by Tezal et al.5 showed a significant association between BMD and ABL. Though the Hausmann’s criteria25 were used in the present study, the quantification of interproximal ABL was done by using the grids and was limited to only the posterior teeth, which could account for the difference in the results of our study and from others.
A score of 0 showing alveolar bone within 2 mm of the cementoenamel junction (CEJ) was assigned to the mesial and distal surfaces of all remaining teeth that formed a part of Hausmann’s criteria. Since it was previously shown to represent the outer limit of normal, a distance of 2 mm was selected. The score that was assigned to each tooth surface when crestal bone was observed >2 mm from the CEJ but when ≤ one-half of the root length (from CEJ to apex) was found to show bone loss was 1. When the alveolar crest height was lost > one-half of the root length, a score of 2 was assigned.
An animal study was performed to determine the association between the pathogenesis of periodontitis and the PMO in ovariectomized rats and a silk ligature was used to induce the experimental periodontitis. The results of the study suggested that changes of bone turnover markers and cytokines in the periodontal tissues contribute to the damage of periodontal tissues of ovariectomized rats.26
Conclusion
The findings of the present study suggest that PMO is associated with an increased incidence and severity of periodontal disease. Hence, there may be a probability for a relationship between periodontal disease and OP, but further prospective and sensitive studies are required in the future to substantiate a definitive evidence of the present study.
With the currently available data, the primary importance of dentists in the early diagnosis of OP is underlined, because of the availability of advanced dental radiography to assess the health of the entire skeleton of the individual. Considering the fact that such advanced dental radiological investigations are routinely performed for diagnosis and treatment of periodontal diseases and other dental diseases, which are particularly frequent in the same population affected by OP, this has a great role in clinical interest. They also are an eye opener for new preventive strategies in the form of an early therapeutic approach resulting in a potential reduction of bone resorption and contributing to maintain bone biomechanical characteristics (e.g., bone architecture, remodeling, quality of matrix collagen, and its mineralization).
Hence, the periodontists seem to have an important role, not only in monitoring/maintaining the oral and periodontal health and its relationships with OP, but at the same time they also play an important role in drafting diagnostic and therapeutic paths and participating in educating patients with OP in collaboration with general practitioners and other specialists.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Wactawski-Wende J. Periodontal diseases and osteoporosis: association and mechanisms. Ann Periodontol. 2001;6(1):197–208. doi: 10.1902/annals.2001.6.1.197. [DOI] [PubMed] [Google Scholar]
- 2.Jeffcoat MK, Lewis CE, Reddy MS, Wang CY, Redford M. Post-menopausal bone loss and its relationship to oral bone loss. Periodontol 2000. 2000;23:94–102. doi: 10.1034/j.1600-0757.2000.2230109.x. [DOI] [PubMed] [Google Scholar]
- 3.Mascarenhas P, Gapski R, Al-Shammari K, Wang HL. Influence of sex hormones on the periodontium. J Clin Periodontol. 2003;30(8):671–81. doi: 10.1034/j.1600-051x.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- 4.Garcia RI, Henshaw MM, Krall EA. Relationship between periodontal disease and systemic health. Periodontol 2000. 2001;25:21–36. doi: 10.1034/j.1600-0757.2001.22250103.x. [DOI] [PubMed] [Google Scholar]
- 5.Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol. 2000;71(9):1492–8. doi: 10.1902/jop.2000.71.9.1492. [DOI] [PubMed] [Google Scholar]
- 6.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–70. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 7.Payne JB, Reinhardt RA, Nummikoski PV, Dunning DG, Patil KD. The association of cigarette smoking with alveolar bone loss in postmenopausal females. J Clin Periodontol. 2000;27(9):658–64. doi: 10.1034/j.1600-051x.2000.027009658.x. [DOI] [PubMed] [Google Scholar]
- 8.Wactawski-Wende J, Hausmann E, Hovey K, Trevisan M, Grossi S, Genco RJ. The association between osteoporosis and alveolar crestal height in postmenopausal women. J Periodontol. 2005;76(11 Suppl):2116–24. doi: 10.1902/jop.2005.76.11-S.2116. [DOI] [PubMed] [Google Scholar]
- 9.Dumitrescu AL, Madalina L. Relationship between systemic osteoporosis and periodontal disease. Int Poster J Dent Oral Med. 2008;8(2):319. [Google Scholar]
- 10.Brennan RM, Genco RJ, Hovey KM, Trevisan M, Wactawski-Wende J. Clinical attachment loss, systemic bone density, and subgingival calculus in postmenopausal women. J Periodontol. 2007;78(11):2104–11. doi: 10.1902/jop.2007.070155. [DOI] [PubMed] [Google Scholar]
- 11.Weyant RJ, Pearlstein ME, Churak AP, Forrest K, Famili P, Cauley JA. The association between osteopenia and periodontal attachment loss in older women. J Periodontol. 1999;70(9):982–91. doi: 10.1902/jop.1999.70.9.982. [DOI] [PubMed] [Google Scholar]
- 12.Kuppuswamy B. Delhi: Manasayan; 1981. Manual of Socioeconomic Status Scale (Urban) [Google Scholar]
- 13.Krall EA, Dawson-Hughes B, Hannan MT, Wilson PW, Kiel DP. Postmenopausal estrogen replacement and tooth retention. Am J Med. 1997;102(6):536–42. doi: 10.1016/s0002-9343(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 14.Pischon N, Pischon T, Kröger J, Gülmez E, Kleber BM, Bernimoulin JP, et al. Association among rheumatoid arthritis, oral hygiene, and periodontitis. J Periodontol. 2008;79(6):979–86. doi: 10.1902/jop.2008.070501. [DOI] [PubMed] [Google Scholar]
- 15.Gomes-Filho IS, Passos Jde S, Cruz SS, Vianna MI, Cerqueira Ede M, Oliveira DC, et al. The association between postmenopausal osteoporosis and periodontal disease. J Periodontol. 2007;78(9):1731–40. doi: 10.1902/jop.2007.070057. [DOI] [PubMed] [Google Scholar]
- 16.Jeffcoat M. The association between osteoporosis and oral bone loss. J Periodontol. 2005;76(11 Suppl):2125–32. doi: 10.1902/jop.2005.76.11-S.2125. [DOI] [PubMed] [Google Scholar]
- 17.Krall EA. The periodontal-systemic connection: implications for treatment of patients with osteoporosis and periodontal disease. Ann Periodontol. 2001;6(1):209–13. doi: 10.1902/annals.2001.6.1.209. [DOI] [PubMed] [Google Scholar]
- 18.Reddy MS. Osteoporosis and periodontitis: discussion, conclusions, and recommendations. Ann Periodontol. 2001;6(1):214–7. doi: 10.1902/annals.2001.6.1.214. [DOI] [PubMed] [Google Scholar]
- 19.Shrout MK, Hildebolt CF, Potter BJ, Brunsden TK, Pilgram TK, Dotson M, et al. Comparison of morphological measurements extracted from digitized dental radiographs with lumbar and femoral bone mineral density measurements in postmenopausal women. J Periodontol. 2000;71(3):335–40. doi: 10.1902/jop.2000.71.3.335. [DOI] [PubMed] [Google Scholar]
- 20.Reinhardt RA, Payne JB, Maze CA, Patil KD, Gallagher SJ, Mattson JS. Influence of estrogen and osteopenia/osteoporosis on clinical periodontitis in postmenopausal women. J Periodontol. 1999;70(8):823–8. doi: 10.1902/jop.1999.70.8.823. [DOI] [PubMed] [Google Scholar]
- 21.Earnshaw SA, Keating N, Hosking DJ, Chilvers CE, Ravn P, McClung M, et al. Tooth counts do not predict bone mineral density in early postmenopausal Caucasian women. EPIC study group. Int J Epidemiol. 1998;27(3):479–83. doi: 10.1093/ije/27.3.479. [DOI] [PubMed] [Google Scholar]
- 22.Jeffcoat MK. Osteoporosis: a possible modifying factor in oral bone loss. Ann Periodontol. 1998;3(1):312–21. doi: 10.1902/annals.1998.3.1.312. [DOI] [PubMed] [Google Scholar]
- 23.Wactawski-Wende J, Grossi SG, Trevisan M, Genco RJ, Tezal M, Dunford RG, et al. The role of osteopenia in oral bone loss and periodontal disease. J Periodontol. 1996;67(10 Suppl):1076–84. doi: 10.1902/jop.1996.67.10s.1076. [DOI] [PubMed] [Google Scholar]
- 24.Jeffcoat MK, Chesnut CH., 3rd Systemic osteoporosis and oral bone loss: evidence shows increased risk factors. J Am Dent Assoc. 1993;124(11):49–56. doi: 10.14219/jada.archive.1993.0225. [DOI] [PubMed] [Google Scholar]
- 25.Wactawski-Wende J, Hausmann E, Hovey K, Trevisan M, Grossi S, Genco RJ. The association between osteoporosis and alveolar crestal height in postmenopausal women. J Periodontol. 2005;76:1123–8. doi: 10.1902/jop.2005.76.11-S.2116. [DOI] [PubMed] [Google Scholar]
- 26.Luo K, Ma S, Guo J, Huang Y, Yan F, Xiao Y. Association between postmenopausal osteoporosis and experimental periodontitis. Biomed Res Int. 2014;2014 doi: 10.1155/2014/316134. 316134. [DOI] [PMC free article] [PubMed] [Google Scholar]


