Abstract
Within the precedent decade, a new field of “tissue engineering” or “tissue regeneration” emerge that offers an innovative and exhilarating substitute for maxillofacial reconstruction. It offers a new option to supplement existing treatment regimens for reconstruction/regeneration of the oral and craniofacial complex, which includes the teeth, periodontium, bones, soft tissues (oral mucosa, conjunctiva, skin), salivary glands, and the temporomandibular joint (bone and cartilage), as well as blood vessels, muscles, tendons, and nerves. Tissue engineering is based on harvesting the stem cells which are having potential to form an organ. Harvested cells are then transferred into scaffolds that are manufactured in a laboratory to resemble the structure of the desired tissue to be replaced. This article reviews the principles of tissue engineering and its various applications in oral and maxillofacial surgery.
Keywords: Reconstruction, regeneration, Scaffolds, signaling molecules, stem cells, tissue engineering
Introduction
The dream is as old as humankind. Injury, disease, and congenital malformation have always been part of the human life. So if damaged body parts can be restored with natural tissue, one would have no complaints of such undesired events. Tissue engineering or tissue regeneration is a multidisciplinary field with the perspective to replace tissue loss as a result of the traumatic defect, oncosurgery, or organ damage.1 The reconstruction of large tissue defects is one of the main challenges to face the modern oral and maxillofacial surgeon. While the transfer of autologous tissue such as bone grafts or tissue free flaps are well-described, they are not without complications.2 With this in mind, the prospect of using principles of tissue engineering to reconstruct defects in soft and hard tissues of the head and neck continues to gain the attention of the reconstructive surgeon.3 As an alternative to current surgical techniques developments in tissue regeneration using the gene therapy and stem cell research endeavor to develop cells, scaffolds and cell signaling molecules to rejuvenate large oral and maxillofacial tissue defect with accurate reproduction of normal tissue. A tissue engineering approach provides numerous prospective benefits, including a declination in donor site morbidity, a decrease in procedural sensitivity of the repair, and the capacity to intimately ape the in vivo tissue environment into recapitulate normal craniofacial development.4
Principles of tissue engineering
Principles of tissue engineering are basically a triad of stem cells, signaling molecules, and scaffolds or extracellular matrix (Figure 1).
Figure 1.
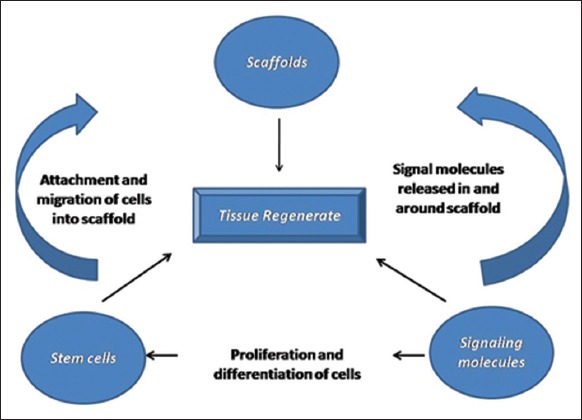
Triad of tissue engineering.
A stem cell is defined as an unspecialized cell that can renew and maintain itself for a longer period of time with the potential to commit to a cell or tissue lineage with specialized functions. The use of stem cells, either embryonic or adult-derived (ADSCs), is a reality of regenerative medicine and dentistry. ADSCs are multipotent cells not derived from embryonic or primordial germ cell lineage, and they have the potential to differentiate into bone, muscle, cartilage, nerve, and vasculature under appropriate conditions.5 These stem cells can differentiate into various cells like chondrocytes, osteoblasts, myoblasts, hematopoietic cells, neural cells as well (Figure 2).
Figure 2.
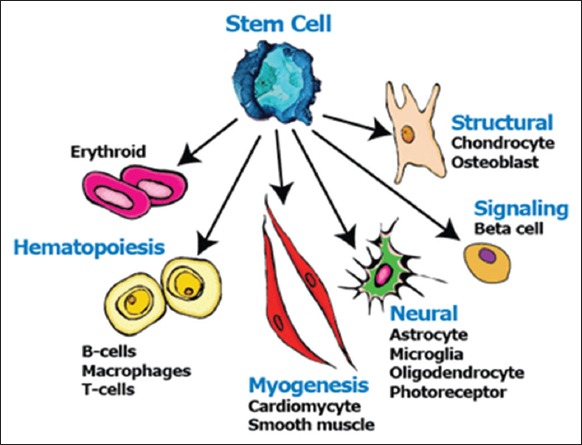
Differentiation of stem cells.
Signaling molecules: Various growth factors and cytokines are mixed to the ECM, like bone morphogenetic proteins (BMP), fibroblast growth factor-2 (FGF-2), interleukin-6, insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), transforming growth factor-β1, etc. This co-localization works as a storage pool of growth factors and may diminish growth factor degradation, protecting them from the local microenvironment while facilitating the presentation of the growth factors to cell surface receptors.6-8
Scaffold: A scaffold (Figure 3) is a permanently or temporarily placed three-dimensional porous and permeable natural or synthetic biomaterial that is biocompatible. It can be natural or synthetic. It acts as a matrix and allows the attachment, migration, and differentiation of progenitor cells. Properties of scaffolds (such as biodegradability, porosity, stiffness, and strength) influence cell adhesion, migration, and proliferation (such as osteoconduction). The greatest challenges faced in tissue-engineered devices, regardless of tissue type, is promoting healing in three dimensions. Scaffolds have been made-up with a variety of innate and synthetic biomaterials, such as ceramics, metals, proteins, and polymers. An appropriate scaffold for tissue engineering will be one that is created with biology in mind. The function of the scaffolds is providing structural support to cells, reservoir for growth factors and provide flexible, physical environment for remodeling.9
Figure 3.
3-D synthetic polycaprolactone scaffold.9
There are three main proposals taken in the branch of tissue engineering: Conduction, induction, and cell transplantation shown in Figure 4. The approach taken to regenerate a tissue relied upon numerous factors, together with the extent of the deficiency, the contribution of cells from adjoining areas, cell resettlement rate, and the available contiguous vasculature. When a pretty small amount of tissue is required, cell conduction and cell induction procedures are frequently used to uphold cell movement from host tissue keen on a scaffold. The alternate of little larger defects habitually requires the straight transplantation of cells. In conductive approach, like guided tissue regeneration, the innately derived or synthetic template simply acts as a submissive 3-D mechanical scaffold to which cells can connect, propagate, transfer, and discriminate. The guided tissue regeneration approach is currently extensively used for the treatment of periodontal diseases. It is over and over again enviable, however, to manage the cell colonization into the scaffold, discrimination of these cells, and consequent tissue fabrication. A potential tissue engineering method is an inductive approach in which bioactive scaffold signaling molecules are used to tempt cell movement and organized cellular behavior. A general inductive technique is the deliverance of soluble signaling molecules s like growth factors to the adjacent tissues. Gene therapy likewise is used to convey specific genetic information to host cells; once introduced, the host cells can then create definite growth factors to sway tissue development. When a huge tissue defect is there or the limited supply of appropriate cells in the tissue environment, cell transplantation is more suitable. This procedure typically includes a biopsy procedure from a donor source, separating and escalating the donor cells in vitro, and implanting the cells directly onto polymers characteristically made up of the physical forms of a fiber-based mesh, a sponge, or a hydrogel. The cells affix to the scaffold, propagate, and eventually form a tissue regenerate. This regenerated tissue is then set in into the individual in the tissue deficient area (Figure 5).10
Figure 4.
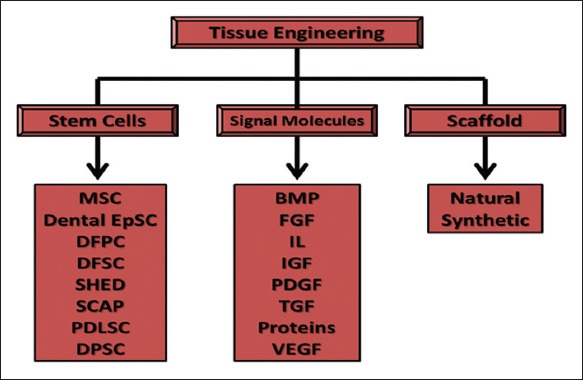
The components of tissue engineering, MSC: Mesenchymal stem cells, EpSC: Epithelial stem cells, DFPC: Dental follicle precursor cell, DFSC: Dental follicle stem cells, SHED: Stem cells from human exfoliated deciduous teeth, SCAP: Stem cells from apical papilla, PDLSC: Periodontal ligament stem cells, DPSC: Dental pulp stem cells, BMP: Bone morphogenic proteins, FGF: Fibroblast growth factor, IL: Interleukins, IGF: Insulin-like growth factor, PDGF: Plasma-derived growth factor, TGF: Transforming growth factor, VEGF: Vascular endothelial growth factor.9
Figure 5.
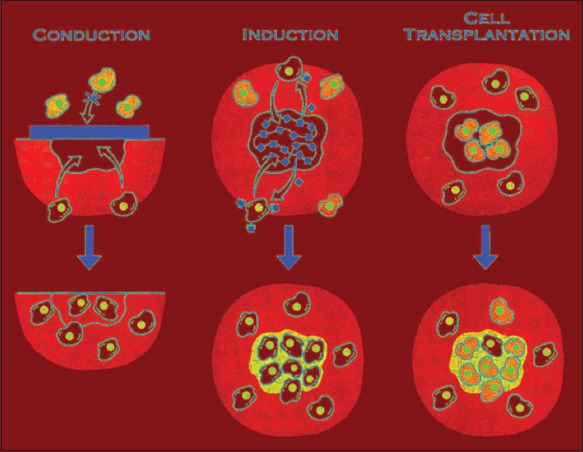
Three approaches to the engineering of a tissue. (a) In conductive approaches, a scaffold can act as a resorbable barrier conniving which cells can penetrate and instigate repair. (b) Inductive approaches can entail the discharge of bioactive molecules that attach to specific host cells with receptors for the molecules. The specific cells migrate into the defect and begin to form a new extracellular matrix. (c) In the cell transplantation approach, cells from a donor source are placed directly into a polymer scaffold in vitro, and the cell-scaffold construct is consequently implanted into the tissue defect site. The transplanted cells, along with host cells form a tissue regenerate that is structurally and functionally incorporated with the host tissue.
Steps involved in tissue regeneration (Figure 6):
Figure 6.
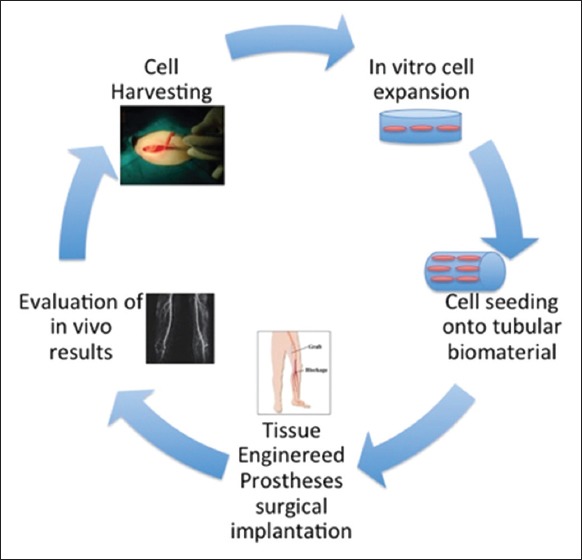
Steps of tissue regeneration and implantation.1
Cell harvesting from body
Isolation, cultivation, and proliferation of cells into scaffold in presence of growth factors or signaling molecules (in vitro)
Implantation of the tissue regenerate.
Application of tissue engineering in oral and maxillofacial surgery:
Bone regeneration
Cartilage regeneration
Soft tissue regeneration
Salivary gland regeneration
Fat, muscle, and nerve regeneration
Bone
The restoration of the large sized bony defect after resection or due to trauma is a big challenge for the maxillofacial surgeon. Rib graft and reconstruction plates are choices among surgeons. Tissue engineered bone is a good available choice too. Mesenchymal stem cells are best cells as they can convert into osteoblasts and osteoclasts.12-14
BMP are the widely used growth factors for bone regeneration by osteoinduction and osteogenesis. BMP 7, BMP 2, PRP, and platelet-derived growth factors are currently researched growth signaling molecules.13 Scaffolds for the bone regeneration are polymers, bovine collagen, hydroxyapatite, tricalcium phosphate.14 This regenerated bone can be used in mandibular small are large defects, in maxillary cleft repair, maxillary and mandibular ridge augmentation, and maxillary sinus augmentation.
Cartilage
In craniofacial region, cartilage present in the articular disc of temporomandibular joint, nasal septum, and ear. The cartilage gives physical and functional support to these parts. Cells with chondrogenic potential like bone marrow-derived mesenchymal cells, and periosteum-derived mesenchymal cells used in concurrence with a diversity of scaffolds such as carbon fiber pads, decalcified bone fibrin glue, collagen gels, and alginate hydrogels in the presence of FGF2, FGF-β, bFGF The need for the cartilage regeneration is for restoration of these body parts as in congenital or traumatic defect.15
Soft tissue
Skin: Skin is composed of superficial epidermal and deeper dermal layer. It acts as a protective barrier and prevents water loss and also helps in temperature regulation. Skin loss most commonly is due to burns followed by trauma and surgical removal in cancer surgery.16 Full thickness skin grafts are the choice for the repair but donor site morbidity, infections, scar formation are the common complications.17 The ideal skin replacement for the facial region shall grant the same aesthetic results as an autograft and it should have all idyllic properties of normal skin. Skin substitutes are available with one that replaces dermis and one which replaces the epidermis. It can be acellular and cellular. Alloderm@ (dead de-epidermized dermis) and Integra@ (collagen sponge) are commercially available skin substitutes which are already in use.11 To generate complex skin grafts poised of both an epidermal and a dermal layer, fibroblasts and keratinocytes have been planted into various scaffolds.18 But none of these substitutes fulfills all fully functional properties of skin. The research to achieve versatile and universal engineered skin tissue is oriented to add vascular endothelial cells (angiogenesis), melanocytes (Melanin pigments) sweat glands, and hair follicles.
Oral mucosa: Oral mucosa is differing from the skin in very few but important aspects. Oral mucosa is thin and more vascular with the absence of hair follicles and sweat glands. Additional properties require keeping the mucosa moist all the time. Oral keratinocytes instead of skin keratinocytes are added. The tissue-engineered oral mucosa can be used for intraoral defects such as cleft lip and palate, traumatic tissue loss, and pathological mucosal loss after surgery. EVPOME@, Alloderm@ are commercially available tissue engineered oral mucosa.19
Salivary gland
Salivary gland hypofunction and dysfunction are common after radiotherapy in cancer. Artificial salivary gland regeneration is quite a challenge due to the complex structure of salivary gland. The secretory function of the salivary gland is vital to treat xerostomia. Joraku et al. cultivated human salivary gland epithelial cells in a polyglutamic acid polymer scaffold. They developed this using an in vitro collagen and Matrigel culture system to reconstitute human salivary gland tissue and showed functional, differentiated salivary units of acini and ducts in a 3-D construct, with the production of secretory granules and expression of aquaporin 5 protein.20 Secretion of saliva in the artificial salivary gland is in research.
Fat, muscle, and nerve cell
Muscles for facial expressions maintain the facial contour and tone. Fat or adipose tissue is also present to support the facial muscles. All the muscles have innervations from the nerves. To reconstruct the facial defects including eye and mouth, all the three mentioned components are required. Muscle has the low regenerative capacity, and traumatic damage may result in fibrous connective tissue formation.21 Adipose tissue, myotubes, and nerves have been regenerated in laboratories with relevant signaling molecules and scaffolds, but motor end plate is must for a muscle to contract and retract. Tissue engineers have failed to regenerate such functional muscle and research is ongoing for the same.22
Conclusion
Maxillofacial rehabilitation after ablative surgery or trauma is a challenging goal for the maxillofacial surgeon. Complex maxillofacial structure creates trouble for the restoration of the defect. As the technology advances, the ability to construct more detailed bioactive tissue will be more sophisticated. Tissue engineering is a highly active field to develop products and devices with all the needful components and following all principles of regenerative medicine. The oral and maxillofacial surgeon should be aware of these advances and should be able to use it for the restoration of the maxillofacial defects. Until now, simple tissue regeneration has been successfully achieved to restore tissue defect but complex tissue structure and its functional restoration are still in the research withstanding challenge. Maxillofacial surgeons and tissue engineers should work as a team by expressing functional need and principles of tissue engineering, respectively. Thus, future developments in the field of tissue engineering will have a significant impact on managing anatomic and physiological changes due to disease process by the most accepted tissue.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Srisuwan T, Tilkorn DJ, Wilson JL, Morrison WA, Messer HM, Thompson EW, et al. Molecular aspects of tissue engineering in the dental field. Periodontol. 2000;41:88–108. doi: 10.1111/j.1600-0757.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang KH, Inman JC, Hayden RE. Modern concepts in mandibular reconstruction in oral and oropharyngeal cancer. Curr Opin Otolaryngol Head Neck Surg. 2011;19(2):119–24. doi: 10.1097/MOO.0b013e328344a569. [DOI] [PubMed] [Google Scholar]
- 3.Susarla SM, Swanson E, Gordon CR. Craniomaxillofacial reconstruction using allotransplantation and tissue engineering: Challenges, opportunities, and potential synergy. Ann Plast Surg. 2011;67(6):655–61. doi: 10.1097/SAP.0b013e31822c00e6. [DOI] [PubMed] [Google Scholar]
- 4.Ward BB, Brown SE, Krebsbach PH. Bioengineering strategies for regeneration of craniofacial bone: A review of emerging technologies. Oral Dis. 2010;16(8):709–16. doi: 10.1111/j.1601-0825.2010.01682.x. [DOI] [PubMed] [Google Scholar]
- 5.Conrad C, Huss R. Adult stem cell lines in regenerative medicine and reconstructive surgery. J Surg Res. 2005;124(2):201–8. doi: 10.1016/j.jss.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Schnaper HW, Kleinman HK. Regulation of cell function by extracellular matrix. Pediatr Nephrol. 1993;7(1):96–104. doi: 10.1007/BF00861587. [DOI] [PubMed] [Google Scholar]
- 7.Flaumenhaft R, Moscatelli D, Rifkin DB. Heparin and heparan sulfate increase the radius of diffusion and action of basic fibroblast growth factor. J Cell Biol. 1990;111(4):1651–9. doi: 10.1083/jcb.111.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts R, Gallagher J, Spooncer E, Allen TD, Bloomfield F, Dexter TM. Heparan sulphate bound growth factors: A mechanism for stromal cell mediated haemopoiesis. Nature. 1988;332(6162):376–8. doi: 10.1038/332376a0. [DOI] [PubMed] [Google Scholar]
- 9.Patil AS, Merchant Y, Nagarajan P. Tissue engineering of craniofacial tissues – A review. J Regen Med Tissue Eng. 2013;2:6. [Google Scholar]
- 10.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 11.Alsberg E, Hill EE, Mooney DJ. Craniofacial tissue engineering. Crit Rev Oral Biol Med. 2001;12(1):64–75. doi: 10.1177/10454411010120010501. [DOI] [PubMed] [Google Scholar]
- 12.DiSilvio L. Bone tissue engineering and biomineralization. In: Boc-Caccini AR, Gough JE, editors. Tissue Engineering Using Ceramics and Polymers. Boca Raton: CRC Press; 2007. pp. 319–34. [Google Scholar]
- 13.Seo S, Na K. Mesenchymal stem cell-based tissue engineering for chondrogenesis. J Biomed Biotechnol. 2011;2011 doi: 10.1155/2011/806891. 806891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scaglione S, Quarto R, Giannoni P. Stem cells and tissue scaffolds for bone repair. In: DiSilvio L, editor. Cellular Response to Biomaterials. Boca Raton: CRC Press; 2008. [Google Scholar]
- 15.Ma PX, Schloo B, Mooney D, Langer R. Development of biomechanical properties and morphogenesis of in vitro tissue engineered cartilage. J Biomed Mater Res. 1995;29(12):1587–95. doi: 10.1002/jbm.820291215. [DOI] [PubMed] [Google Scholar]
- 16.Dougherty WR, Chalabian JR. Skin substitutes. West J Med. 1995;162(6):540–1. [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh MM, Boyce S, Layton C, Freedlander E, Mac Neil S. A comparison of methodologies for the preparation of human epidermal-dermal composites. Ann Plast Surg. 1997;39(4):390–404. doi: 10.1097/00000637-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 18.MacFarlane DF. Current techniques in skin grafting. Adv Dermatol. 2006;22:125–38. doi: 10.1016/j.yadr.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Feinberg SE, Aghaloo TL, Cunningham LL., Jr Role of tissue engineering in oral and maxillofacial reconstruction: Findings of the 2005 AAOMS research summit. J Oral Maxillofac Surg. 2005;63(10):1418–25. doi: 10.1016/j.joms.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Joraku A, Sullivan CA, Yoo J, Atala A. In-vitro reconstitution of three-dimensional human salivary gland tissue structures. Differentiation. 2007;75(4):318–24. doi: 10.1111/j.1432-0436.2006.00138.x. [DOI] [PubMed] [Google Scholar]
- 21.Tardy ME, Kastenbauer ER. 2nd rev. ed. I. New York, NY: Thieme Medical Publishers, Inc; 1995. Head and Neck Surgery. [Google Scholar]
- 22.Bach AD, Beier JP, Stern-Staeter J, Horch RE. Skeletal muscle tissue engineering. J Cell Mol Med. 2004;8(4):413–22. doi: 10.1111/j.1582-4934.2004.tb00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


