Abstract
Background:
The objective of this study was to compare the efficiency of four commonly used chemicals in their ability to remove smear layer after instrumentation using scanning electron microscope (SEM).
Materials and Methods:
Seventy-five extracted single canaled teeth of roots ranging 10-12 mm in length were used for the study. Teeth were divided into 4 study groups and 1 control group of 15 teeth each. Standard access to the pulp chambers were performed with diamond burs. The lengths of the teeth were determined by the introduction of a size 15 K-file into the root canal until the tip reached the apical foramen. The working length for preparation of the canal is set 0.5 mm shorter than the measurement. Irrigation was performed using 2 ml of irrigant for every instrument change and finally rinsed using 5 ml of the respective solutions. The roots were then split with a chisel and hammer. One-half of each tooth was selected and prepared for SEM examination. After assembly on coded stubs, the specimens were placed in a vacuum chamber and sputter-coated with a 300 Å gold layer. The specimens were then analyzed using a Philips SEM XL 30. The dentinal wall of the cervical, middle and apical thirds was observed at magnifications of up to ×1000 for the presence/absence of smear layer and visualization of the entrance to dentinal tubules. Photomicrographs (×1000) of these areas on each of the coronal, middle and apical thirds were made Data were analyzed using Kruskal–Wallis test and Mann–Whitney U test.
Results:
SEM study done on these prepared teeth with the popularly used four chemicals, namely, 3% NaOCl (Group A), 3% NaOCl followed by 17% ethylene diamine-tetra-acetic acid (Group B), 0.2% chlorhexidine (Group C) and 3% NaOCl followed by MTAD (Group D), with distilled water (Group E) which is used as control, revealed that NaOCl showed statistically significant, better cleansing effect than distilled water. Chlorhexidine and NaOCl showed equal kind of efficacy but were statistically significant, with lower efficacy than MTAD. It may be concluded that MTAD appears to be the most effective solution compared to the rest.
Conclusion:
The study demonstrated that MTAD as a final rinse after the entire instrumentation with 3% NaOCl as irrigant provided the best cleansing in all parts of the root canal system. The smear layer has been shown to hinder the penetration of intracanal disinfectants and sealers into dentinal tubules and has the potential of compromising the seal of the root filling. Degradation of the smear layer after treatment may contribute to leakage and reinfection of the root canal space. Removal of the smear layer reduced the penetration of bacteria through the root canal system after root filling.
Keywords: Dentinal tubule cleansing, intra-canal disinfectants, irrigants, smear layer
Introduction
The endodontic procedure is aimed toward complete elimination of microorganism from the root canal system and prevention of re-infection. To achieve this objective, the root canals are thoroughly cleaned before filling, using mechanical instrumentation, supplemented with irrigants, and intracanal medications.1
An amorphous, irregular layer known as smear layer has been shown to form on root canal walls following mechanical preparation. Studies have proved that smear layer is a by-product of mechanical preparation as it is not found in uninstrumented canals.2
Smear layer is shown to consist of not only dentin but also necrotic and viable tissue, including remnants of odontoblastic processes, pulp tissue microorganisms and their by-products.3 Smear layer has been shown to hinder the penetration of intracanal disinfectants and sealers into dentinal tubules and has the potential of compromising the seal of the root filling. Although it has been suggested that an intact smear layer may prevent initial bacterial penetration into dentinal tubules, degradation of the smear layer after treatment may contribute to leakage and reinfection of the root canal space. It has been shown that removal of the smear layer reduced the penetration of bacteria through the root canal system after root filling.3,4
The components of the smear layer were very small particles with a large surface-mass ratio, which makes them very soluble in acids. Mc Comb and Smithwere the first investigators to show that R ethylene diamine-tetra-acetic acid (EDTA) (a commercial brand of EDTA) can remove the smear layer. Goldman et al.5 showed that when used alone, REDTA removed the inorganic portion and left an organic layer intact in the tubules. Sodium hypochlorite (NaOCl) has been shown to be very effective for this purpose. When used alone, NaOCl can dissolve pulpal remnants and predentin. However, many studies have shown its ineffectiveness in removing the entire smear layer when used alone. Other studies showed that alternating the use of EDTA and NaOCl is an effective method for smear layer removal.5-7
Tetracycline has been shown to remove smear layer formed on root canal walls. Low pH of tetracycline in a concentrated solution stimulates superficial demineralization of dentine in a similar way as citric acid. Tetracycline solution in the root canal is shown to behave like a chelator (bonds calcium ions) and also be absorbed in canal wall and subsequently released from dental tissues (dentine, cement).8 A new irrigation solution (MTAD), introduced in 2003 by Mahmoud Torabinejad of Loma Linda University, containing a mixture of a tetracycline isomer, an acid, and a detergent is claimed to remove smear layer.9
The present study evaluates and compares the efficiency of four commonly used chemicals in their ability to remove smear layer after instrumentation using scanning electron microscope.
Materials and Methods
Seventy-five extracted single canaled teeth of roots ranging 10-12 mm in length were stored in 10% formalin solution at room temperature until the root canal preparation is performed.
The samples were divided into Groups A, B, C, D, and E containing 15 samples each.
Group A: Samples rinsed with 3% NaOCl during instrumentation and finally rinsed with the same solution (Figure 5).
Figure 1.
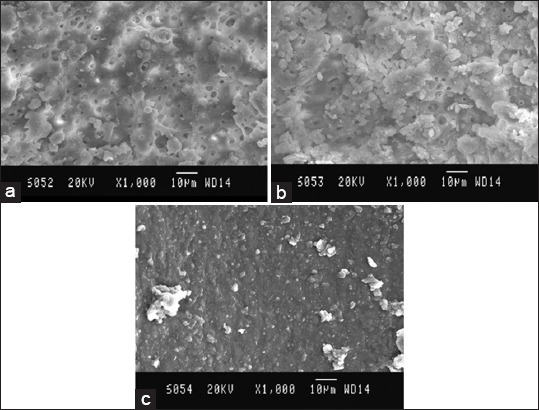
Scanning electron microscopy (SEM) picture of Group A, (a) Coronal third: SEM picture of the root canal wall of sample in Group A, (b), (c) Apical third: SEM picture of the root canal wall of sample in Group A coronal third.
Figure 2.
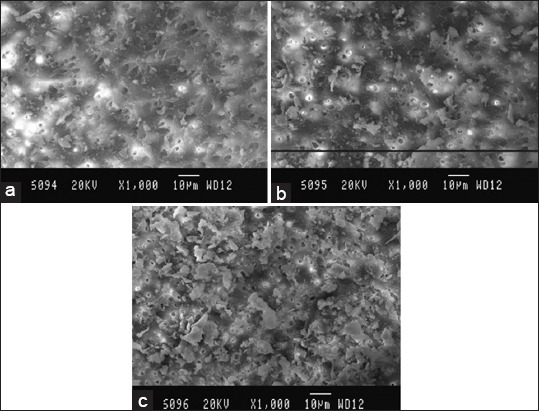
Scanning electron microscopy (SEM) picture of Group B, (a) Coronal third: SEM picture of the root canal wall of sample in Group B, (b), (c) Apical third: SEM picture of the root canal wall of sample in Group B.
Figure 3.
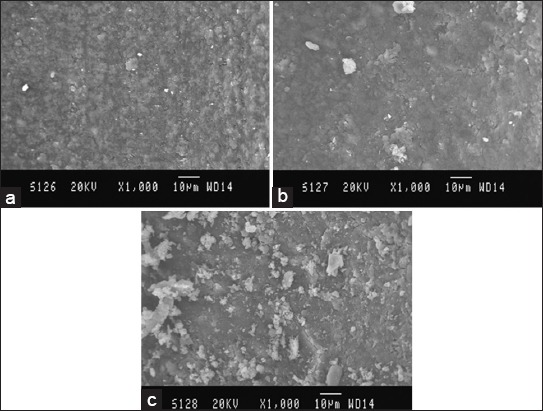
Scanning electron microscopy (SEM) picture of Group C, (a) Coronal third: SEM picture of the root canal wall of sample in Group C, (b) Middle third: SEM picture of the root canal wall of sample in Group C, (c) Apical third: SEM picture of the root canal wall of sample in Group C.
Figure 4.
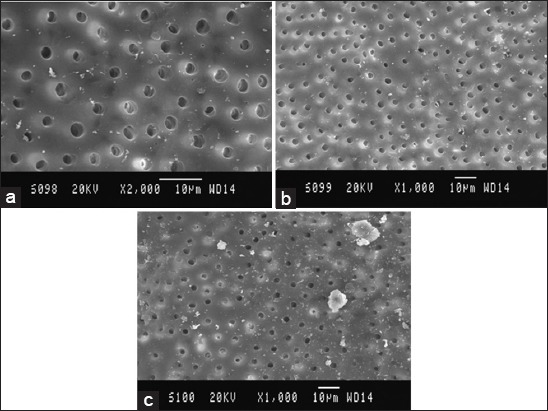
Scanning electron microscopy (SEM) picture of Group D, (a) Coronal third: SEM picture of the root canal wall of sample in Group D, (b) Middle third: SEM picture of the root canal wall of sample in Group D, (c) Apical third: SEM picture of the root canal wall of sample in Group D.
Figure 5.
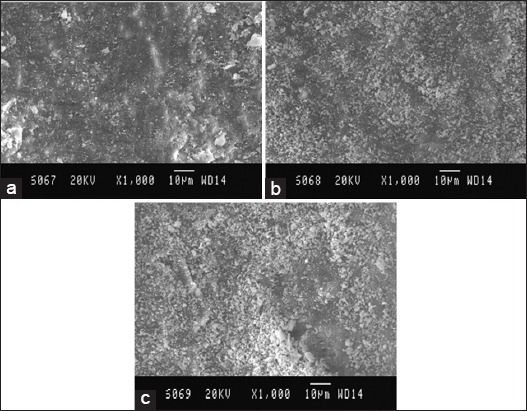
Scanning electron microscopy (SEM) picture of Group E, (a) Coronal third: SEM picture of the root canal wall of sample in Group E, (b) Middle third: SEM picture of the root canal wall of sample in Group E, (c) Apical third: SEM picture of the root canal wall of sample in Group E.
Group B: Samples rinsed with 3% NaOCl during instrumentation and finally rinsed with 5 ml solution of 17% EDTA for 1 min.
Group C: Samples rinsed with 0.2% chlorhexidine during instrumentation and finally rinsed with the same solution.
Group D: Samples rinsed with 3% NaOCl during instrumentation and finally rinsed with 5 ml MTAD for 1 min.
Group E/Control: Samples rinsed with distilled water during instrumentation and finally rinsed with the same solution.
Group A and B were finally irrigated with 10 ml of distilled water to remove any precipitates that may have formed from the irrigants used. Irrigation was performed using 2 ml of irrigant for every instrument change and finally rinsed using 5 ml of the respective solutions. The irrigants were delivered with an endodontic irrigating needle supplied along with MTAD, pro-rinse, dentsply.
The canals were dried with absorbent paper points and the entrance to each of the canals was protected with a cotton pellet. Using diamond discs, the crowns were removed at the cement-enamel junction. Deep grooves were cut on the buccal and palatal surfaces of the roots, without perforating the root canals. The roots were then split with a chisel and hammer. One-half of each tooth was selected and prepared for SEM examination. After assembly on coded stubs, the specimens were placed in a vacuum chamber and sputter-coated with a 300 Å gold layer.
The specimens were then analyzed using a Philips SEM XL 30. The dentinal wall of the cervical, middle and apical thirds was observed at magnifications of up to ×1000 for the presence/absence of smear layer and visualization of the entrance to dentinal tubules. Photomicrographs (×1000) of these areas on each of the coronal, middle and apical thirds were made.
Statistical analysis
Null hypothesis: There is no significant difference between the median scores, i.e. η1 = η2 = η3 = η4 = η5
Alternate hypothesis: There is a significant difference between the median scores, i.e. η1 ≠ η2 ≠ η3≠ η4≠ η5
Level of significance: α=0.05
Statistical technique used: Kruskal–Wallis test, Chi-square test, Mann–Whitney test
Decision criterion: The decision criterion is to reject the null hypothesis if the P < 0.05. Otherwise we accept the null hypothesis. If there is a significant difference, we carry out multiple comparisons between different pairs of groups using Mann–Whitney test.
Results
SEM study done on these prepared teeth with the popularly used four chemicals, namely, 3% NaOCl (Group A), 3% NaOCl followed by 17% EDTA (Group B), 0.2% chlorhexidine (Group C) and 3% NaOCl followed by MTAD (Group D), with distilled water (Group E) which is used as control, revealed interesting findings.(Tables 1 and 2) At the outset, it can be stated that distilled water showed the least cleaning effect, which is confirmed statistically, as is given below. (Figures 1-4)
Table 1.
Inclusion and exclusion criteria.
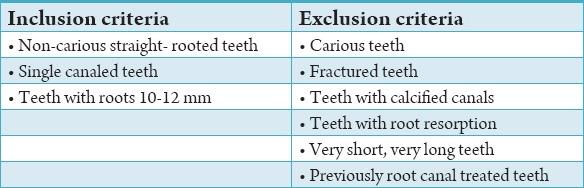
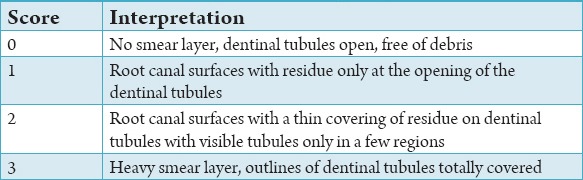
Table 2.
Scoring criteria developed by Rome et al.10
Coronal third
MTAD (Group D) showed the best effect. This was followed by NaOCl + EDTA (Group B), NaOCl (Group A) and least with chlorhexidine (Group C). NaOCl showed statistically significant, better cleansing effect than distilled water.
Middle third
Distilled water has shown the least cleansing effect compared to all the other groups. This was also statistically significant. The order of cleansing efficacy was, first NaOCl + MTAD (Group D), followed by NaOCl + EDTA (Group B), which was followed by NaOCl and chlorhexidine (Group C). Chlorhexidine (Group C) and NaOCl (Group A) seem to have equal kind of efficacy but were statistically significant, with lower efficacy than MTAD.
Apical third
The control group (Group E) showed the least cleansing effect. Interestingly NaOCl (A) appeared to be almost same as distilled water (Group E). The best cleansing was seen with MTAD (Group D). EDTA (Group B) was found to be better than chlorhexidine (Group C), but inferior to MTAD (Group D).
In General, it may be concluded that MTAD appears to be the most effective solution compared to the rest.
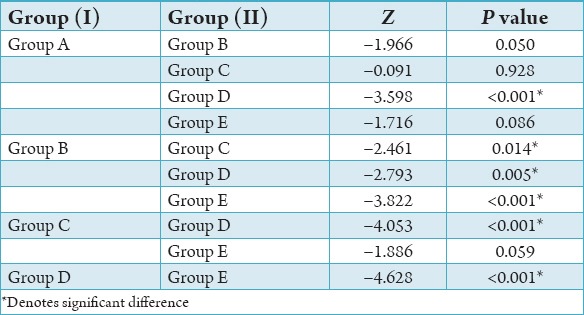
There was a statistically significant difference between the groups with respect to the median smear layer scores (P < 0.001). Group E recorded a higher mean and median smear layer score compared to the other groups. The next highest mean and median smear layer score was found in Group C followed by Group A and Group B, respectively. The lowest mean smear layer score was found in Group D. In order to find out among which pair of groups there exist a significant difference; we carry out multiple comparisons using Mann–Whitney test. The results were given below: The difference in median smear layer scores between Group A and Group D is found to be statistically significant (P < 0.001). Group A has a higher mean and median score compared to Group D. No significant difference in median smear layer scores between Group A and the remaining Groups (P > 0.05).
Statistically significant difference is observed between Group B and Group C (P < 0.05), Group B and Group D (P < 0.01), as well as Group B and Group E (P < 0.001) with respect to median smear layer score. Group B has a higher mean and median smear layer score compared to Group D and a lower mean smear layer score compared to Group C and Group E. The difference in median smear layer scores between Group C and Group D is found to be statistically significant (P < 0.001). Group C has a higher mean and median score compared to Group D. No significant difference is observed in the median smear layer score between Group C and Group E (P > 0.05). Statistically, significant difference is observed between Group D and Group E with respect to the median smear layer score (P < 0.001). The mean and median smear layer score in Group D is found to be lower than that of Group E.
Discussion
One of the important objectives of endodontic therapy is to debride the root canals, rendering them free of the pulp tissue, necrotic debris, microorganisms and their toxins. Root canal irrigants help in chemo-mechanical preparation by removing thus formed by products.1-3 Various irrigants have been tried for the removal of smear layer of which the commonly used ones were citric acid, tannic acid, maleic acid, polyacrylic acid, tetracyclines, chlorhexidine, EDTA, and sodium hypochlorite.10,11
In the present study 3% Sodium hypochlorite (NaOCl), 17% EDTA, 0.2% chlorhexidine and MTAD have been used with distilled water as control. Sodium hypochlorite is both an oxidizing and hydrolyzing agent. It has a strong proteolytic effect and therefore, serves as an excellent aid during instrumentation. Necrotic tissue and debris were dissolved through a complex biochemic process. The amount of free chlorine is important for the breakdown of the proteins into amino Groups. The original concentration suggested by Dakin was 0.5%, but the concentration used in dentistry has been as high as 5.25%.12,13
The effectiveness of NaOCl to remove the organic part of the smear layer becomes evident and significant at higher concentrations (1.3-5.25%). Chelating agents were first introduced into endodontics as an aid for the preparation of narrow and calcified canals. EDTA is often suggested as an irrigation solution, because it has the capability to chelate and remove the mineralized portion of smear layer.7,14,15 Among first researchers, McComb and Smith have reported that REDTA (brand mark of EDTA: ethylene diamine-tetra-acetate) is potent in removing smear layer from root canal walls. Numerous studies have reported that irrigation with a 17% EDTA solution has a good cleaning effect on the root canal walls.16 Therefore in the present study 17% EDTA solution has been used for 1 min for rinsing after the completion of instrumentation.
Chlorhexidine in the chemical form is a cationic bisbiguanide that is primarily marketed as a gluconate salt. A commercially available oral rinse contains 0.12% chlorhexidine gluconate is a base containing water, 11.6% alcohol, glycerine, flavoring agents, and saccharin.17 In the endodontic literature, chlorhexidine has been shown to be tested in the concentrations of 0.2% w/v and 2%. There is no strong recommendation either in favor or against either of these concentrations. Since 0.2 % w/v is the most common available type, the same is used for this study for its cleaning ability.18-20
The ability of the tetracycline family of antibiotics to remove smear layers has been studied in the past. They have been used to demineralize dentin surfaces, uncover and widen the orifices of dentinal tubules, and expose the dentin collagen matrix.9 Torabinejad et al. showed that MTAD is an effective solution for the removal of the smear layer which does not significantly change the structure of the dentinal tubules when used as a final irrigant in conjunction with 5.25% NaOCl as a root canal irrigant. MTAD is an acidic solution with a pH of 2.15 that is capable of removing inorganic substances.9 SEM examinations of the surfaces of root canals treated with MTAD showed the presence of severe erosion at all levels of the root canal. Thereby, MTAD reacts with the surface of dentin differently compared with citric acid or EDTA and should be used as the final rinse in conjunction with NaOCl. Therefore in the current study 3% NaOCl was used during instrumentation and finally rinsed with MTAD.21,22
In this study, care was taken to use needles specific for irrigation purpose. Prorinse (Dentsply) was the needle used for irrigation. Studies have shown more significant stagnation of smear layer following irrigation with standard needle and syringe, while more efficient cleansing when blunt perforated needle was used. Blunt needles have shown to detach smear layer from root canal walls under pressure, thus making debridement of canal more efficient.
The results obtained from our study seems to indicate that rinsing the canal with MTAD for 1 min following thorough preparation using 3% NaOCl can be used in routine clinical practice (Table 3). As it has been shown and proved in the earlier studies that the removal of smear layer eliminates the remnant bacteria and their by-products, enable better penetration of intracanal medicaments and offer better sealing of sealer cement on the canal wall, MTAD probably is the best choice among the commonly used chemicals to remove the smear layer (Table 4).
Table 3.
Overall score of all the groups.
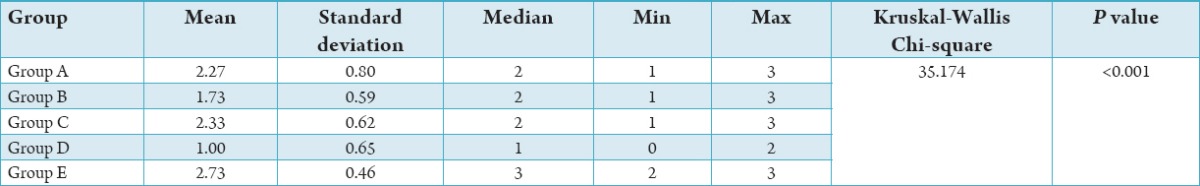
Table 4.
Multiple comparison of Mann-Whitney test.
From this study it may also be inferred that MTAD may be made a routine chemical to be used at the end of the preparation, particularly in the cases where the root canal system is found to be heavily infected (Graph 4). The fact that the tetracycline has got the ability of substantivity makes it more favorable for the same. Despite its effectiveness in its anti-bacterial property, substantivity, and cleansing ability, it may be pointed out that the potential adverse possibility of local application of a systemic drug be studied further in clinical conditions.
In all the SEM analysis, the ability of all the chemicals, excepting MTAD was least in the apical third of the root canals. This gives a clue for applying caution to conduct the study in a clinical environment. In real in-vivo situation intrusion of body fluid into the root canal system may interfere with the nature and effectiveness of the chemicals. It may be concluded that smear layer removal may be routinely practised, particularly in cases of teeth with established infection in the apical part of the root canal.
Conclusion
Thorough cleaning and hermetic sealing of the apical third of the root canal system is of vital importance. Interestingly, the SEM results of the study revealed that all the chemicals were relatively more effective in cleansing the coronal third, but comparatively less effective in the apical third. Compared to all the chemicals, MTAD as a final rinse after the entire instrumentation with 3% NaOCl as irrigant provided the best cleansing in all parts of the root canal system. From this study and the SEM analysis it may be concluded that using MTAD as a final rinse may be made a part of routine cleansing procedure in the root canal treatment, particularly in cases of teeth with established infection in the apical part of the root canal.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Peters OA, Peters CI. Pathways of the Pulp. 9th ed. St. Louis: Mosby, Inc; 2006. Cleaning and shaping the root canal system; p. 290. [Google Scholar]
- 2.Takeda FH, Harashima T, Kimura Y, Matsumoto K. A comparative study of the removal of smear layer by three endodontic irrigants and two types of laser. Int Endod J. 1999;32(1):32–9. doi: 10.1046/j.1365-2591.1999.00182.x. [DOI] [PubMed] [Google Scholar]
- 3.Pashley DH. Smear layer: Overview of structure and function. Proc Finn Dent Soc. 1992;88(Suppl 1):215–24. [PubMed] [Google Scholar]
- 4.Pashley DH, Livingston MJ. Effect of molecular size on permeability coefficients in human dentine. Arch Oral Biol. 1978;23(5):391–5. doi: 10.1016/0003-9969(78)90098-5. [DOI] [PubMed] [Google Scholar]
- 5.Goldman M, Goldman LB, Cavaleri R, Bogis J, Lin PS. The efficacy of several endodontic irrigating solutions: A scanning electron microscopic study: Part 2. J Endod. 1982;8(11):487–92. doi: 10.1016/s0099-2399(82)80073-3. [DOI] [PubMed] [Google Scholar]
- 6.Mader CL, Baumgartner JC, Peters DD. Scanning electron microscopic investigation of the smeared layer on root canal walls. J Endod. 1984;10(10):477–83. doi: 10.1016/S0099-2399(84)80204-6. [DOI] [PubMed] [Google Scholar]
- 7.Yamada RS, Armas A, Goldman M, Lin PS. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions: Part 3. J Endod. 1983;9(4):137–42. doi: 10.1016/S0099-2399(83)80032-6. [DOI] [PubMed] [Google Scholar]
- 8.Cameron JA. The use of ultrasonics in the removal of the smear layer: A scanning electron microscope study. J Endod. 1983;9(4):289–92. doi: 10.1016/S0099-2399(83)80119-8. [DOI] [PubMed] [Google Scholar]
- 9.Torabinejad M, Khademi AA, Babagoli J, Cho Y, Johnson WB, Bozhilov K, et al. A new solution for the removal of the smear layer. J Endod. 2003;29(3):170–5. doi: 10.1097/00004770-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Rome WJ, Doran JE, Walker WA., 3rd The effectiveness of Gly-Oxide and sodium hypochlorite in preventing smear layer formation. J Endod. 1985;11(7):281–8. doi: 10.1016/S0099-2399(85)80158-8. [DOI] [PubMed] [Google Scholar]
- 11.Drake DR, Wiemann AH, Rivera EM, Walton RE. Bacterial retention in canal walls in vitro: Effect of smear layer. J Endod. 1994;20(2):78–82. doi: 10.1016/S0099-2399(06)81186-6. [DOI] [PubMed] [Google Scholar]
- 12.Clark-Holke D, Drake D, Walton R, Rivera E, Guthmiller JM. Bacterial penetration through canals of endodontically treated teeth in the presence or absence of the smear layer. J Dent. 2003;31(4):275–81. doi: 10.1016/s0300-5712(03)00032-0. [DOI] [PubMed] [Google Scholar]
- 13.Brännström M. Smear layer: Pathological and treatment considerations. Oper Dent Suppl. 1984;3:35–42. [PubMed] [Google Scholar]
- 14.McComb D, Smith DC. A preliminary scanning electron microscopic study of root canals after endodontic procedures. J Endod. 1975;1(7):238–42. doi: 10.1016/S0099-2399(75)80226-3. [DOI] [PubMed] [Google Scholar]
- 15.Sajedeh Ghorbanzadeh, Sara Arab Loodaricheh, Sara Samizade, Saeede Zadsirjan. “Irrigants in endodontic treatment,”. Int J Contemp Dent Med Rev. 2015 Article ID: 030515, 2015. doi: 10.15713/ins.ijcdmr.77. [Google Scholar]
- 16.Martin H, Cunningham WT, Norris JP, Cotton WR. Ultrasonic versus hand filing of dentin: A quantitative study. Oral Surg Oral Med Oral Pathol. 1980;49(1):79–81. doi: 10.1016/0030-4220(80)90034-1. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad M, Pitt Ford TJ, Crum LA. Ultrasonic debridement of root canals: Acoustic streaming and its possible role. J Endod. 1987;13(10):490–9. doi: 10.1016/s0099-2399(87)80016-x. [DOI] [PubMed] [Google Scholar]
- 18.Goodis HE, White JM, Moskowitz E, Marshall SY. Root canal system preparation: Conventional versus laser method in vitro. J Dent Res. 1992;71:162. [Google Scholar]
- 19.Takeda FH, Harashima T, Kimura Y, Matsumoto K. Efficacy of Er: YAG laser irradiation in removing debris and smear layer on root canal walls. J Endod. 1998;24(8):548–51. doi: 10.1016/S0099-2399(98)80075-7. [DOI] [PubMed] [Google Scholar]
- 20.Dederich DN, Zakariasen KL, Tulip J. Scanning electron microscopic analysis of canal wall dentin following neodymium-yttrium-aluminum-garnet laser irradiation. J Endod. 1984;10(9):428–31. doi: 10.1016/S0099-2399(84)80264-2. [DOI] [PubMed] [Google Scholar]
- 21.Bjorvatn K. Antibiotic compounds and enamel demineralization. An in vitro study. Acta Odontol Scand. 1982;40(5):341–52. doi: 10.3109/00016358209024079. [DOI] [PubMed] [Google Scholar]
- 22.Wikesjö UM, Baker PJ, Christersson LA, Genco RJ, Lyall RM, Hic S, et al. A biochemical approach to periodontal regeneration: Tetracycline treatment conditions dentin surfaces. J Periodontal Res. 1986;21(4):322–9. doi: 10.1111/j.1600-0765.1986.tb01466.x. [DOI] [PubMed] [Google Scholar]


