Abstract
Oncolytic engineered herpes simplex viruses (HSVs) possess many biologic and functional attributes that support their use in clinical trials in children with solid tumors. Tumor cells, in an effort to escape regulatory mechanisms that would impair their growth and progression, have removed many mechanisms that would have protected them from virus infection and eventual virus-mediated destruction. Viruses engineered to exploit this weakness, like mutant HSV, can be safely employed as tumor cell killers, since normal cells retain these antiviral strategies. Many preclinical studies and early phase trials in adults demonstrated that oncolytic HSV can be safely used and are highly effective in killing tumor cells that comprise pediatric malignancies, without generating the toxic side effects of nondiscriminatory chemotherapy or radiation therapy. A variety of engineered viruses have been developed and tested in numerous preclinical models of pediatric cancers and initial trials in patients are underway. In Part II of this review series, we examine the preclinical evidence to support the further advancement of oncolytic HSV in the pediatric population. We discuss clinical advances made to date in this emerging era of oncolytic virotherapy.
Oncolytic herpes simplex viruses (oHSVs) retain their susceptibility to innate antiviral mechanisms in normal cells that quickly limit the ability of the virus to yield a productive infection. The pathogenesis of tumor cells has evolved to mutate or outright delete most of these mechanisms to select for the most replicative, invasive, and progressive cancer cell, creating a significant vulnerability to virus infection. The current wisdom holds that among the cells comprising a tumor, there resides a subpopulation resistant to radiation and chemotherapies and from which more aggressive malignancies arise. Strategies to target this tumor-initiating cell or tumor stem cell subpopulation are being developed and tested. However, as described below, preclinical evidence suggests that oHSV is an equal opportunity tumor killer that is not constrained by some of the limitations of chemotherapy or radiation, such as the requirement for cells to be dividing. As described in Part I of this review series, a number of unique challenges to the successful application of oHSV in children exist; however multiple lines of evidence in preclinical and clinical studies define most of the consequential barriers to a rational deployment of oHSV for cancer therapy.1 In this Part II, we review the preclinical evidence to support the further advancement of oHSV in the pediatric population. We discuss clinical advances made to date in this emerging era of oncolytic virotherapy.
Antitumor Efficacy by Disease
Neuroblastoma
Neuroblastoma is the most common non-central nervous system (CNS) solid tumor in children and contributes to 10–15% of all pediatric cancer mortality. Average age at diagnosis is 19 months and 90% are less than 5 years old.2 Children with high-risk disease urgently require novel therapy as they continue to have poor outcomes despite intensive chemotherapy, surgery, radiation, immunotherapy, and retinoic acid. In fact, we are now finding that among those who survive this disease, about 30% of children develop secondary cancers as a result of the intensive treatments 10–15 years later.3 Dr. Alice Moore is one of the earliest known physicians to study virotherapy for neuroblastoma in the early 1950s when she utilized a Russian Far East encephalitis virus to treat neuroblastoma with dramatic in vivo responses in mouse models, but with limited success overall as the mice died from the viral infection.4–7 Neuroblastoma cells are moderately susceptible to G207 (see Table 1 for summary of viruses and Figure 1 for structural schematics for preclinical and therapeutic HSVs discussed in the text) in vitro but demonstrated tumor growth reduction and even cures in immunocompetent murine models. Additionally, intratumoral injections of G207 into subcutaneous N18 tumors demonstrated regression of a remote intracranial neuroblastoma. The enhanced antitumor effects in vivo are hypothesized to be secondary to stimulation of the host systemic antitumor response. Elevations of specific cytotoxic T-cell activity against neuroblastoma cells have been noted to persist for over 1 year. The increased specific cytotoxic T-cell activity is also theorized by Todo et al.8 to further elicit systemic antitumor immunity as demonstrated by lack of tumor growth upon tumor rechallenge. The Cripe lab demonstrated the antitumor efficacy of NV1066 against human neuroblastoma xenograft models.9 Because increased matrix metalloproteinase expression and activity correlates with poor prognosis in several human malignancies, including neuroblastoma,10 they sought to create an oHSV, rQT3, which expressed human tissue inhibitor of metalloproteinases 3 and compared its antitumor effects with an oHSV expressing firefly luciferase (rQLuc).11 Neuroblastoma cells demonstrated equivalent viral replication with these two oHSVs, but rQT3 enhanced cytotoxicity by 65% and reduced matrix metalloproteinase activity. In xenograft neuroblastoma models subcutaneously implanted in mice, rQT3 and rQLuc inhibited tumor growth by 85 and 82% respectively, with rQT3 demonstrating a greater inhibition of tumor growth at later time points. However, rQT3 was significantly more effective against neuroblastoma in vitro than in vivo. Neuroblastoma cells, as embryonal tumors derived from the neural crest, frequently express neural precursor markers, such as nestin. The Cripe lab confirmed nestin expression in multiple neuroblastoma cells lines and demonstrated that intratumoral rQNestin34.5, a nestin-targeted oHSV, infected, replicated, and killed neuroblastoma tumor-initiating cells grown as tumorspheres in vitro.12 In mouse models, ex vivo rQNestin34.5 infection of neuroblastoma cells prevented tumor formation for more than 60 days compared to the control oHSV, which significantly delayed tumor formation from 25 to 35 days. Recently, Gillory et al.13 showed the M002 virus, which expresses murine interleukin-12 (IL-12) in physiologically relevant amounts, infected and replicated in, and effectively produced IL-12 in neuroblastoma cell lines. A single dose of intratumoral M002 resulted in tumor growth delay in neuroblastoma xenograft models in mice. When combined with repeated radiation doses of 3 Gy, a single intratumoral dose of M002 improved survival in immunocompromised and immunocompetent neuroblastoma murine models. Taken together, these data indicate that oHSV holds significant promise for targeting high-risk neuroblastoma.
Table 1. Summary of oncolytic HSVs discussed in the text.
| Virus | Deletions | Foreign gene/promoter insertion | References |
|---|---|---|---|
| C134 | Deletions in both copies of γ134.5 gene | IRS1 gene under control of an HCMV immediate early promoter | 20,74 |
| d12.CALP | ICP4 deletion | Calponin promoter | |
| G207 | Deletions in both copies of γ134.5 gene and disabling lacZ insertion within ICP6 gene | LacZ | 8,20,22,23,41,42,72 |
| G47Δ | Deletions of the γ134.5 and α47 genes and a disabling lacZ insertion within ICP6 gene | LacZ | 29,30 |
| hrR3 | In-frame insertion of the bacterial lacZ gene within ICP6 | LacZ | 22,23 |
| HSV1716 | Deletions in both copies of γ134.5 gene | None | 17,68 |
| M002 | Deletions in both copies of γ134.5 gene | Murine IL-12 under the transcriptional control of the murine early-growth response-1 promoter (Egr-1) | 13,45,60,70,72 |
| NV1020 | Deletion in thymidine kinase (tk) locus and across the joining region of the long and short components of the HSV-1 genome | HSV-1 DNA fragment encoding the tk gene fused to the α gene promoter | 41,43 |
| NV1066 | Same NV1020 | Enhanced GFP, CMV promoter | 9,43 |
| oHSV-MDK-34.5 | Deletions of ICP6 and ICP34.5 | ICP34.5 expression driven by the midkine promoter | 24 |
| rRp450 | Deletion of ICP6 | Rat CYP2B1 | 44,49,50 |
| rQNestin-34.5 | Deletions in γ134.5 gene and in-frame gene-disrupting insertion of GFP within ICP6 gene | ICP34.5 under control of a synthetic nestin promoter | 11 |
| rQT3 | Deletions in ICP6 and γ134.5 | Tissue inhibitor of metalloproteinases 3, HSV-1 immediate early 4/5 promoter | 11 |
CMV, cytomegalovirus; GFP, green fluorescent protein; HCMV, human cytomegalovirus; HSV, herpes simplex virus.
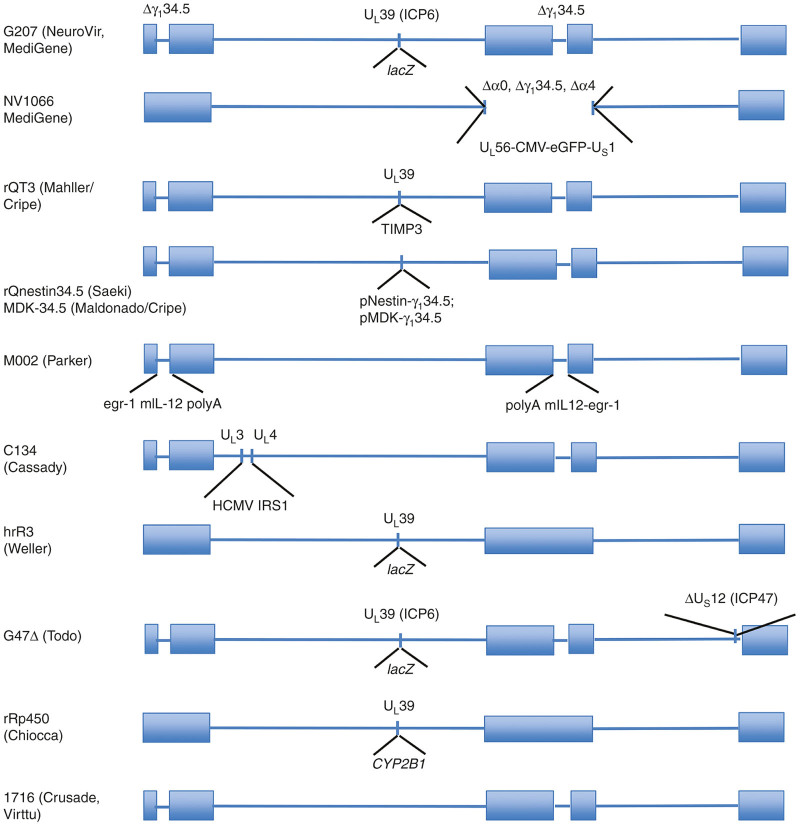
Figure 1.
Structural schematics of mutant herpes simplex virus (HSV)-1 constructs that have been generated for both preclinical proof of principle studies and for clinical applications in pediatric tumors. The HSV-1 DNA genome is circular but is depicted here in a linear format to show the relevant locations of the deletions and insertions for each of the viruses described in the text. Most of these constructs have demonstrated safety and efficacy in HSV-sensitive animal models and some have been advanced to clinical trials. Neuroattenuation has been achieved primarily by deletion of one or both copies of the neurovirulence gene, γ134.5, or by other deletions in essential genes that are complemented in malignant cells. Attempts to enhance virus replication without increasing toxicity are shown using tumor-specific transcriptional targeting where γ134.5 gene expression is driven by a gene promoter expressed primarily in tumor cells. Intratumoral delivery of antitumor therapeutic genes has been tested in distinct formats, as indicated in this schematic for several of the viruses described in the text. CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein; HCMV, human cytomegalovirus.
MPNSTs
Malignant peripheral nerve sheath tumors (MPNSTs) are a heterogeneous group of soft tissue sarcomas that are also known as malignant schwannomas, neurofibrosarcomas, and neurogenic sarcomas. These tumors arise from the Schwann cells that insulate peripheral nerves and are frequently associated with genetic alterations in the tumor suppressor protein known as neurofibromin-1.14 While MPNSTs can technically develop in any peripheral nerve, they are most commonly found in the brachial plexus, sacral plexus, and sciatic nerve.15 Pediatric MPNSTs are rare cancers, and few data exist regarding their demographics and treatment.16 Surgical resection, radiation, and chemotherapy are the mainstays of MPNST management, but achieving adequate surgical margins are difficult with these tumors and many MPNST patients are hypersensitive to therapy-induced malignancies.17,18 According to one recent, multi-institution study, the 5-year overall survival rate for MPNST patients ranges from 43 to 59%.19
It has been noted that cell lines derived from human MPNSTs display variable sensitivity to oncolytic HSVs with one explanation centering on expression of critical HSV entry molecules, such as nectin-1 (CD111). However, a survey of 8 human MPNS cell lines for infectivity by several oHSVs including G207 and C134, a chimeric virus which expresses the human cytomegalovirus IRS-1 gene to improve virus replication without restoring wild-type neurovirulence, found no correlation between CD111 expression and virus yields.20 Like many cancers, MPNSTs show hyperactive signaling of Ras, a factor that has previously been positively correlated with oHSV permissivity, replication, and oncolysis.21 Initial studies by the Cripe lab with G207 and hrR3 revealed that human MPNST lines were indeed susceptible to infection and oncolysis.22 Virus replication benefited by the presence of the intact ICP34.5 gene in hrR3, but both viruses were capable of triggering apoptosis in infected MPNST cells, albeit to varying degrees. Importantly, normal human Schwann cells, which have low basal Ras activity and intact PKR defense pathways, failed to support replication of either oHSV mutant. A follow-up study investigated the use of G207 and hrR3 in xenograft models of MPNST as single agents and in combination with erlotinib, an inhibitor of the epidermal growth factor receptor.23 Overall, both oHSVs showed highly potent antitumor effects, leading to a marked decrease tumor burden and significantly improved survival times in mice. Although the combination of oHSV with erlotinib failed to demonstrate synergy in vivo, both agents were found to exhibit antiangiogenic effects within the tumor, and trended toward increased survival.
In light of the increased efficacy witnessed in an oHSV with an intact γ134.5, the Cripe and Crombleholme labs engineered oHSV-MDK-34.5, an oHSV with ICP34.5 expression driven by the midkine (MDK) promoter.24 MDK is a heparin-binding growth factor that has important roles in development. Although its expression is normally repressed postnatally, approximately 80% of adult carcinomas and pediatric cancers (including MPNSTs) over-express MDK.25–28 Compared to a control virus, oHSV-MDK-34.5 exhibited increased replication, propagation and cytotoxicity in MPNST cells, but remained attenuated against nontransformed fibroblasts. Likewise, oHSV-MDK-34.5 showed superior efficacy in the STS26T mouse xenograft model of MPNST.
Similar observations were reported by Prabhakar et al.29, who evaluated the G47Δ oHSV in xenografts from two immortalized schwannoma cell lines: the patient-derived HEI193 cell line and the spontaneous mouse schwannoma NFS2-1. Both schwannoma lines were highly susceptible to G47Δ, as evidenced by rapid tumor regression following direct injection. Greater efficacy was observed in HEI193 xenografts, presumably due to a higher burst size of virus and the increased susceptibility of human cells versus mouse cells to HSV-1 infection. While the mouse NFS2-1 line could conceivably be implanted in a syngeneic, immune competent host, these additional studies fell beyond the scope of the paper and only athymic nude mice were utilized.
More recently, Antoszczyk et al.30 used G47Δ and armed G47Δ derivatives expressing platelet factor 4 (PF4) and IL-12 to treat an orthotopic model of MPNST in immunocompetent mice. A single intratumoral dose of G47Δ in sciatic nerve tumors significantly inhibited tumor growth and prolonged survival, and was further enhanced by the expression of PF4 and IL-12 in the armed G47Δ variants. Whereas inoculation of the sciatic nerve with wild-type HSV-1 causes demyelination and inevitably leads to lethal encephalitis,31,32 no virus pathology was observed in the sciatic nerves of the “cured” animals following G47Δ treatment.
Rhabdomyosarcoma
Rhabdomyosarcoma (RMS), a tumor that develops from primitive muscle cells, is the most common soft tissue sarcoma of childhood, representing over half of all soft tissue sarcoma diagnoses in children and adolescents33 and affecting approximately 300 children per year in the United States.34 It has the potential to occur at almost any anatomical location, and signs and symptoms vary based upon tumor site. RMS occurs in multiple distinct histological phenotypes, with the two most common being the embryonal (E-RMS) and alveolar (A-RMS) subtypes with A-RMS portending a worse prognosis.35 Current RMS treatment includes multiagent chemotherapy, with additional local control measures of surgery and radiation.36,37 Though progressive advances in chemotherapy regimens have steadily improved survival for some patients with localized disease and favorable histology, progress has stalled over the last several decades and 5-year overall survival rates have plateaued at around 70% since 1990, despite the advent of new targeted agents.35,37,38 The treatments successes seen with dose-escalation and intensification of standard therapies are unfortunately accompanied by a heightened risk of bone growth deformities, endocrinopathies,39 and secondary cancers.40 Relapse and metastasis remain a significant challenge, and cure rates for these patients remain less than 30%. There is, therefore, a significant need for novel sarcoma therapies for both localized and metastatic disease.
Preclinical studies suggest that oHSV may benefit patients with RMS. The first studies, conducted by the Cripe lab, tested a panel of several cell lines to the attenuated HSV vectors NV1020 and G207 and found that RMS were sensitive to oncolysis by both HSV recombinants.41 Shortly thereafter, Cinatl et al.42 showed significant in vitro activity of oHSV G207 against both embryonal and alveolar RMS cells, with high sensitivity to cytotoxic and replicative effects, even at low viral doses. In vivo studies revealed significant tumor growth inhibition when G207 was given intravenously, whereas intratumoral G207 treatment further resulted in complete tumor disappearance in 25% of animals. It was noted that no difference was found between the E-RMS and A-RMS. The in vivo combination of the chemotherapeutic agent vincristine with intravenous G207 resulted in the complete regression of A-RMS in five of eight animals, while causing significant growth inhibition of E-RMS. To explore whether oHSV could be used for local sarcoma control, the Cripe lab tested NV1020 and NV1066 on human RMS cells and xenografts. Cell death correlated with virus replication and apoptosis in both cultured cells and tumors. Fractionated virus administration resulted in a more widespread virus infection and better tumor control.43 They later studied the safety and efficacy profile of a different oHSV vector, rRp450, which is ICP6-deleted and expresses a prodrug enzyme for cyclophosphamide (rat CYP2B1). Cyclophosphamide enhanced the antitumor efficacy of rRp450.44 Recently, Pressey et al.45 demonstrated that CD133 marks a myogenically primitive subpopulation of rhabdomyosarcoma cell lines, thought to be representative of cancer stem cells or cancer-initiating cells, and theorized to be responsible for treatment resistance. The CD133+ subpopulation, though resistant to chemotherapy, was equally sensitive as CD133 negative tumor cells to oHSV oncolysis with the M002 vector.
Ewing sarcoma
Ewing sarcoma (EWS), named after Dr. James R. Ewing who identified this disease in the 1920s,46 is the second most common form of childhood bone cancer. It develops in bones or the surrounding soft tissue and appears as small-round-blue tumor cells. The molecular basis of the disease is characterized by a chromosomal translocation at locus (11; 22)(q24; q12), rearranging the EWS gene with FLI1, a member of the ETS family of transcription factors. The resulting EWS-FLI1 fusion gene encodes a potent transformation protein that triggers disease development.47 Current treatments for EWS patients include surgery, radiation and chemotherapy. Despite tremendous advances in the 5-year survival rate of localized disease (70~80%), overall survival for patients with relapse or metastasis remains poor (<30%),48 suggesting more effective therapy is needed. Using attenuated oHSV against EWS has shown promising results in recent studies. Although EWS cell lines were not as sensitive as RMS or osteosarcoma (OS) cell lines to HSV-mediated oncolysis in vitro,41 modulation of the tumor microenvironment further enhanced oHSV therapy in vivo.49,50 In a subcutaneous model of A673 human sarcoma xenograft in nude mice, two doses of intratumoral rRp450 significantly slowed the tumor growth. The oHSV therapeutic effect was further enhanced by blocking recruitment of CD11b+ cells, presumably tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs), without affecting in vivo virus replication.49 In addition, combining rRp450 treatment with blockade of vascular endothelial growth factor (VEGF), an important cytokine for tumor growth and angiogenesis, produced superior therapeutic outcomes compared to each agent as a monotherapy.49,50 These data not only highlight the impact of tumor microenvironment on oHSV therapy, but also inform the design of novel combination virotherapy against refractory EWS in clinical trials.
Osteosarcoma
Osteosarcoma is the most common type of bone cancer in childhood and adolescence.51 The disease most often occurs in longer bones such as the femur or tibia, but tumor cells can spread or metastasize to the lung or other bones.52,53 Alternations of p53 and RB1 tumor suppressor genes are a frequent occurrence in osteosarcoma.54,55 Current treatments, including surgery and chemotherapy, have improved outcome for localized disease with a 5-year survival rate of approximately 70%. However, the survival rate for patients with metastatic disease remains only 10–30%.56 Results from recent studies support oHSV therapy as a potentially effective treatment against osteosarcoma. Osteosarcoma cells were susceptible to NV1020 or G207 treatment in vitro41 and responded to rRp450 therapy in a human osteosarcoma xenograft mouse model.49 Two intratumoral injections of rRp450 into the flanks of 143.98.2 osteosarcoma tumor-bearing mice significantly prolonged their survival, with 2 out of 10 complete responses and 8 partial responses observed at 30 days. By comparison, all untreated control mice required euthanization by day 13.49 Yamamura et al.57 tested transcriptional targeting of HSV to provide specificity for treating osteosarcoma cells that expressed an actin-associated protein, calponin. The promoter for calponin was used to drive ICP4 from a heterotopic locus, UL23 (the deleted thymidine kinase gene locus). The backbone virus was d120, a highly attenuated HSV that had ICP4 deleted from both sites in the repeat elements and can only be grown in Vero cells that complement the ICP4 deletion. Osteosarcoma cells that expressed calponin easily supported d12.CALP HSV replication and were killed efficiently; moreover, tumors induced in nude mice with the human osteosarcoma cells were effectively eliminated by this virus. Calponin-expressing normal cells (perivascular smooth muscle cells) were not affected, suggesting both calponin expression and cell proliferation were needed to support d12.CALP HSV replication. Overall, these data in various sarcoma models suggest that pediatric sarcomas, as a group, are sensitive to HSV mediated infection/oncolysis and may be an excellent target for oHSV.
Hepatoblastoma
Malignant liver tumors are rare in the pediatric population and only compromise 1% of all pediatric malignancies. Hepatoblastoma, which originates from immature liver cells, is the most commonly diagnosed liver tumor in children. The median age at diagnosis is 18 months, and the 5-year disease-free survival is approximately 50%.58,59 Recently, Megison et al.60 confirmed that the primary HSV-entry receptor CD111 was present in most HuH6 hepatoblastoma cells and in the majority of the human hepatoblastoma specimens examined by immunohistochemistry. The IL-12 producing M002 virus was able to infect and replicate in hepatoblastoma tumor cells leading to decreased cell viability. In vivo experiments demonstrated a significant decrease in tumor growth and increase in animal survival in those animals treated with M002 versus controls.
Pediatric renal tumors
High-grade pediatric kidney tumors, such as malignant rhabdoid kidney tumors (MRKT) and nonosseous renal Ewing sarcoma, continue to provide a therapeutic challenge. MRKT are a rare, aggressive tumors comprising 2% of pediatric kidney tumors; they occur predominately in young children with a median age at diagnosis of 13 months, and the majority of children have advanced stage disease at presentation.61 Patients with MRKT have a dismal prognosis, with an overall 10-year survival rate of less than 30%. Renal Ewing sarcoma is another highly aggressive pediatric renal neoplasm with 25–50% of patients presenting with advanced disease. Response to medical and surgical management modalities is poor with a 5-year disease-free survival rate of 45–55%.62 Preclinical studies suggest that these aggressive renal tumors may be highly susceptible to oHSV. CD111 was present on 92% of the MRKT specimens examined,60 and flow cytometry confirmed high CD111 expression in both a MRKT and renal Ewing sarcoma cell line.60 M002 successfully infected and replicated in both kidney tumor cell lines, leading to decreased cell viability at low virus doses. In animals bearing MKRT or renal Ewing sarcoma, survival was significantly increased with M002 treatment compared to vehicle.
CNS tumors
CNS malignancies represent the most common solid tumor and a leading cause of cancer morbidity and mortality in children with an overall survival rate of approximately 70%.63 Outcomes for children with high-risk, metastatic, or unresectable disease are unacceptably poor, and current therapies like surgery, radiation, and chemotherapy are very damaging to the developing brain of a child resulting in endocrinopathies and neurosensory and neurocognitive impairment.64–66 Preclinical evidence suggests that oHSV may benefit this group of patients. While an initial study found that human medulloblastoma, the most common malignant pediatric brain tumor, was negative for CD111, a few prior studies indicated that oHSV can infect and kill medulloblastoma cells.67 Lasner et al.68 demonstrated the medulloblastoma cell line D283 was sensitive to HSV1716 in vitro, and survival was increased in tumor bearing mice with evidence of viral replication within tumors for several weeks. Similarly, Pyles et al.69 found that the long-term cultured desmoplastic medulloblastoma cell line DAOY was sensitive to a first-generation oHSV. More recently, Friedman et al.70 showed that four medulloblastoma xenografts, including three molecular subgroup 3 tumors, which portend the worst prognosis with survival rates <50%, expressed CD111 (>80% of cells)) and were highly sensitive to oHSV G207 or M002 both in vitro when cells were grown as neurospheres in stem cell–defined medium and in vivo in mice bearing intracranial tumors. Importantly, the CD133+ and CD15+ cancer stem-like cells displayed no inherent resistance to the viruses and were likewise highly sensitive to killing, indicating that chemotherapy and radiation resistant medulloblastomas may be an excellent target for HSV virotherapy.
While high-grade gliomas, which represent 5–10% of pediatric brain tumors, are not as common in children as they are in adults, they are just as deadly with an abysmal 7 ± 4% 3-year event-free survival for children with glioblastoma multiforme.71 Similar to adult studies, preclinical studies evaluating oHSV in pediatric glioblastoma multiforme xenograft lines (xenolines) demonstrated efficacy. Friedman et al.72 found that pediatric glioblastoma multiforme brain tumor stem cells, marked by the surface marker CD133, were sensitive to several γ134.5-deleted viruses including G207 and M002, and the cells had no inherent resistance when compared to the CD133-negative cells. In a subsequent study, they discovered that while hypoxia moderated the efficacy of the first-generation γ134.5-deleted virus, CD133+ cells were not more resistant to oHSV than CD133-negative tumor cells regardless of oxygen tension.73 Furthermore, the chimeric virus C134 was less affected by hypoxia than the γ134.5-deleted virus, suggesting that the next-generation virus will have improved efficacy at targeting chemotherapy and radiotherapy resistant cells in the hypoxic microenvironment.74
Current and Upcoming Trials
Two trials of oncolytic HSV using the 1716 strain (Seprehvir) have been initiated in pediatric patients. The first was designed to test safety of a single intratumoral or intravenous injection into patients with relapsed or refractory solid tumors excluding brain and spinal cord tumors (ClinicalTrials.gov Identifier: NCT00931931), and the second was designed to test intratumoral or peritumoral injection into the residual tumor or resected cavity of relapsed high-grade gliomas (NCT02031965). As the initial studies in young patients, both have a lower limit restriction on age (7 and 13 years old, respectively). Both studies are currently recruiting patients and it is thus too early for published results. A trial of G207 alone or combined with a single low-dose of radiation, which has been shown to enhance virus replication preclinically,75 in children 3–18 years old with recurrent or progressive supratentorial tumors has received FDA IND approval and is forthcoming (NCT02457845).
Future Perspectives
There is a growing body of literature reporting preclinical evidence for antitumor efficacy of oncolytic HSV in pediatric cancer models. Engineered oHSV may offer a less-toxic approach for children with incurable solid tumors and may be beneficial as an adjuvant therapy for curable tumors allowing for lower doses, and therefore, less toxicity from traditional therapies. Although initial studies are underway employing viruses as single agents, testing them in combination with either traditional therapeutic regimens or novel targeted inhibitors will likely be necessary to reveal their full potential.
Acknowledgments
St. Baldrick’s Foundation, The Rally Foundation for Childhood Cancer Research, Hyundai Hope on Wheels, Vs. Cancer Foundation, and the Kaul Pediatric Research Institute to G.K.F.; the National Institutes of Health (CA071933, CA097247, CA151129) and the Department of Defense (W81XWH-11-1-0498) to G.Y.G. and J.M.M. and 1R21CA191544-01 (T. Cripe, PI), 5R01FD003717-04 (T. Cripe, PI), 1R21NS084885-01A1 (N. Ratner, PI), 5P01CA163205-03 (M. Caligiuri, PI); and Nationwide Children’s Hospital, Solvingkidscancer.org, the Katie Linz Foundation, Alex’s Lemonade Stand Foundation to T.P.C.
Footnotes
All funding sources are listed below in the acknowledgements. J.M.M. and G.Y.G. are founders of and own stock and stock options (<7% interest) in Catherex, Inc., and in Aettis, Inc., biotechnology companies that are developing oncolytic HSV. They serve as consultants for Catherex, Inc. G.Y.G. currently serves as one of five unpaid members of the Board of Directors for Catherex, Inc, and has served as a paid advisor to the Program Project at the Ohio State University that seeks to find improved methods for application of oncolytic HSV to treat localized and metastatic cancers. T.P.C. is the sponsor of NCT00931931, and he received some limited funding for said clinical trial from Virttu Biologics, Ltd (Glasgow, UK).
References
- Cripe, TP, Chen, C-Y, Denton, NL, Haworth, KB, Hutzen, B, Leddon, JL et al. (2015). Pediatric cancer gone viral part I: strategies for utilizing oncolytic herpes simplex virus-1 in children. Molecular Therapy — Oncolytics 2, 15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London, WB, Castleberry, RP, Matthay, KK, Look, AT, Seeger, RC, Shimada, H et al. (2005). Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children’s Oncology Group. J Clin Oncol 23: 6459–6465. [DOI] [PubMed] [Google Scholar]
- Applebaum, MA, Henderson, TO, Lee, SM, Pinto, N, Volchenboum, SL and Cohn, SL (2015). Second malignancies in patients with neuroblastoma: the effects of risk-based therapy. Pediatr Blood Cancer 62: 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, AE (1951). Inhibition of growth of five transplantable mouse tumors by the virus of Russian Far East encephalitis. Cancer 4: 375–382. [DOI] [PubMed] [Google Scholar]
- Moore, AE (1951). Enhancement of oncolytic effect of Russian encephalitis virus. Proc Soc Exp Biol Med 76: 749–754. [DOI] [PubMed] [Google Scholar]
- Moore, AE and O’Connor, S (1950). Further studies on the destructive effect of the virus of Russian Far East encephalitis on the transplantable mouse sarcoma 180. Cancer 3: 886–890. [DOI] [PubMed] [Google Scholar]
- Moore, AE (1949). The destructive effect of the virus of Russian Far East encephalitis on the transplantable mouse sarcoma 180. Cancer 2: 525–534. [DOI] [PubMed] [Google Scholar]
- Todo, T, Rabkin, SD, Sundaresan, P, Wu, A, Meehan, KR, Herscowitz, HB et al. (1999). Systemic antitumor immunity in experimental brain tumor therapy using a multimutated, replication-competent herpes simplex virus. Hum Gene Ther 10: 2741–2755. [DOI] [PubMed] [Google Scholar]
- Parikh, NS, Currier, MA, Mahller, YY, Adams, LC, Di Pasquale, B, Collins, MH et al. (2005). Oncolytic herpes simplex virus mutants are more efficacious than wild-type adenovirus Type 5 for the treatment of high-risk neuroblastomas in preclinical models. Pediatr Blood Cancer 44: 469–478. [DOI] [PubMed] [Google Scholar]
- Sakakibara, M, Koizumi, S, Saikawa, Y, Wada, H, Ichihara, T, Sato, H et al. (1999). Membrane-type matrix metalloproteinase-1 expression and activation of gelatinase A as prognostic markers in advanced pediatric neuroblastoma. Cancer 85: 231–239. [DOI] [PubMed] [Google Scholar]
- Mahller, YY, Vaikunth, SS, Ripberger, MC, Baird, WH, Saeki, Y, Cancelas, JA et al. (2008). Tissue inhibitor of metalloproteinase-3 via oncolytic herpesvirus inhibits tumor growth and vascular progenitors. Cancer Res 68: 1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahller, YY, Williams, JP, Baird, WH, Mitton, B, Grossheim, J, Saeki, Y et al. (2009). Neuroblastoma cell lines contain pluripotent tumor initiating cells that are susceptible to a targeted oncolytic virus. PLoS One 4: e4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillory, LA, Megison, ML, Stewart, JE, Mroczek-Musulman, E, Nabers, HC, Waters, AM et al. (2013). Preclinical evaluation of engineered oncolytic herpes simplex virus for the treatment of neuroblastoma. PLoS One 8: e77753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, SL and Stonecypher, MS (2005). Tumor suppressor mutations and growth factor signaling in the pathogenesis of NF1-associated peripheral nerve sheath tumors: II. The role of dysregulated growth factor signaling. J Neuropathol Exp Neurol 64: 1–9. [DOI] [PubMed] [Google Scholar]
- Bates, JE, Peterson, CR, Dhakal, S, Giampoli, EJ and Constine, LS (2014). Malignant peripheral nerve sheath tumors (MPNST): a SEER analysis of incidence across the age spectrum and therapeutic interventions in the pediatric population. Pediatr Blood Cancer 61: 1955–1960. [DOI] [PubMed] [Google Scholar]
- Casanova, M, Ferrari, A, Spreafico, F, Luksch, R, Terenziani, M, Cefalo, G et al. (1999). Malignant peripheral nerve sheath tumors in children: a single-institution twenty-year experience. J Pediatr Hematol Oncol 21: 509–513. [PubMed] [Google Scholar]
- Topal, O, Yilmaz, T and Oğretmenoğlu, O (2004). Giant malignant peripheral nerve sheath tumor of the neck in a patient with neurofibromatosis-1. Int J Pediatr Otorhinolaryngol 68: 1465–1467. [DOI] [PubMed] [Google Scholar]
- Maris, JM, Wiersma, SR, Mahgoub, N, Thompson, P, Geyer, RJ, Hurwitz, CG et al. (1997). Monosomy 7 myelodysplastic syndrome and other second malignant neoplasms in children with neurofibromatosis type 1. Cancer 79: 1438–1446. [PubMed] [Google Scholar]
- Carli, M, Ferrari, A, Mattke, A, Zanetti, I, Casanova, M, Bisogno, G et al. (2005). Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol 23: 8422–8430. [DOI] [PubMed] [Google Scholar]
- Jackson, JD, McMorris, AM, Roth, JC, Coleman, JM, Whitley, RJ, Gillespie, GY et al. (2014). Assessment of oncolytic HSV efficacy following increased entry-receptor expression in malignant peripheral nerve sheath tumor cell lines. Gene Ther 21: 984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farassati, F, Yang, AD and Lee, PW (2001). Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat Cell Biol 3: 745–750. [DOI] [PubMed] [Google Scholar]
- Mahller, YY, Rangwala, F, Ratner, N and Cripe, TP (2006). Malignant peripheral nerve sheath tumors with high and low Ras-GTP are permissive for oncolytic herpes simplex virus mutants. Pediatr Blood Cancer 46: 745–754. [DOI] [PubMed] [Google Scholar]
- Mahller, YY, Vaikunth, SS, Currier, MA, Miller, SJ, Ripberger, MC, Hsu, YH et al. (2007). Oncolytic HSV and erlotinib inhibit tumor growth and angiogenesis in a novel malignant peripheral nerve sheath tumor xenograft model. Mol Ther 15: 279–286. [DOI] [PubMed] [Google Scholar]
- Maldonado, AR, Klanke, C, Jegga, AG, Aronow, BJ, Mahller, YY, Cripe, TP et al. (2010). Molecular engineering and validation of an oncolytic herpes simplex virus type 1 transcriptionally targeted to midkine-positive tumors. J Gene Med 12: 613–623. [DOI] [PubMed] [Google Scholar]
- Tsutsui, J, Kadomatsu, K, Matsubara, S, Nakagawara, A, Hamanoue, M, Takao, S et al. (1993). A new family of heparin-binding growth/differentiation factors: increased midkine expression in Wilms’ tumor and other human carcinomas. Cancer Res 53: 1281–1285. [PubMed] [Google Scholar]
- Muramatsu, T (2002). Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem 132: 359–371. [DOI] [PubMed] [Google Scholar]
- Mashour, GA, Ratner, N, Khan, GA, Wang, HL, Martuza, RL and Kurtz, A (2001). The angiogenic factor midkine is aberrantly expressed in NF1-deficient Schwann cells and is a mitogen for neurofibroma-derived cells. Oncogene 20: 97–105. [DOI] [PubMed] [Google Scholar]
- Lucas, S, Reindl, T, Henze, G, Kurtz, A, Sakuma, S and Driever, PH (2009). Increased midkine serum levels in pediatric embryonal tumor patients. J Pediatr Hematol Oncol 31: 713–717. [DOI] [PubMed] [Google Scholar]
- Prabhakar, S, Messerli, SM, Stemmer-Rachamimov, AO, Liu, TC, Rabkin, S, Martuza, R et al. (2007). Treatment of implantable NF2 schwannoma tumor models with oncolytic herpes simplex virus G47Delta. Cancer Gene Ther 14: 460–467. [DOI] [PubMed] [Google Scholar]
- Antoszczyk, S, Spyra, M, Mautner, VF, Kurtz, A, Stemmer-Rachamimov, AO, Martuza, RL et al. (2014). Treatment of orthotopic malignant peripheral nerve sheath tumors with oncolytic herpes simplex virus. Neuro Oncol 16: 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend, JJ and Collins, PK (1986). Peripheral nervous system demyelination with herpes simplex virus. J Neuropathol Exp Neurol 45: 419–425. [DOI] [PubMed] [Google Scholar]
- Gil, Z, Rein, A, Brader, P, Li, S, Shah, JP, Fong, Y et al. (2007). Nerve-sparing therapy with oncolytic herpes virus for cancers with neural invasion. Clin Cancer Res 13: 6479–6485. [DOI] [PubMed] [Google Scholar]
- Sultan, I, Qaddoumi, I, Yaser, S, Rodriguez-Galindo, C and Ferrari, A (2009). Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol 27: 3391–3397. [DOI] [PubMed] [Google Scholar]
- Ries, L, Smith, M, Gurney, J, Linet, M, Tamra, T, Young, J et al. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. National Cancer Institute, SEER Program. NIH Pub No 99–4649. Bethesda, MD, 1999. [Google Scholar]
- Perez, EA, Kassira, N, Cheung, MC, Koniaris, LG, Neville, HL and Sola, JE (2011). Rhabdomyosarcoma in children: a SEER population based study. J Surg Res 170: e243–e251. [DOI] [PubMed] [Google Scholar]
- Huh, WW and Skapek, SX (2010). Childhood rhabdomyosarcoma: new insight on biology and treatment. Curr Oncol Rep 12: 402–410. [DOI] [PubMed] [Google Scholar]
- Dasgupta, R and Rodeberg, DA (2012). Update on rhabdomyosarcoma. Semin Pediatr Surg 21: 68–78. [DOI] [PubMed] [Google Scholar]
- Smith, MA, Seibel, NL, Altekruse, SF, Ries, LA, Melbert, DL, O’Leary, M et al. (2010). Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 28: 2625–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, KE, Derdak, J, Bernstein, D, Reynolds, JC, Avila, NA, Gerber, L et al. (2008). Metabolic syndrome traits in long-term survivors of pediatric sarcoma. Pediatr Blood Cancer 50: 341–346. [DOI] [PubMed] [Google Scholar]
- Mansky, P, Arai, A, Stratton, P, Bernstein, D, Long, L, Reynolds, J et al. (2007). Treatment late effects in long-term survivors of pediatric sarcoma. Pediatr Blood Cancer 48: 192–199. [DOI] [PubMed] [Google Scholar]
- Bharatan, NS, Currier, MA and Cripe, TP (2002). Differential susceptibility of pediatric sarcoma cells to oncolysis by conditionally replication-competent herpes simplex viruses. J Pediatr Hematol Oncol 24: 447–453. [DOI] [PubMed] [Google Scholar]
- Cinatl, J Jr, Cinatl, J, Michaelis, M, Kabickova, H, Kotchetkov, R, Vogel, JU et al. (2003). Potent oncolytic activity of multimutated herpes simplex virus G207 in combination with vincristine against human rhabdomyosarcoma. Cancer Res 63: 1508–1514. [PubMed] [Google Scholar]
- Currier, MA, Adams, LC, Mahller, YY and Cripe, TP (2005). Widespread intratumoral virus distribution with fractionated injection enables local control of large human rhabdomyosarcoma xenografts by oncolytic herpes simplex viruses. Cancer Gene Ther 12: 407–416. [DOI] [PubMed] [Google Scholar]
- Currier, MA, Gillespie, RA, Sawtell, NM, Mahller, YY, Stroup, G, Collins, MH et al. (2008). Efficacy and safety of the oncolytic herpes simplex virus rRp450 alone and combined with cyclophosphamide. Mol Ther 16: 879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey, JG, Haas, MC, Pressey, CS, Kelly, VM, Parker, JN, Gillespie, GY et al. (2013). CD133 marks a myogenically primitive subpopulation in rhabdomyosarcoma cell lines that are relatively chemoresistant but sensitive to mutant HSV. Pediatr Blood Cancer 60: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripe, TP (2011). Ewing sarcoma: an eponym window to history. Sarcoma 2011: 457532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucman, J, Delattre, O, Desmaze, C, Plougastel, B, Joubert, I, Melot, T et al. (1992). Cloning and characterization of the Ewing’s sarcoma and peripheral neuroepithelioma t(11;22) translocation breakpoints. Genes Chromosomes Cancer 5: 271–277. [DOI] [PubMed] [Google Scholar]
- Potratz, J, Dirksen, U, Jürgens, H and Craft, A (2012). Ewing sarcoma: clinical state-of-the-art. Pediatr Hematol Oncol 29: 1–11. [DOI] [PubMed] [Google Scholar]
- Currier, MA, Eshun, FK, Sholl, A, Chernoguz, A, Crawford, K, Divanovic, S et al. (2013). VEGF blockade enables oncolytic cancer virotherapy in part by modulating intratumoral myeloid cells. Mol Ther 21: 1014–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshun, FK, Currier, MA, Gillespie, RA, Fitzpatrick, JL, Baird, WH and Cripe, TP (2010). VEGF blockade decreases the tumor uptake of systemic oncolytic herpes virus but enhances therapeutic efficacy when given after virotherapy. Gene Ther 17: 922–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddox, CL, Han, G, Anijar, L, Binitie, O, Letson, GD, Bui, MM et al. (2014). Osteosarcoma in pediatric patients and young adults: a single institution retrospective review of presentation, therapy, and outcome. Sarcoma 2014: 402509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya, H, Kanazawa, Y, Abdel-Wanis, ME, Asada, N, Abe, S, Isu, K et al. (2002). Effect of timing of pulmonary metastases identification on prognosis of patients with osteosarcoma: the Japanese Musculoskeletal Oncology Group study. J Clin Oncol 20: 3470–3477. [DOI] [PubMed] [Google Scholar]
- Corradi, D, Wenger, DE, Bertoni, F, Bacchini, P, Bosio, S, Goldoni, M et al. (2011). Multicentric osteosarcoma: clinicopathologic and radiographic study of 56 cases. Am J Clin Pathol 136: 799–807. [DOI] [PubMed] [Google Scholar]
- Hansen, MF, Koufos, A, Gallie, BL, Phillips, RA, Fodstad, O, Brøgger, A et al. (1985). Osteosarcoma and retinoblastoma: a shared chromosomal mechanism revealing recessive predisposition. Proc Natl Acad Sci USA 82: 6216–6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, CW, Aslo, A, Tsay, C, Slamon, D, Ishizaki, K, Toguchida, J et al. (1990). Frequency and structure of p53 rearrangements in human osteosarcoma. Cancer Res 50: 7950–7954. [PubMed] [Google Scholar]
- Ferrari, S and Palmerini, E (2007). Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr Opin Oncol 19: 341–346. [DOI] [PubMed] [Google Scholar]
- Yamamura, H, Hashio, M, Noguchi, M, Sugenoya, Y, Osakada, M, Hirano, N et al. (2001). Identification of the transcriptional regulatory sequences of human calponin promoter and their use in targeting a conditionally replicating herpes vector to malignant human soft tissue and bone tumors. Cancer Res 61: 3969–3977. [PubMed] [Google Scholar]
- Darbari, A, Sabin, KM, Shapiro, CN and Schwarz, KB (2003). Epidemiology of primary hepatic malignancies in U.S. children. Hepatology 38: 560–566. [DOI] [PubMed] [Google Scholar]
- Litten, JB and Tomlinson, GE (2008). Liver tumors in children. Oncologist 13: 812–820. [DOI] [PubMed] [Google Scholar]
- Megison, ML, Gillory, LA, Stewart, JE, Nabers, HC, Mroczek-Musulman, E, Waters, AM et al. (2014). Preclinical evaluation of engineered oncolytic herpes simplex virus for the treatment of pediatric solid tumors. PLoS One 9: e86843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel-Eibrink, MM, van Tinteren, H, Rehorst, H, Coulombe, A, Patte, C, de Camargo, B et al. (2011). Malignant rhabdoid tumours of the kidney (MRTKs), registered on recent SIOP protocols from 1993 to 2005: a report of the SIOP renal tumour study group. Pediatr Blood Cancer 56: 733–737. [DOI] [PubMed] [Google Scholar]
- Kushner, BH, Hajdu, SI, Gulati, SC, Erlandson, RA, Exelby, PR and Lieberman, PH (1991). Extracranial primitive neuroectodermal tumors. The Memorial Sloan-Kettering Cancer Center experience. Cancer 67: 1825–1829. [DOI] [PubMed] [Google Scholar]
- Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, et al. (eds). SEER Cancer Statistics Review, 1975–2004, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER web site, 2007.
- Diller, L, Chow, EJ, Gurney, JG, Hudson, MM, Kadin-Lottick, NS, Kawashima, TI et al. (2009). Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol 27: 2339–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribi, K, Relly, C, Landolt, MA, Alber, FD, Boltshauser, E and Grotzer, MA (2005). Outcome of medulloblastoma in children: long-term complications and quality of life. Neuropediatrics 36: 357–365. [DOI] [PubMed] [Google Scholar]
- Packer, RJ, Sutton, LN, Atkins, TE, Radcliffe, J, Bunin, GR, D’Angio, G et al. (1989). A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg 70: 707–713. [DOI] [PubMed] [Google Scholar]
- Guzman, G, Oh, S, Shukla, D, Engelhard, HH and Valyi-Nagy, T (2006). Expression of entry receptor nectin-1 of herpes simplex virus 1 and/or herpes simplex virus 2 in normal and neoplastic human nervous system tissues. Acta Virol 50: 59–66. [PubMed] [Google Scholar]
- Lasner, TM, Kesari, S, Brown, SM, Lee, VM, Fraser, NW and Trojanowski, JQ (1996). Therapy of a murine model of pediatric brain tumors using a herpes simplex virus type-1 ICP34.5 mutant and demonstration of viral replication within the CNS. J Neuropathol Exp Neurol 55: 1259–1269. [DOI] [PubMed] [Google Scholar]
- Pyles, RB, Warnick, RE, Chalk, CL, Szanti, BE and Parysek, LM (1996). A novel, multiply-mutated HSV-1 strain for the treatment of human brain tumors. Cancer Gene Ther 3: O35–O35. [DOI] [PubMed] [Google Scholar]
- Friedman, GK, Moore, BP, Nan, L, Kelly, VM, Etminan, T, Langford, CP et al. (2015). Pediatric medulloblastoma xenografts including molecular subgroup 3 and CD133+ and CD15+ cells are sensitive to killing by oncolytic herpes simplex viruses. Neuro Oncol (e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Cohen, KJ, Pollack, IF, Zhou, T, Buxton, A, Holmes, EJ, Burger, PC et al. (2011). Temozolomide in the treatment of high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro Oncol 13: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, GK, Langford, CP, Coleman, JM, Cassady, KA, Parker, JN, Markert, JM et al. (2009). Engineered herpes simplex viruses efficiently infect and kill CD133+ human glioma xenograft cells that express CD111. J Neurooncol 95: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, GK, Haas, MC, Kelly, VM, Markert, JM, Gillespie, GY and Cassady, KA (2012). Hypoxia Moderates γ(1)34.5-Deleted Herpes Simplex Virus Oncolytic Activity in Human Glioma Xenoline Primary Cultures. Transl Oncol 5: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, GK, Nan, L, Haas, MC, Kelly, VM, Moore, BP, Langford, CP et al. (2015). γ₁34.5-deleted HSV-1-expressing human cytomegalovirus IRS1 gene kills human glioblastoma cells as efficiently as wild-type HSV-1 in normoxia or hypoxia. Gene Ther 22: 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advani, SJ, Markert, JM, Sood, RF, Samuel, S, Gillespie, GY, Shao, MY et al. (2011). Increased oncolytic efficacy for high-grade gliomas by optimal integration of ionizing radiation into the replicative cycle of HSV-1. Gene Ther 18: 1098–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]