Abstract
Although the disorder of sex development in dogs with female karyotype (XX DSD) is quite common, its molecular basis is still unclear. Among mutations underlying XX DSD in mammals are duplication of a long sequence upstream of the SOX9 gene (RevSex) and duplication of the SOX9 gene (also observed in dogs). We performed a comparative analysis of 16 XX DSD and 30 control female dogs, using FISH and MLPA approaches. Our study was focused on a region harboring SOX9 and a region orthologous to the human RevSex (CanRevSex), which was located by in silico analysis downstream of SOX9. Two highly polymorphic copy number variable regions (CNVRs): CNVR1 upstream of SOX9 and CNVR2 encompassing CanRevSex were identified. Although none of the detected copy number variants were specific to either affected or control animals, we observed that the average number of copies in CNVR1 was higher in XX DSD. No copy variation of SOX9 was observed. Our extensive studies have excluded duplication of SOX9 as the common cause of XX DSD in analyzed samples. However, it remains possible that the causative mutation is hidden in highly polymorphic CNVR1.
The most common canine disorder of sex development (DSD) manifests as testes or ovotestes without gametogenic activity, normal female karyotype (78,XX) and lack of the SRY gene1. This abnormality, termed testicular or ovotesticular XX DSD, is also quite common in other mammals, including humans2 and livestock species – goat, pig, horse3.
The genetic basis of XX DSD phenotype is not uniform between mammalian species. In humans heterozygous duplication and triplication of a long sequence (approx. 78 kb) located 0.5 Mb upstream of SOX9, called RevSex4,5,6 (in this study called HumRevSex) or testis specific SOX9 enhancer candidate region7 are considered as the causative mutations. Recently the XX DSD HumRevSex region was delimited to 68 kb and distinguished from the XY DSD RevSex region8. This region likely contains an enhancer regulatory sequence which duplicated may trigger SOX9 expression in the absence of the SRY gene product9. In a single case of testicular XX DSD in roe deer three copies of the entire SOX9 gene, including 5′- and 3′-UTR, were detected by quantitative PCR (qPCR)10. In pigs, genome wide association study (GWAS) of related animals with XX DSD implicated the SOX9 region, but did not pinpoint the causative mutation11.
Other regions have also been implicated in XX DSD. The first causative mutations were identified in human RSPO1 gene, which is involved in ovarian development in humans12. In goats a 11.7 kb deletion near another gene (FOXL2), also important for ovarian development, is responsible for this disorder13,14. Finally, the duplication of SOX3 gene has been identified in humans with XX DSD phenotype15.
There have been numerous attempts to identify the causative mutation or linked genetic markers in dogs, but so far without success [reviewed by1,16,17]. It has recently been claimed that some canine XX DSD cases are caused by duplication of a 577 kb region containing the SOX9 gene, as detected by array comparative genome hybridization (aCGH) and confirmed by real time qPCR. The mutation was detected in two of seven XX DSD dogs analyzed and study of control samples was not performed18. Although, it has to be noted that duplication of SOX9 in control samples was not detected in any of copy number variants (CNVs) discovery studies19,20,21,22,23.
Dogs show exceptional phenotypic variability and a high frequency of hereditary diseases, some of which are known to be due to structural variations in the genome24. Identification of copy number variable regions (CNVRs) in the dog is thus of particular interest. Rossi et al.18 suggested a potential association between XX DSD and a duplication in the SOX9 region, and we considered this worthy of further investigation. Here we describe the use of cytogenetic mapping (fluorescence in situ hybridization, FISH) and multiplex ligation-dependent probe amplification (MLPA) approaches to identify two highly variable CNVRs, and show that SOX9 is not duplicated in any of the samples analyzed.
Results
In silico analysis of the SOX9 region
Since it is known that critical regulatory region for human SOX9 is located approx. 0.5 Mb upstream of the gene we anticipated a similar location for regulatory region in the dog genome. Unexpectedly, sequence similarity analysis of HumRevSex and canine reference genome (http://genome.ucsc.edu/cgi-bin/hgBlat, BLAT, UCSC GB) revealed that the predicted position of the canine sequence, termed CanRevSex (chr9:17605057-17642567, CanFam3.1), is very different, being located more than 9 Mb downstream of SOX9 (Fig. 1). CanRevSex also spans only about 37 kb (part of HumRevSex) and shows relatively low homology to HumRevSex (86.1%). CanRevSex and HumRevSex differ in their sequence and genomic location, but it is not clear whether they are similar in function. We thus decided to analyze the region upstream of SOX9 and the downstream CanRevSex region, using FISH and MLPA for locus-specific CNVs analysis.
Figure 1. Map of the region studied.
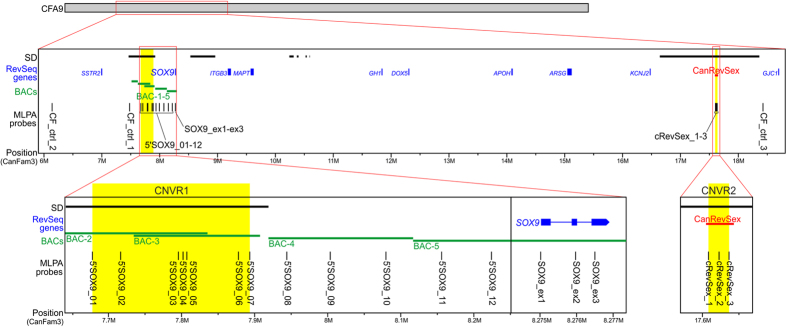
From top to bottom are indicated: segmental duplication (SD) according to Nicholas et al.20, RevSeq genes (blue), FISH probes (green) and MLPA probes (black) with IDs indicated next to or below. The SOX9 with the upstream region and CanRevSex region are shown at higher resolution. Regions highlighted in yellow correspond to CNVRs detected in this study. The position of selected elements is indicated under the panels (please note that the scale is different for each panel).
Analysis of the SOX9 region by FISH
Cytogenetic study of the SOX9 upstream region commenced with verification of BAC clones on one animal with XX DSD and one healthy reference female dog (data not shown). The BAC-5 clone, located closest to SOX9, produced specific and typical hybridization signals on CFA9. The second clone (BAC-4) in the contig order, upstream of the SOX9 gene (Fig. 1), also produced typical hybridization signals on CFA9, but additional signals were found on a long arm of the chromosome X (CFAXq), where there is a block of constitutive heterochromatin. The BAC-3 clone showed variation of the hybridization signals. A typical signal was present on CFA9 in a reference female dog, while in the XX DSD case the signal appeared in only one copy of the CFA9. Signals were also detected on one small autosome, which was identified as CFA18 by in silico analysis of sequence homology. Signals on CFA18 were observed in XX DSD and reference dogs. Another two clones (BAC-1 and BAC-2) showed hybridization signal at the same position as the BAC-4 clone. BAC-1 and BAC-2 signals were observed at the expected position on CFA9, and additional signals on the long arm of the X chromosome. The BAC-2 signal intensity showed variability, but because the probe bound to other chromosomes, it was not deemed a reliable indicator for CNV analysis.
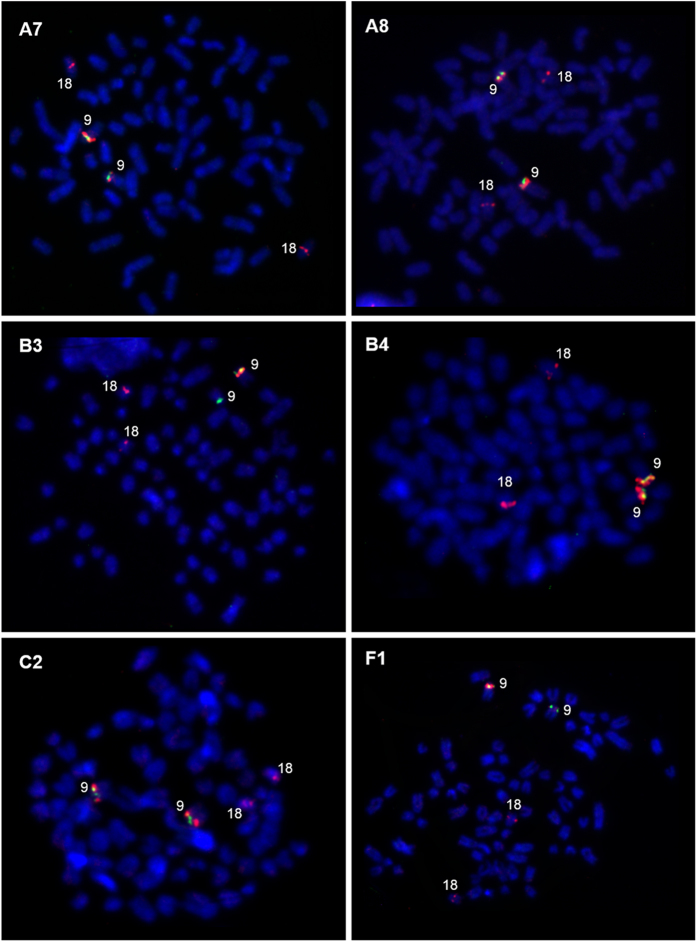
Results of the FISH experiment indicated a potential CNVR in the region detected by BAC-3. To investigate this further, four groups of animals were included in the FISH study: XX DSD dogs (A5, A6, A7, A8, B3, B4), healthy relatives of A6 and B4 XX DSD dogs (C2, C3, C4 and C5) and 4 reference, fertile female dogs (F1–F4). Dual-colour FISH was applied, using two probes, BAC-3 and BAC-5. The BAC-3 clone was used as a probe for CNV detection (labelled in red), while BAC-5 was used as a CFA9-specific reference probe (labelled in green). The hybridization signal produced by the BAC-3 probe showed variation in the animals studied (Fig. 2). Two large signals on CFA9 were found in A5, A6, A8, B4 and C2 animals, a large signal on one copy of CFA9 and a weak signal on the second copy of CFA9 were observed in A7, C3, C4 and C5, while in B3 the signal was visible on only one copy of CFA9. Similar variation was observed on CFA9 in reference animals (F1–F4). These results suggested that hybridization signal variability is a common variation in this species. Inheritance of the CNV was confirmed in Pug family.
Figure 2. Representative images of FISH analysis with two BAC clones: BAC-5 (green) and BAC-3 (red), for 6 animals.
Red signals were observed on CFA9 and CFA18, while the green signal was present only on CFA9. Size variation of the red FISH signal in CFA9 was described as: large (++), normal (+) and lack of the signal (−). The following FISH patterns are visible: A7 (++/+), A8 (++/++), B3 (++/−), B4 (++/++), C2 (++/++) and F1 (++/+).
Analysis of the SOX9 and CanRevSex regions by MLPA
To investigate the canine SOX9 region in more detail, we developed a custom-made CanSOX9+ MLPA assay composed of 21 probes and covering the following sub-regions of interest: i) SOX9 gene, ii) the region of ~620 kb in length upstream of the SOX9 gene and iii) the 37,5 kb CanRevSex region (Fig. 1).
MLPA analysis was performed on 45 dog samples from 21 different breeds (for details see Table 1). Results for individual samples are shown as bar plots in Suppl. Fig. S1 and summarized in the column scatter plot in Fig. 3. As shown in Fig. 3, three probes located in the CanRevSex region (cRevSex_1-cRevSex_3) and seven probes located in the region 0.4 Mb upstream of SOX9 (5′SOX9_01-5′SOX9_07) show very high signal variation (average coefficient of variation (aCV) = 0.55, compared to average CV = 0.14 and CV = 0.11 in non-polymorphic and control regions, respectively), indicating the extensive copy number (CN) variation, including homozygous deletions, in these regions. This result shows that two CNVRs are present in the studied SOX9 region: CNVR1 upstream of SOX9 gene of a minimal size of 214 433 bp, and CNVR2 in the CanRevSex region of a minimal size of 27 961 bp. The relative CN of these regions ranges from 0 to over 8 copies. Also, as shown in Fig. 3 and S1, there is no sign of CNVs within the SOX9 gene (average CV = 0.14). To confirm this result, we replicated our analysis with the modified CanSOX9+ assay excluding probes located in detected CNVRs. As Suppl. Fig. S2 shows, this confirmed the absence of CNV in SOX9 gene in the analyzed samples.
Table 1. Characterization of the dogs studied.
| Animal | Breed | Reference | Technique applied |
|
|---|---|---|---|---|
| FISH | MLPA | |||
| Group A – testicular or ovotesticular 78,XX and SRY-negative DSD phenotypical females with enlarged clitoris | ||||
| A1 | Cocker spaniel | 47 | − | + |
| A2 | Bernese Mountain Dog | 48 | − | + |
| A3 | American Staffordshire Terrier | 16 | − | + |
| A4 | American Staffordshire Terrier | 49 | − | + |
| A5 | Leonberger | 16 | + | + |
| A6 | Pug | This study | + | + |
| A7 | Beagle | This study | + | + |
| A8 | Cocker spaniel | This study | + | + |
| Group B – 78,XX and SRY-negative DSD phenotypical females with enlarged clitoris but with unknown histology of gonads | ||||
| B1 | American Staffordshire Terrier | 16 | − | + |
| B2 | Tibetan Terrier | 16 | − | + |
| B3 | French Bulldog | 16 | + | + |
| B4 | Pug | This study | + | + |
| B5 | American Staffordshire Terrier | 49 | − | + |
| B7 | Miniature Pinscher | 49 | − | + |
| B8 | German Shepherd | 50 | − | + |
| B9 | Yorkshire Terrier | 16 | − | + |
| Group C – Ancestors of 2 fullsibs with DSD (A6 and B4) | ||||
| C2 | Pug, father of A6 and B4 | This study | + | + |
| C3 | Pug, mother of A6 and B4 | This study | + | + |
| C4 | Pug, father of C2 and C3 | This study | + | + |
| C5 | Pug, mother of C2 | This study | + | − |
| Group D - 16 control females representing breeds in which DSD has been reported: American Staffordshire Terrier (4), Beagle (2), German Shepherd, Cocker Spaniel (2), Pug (2), Bernese Mountain Dog, Yorkshire Terrier (3) and French Bulldog | − | + | ||
| Group E - 10 control females representing breeds in which DSD has not so far been reported: Rottweiler, Poodle, Mastiff, Pekingese, Bichon Friese, Chihuahua, Boxer, Dachshund, Briard, Labrador | − | + | ||
| Group F – 4 control females used for FISH analysis: Akita, Irish Setter, Shih Tzu and mongrel | + | − | ||
Figure 3. MLPA results for all tested samples.
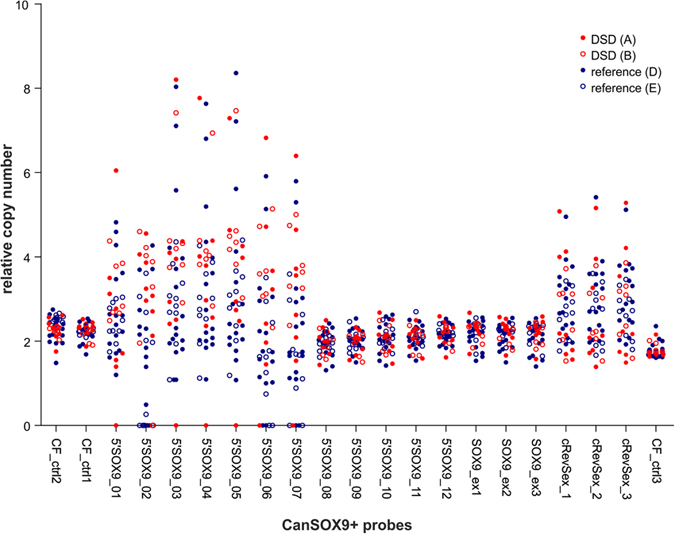
The column scatter plot represents the relative copy number (y-axis) of each CanSOX9+ probe (x-axis). Each dot represents one sample. Colored dots represent samples of XX, SRY-negative, testicular or ovotesticular DSD females (group A, solid red dots) and XX, SRY-negative DSD females with unknown gonad histology (group B, open red dots). Blue dots represent reference females (groups D and E).
As shown in Suppl. Fig. S1 CNVs do not occur at random in the variable regions, but the identified CNVs often extend over several consecutive probes. Among the most common CNVs observed are: i) gains of the region from probe 5′SOX9_03 to probe 5′SOX9_07 (5′SOX9_03-07); ii) deletions of probes 5′SOX9_02 and 5′SOX9_06-07; or iii) gains of CanRevSex region (cRevSex_1-3). Although the pattern of the observed variants is relatively conservative, it has to be noted that the determined CN values are relative and may differ somewhat depending on the reference samples used.
Detailed analysis of the variation observed in the regions tested showed that extensive CN variation occurs both in affected and control samples and that there is no unambiguous relationship between observed CNVs and the XX DSD phenotype (Fig. 3). Although we observed some specific CNV patterns for different breeds, especially for the CanRevSex region (Suppl. Fig. S3), most CNVs occurred in more than one breed and were not breed-specific.
Due to the lack of a simple relationship between CNVs and XX DSD phenotype, we decided to compare the relative signal distribution between samples from two groups of animals: XX DSD females (group A + B) and healthy females (group D + E) (Suppl. Fig. S4). This analysis demonstrated that in the region covered by six consecutive probes (5′SOX9_02-07) XX DSD animals showed increased average CN values compared to control samples. The strongest association (the highest signal difference between groups) was observed for probe 5′SOX9_02, which showed a significantly higher average relative signal in XX DSD females than in healthy females (3.23 vs 1.54, respectively; Mann-Whitney U test, p value = 0.0008). Similar CN differences were observed when affected animals (group A + B) were compared only with animals from group D, and no substantial differences were observed between groups D and E. These results suggest that the causative mutation for XX DSD may be located in this region. It has to be noted, however, that the observed association may result from heterogeneity and different breed composition of compared groups.
We then analyzed the segregation of copy number changes in Pug family, which consisted of two affected siblings and their healthy parents (Fig. 4). We did not analyze grandparents of the affected siblings, due to the lack of grandfather DNA (C5) and the repeatedly poor quality of MLPA (non interpretable) results of the grandmother sample (C4). Both XX DSD animals (A6 and B4) showed a clear signal increase for probes 5′SOX9_01-07. A similar signal increase was observed in the father (C2), but not the mother (C3). The increased MLPA signal correlated with results of the FISH analysis. Unfortunately, we could not analyze other littermates, and were thus unable to conclude, whether the increase of copies in XX DSD animals represents an association or is coincidental.
Figure 4. The MLPA and FISH analysis in the Pug family.
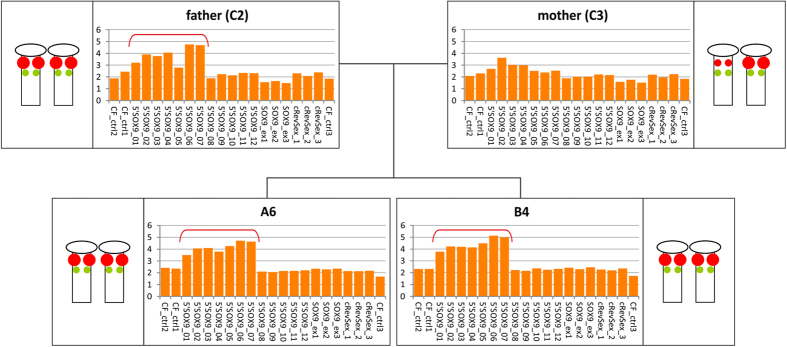
The family consisted of two XX, SRY-negative DSD females (A6 and B4) and their healthy parents: father (C2) and mother (C3). Bar plots represent the relative copy number (y-axis) of each CanSOX9+ probe (x-axis). The red bracket marks probes, where the signal is increased (5′SOX9_01–5′SOX9_07). Corresponding diagrams of the results of FISH analysis are shown next to the MLPA bar plots.
Discussion
Mutations in the region upstream of SOX9 are responsible for several human genetic diseases25, including XX DSD, which is associated with duplications or triplications of the HumRevSex region located approx. 0.5 Mb upstream of SOX95,6. Different techniques can be used to detect CNVRs26,27 and we applied two of them (FISH and MLPA) to compare region harboring SOX9 in XX DSD dogs and normal, fertile female dogs.
FISH analyses revealed differences of the hybridization signal size for one of five BAC probes tested for 5′-flanking region of the dog SOX9 gene. To visualize or confirm variation in this CNVR different types of FISH approaches can be applied using BAC or fosmid probes to detect FISH signal intensity28,29,30,31. We used a standard FISH procedure with BAC clones to find out whether different signal intensity variants exist among the XX DSD and control dogs. The size of inserts in the used BAC clones varied from 165–203 kb. The clone which revealed a very polymorphic fragment upstream of the SOX9 (BAC-3) carried the 165 kb long insert. Due to a visible variation of the FISH signal we assumed that there is an extensive polymorphism in the region studied. An advantage of the FISH approach is visualization of CNVs size differences in homologous chromosomes, however, it does not indicate the precise CN in a locus of interest.
To precisely characterize CNVR detected by FISH, we employed the MLPA technique with a set of 21 probes designed to study the SOX9 region (~650 kb) and the CanRevSex region (~40 kb), located ~9 Mb downstream of SOX9. This approach allowed us to conclude that there was no SOX9 duplication in any of the samples tested. This observation contrasts with results of Rossi et al.18 who claimed that duplication of a large region, including entire SOX9 gene, is a relevant cause of XX DSD. A similar association between SOX9 duplication and XX DSD has also been reported for a roe deer10 and a boy32. However, both were single cases analyzed by qPCR. The lack of SOX9 duplication in our relatively large cohort of XX DSD females at least suggests it is not a common cause of XX DSD in dogs.
The MLPA approach also allowed us to identify two highly variable regions: CNVR1 upstream of SOX9, and CNVR2 in the CanRevSex region downstream of SOX9. The location and observed variation of CNVR1 correlate well with the FISH analysis (BAC-3 probe). Because CNVs in both CNVRs were observed in affected and healthy animals they do not provide a simple explanation of the disorder. However, the association of the higher CN in the CNVR1 (from probes 5′SOX9_01 to 5′SOX9_07) with XX DSD phenotype suggests that this region may harbor CNV or some other genetic variation associated with XX DSD. This association is in line with our FISH results (BAC-3 probe) and with results of Pug family analysis in which high CN value in CNVR1 are observed in two affected siblings but not in the normal mother. The detection of two CNVRs in the SOX9 region generally confirms previous reports. CNVR1 has been described previously in two genome-wide studies20,33 and CNVR2 in six genome-wide studies19,20,22,23,33,34 (Suppl. Fig. S5). Both CNVRs detected in this study co-localize with previously identified segmental duplications (SDs)20, which suggests they may have been formed by non-allelic homologous recombination (NAHR).
CNVR1 is located ~0.4–0.6 Mb upstream of SOX9 and its approximate length is greater than 214 kb. It well overlaps with the size and the location of HumRevSex, which is located more than 500 kb upstream of SOX9 and the length of this region is at least 67 kb6. However the in silico predicted position of CanRevSex, based on homology analysis, is ~9 Mb downstream of SOX9. This may suggest that the region critical for XX DSD is characterized not by sequence, but by its location. Interestingly, a very recent report of Rossi et al.35, based on FISH study and in silico analysis, suggested that canine genome assembly (canFam3) in the region of SOX9 is not correct and the CanRevSex is in fact localized upstream of SOX9.
Our findings are consistent with previous studies performed on mouse models. The regulatory role of the upstream region (approx. 1 Mb) of Sox9 was revealed in transgenic mouse, called the Odds sex mouse, which also carries an ~150 kb deletion upstream of Sox9. This modification triggered upregulation of Sox9 in fetal gonads and XX mice, which developed as sterile males36. Further studies showed that promoter of the transgene, not the deletion itself, was responsible for the upregulation in XX Odds sex mice37. Extensive study on murine Sox9 gene regulation revealed that its expression is crucial for Sertoli cell differentiation and is controlled by positive and negative regulators interacting with a 3.3 kb testis-specific enhancer (TES) that contains a highly conserved 1.4 kb sequence. The main positive regulators are SF1 and SRY transcription factors, and among crucial regulators are DAX1, WNT4, FOXL2, RESPO1 and β-catenin38.
Analysis of families in which the disorder segregates is a crucial step towards identifying its mode of inheritance and molecular characterization of the causative mutation. Extensive studies performed in goats revealed autosomal recessive inheritance13. Similar approach was applied to show the causative role of the RSPO1 gene mutations in some cases of human XX DSD12. In pigs autosomal recessive inheritance was also proposed39 and candidate region containing the SOX9 gene was indicated11. Interestingly, in XX DSD pigs no CNV was found in the 3.14 Mb region containing the SOX9 gene. In humans pedigree analysis, associated with a causative 178 kb duplication located 600 kb upstream of SOX9, revealed autosomal dominant sex-limited (females only) inheritance. The inheritance mode of canine XX DSD is unclear. Originally, it was hypothesized that this disorder is inherited according to an autosomal recessive, sex-limited model40. The deleterious effect of SOX9 duplication suggested by Rossi et al.18 does not fit this model and indicated that also dominant, sex-limited mode of inheritance is possible, as it was earlier demonstrated in humans18. In our study we could only analyze parents of two full sibling XX DSD cases. Since other littermates were unavailable we cannot draw any conclusion concerning the inheritance model.
Concluding, in our study on the molecular background of XX DSD we analyzed a relatively large cohort of cases and controls. We did not observe CN variation of the SOX9 gene, however we found two highly polymorphic CNVRs located upstream (CNVR1) and downstream (CNVR2) of SOX9. Although higher copy number in CNVR1 may be associated with XX DSD phenotype, it was detected in both cases and controls, and therefore cannot be considered as a causative mutation. We hypothesize that within CNVR1 may occur mutation site/sites responsible for ablation of binding sites for repressor or enhancer of the SOX9.
Material and Methods
Ethics statement
Tissue sampling and clinical studies were carried out according to standard Polish veterinary protocols. All animal experiments were approved by a local Bioethical Commission for Animal Care and Use in Poznan (Poland).
Material
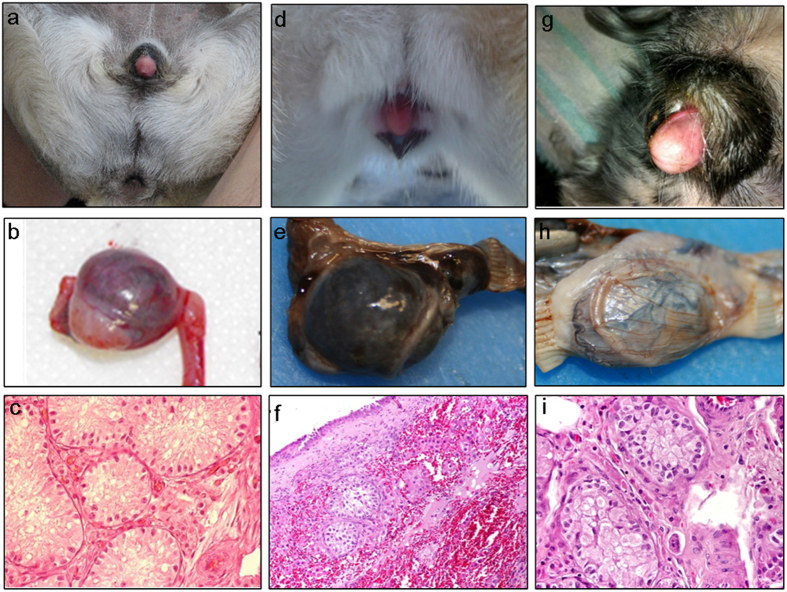
A total of 50 dogs were included in the study. These were grouped as follows: (A) 8 cases of unrelated XX DSD phenotypic females with enlarged clitoris and testis or ovotestis, this group was crucial in our study due to the knowledge of the gonads histology, (B) 8 cases of unrelated XX DSD phenotypic females with enlarged clitoris, but unknown gonad histology; (C) 4 ancestors of two testicular XX DSD full sibs, (D) 16 control females of the same breed as the XX DSD cases studied, (E) 10 control females of breeds where XX DSD has not yet been reported, and (F) 4 control females for the FISH study. Some XX DSD dogs were described earlier in terms of clinical examination, chromosome complement, presence of the SRY gene and histology of gonads, but were not studied by FISH and MLPA techniques (Table 1). Four new XX DSD cases, included in groups A (3) or B (1), were described for the first time (Fig. 5).
Figure 5. Characteristics of three new testicular (A6 and A8) or ovotesticular (A7) XX DSD cases.
The following breeds were represented: Pug – case A6 (a–c), Beagle – case A7 (d–f) and Cocker Spaniel – case A8 (g–i). External genitalia (a,d,g), gonad morphology (b,e,h) and histology (c,f,i) are shown.
Fluorescence in Situ Hybridization (FISH)
Chromosome preparations were obtained from short-term lymphocyte culture by standard procedures41. BAC clones covering the 5′ region of SOX9 gene on canine chromosome 9 (CFA9) were selected based on localization upstream of SOX9, in a region which position corresponds to HumRevSex. The BACs were selected from CHORI-82 Canine Boxer (F) (Canis familiaris) BAC library (https://bacpac.chori.org/) with the use of UCSC Genome Browser (http://www.genome.ucsc.edu/). The following BAC clones were used in FISH experiments: BAC-1 (CH82-405G24), BAC-2 (CH82-496F06), BAC-3 (CH82-26L13), BAC-4 (CH82-135B15) and BAC-5 (CH82-116C01). The locations of the BAC probes are shown in Fig. 1.
DNA from BAC clones was isolated by alkaline lysis and labelled by random priming with biotin-16-dUTP or digoxigenin-11-dUTP. FISH hybridization was performed according to Szczerbal et al.42. Briefly, denatured probes were applied to a denatured chromosome preparation and hybridized overnight at 37 °C. Signal detection and amplification were performed using streptavidin-Cy3 or anti-digoxigenin-fluorescein. Chromosomes were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and the standard chromosome nomenclature of the dog43 was applied for localization of the probes on CFA9. Hybridization signal of BAC5 probe, specific to the region of interest, was used as a marker of CFA9.
Slides were analyzed under a Nikon E 600 Eclipse fluorescence microscope, equipped with a cooled digital CCD camera, driven by the computer-aided software LUCIA.
Multiplex Ligation-dependent Probe Amplification (MLPA)
A custom-made MLPA assay (CanSOX9+ assay) was designed and generated for detailed analysis of CNVRs in the canine region harboring SOX9. The CanSOX9+ assay was composed of 21 probes (Fig. 1, Suppl. Table S1) including: i) three control probes (CF_ctrl1-CF_ctrl3) located on CFA9, outside the investigated region, in locations with no evidence of common CNVRs19,20,22,23,33,34 and 18 probes covering the investigated SOX9 region, including: ii) three probes located in the SOX9 gene, one probe in each exon (SOX9_ex1-SOX9_ex3); iii) 12 probes evenly distributed along the 620 kb-long region upstream of SOX9 (5′SOX9_01-5′SOX9_12) (the less even distribution of probes in the fragment overlapped by BAC-3 is a consequence of segmentally duplicated sequences with high homology to sequences on CFA18); and iv) three probes located in the region homologous to HumRevSex region (CanRevSex) (cRevSex_1-cRevSex_3). The MLPA probes and general probe layout were designed according to the strategy previously proposed by Kozlowski et al.44 and by Marcinkowska et al.45, which involves short oligonucleotide probes easily generated via standard chemical synthesis. The total probe length ranged from 93 to 164 nt. Target sequences for all probes were designed according to the CanFam2 and CanFam3.1 dog genome assemblies and were selected to avoid SNPs, repeat elements and sequences of extremely high or low GC content. All MLPA probes were designed to be specific for the unique genomic sequence. In some cases (7 probes), in regions with high homology to other chromosomes, the MLPA probes were designed to specifically recognize only the region of interest on CFA9. Suppl. Table S1 shows detailed characteristics and sequences of the probes used. All MLPA probes were synthesized by Integrated DNA Technologies (Skokie, IL, USA).
MLPA reactions were run according to the manufacturer’s general recommendations (MRC-Holland, Amsterdam, Netherlands), as described previously in46 and44. Briefly, approximately 100 ng DNA was denatured and hybridized with MLPA probe-mix for 16 hours. In the next step, all properly hybridized probes were ligated and then amplified using the pair of universal primers. PCR products were separated via capillary electrophoresis on an ABI Prism 3130XL apparatus (Applied Biosystems, Carlsbad, CA, USA) and electropherograms analyzed using GeneMarker software (v1.91). The signal intensities (peak heights) of all probes were transferred to the prepared Excel spreadsheets in which further analyses were performed. To avoid run-to-run signal variation, signals of each probe in each sample were normalized by division by the average signal of control probes. The normalized MLPA signal of all samples was then compared to the normalized signal of samples selected as references. Reference samples were randomly selected from the group of healthy females (D) that showed the lowest normalized signal variation for all probes and no homozygous deletions for any of the regions investigated.
All MLPA reagents, except for the probe mix, were purchased from MRC-Holland (Amsterdam, the Netherlands). All graphs shown were generated using Prism v. 4.0 (GraphPad, San Diego, CA, USA) and Microsoft Excel.
Additional Information
How to cite this article: Marcinkowska-Swojak, M. et al. Copy number variation in the region harboring SOX9 gene in dogs with testicular/ovotesticular disorder of sex development (78,XX; SRY-negative). Sci. Rep. 5, 14696; doi: 10.1038/srep14696 (2015).
Supplementary Material
Acknowledgments
This study was financed by the National Science Center in Poland - grant 2012/05/B/NZ9/00907 and 2011/01/B/NZ5/02773. We wish to thank Dr. Alex Kind for his comments on the manuscript and Mrs Sylwia Salamon, M.Sc. for technical assistance.
Footnotes
Author Contributions M.M.S. performed MLPA experiments and analyses, and contributed to the manuscript preparation. I.S. performed FISH studies and cytogenetic diagnosis of new DSD cases, was responsible for DNA sample preparation for further analysis and contributed to the results interpretation and manuscript preparation. H.P., K.F. and R.F. contributed to molecular analysis and the manuscript preparation. J.N.W. was responsible for DNA sample preparation for further analysis and contributed to the results interpretation. S.D., W.N. and R.P.C. performed clinical, anatomical and histological studies of new DSD cases. P.K. contributed to MLPA design and interpretation of the results, performed in silico analysis and contributed to preparation of the manuscript. M.S. was responsible for study design and supervision of the whole experiment, interpretation of the obtained results and was responsible for preparation and submission of the manuscript. All authors read and approved the final manuscript.
References
- Meyers-Wallen V. N. Gonadal and sex differentiation abnormalities of dogs and cats. Sex Dev. 6, 46–60 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson A., Nokoff N. J. & Travers S. Disorders of sex development: clinically relevant genes involved in gonadal differentiation. Discov Med. 14, 301–309 (2012). [PubMed] [Google Scholar]
- Favetta L. A. et al. Disorders of sexual development and abnormal early development in domestic food-producing mammals: the role of chromosome abnormalities, environment and stress factors. Sex Dev. 6, 18–32 (2012). [DOI] [PubMed] [Google Scholar]
- Benko S. et al. Disruption of a long distance regulatory region upstream of SOX9 in isolated disorders of sex development. J Med Genet. 48, 825–830 (2011). [DOI] [PubMed] [Google Scholar]
- Cox J. J., Willatt L., Homfray T. & Woods C. G. A SOX9 duplication and familial 46,XX developmental testicular disorder. N Engl J Med. 364, 91–93 (2011). [DOI] [PubMed] [Google Scholar]
- Xiao B., Ji X., Xing Y., Chen Y. W. & Tao J. A rare case of 46, XX SRY-negative male with approximately 74-kb duplication in a region upstream of SOX9. Eur J Med Genet. 56, 695–698 (2013). [DOI] [PubMed] [Google Scholar]
- Fonseca A. C. et al. The clinical impact of chromosomal rearrangements with breakpoints upstream of the SOX9 gene: two novel de novo balanced translocations associated with acampomelic campomelic dysplasia. BMC Med Genet. 14, 50 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G. J. et al. Copy number variation of two separate regulatory regions upstream of SOX9 causes isolated 46,XY or 46,XX disorder of sex development. J Med Genet. 52, 240–247 (2015). [DOI] [PubMed] [Google Scholar]
- Warr N. & Greenfield A. The molecular and cellular basis of gonadal sex reversal in mice and humans. Wiley Interdiscip Rev Dev Biol. 1, 559–577 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropatsch R. et al. SOX9 duplication linked to intersex in deer. PLoS One. 8, e73734 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau S. et al. A genome-wide association study points out the causal implication of SOX9 in the sex-reversal phenotype in XX pigs. PLoS One. 8, e79882 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma P. et al. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet. 38, 1304–1309 (2006). [DOI] [PubMed] [Google Scholar]
- Pailhoux E. et al. A 11.7-kb deletion triggers intersexuality and polledness in goats. Nat Genet. 29, 453–458 (2001). [DOI] [PubMed] [Google Scholar]
- Pannetier M., Elzaiat M., Thepot D. & Pailhoux E. Telling the story of XX sex reversal in the goat: highlighting the sex-crossroad in domestic mammals. Sex Dev. 6, 33–45 (2012). [DOI] [PubMed] [Google Scholar]
- Moalem S. et al. XX male sex reversal with genital abnormalities associated with a de novo SOX3 gene duplication. Am J Med Genet A. 158A, 1759–1764 (2012). [DOI] [PubMed] [Google Scholar]
- Salamon S. et al. A lack of association between polymorphisms of three positional candidate genes (CLASP2, UBP1, and FBXL2) and canine disorder of sexual development (78,XX; SRY -negative). Sex Dev. 8, 160–165 (2014). [DOI] [PubMed] [Google Scholar]
- Salamon S., Nowacka-Woszuk J. & Switonski M. Exclusion of the CTNNB1 and FOXL2 genes for canine XX testicular/ovotesticular disorde of sex development. Folia Biologica- Krakow. 63, 57–62 (2015). [DOI] [PubMed] [Google Scholar]
- Rossi E. et al. Sox9 duplications are a relevant cause of Sry-negative XX sex reversal dogs. PLoS One. 9, e101244 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. K., Swartz J. D., Rush L. J. & Alvarez C. E. Mapping DNA structural variation in dogs. Genome Res. 19, 500–509 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas T. J. et al. The genomic architecture of segmental duplications and associated copy number variants in dogs. Genome Res. 19, 491–499 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilez J. et al. A selective sweep of >8 Mb on chromosome 26 in the Boxer genome. BMC Genomics. 12, 339 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund J. et al. Novel origins of copy number variation in the dog genome. Genome Biol. 13, R73 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin A. M., Berglund J., Webster M. T. & Lindblad-Toh K. Genome-wide copy number variant discovery in dogs using the CanineHD genotyping array. BMC Genomics. 15, 210 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez C. E. & Akey J. M. Copy number variation in the domestic dog. Mamm Genome. 23, 144–163 (2012). [DOI] [PubMed] [Google Scholar]
- Gordon C. T. et al. Long-range regulation at the SOX9 locus in development and disease. J Med Genet. 46, 649–656 (2009). [DOI] [PubMed] [Google Scholar]
- Stuppia L., Antonucci I., Palka G. & Gatta V. Use of the MLPA assay in the molecular diagnosis of gene copy number alterations in human genetic diseases. Int J Mol Sci. 13, 3245–3276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantsilieris S., Baird P. N. & White S. J. Molecular methods for genotyping complex copy number polymorphisms. Genomics. 101, 86–93 (2013). [DOI] [PubMed] [Google Scholar]
- Mkrtchyan H. et al. Early embryonic chromosome instability results in stable mosaic pattern in human tissues. PLoS One. 5, e9591 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y. et al. Large-scale copy number variants (CNVs): distribution in normal subjects and FISH/real-time qPCR analysis. BMC Genomics. 8, 167 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvelyan M. et al. New cytogenetically visible copy number variant in region 8q21.2. Mol Cytogenet. 4, 1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S. et al. Copy number variation in the horse genome. PLoS Genet. 10, e1004712 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. M., Ko J. M., Shin C. H. & Yang S. W. A Korean boy with 46,XX testicular disorder of sex development caused by SOX9 duplication. Ann Pediatr Endocrinol Metab. 19, 108–112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas T. J., Baker C., Eichler E. E. & Akey J. M. A high-resolution integrated map of copy number polymorphisms within and between breeds of the modern domesticated dog. BMC Genomics. 12, 414 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgul A. et al. General assessment of copy number variation in normal and tumor tissues of the domestic dog (Canis lupus familiaris). J Appl Genet. 55, 353–363 (2014). [DOI] [PubMed] [Google Scholar]
- Rossi E. et al. A revised genome assembly of the region 5’ to canine SOX9 includes the RevSex orthologous region. Sex Dev. 9, 155–161 (2015). [DOI] [PubMed] [Google Scholar]
- Bishop C. E. et al. A transgenic insertion upstream of sox9 is associated with dominant XX sex reversal in the mouse. Nat Genet. 26, 490–494 (2000). [DOI] [PubMed] [Google Scholar]
- Qin Y. et al. Long-range activation of Sox9 in Odd Sex (Ods) mice. Hum Mol Genet. 13, 1213–1218 (2004). [DOI] [PubMed] [Google Scholar]
- Jakob S. & Lovell-Badge R. Sex determination and the control of Sox9 expression in mammals. FEBS J. 278, 1002–1009 (2011). [DOI] [PubMed] [Google Scholar]
- Pailhoux E. et al. Relevance of intersexuality to breeding and reproductive biotechnology programs; XX sex revarsal in pigs. Theriogenology. 47, 93–102 (1997). [Google Scholar]
- Meyers-Wallen V. N. & Patterson D. F. XX sex reversal in the American cocker spaniel dog: phenotypic expression and inheritance. Hum Genet. 80, 23–30 (1988). [DOI] [PubMed] [Google Scholar]
- Iannuzzi L. & Di Berardino D. Tools of the trade: diagnostics and research in domestic animal cytogenetics. J Appl Genet. 49, 357–366 (2008). [DOI] [PubMed] [Google Scholar]
- Szczerbal I. et al. Comparative chromosomal localization of the canine-derived BAC clones containing LEP and IGF1 genes in four species of the family Canidae. Cytogenet Genome Res. 102, 264–266 (2003). [DOI] [PubMed] [Google Scholar]
- Switonski M. et al. Report on the progress of standardization of the G-banded canine (Canis familiaris) karyotype. Committee for the Standardized Karyotype of the Dog (Canis familiaris). Chromosome Res. 4, 306–309 (1996). [DOI] [PubMed] [Google Scholar]
- Kozlowski P. et al. Identification of 54 large deletions/duplications in TSC1 and TSC2 using MLPA, and genotype-phenotype correlations. Hum Genet. 121, 389–400 (2007). [DOI] [PubMed] [Google Scholar]
- Marcinkowska M., Wong K.-K., Kwiatkowski D. J. & Kozlowski P. Design and generation of MLPA probe sets for combined copy number and small-mutation analysis of human genes: EGFR as an example. Sci World J. 10, 2003–2018 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten J. P. et al. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 30, e57 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switonski M. et al. Hypospadias in a male (78,XY; SRY-positive) dog and sex reversal female (78,XX; SRY-negative) dogs: clinical, histological and genetic studies. Sex Dev. 6, 128–134 (2012). [DOI] [PubMed] [Google Scholar]
- Switonski M. et al. Robertsonian translocation in a sex reversal dog (XX, SRY negative) may indicate that the causative mutation for this intersexuality syndrome resides on canine chromosome 23 (CFA23). Sex Dev. 5, 141–146 (2011). [DOI] [PubMed] [Google Scholar]
- Nowacka J., Nizanski W., Klimowicz M., Dzimira S. & Switonski M. Lack of the SOX9 gene polymorphism in sex reversal dogs (78,XX; SRY negative). J Hered. 96, 797–802 (2005). [DOI] [PubMed] [Google Scholar]
- Switonski M., Nowacka J., Skorczyk A., Chmurzynska A. & Nizanski W. Hereditary sex-reversal syndrome (78,XX, SRY-negative) in German shepherd puppies. Med Wet. 60, 705–707 (2004). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.