Abstract
The ability to divide one's attention deteriorates in patients with childhood chronic fatigue syndrome (CCFS). We conducted a study using a dual verbal task to assess allocation of attentional resources to two simultaneous activities (picking out vowels and reading for story comprehension) and functional magnetic resonance imaging. Patients exhibited a much larger area of activation, recruiting additional frontal areas. The right middle frontal gyrus (MFG), which is included in the dorsolateral prefrontal cortex, of CCFS patients was specifically activated in both the single and dual tasks; this activation level was positively correlated with motivation scores for the tasks and accuracy of story comprehension. In addition, in patients, the dorsal anterior cingulate gyrus (dACC) and left MFG were activated only in the dual task, and activation levels of the dACC and left MFG were positively associated with the motivation and fatigue scores, respectively. Patients with CCFS exhibited a wider area of activated frontal regions related to attentional resources in order to increase their poorer task performance with massive mental effort. This is likely to be less efficient and costly in terms of energy requirements. It seems to be related to the pathophysiology of patients with CCFS and to cause a vicious cycle of further increases in fatigue.
Keywords: Children and adolescents, Chronic fatigue syndrome, Cognitive compensation, Divided attention, Frontal cortex, Functional magnetic resonance imaging
Highlights
-
•
Decrease in divided attention was related to fatigue in childhood and adolescence.
-
•
Left frontal cortex of healthy students activated in verbal divided attention task
-
•
Right MFG and ACG were additionally activated in CCFS patients.
-
•
CCFS is characterized as an energy-inefficient process in frontal cortex.
1. Introduction
Up to 8% of children and adolescents have experienced fatigue for a duration of more than 1 month, and nearly 2% have experienced chronic fatigue lasting more than 6 months (Miike and Bell, 2008). Because fatigue in students corresponds to a decrease in academic performance (Garralda and Rangel, 2002), clarification of the precise mechanisms of fatigue and identification of ways to overcome fatigue are very important. Fatigue induces difficulty in initiating or sustaining voluntary activities (Chaudhuri and Behan, 2004). In fact, fatigued children and adolescents and patients with childhood chronic fatigue syndrome (CCFS), which is characterized by profound and disabling fatigue for 6 months (Fukuda et al., 1994), show poor performance on cognitive tasks related to memory and attention (Haig-Ferguson et al., 2009; Kawatani et al., 2011; Mizuno et al., 2013a; Tomoda et al., 2007).
Along with structural changes in the brain from childhood to adolescence, executive function, defined as the set of mental cognitive control processes that permit goal-directed behavior, develops dramatically between childhood and adolescence (Travis, 1998). Recently, we reported that task performance on a divided attention task using the Kana Pick-out Test (KPT), which assesses participants' allocation of attentional resources to two simultaneous activities [picking out vowels (PV) and reading for story comprehension (SC)] improved as children progressed from elementary to junior high school (Mizuno et al., 2011a), and decreased with fatigue (Mizuno et al., 2011b) and lower academic motivation (Mizuno et al., 2011c). Poor performance of the KPT also relates to poor lifestyle choices (skipping breakfast, too much time watching television) and family conditions (little time spent with family and little praise from family members) in junior high school students (Mizuno et al., 2013b). CCFS patients also have lower performance on the KPT (Tomoda et al., 2007). These findings suggest that although development of the ability to divide attention is crucial for good academic performance in adolescence, it is inhibited by fatigue, not only in healthy children and adolescents (HCA), but also in CCFS patients. Therefore, clarifying the neural relationship between fatigue and divided attention is critical for the evaluation of cognitive development and interventional efforts in both fatigued children and adolescents and CCFS patients. However, there are currently no studies designed to investigate the neural substrates of the relationship between fatigue and divided attention in any age group.
Recently, we demonstrated the neural substrates associated with the KPT in healthy young adults using functional magnetic resonance imaging (fMRI) (Mizuno et al., 2012). We compared patterns of activation in the brain obtained during performance of the individual tasks of PV and SC to levels of activation when participants performed the two tasks simultaneously during the KPT (Fig. 1). Activations of the left dorsal inferior frontal gyrus (lDIFG) and left superior parietal lobule (lSPL) in the dual task (PV + SC) condition were greater than in the two single task (PV and SC) conditions alone, suggesting that these increased activations during the KPT reflect the extent of utilization of attentional resources (Mizuno et al., 2012). In contrast, decreased activations of the left fusiform gyrus (lFFG) and the left middle temporal gyrus (lMTG), which are domain regions for processing of the PV (Murray and He, 2006) and SC (Grossman et al., 2002), respectively, were observed during the dual task conditions in comparison with the two single task conditions, suggesting that the reduced activations during the KPT reflect the difficulty of concurrent processing of the two tasks (Mizuno et al., 2012).
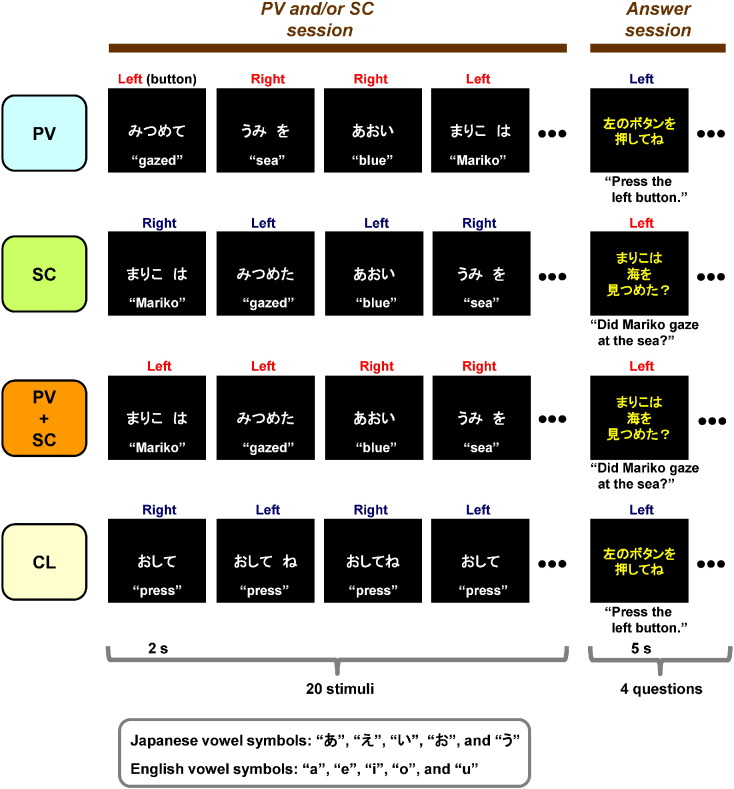
Fig. 1.
Time course of stimulus display sequences.The PV and/or SC session involved picking out vowels (PV), story comprehension (SC), the concurrent processes of both PV and SC (PV + SC) and control (CL) conditions. In the PV condition, participants judged whether a target word included vowels (/a/, /e/, /i/, /o/, and /u/). In the SC condition, participants read each word presented in sequence on the screen and were later tested for comprehension of the short story. In the PV + SC condition, participants concurrently performed both picking out vowels and story comprehension. In the CL condition, participants pressed either the right or left button in alternate trials. The word “press” appeared on the screen for every control trial. After the PV and/or SC session, participants completed an answer session. In the SC and PV + SC conditions, participants answered four questions, designed to require “yes” or “no” answers. Although the Japanese version of the task was used in the present study, an English version was also developed.
Based on these findings in the adult study, we tried to identify the neural relationship between fatigue and divided attention in HCA and CCFS patients. We performed the fMRI experiment in the HCA group, which had an age range of 11–14 years. A follow-up study was conducted in the HCA group for a period of 2 years to identify fatigue-related neural processing during the KPT. We chose this age range because the number of fatigued students increases from elementary to junior high school in Japan (Mizuno et al., 2011b; BRED, 2006). The increase in the number of fatigued students from elementary to junior high school indicates that the variance in fatigue severity in HCA increases; thus, correlation analysis between fatigue and brain activation proves useful for identifying features of the neural basis of divided attention in fatigued children and adolescents. In addition, to specify the neural substrates of divided attention in CCFS patients, we compared the blood oxygenation level dependent signal during the KPT between HCA and CCFS patients.
2. Materials and methods
2.1. Participants
Twenty-three HCA participated in the three fMRI experiments. They had normal or corrected-to-normal visual acuity, no history of medical illness, and were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971). Ten out of 23 HCA could not completely perform the experiments three times due to a lack of understanding of the task and motion artifacts during fMRI scans. A total of 13 HCA (9 females and 4 males) completely performed the experiments, and we analyzed the data.
Regarding CCFS patients recruited for the study, patients who had undergone treatment with antidepressants or hypotension drugs or who had diagnoses of neurological illness, migraine, obstructive sleep apnea, below average intelligence, or severe psychopathology were excluded from the study. Serious psychopathology was evaluated as referral to at least one pediatric psychiatrist if the patient presented with indicative symptoms. No patients or healthy participants had any history of Diagnostic and Statistical Manual of Mental Disorders Fourth Edition, Text Revision (DSM-IVTR) Axis I Disorder (based on Structured Clinical Interview for DSM-IV Axis I Disorders), drug abuse, head injury, or fetal drug exposure that may have influenced brain development. Seventeen patients with CCFS participated in the fMRI experiments, were right-handed, and scored more than 80 on the full scale intelligence quotient derived from the Wechsler Intelligence Scale for Children (Wechsler, 1991). Two patients were excluded from analysis because they misunderstood the task. Therefore, we analyzed the data obtained from 15 patients (6 females and 9 males), all of whom fulfilled the diagnostic criteria for CCFS (Fukuda et al., 1994). Although two patients had been administered antidepressants, the drugs were discontinued 2 weeks before the fMRI experiments. The protocol was approved by the Ethics Committee of the National Institute for Physiological Sciences (NIPS), and all participants and their parents gave written informed consent for participation in the study. The experiments were undertaken in compliance with national legislation and the Code of Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association (Declaration of Helsinki).
2.2. Fatigue and motivation scales
The severity of fatigue was measured using the Chalder Fatigue Scale (Chalder et al., 1993); this questionnaire was distributed to participants before the fMRI experiments. The reliability and validity of the Japanese version of the Chalder Fatigue Scale for evaluation of the severity of fatigue in students have been previously confirmed (Tanaka et al., 2008). The fatigue scale consists of 11 questions using a 4-level (0–3) scale that allows the following responses: 0 = less than usual; 1 = no more than usual; 2 = more than usual; and 3 = much more than usual during the past several weeks. The total score for the 11-item fatigue scale ranges from 0 to 33, with higher scores indicating a greater degree of fatigue. To identify the mental effort level of participants for the dual and single tasks, a visual analogue scale (VAS) for the subjective experience of motivation to each task condition was measured after the fMRI experiments (Mizuno et al., 2008). The VAS scores ranged from 0 (compete lack of motivation) to 100 (maximum motivation).
2.3. Experimental paradigms for functional imaging
The fMRI experimental design was identical with that of the previous study (Mizuno et al., 2012), and is shown in Fig. 1. The participants performed the modified version of the KPT, which included single and dual task conditions presented on a computer screen for use with fMRI. The single task conditions were PV and SC, and the dual task required participants to perform PV and SC tasks concurrently (PV + SC). In addition, to control for the normal activation of brain areas due to visual and motor processing, the participants performed a test under control (CL) conditions. Although we used the Japanese version of the modified KPT in the present study, an English version has also been developed.
Hereafter, this part of the KPT is referred to as the PV and/or SC session. In the PV condition, participants judged whether vowels included in the words were presented in the center of the screen. If the target letters were presented in the center of the screen, participants were instructed to press the right button. If the target letters did not appear in the center of the screen, participants were instructed to press the left button.
In the SC condition, participants read silently each presented word as it appeared in sequence on the screen. An example sentence was “Mariko gazed at the blue sea and watched the white bird, and Takashi gazed at the blue mountain and watched the red bird.” The participants pressed the right and left buttons alternately for each word presented.
In the PV + SC condition, the participants were required to simultaneously pick out vowels and understand the story. Thus, when the target letters (vowels) were presented in the center of the screen, the participants pressed the right button. If target letters did not appear in the center of the screen, the participants were instructed to press the left button. These judgments about the individual vowels and the direction of the button press were performed while reading the story for comprehension.
In the CL condition, the participants were not required to perform either task and were instructed to simply press the right and left buttons alternately when presented with the word “press” on every trial.
Each condition consisted of 20 trials during which 20 word stimuli were displayed for 1 s each, followed by a blank screen displayed for 1 s, for a total of 40 s per condition for the PV and/or SC session. Before the first word stimulus was presented, the name of the stimulus condition appeared on the screen for 5 s (“PV”, “SC”, “PV + SC” or “CL”) to instruct the participants. The probability of a target letter appearing in the PV and PV + SC conditions was 50%. The sequence of presented words was pseudorandom in the PV condition, and the presented words were chosen from those used in the SC and PV + SC conditions. In order to control the difficulty of comprehension of the story between the SC and PV + SC conditions, sentences from the SC condition were alternately replaced with sentences from the PV + SC condition for each participant.
After all conditions, the participants completed an answer session. In the SC and PV + SC conditions, this comprised a series of four “yes” or “no” questions to assess story comprehension. Example questions were “Did Mariko gaze at the sea?”, “Did Takashi gaze at the sea?”, “Did Mariko watch the red bird?”, and “Did Takashi watch the red bird?”. Participants were instructed to press the right button if the answer was “yes” and the left button if the answer was “no”.
In the PV and CL conditions, participants were not required to answer questions and were simply directed to press the right or left button (e.g., “Press the left button.”). The questions for each condition consisted of four trials, each of which lasted 4 s followed by a blank screen that lasted 1 s, for a total of 20 s in the answer session.
The probability of a “yes” question appearing in the SC and PV + SC conditions was 50%. The total time for each condition, including the answer session, was 60 s. Each condition was repeated twice per run, in counter-balanced order, and the time interval between conditions was 20 s. The participants were instructed to perform each task as quickly and accurately as possible. The direction of the button press was inverted for half of the participants. Before scanning, participants practiced a series of CL, PV, SC, and PV + SC conditions for approximately 15 min, to ensure that all participants understood the task. The visual stimuli and the duration of each stimulus presentation were developed and presented using Presentation® software (Neurobehavioral Systems, Albany, CA, USA).
2.4. Functional imaging and data analyses
All images were obtained using a 3-Tesla MR scanner (Allegra; Siemens, Erlangen, Germany) located at the National Institute for Physiological Sciences (Okazaki, Aichi, Japan). For functional imaging, a series of 272 volumes (136 volumes per run) were acquired using T2-weighted, gradient echo, echo planar imaging (EPI) sequences. Each volume consisted of 34 transaxial slices, each having a thickness of 3.0 mm with a 0.5-mm gap between slices to include the entire cerebrum and cerebellum [repetition time (TR), 2500 ms; echo time, 30 ms; flip angle (FA), 75°; field of view (FoV), 19.2 cm; in-plane matrix size, 64 × 64 pixels; voxel dimensions, 3.0 × 3.0 × 3.0 mm]. Oblique scanning was used to exclude the eyeballs from the images. Tight but comfortable foam padding was placed around the participant's head to minimize head movement. To acquire a fine structural whole-brain image, magnetization-prepared rapid-acquisition gradient-echo (MP-RAGE) images were obtained [repetition time (TR), 2500 ms; echo time (TE), 4.38 ms; flip angle = 8°; FoV, 230 mm; one slab; number of slices per slab = 192; voxel dimensions = 0.9 × 0.9 × 1.0 mm].
The first two volumes acquired in each MRI run were discarded due to unsteady magnetization, and the remaining 134 volumes per run were used for analysis. Data were analyzed using Statistical Parametric Mapping 5 (The Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB 7.7.0 (Mathworks, Natick, MA, USA). Following realignment for motion correction of all EPI images, a high-resolution whole-brain T1-weighted image was co-registered with the first volume of EPI images. The whole-head MP-RAGE images were then normalized to the Montréal Neurological Institute (MNI) T1 image template. These parameters were applied to all EPI images. The EPI images were spatially smoothed in three dimensions using an 8-mm full-width half-maximum Gaussian kernel.
Statistical analyses were performed at two levels. First, individual task-related activation was evaluated. We used the modified version of the KPT, which was a block-design version of the task used in a previous study (Mizuno et al., 2012). In each task condition (i.e., CL, PV, SC, and PV + SC), the event onset was designated as the presentation of the first stimulus of the first trial in a block in the PV and/or SC session or answer session. We modeled each of the four regressors (CL, PV, SC, and PV + SC), which were convolved with a canonical hemodynamic response function to obtain the expected signal changes caused by the tasks. The data were high-pass filtered with a cut-off period of 160 s to remove low-frequency signal drifts. An autoregressive model was used for whitening the residuals to meet the assumptions for application of a general linear model (GLM). The effect of each condition was evaluated with a GLM. The weighted sum of the parameters estimated in the individual analyses consisted of “contrast” images. Specifically, for each participant, the following first-level contrast images were generated: (PV, SC, or PV + SC minus CL), (PV minus SC), (SC minus PV), and [2 (PV + SC) minus (SC plus PV)] in the PV and/or SC session and answer session (Mizuno et al., 2012).
Second, the contrast images corresponding to each condition in each participant were used for group analyses with a random-effects model to obtain population inferences (Friston et al., 1999). We used a flexible factorial design that can compare the activities of task level contrasts within all the above contrast images, and among the 1st, 2nd, and 3rd trials of HCA trial groups by repeated measures, as well as compare those between the HCA and CCFS groups by non-repeated measures. The resulting set of voxel values for each comparison constituted a statistical parametric map of t statistics [SPM(t)]. Significant signal changes for each contrast were assessed by means of t statistics on a voxel-by-voxel basis. The threshold for the SPM(t) of group analyses was set at p < 0.005 at the voxel level and p < 0.05 with a correction for multiple comparisons at the cluster level for the entire brain (Mizuno et al., 2012).
Comparisons of PV, SC, and PV + SC conditions with the CL condition (PV, SC, or PV + SC minus CL) were performed in order to obtain the activation pattern of the two types of single task processing and dual task processing. To specify the brain areas involved in the processing of PV, we used the contrast of (PV minus SC). Likewise, to identify the brain areas involved in the processing of SC, we used the contrast of (SC minus PV). In addition, to specify the brain areas involved in the processing of PV + SC, we used the contrast of [2 (PV + SC) minus (SC plus PV)]. Anatomic localization of significant voxels within clusters was done using the Wake Forest University Pick-Atlas (Maldjian et al., 2003) and a probabilistic cytoarchitectonic map (Eickhoff et al., 2005). The effects of task condition or group (HCA and CCFS) on activation of brain region and task performance (reaction time and accuracy) in single and dual trials were analyzed using one-way repeated-measures analysis of variance (ANOVA) or two-way ANOVA. When statistically significant effects were found, intergroup differences were evaluated using a paired t-test with the Bonferroni correction or Student's t-test. All p values were two-tailed, and p values less than 0.05 were considered significant. These analyses were performed with the SPSS 17.0 software package (IBM SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Questionnaire
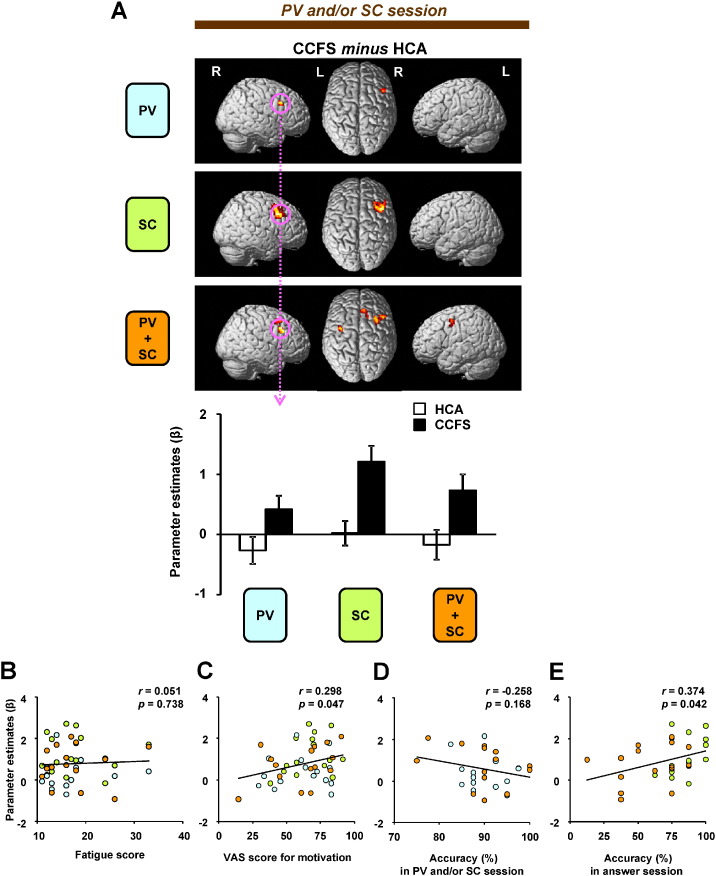
Table 1 summarizes the results for the fatigue score of the Chalder Fatigue Scale and the VAS scores for motivation to single or dual tasks. The fatigue scores of HCA gradually increased at each measurement point [F(2, 24) = 3.47, p = 0.048]. Fatigue scores of CCFS patients were higher than those of HCA. The VAS scores for the motivation of each condition in HCA among all the measurement points were not significantly different, and those of all the conditions between HCA and CCFS were nearly equal.
Table 1.
Demographic characteristics of participants.
| HCA |
CCFS | ||||
|---|---|---|---|---|---|
| 1st trial | 2nd trial | 3rd trial | All | ||
| Age (years) | 12.2 ± 0.8 | 13.5 ± 0.9 | 14.4 ± 0.9 | 13.4 ± 1.2 | 13.5 ± 1.0 |
| BMI (kg/m2) | 18.6 ± 1.7 | 19.6 ± 2.3 | 20.2 ± 2.2 | 19.5 ± 2.1 | 20.1 ± 4.2 |
| Disease duration (month) | 12.3 ± 6.4 | ||||
| WISC-III, FIQ score | 96.9 ± 12.2 | ||||
| Fatigue score | 11.0 ± 6.3 | 12.4 ± 7.6 | 14.0 ± 9.2 | 12.5 ± 7.7 | 17.5 ± 6.1* |
| VAS score for motivation | |||||
| CL | 64.4 ± 27.1 | 71.8 ± 12.3 | 73.5 ± 19.7 | 69.9 ± 20.4 | 65.6 ± 15.9 |
| PV | 68.1 ± 20.5 | 71.5 ± 14.4 | 78.8 ± 13.4 | 72.8 ± 16.6 | 61.3 ± 19.5 |
| SC | 66.4 ± 25.4 | 70.3 ± 16.8 | 75.6 ± 10.7 | 70.8 ± 18.5 | 66.8 ± 15.3 |
| PV + SC | 61.5 ± 28.1 | 68.0 ± 21.5 | 78.8 ± 24.7 | 69.5 ± 25.3 | 61.0 ± 22.0 |
HCA, healthy children and adolescents; CCFS, childhood chronic fatigue syndrome; BMI, body mass index; WISC-III, Wechsler intelligence scale for children-third edition; FIQ, full scale intelligence quotient; VAS, visual analogue scale; CL, control condition; PV, picking out vowels condition; SC, story comprehension condition; PV + SC, concurrent processes of PV and SC conditions. Values are the number or mean ± SD.
p < 0.05, significantly different from the corresponding values for the healthy adolescents (All).
3.2. Task performance
The task performance results are summarized in Table 2. In HCA, the reaction times and accuracies of each condition in both sessions among all the measurement points were not significantly different. The reaction times and accuracies of each condition in the PV and/or SC session between CCFS patients and HCA were similar. The reaction times of each condition in the answer session (questions to assess story comprehension) between CCFS patients and HCA were also nearly equal. In the answer session, although accuracies of the CL, PV, and SC conditions between CCFS patients and HCA were not different, accuracy of the PV + SC condition in CCFS patients was lower than that in HCA.
Table 2.
Task performances of control (CL), picking out vowels (PV), story comprehension (SC) and the concurrent processes of PV and SC (PV + SC) conditions.
| HCA |
CCFS | ||||
|---|---|---|---|---|---|
| 1st trial | 2nd trial | 3rd trial | All | ||
| VAS score for motivation | |||||
| CL | 64.4 ± 27.1 | 71.8 ± 12.3 | 73.5 ± 19.7 | 69.9 ± 20.4 | 65.6 ± 15.9 |
| PV | 68.1 ± 20.5 | 71.5 ± 14.4 | 78.8 ± 13.4 | 72.8 ± 16.6 | 61.3 ± 19.5 |
| SC | 66.4 ± 25.4 | 70.3 ± 16.8 | 75.6 ± 10.7 | 70.8 ± 18.5 | 66.8 ± 15.3 |
| PV + SC | 61.5 ± 28.1 | 68.0 ± 21.5 | 78.8 ± 24.7 | 69.5 ± 25.3 | 61.0 ± 22.0 |
| PV and/or SC session | |||||
| Reaction time (ms) | |||||
| CL | 340.2 ± 64.7 | 314.5 ± 58.9 | 345.1 ± 73.0 | 333.3 ± 65.5 | 385.6 ± 130.8 |
| PV | 746.9 ± 69.0 | 700.7 ± 72.3 | 648.5 ± 118.6 | 698.7 ± 110.9 | 760.0 ± 92.0 |
| SC | 366.5 ± 120.0 | 407.8 ± 72.3 | 358.7 ± 90.4 | 377.7 ± 78.8 | 499.3 ± 140.1 |
| PV + SC | 742.0 ± 86.3 | 765.6 ± 96.6 | 719.3 ± 104.7 | 742.3 ± 95.6 | 778.9 ± 114.7 |
| Accuracy (%) | |||||
| CL | 98.7 ± 1.9 | 97.1 ± 4.2 | 95.2 ± 5.8 | 97.0 ± 4.4 | 97.3 ± 3.5 |
| PV | 83.1 ± 8.1 | 86.9 ± 10.2 | 87.9 ± 5.8 | 86.0 ± 8.3 | 89.7 ± 4.3 |
| SC | 95.8 ± 5.0 | 95.2 ± 6.2 | 95.8 ± 4.8 | 95.6 ± 5.3 | 94.2 ± 7.9 |
| PV + SC | 82.9 ± 7.8 | 90.4 ± 7.3 | 93.3 ± 4.7 | 88.8 ± 7.9 | 90.2 ± 7.1 |
| Answer session | |||||
| Reaction time (ms) | |||||
| CL | 826.7 ± 144.8 | 798.8 ± 238.0 | 781.0 ± 149.7 | 802.2 ± 178.7 | 807.1 ± 229.2 |
| PV | 979.4 ± 375.2 | 932.7 ± 199.4 | 825.5 ± 162.9 | 912.5 ± 216.9 | 911.3 ± 203.2 |
| SC | 2077.8 ± 263.1 | 1970.1 ± 325.0 | 1744.3 ± 495.0 | 1930.8 ± 418.3 | 1926.5 ± 427.6 |
| PV + SC | 2258.6 ± 440.7 | 2098.4 ± 413.9 | 1978.4 ± 590.0 | 2111.8 ± 488.7 | 2052.9 ± 353.7 |
| Accuracy (%) | |||||
| CL | 100.0 ± 0.0 | 98.1 ± 4.7 | 97.1 ± 10.4 | 98.4 ± 6.5 | 98.3 ± 4.4 |
| PV | 99.0 ± 3.5 | 95.2 ± 14.0 | 97.1 ± 7.5 | 97.1 ± 9.3 | 99.2 ± 3.2 |
| SC | 69.2 ± 18.1 | 78.8 ± 12.9 | 84.6 ± 14.6 | 77.6 ± 16.3 | 85.0 ± 11.8 |
| PV + SC | 71.2 ± 21.9 | 72.1 ± 14.6 | 83.7 ± 18.0 | 75.6 ± 18.8 | 58.3 ± 22.5** |
HCA, healthy children and adolescents; CCFS, childhood chronic fatigue syndrome. Values are the mean ± SD.
p < 0.01, significantly different from the corresponding values for the HCA (All).
3.3. fMRI
3.3.1. Single and dual task sessions
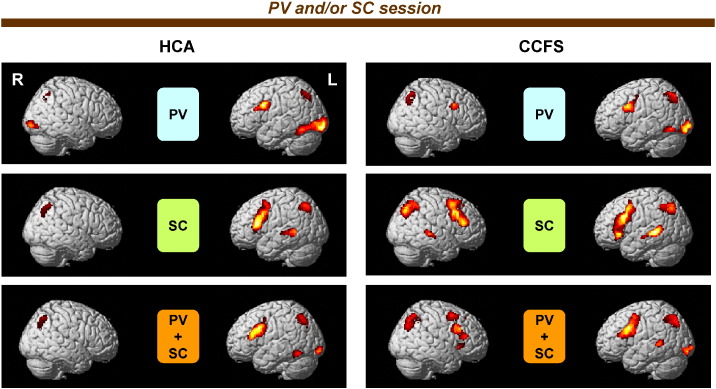
The imaging results for each condition in the PV and/or SC sessions are shown in Fig. 2 and Table 3. In HCA, the lIFG, bilateral SPL, lFFG, left inferior occipital gyrus (lIOG), left middle occipital gyrus, and cerebellum were significantly activated in the PV condition. The left middle frontal gyrus (lMFG), lIFG, lMTG, left superior temporal gyrus, bilateral SPL, bilateral inferior parietal lobules (IPL), and bilateral precuneus were activated in the SC condition. The activated brain regions in the PV + SC condition almost overlapped with those in the PV or SC conditions. However, additional activated regions were not observed. In comparison with activation levels of the activated regions among all the measurement points in HCA (Table 4), we did not find any differences. Likewise, we found similar results obtained by each comparison among them, discussed in the following paragraphs, indicating that activation levels and patterns in each condition were not changed by development in HCA. In CCFS patients, these activated regions were also observed in each condition. However, in addition to these brain regions, the right MFG (rMFG) was specifically activated in all conditions in CCFS patients. In the PV + SC condition, specific activation of the anterior cingulate cortex (ACC) was also observed in CCFS patients.
Fig. 2.
Statistical parametric maps (SPM) of activation for each condition in the PV and/or SC session.The SPM of picking out vowels (PV minus CL), story comprehension (SC minus CL), and concurrent processes of both PV and SC (PV + SC minus CL) conditions in the PV and/or SC session. The extent threshold was set at p = 0.05 with a correction for multiple comparisons at the cluster level for the entire brain. The height threshold was set at p = 0.005 (uncorrected) at the voxel level. Statistical parametric maps are superimposed on surface-rendered high-resolution MRIs. Right (R) and left (L) sides are indicated. HCA, healthy children and adolescents; CCFS, childhood chronic fatigue syndrome.
Table 3.
Activated brain regions associated with picking out vowels (PV), story comprehension (SC) and the concurrent processes of PV and SC (PV + SC) during the PV and/or SC session.
| HCA (All) |
CCFS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Brain region | Side | BA | MNI coordinates | Z |
MNI coordinates | Z |
||||
| Value | Value | |||||||||
| PV minus CL | ||||||||||
| Middle frontal gyrus | R | 9 | 44 | 14 | 32 | 5.39 | ||||
| L | 6 | −34 | −6 | 48 | 3.64 | |||||
| Inferior frontal gyrus | L | 9/44 | −44 | 8 | 30 | 7.55 | −40 | 12 | 28 | 6.98 |
| Superior parietal lobule | L | 7 | −24 | −66 | 42 | Inf | −22 | −64 | 46 | 6.65 |
| R | 7 | 28 | −64 | 42 | 4.92 | 28 | −60 | 44 | 4.91 | |
| Fusiform gyrus | L | 37 | −46 | −56 | −16 | 6.02 | −46 | −66 | −10 | 4.44 |
| Inferior occipital gyrus | L | 18 | −30 | −90 | −10 | 4.93 | −30 | −92 | −10 | 4.78 |
| Middle occipital gyrus | L | 18 | −38 | −84 | −12 | 3.89 | −38 | −82 | −16 | 3.32 |
| Cerebellum | R | 8 | −76 | −26 | 4.88 | 4 | −78 | −30 | 4.44 | |
| SC minus CL | ||||||||||
| Middle frontal gyrus | R | 9 | 42 | 14 | 34 | 6.20 | ||||
| L | 6 | −46 | 4 | 52 | 5.30 | −44 | 4 | 50 | 4.08 | |
| R | 6 | 34 | 10 | 56 | 4.92 | |||||
| Inferior frontal gyrus | L | 44/45 | −56 | 24 | 16 | 6.31 | −58 | 24 | 20 | 5.15 |
| L | 9/44 | −44 | 8 | 34 | 5.59 | −52 | 20 | 30 | 5.63 | |
| Middle temporal gyrus | L | 21 | −66 | −28 | 0 | 4.61 | −56 | −18 | −8 | 3.97 |
| Superior temporal gyrus | L | 22 | −52 | −42 | 2 | 4.72 | −58 | −40 | 2 | 7.02 |
| R | 22 | 54 | −28 | −2 | 4.73 | |||||
| Inferior parietal lobule | L | 40 | −32 | −54 | 42 | 3.54 | −32 | −54 | 44 | 6.17 |
| R | 40 | 34 | −56 | 46 | 3.33 | 34 | −54 | 46 | 5.71 | |
| Superior parietal lobule | L | 7 | −30 | −64 | 46 | 5.92 | −30 | −68 | 46 | 5.88 |
| R | 7 | 32 | −64 | 46 | 4.11 | 34 | −62 | 54 | 5.46 | |
| Precuneus | L | 7 | −32 | −68 | 32 | 3.17 | −32 | −66 | 42 | 6.39 |
| R | 7 | 32 | −68 | 34 | 4.03 | 32 | −68 | 40 | 5.88 | |
| PV + SC minus CL | ||||||||||
| Middle frontal gyrus | R | 9 | 38 | 16 | 34 | 5.08 | ||||
| L | 6 | −34 | −4 | 50 | 4.57 | |||||
| R | 6 | 36 | 6 | 56 | 4.36 | |||||
| Inferior frontal gyrus | L | 44/45 | −58 | 24 | 20 | 3.96 | −46 | 24 | 20 | 4.39 |
| L | 9/44 | −44 | 10 | 28 | 7.56 | −40 | 14 | 28 | 7.47 | |
| Medial frontal gyrus | L | 8 | −4 | 18 | 50 | 5.18 | −4 | 18 | 48 | 5.13 |
| R | 8 | 2 | 18 | 48 | 5.51 | |||||
| Anterior cingulate gyrus | L | 32 | −12 | 16 | 40 | 3.28 | ||||
| R | 32 | 12 | 20 | 40 | 5.44 | |||||
| Superior temporal gyrus | L | 22 | −56 | −42 | 4 | 4.64 | ||||
| Inferior parietal lobule | L | 40 | −32 | −54 | 42 | 5.00 | −30 | −54 | 44 | 5.02 |
| R | 40 | 32 | −56 | 46 | 4.11 | 32 | −54 | 42 | 4.77 | |
| Superior parietal lobule | L | 7 | −26 | −64 | 40 | 7.84 | −26 | −64 | 42 | 6.47 |
| R | 7 | 30 | −64 | 44 | 5.25 | 28 | −66 | 44 | 5.59 | |
| Precuneus | L | 7 | −30 | −68 | 32 | 5.34 | −30 | −68 | 30 | 3.42 |
| R | 7 | 34 | −66 | 30 | 3.50 | 34 | −68 | 34 | 4.68 | |
| Fusiform gyrus | L | 37 | −48 | −56 | −16 | 5.01 | ||||
| Inferior occipital gyrus | L | 18 | −28 | −92 | −10 | 3.93 | −30 | −92 | −12 | 4.23 |
| Cerebellum | R | 8 | −78 | −26 | 5.54 | 4 | −78 | −30 | 4.79 | |
HCA, healthy children and adolescents; CCFS, childhood chronic fatigue syndrome; L, left; R, right; BA, Brodmann's area; MNI, Montréal Neurological Institute; inf, infinity. The extent threshold was set at p = 0.05 with a correction for multiple comparisons at the cluster level for the entire brain. The height threshold was set at p = 0.005 (uncorrected) at the voxel level.
Table 4.
Comparisons for activation patterns of each task condition among 1st, 2nd, and 3rd trials in healthy children and adolescents (HCA) using six contrasts.
| HCA |
|||
|---|---|---|---|
| Contrast | 1st trial | 2nd trial | 3rd trial |
| 1st –2nd | 1 | −1 | 0 |
| 1st –3rd | 1 | 0 | −1 |
| 2nd –1st | −1 | 1 | 0 |
| 2nd –3rd | 0 | 1 | −1 |
| 3rd –1st | −1 | 0 | 1 |
| 3rd –2nd | 0 | −1 | 1 |
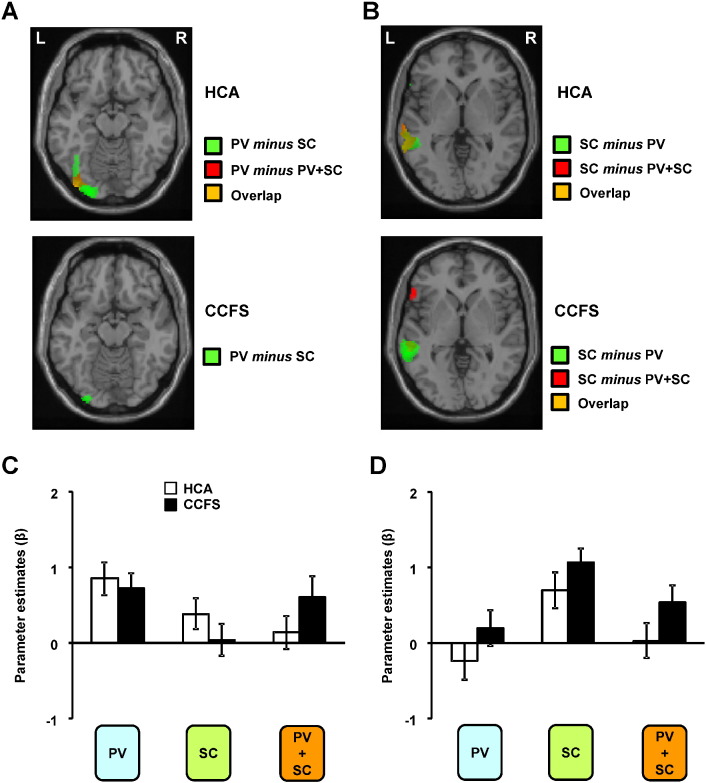
Unique or more strongly activated brain regions during single tasks (Fig. 3) and dual tasks (Fig. 4) in the PV and/or SC session were identified. The activations of the lFFG and lIOG of the PV condition were higher than those of the SC condition in HCA (Fig. 3A). In the SC condition, unique activation of the lMTG was observed in comparison with the PV condition in HCA and CCFS patients (Fig. 3B). In addition, we found that activation of the lIOG of the PV condition was greater than that of the PV + SC condition in HCA (Fig. 3A), and the activation of the lMTG of the SC condition was higher than that of the PV + SC condition in HCA and CCFS patients (Fig. 3B). The activation levels of these regions between HCA and CCFS patients were not significantly different (Fig. 3C, D). In both HCA and CCFS patients, although the lDIFG and lSPL were commonly activated in the PV, SC, and PV + SC conditions (Fig. 2), activations of these regions in the PV + SC condition were higher than those in the PV or SC conditions (Fig. 4A). Activation levels of the lDIFG [Brodmann area (BA) 9: x = −46, y = 12, z = 26 (common activated coordinates between HCA and CCFS patients)] and lSPL (BA 7: x = −28, y = −66, z = 38) between HCA and CCFS patients were not significantly different. Consistent with our results from HCA and CCFS patients, the lDIFG and lSPL were strongly activated, whereas the lFFG, lIOG, and lMTG were deactivated during the divided attention task relative to the two single tasks using the KPT in our previous study of healthy adults (Mizuno et al., 2012).
Fig. 3.
Decrease in activities of dual-task sessions.Statistical parametric maps of higher activation of the (A) left inferior occipital gyrus of the healthy children and adolescents (HCA) and patients with childhood chronic fatigue syndrome (CCFS) in the condition of picking out vowels (PV minus SC or PV minus PV + SC) and (B) left middle temporal gyrus of the healthy adolescents and patients in the condition of story comprehension (SC minus PV or SC minus PV + SC) during the PV and/or SC session. The extent threshold was set at p = 0.05 with a correction for multiple comparisons at the cluster level for the entire brain. The height threshold was set at p = 0.005 (uncorrected) at the voxel level. Right (R) and left (L) sides are indicated. The extent of activations of (C) left inferior occipital gyrus (x = −38, y = −86, z = −12) and (D) left middle temporal gyrus (x = −56, y = −36, z = −2) among the PV, SC, and PV + SC conditions (PV minus CL, SC minus CL, and PV + SC minus CL). Values are expressed as the mean and SD.
Fig. 4.
Increase in activities of dual-task sessions and correlations.(A) Statistical parametric maps of greater activations of the left dorsal inferior frontal gyrus (lDIFG) and left superior parietal lobule (lSPL) in the PV + SC condition compared with the PV and SC conditions during the PV and/or SC session. The extent threshold was set at p = 0.05 with a correction for multiple comparisons at the cluster level, and the height threshold was set at p = 0.005 (uncorrected) at the voxel level. Right (R) and left (L) sides are indicated. The extent of activations are shown of the lDIFG (x = −46, y = 12, z = 26) and lSPL (x = −28, y = −66, z = 38) among the PV, SC, and PV + SC conditions (PV minus control (CL), SC minus CL, and PV + SC minus CL). Values are the mean ± SD. Correlations are shown between the Chalder Fatigue Score and activation levels in the lDIFG in the healthy children and adolescents (HCA) (B) and childhood chronic fatigue syndrome (CCFS) groups (C) and in the lSPL in the HCA (D) and CCFS groups (E). Correlations are shown between accuracy in the dual task condition in the answer session and activation levels of the lDIFG in the HCA (F) and CCFS groups (G) and of the lSPL in the HCA (H) and CCFS groups (I). The linear regression line, p value, and Pearson's correlation coefficient are shown.
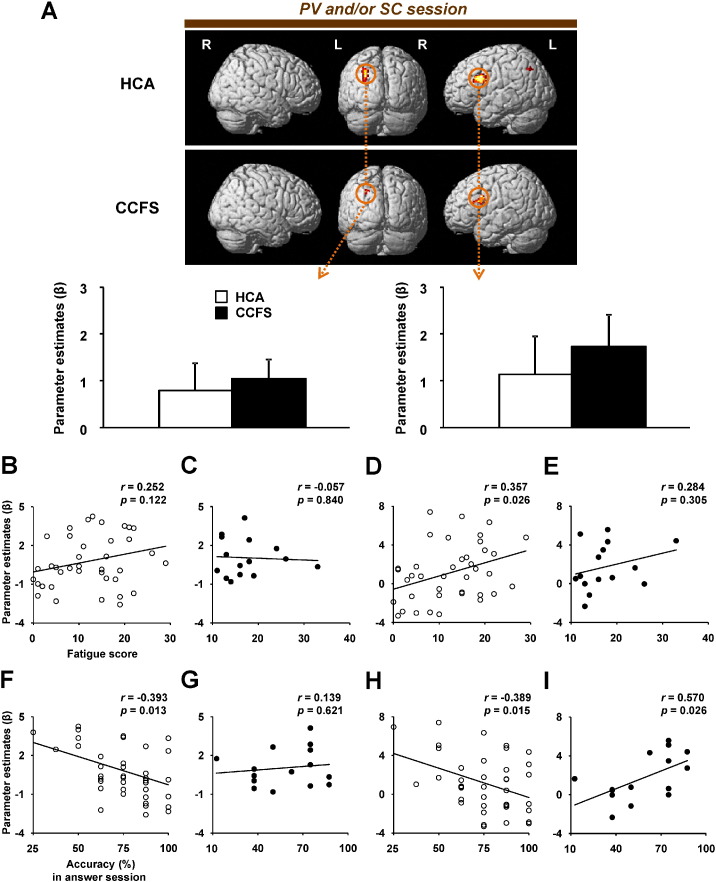
3.3.2. Correlations between fatigue and activity
To clarify fatigue-related neural processing during divided attention in HCA and CCFS patients, we performed correlation analyses between the fatigue scores and activation levels in these brain regions. Although activation of the lSPL was not correlated with the fatigue score in HCA (Fig. 4B) or CCFS patients (Fig. 4C), activation of the lDIFG was positively correlated with the fatigue score in HCA (Fig. 4D), but not in CCFS patients (Fig. 4E). The activation of the lDIFG was negatively correlated with the accuracy of outcomes in the PV + SC condition in the answer session in HCA (Fig. 4F). However, this correlation was not observed in CCFS patients (Fig. 4G). Although there was a negative correlation between activation of the lDIFG and accuracy of the PV + SC condition in the answer session in HCA (Fig. 4H), a positive correlation between them was observed in CCFS patients (Fig. 4I). We also performed correlation analyses between VAS scores for motivation and activation levels in these brain regions. Only in CCFS patients, the VAS score tended to be positively correlated with activation of the lDIFG (Table 5).
Table 5.
Correlations between visual analogue scale score for motivation and activations of the left dorsal inferior frontal gyrus (lDIFG) or left superior parietal lobule (lSPL) of the dual task (PV + SC) condition during the PV and/or SC session.
| HCA |
CCFS |
|||
|---|---|---|---|---|
| r | p value | r | p value | |
| lDIFG (x = −46, y = 12, z = 26) | −0.189 | 0.250 | 0.460 | 0.085 |
| lSPL (x = −28, y = −66, z = 38) | 0.022 | 0.894 | 0.431 | 0.108 |
PV, picking out vowels; SC, story comprehension; PV + SC, concurrent processes of PV and SC; r, Pearson's correlation coefficient. Montréal Neurological Institute coordinates (x, y, z) are shown.
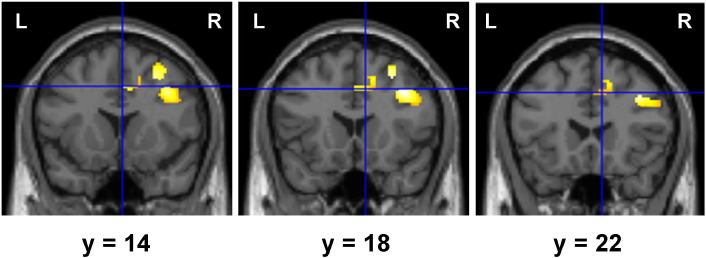
A comparison of the results between HCA and CCFS patients for each condition in the PV and/or SC session is shown in Fig. 5. In all conditions, activations of the rMFG, which is included in the dorsolateral prefrontal cortex (DLPFC) [BA 9: x = 38, y = 16, z = 36 (commonly activated coordinates among PV, SC, and PV + SC conditions)], in CCFS patients were higher than those in HCA (Fig. 5A). Furthermore, only in the PV + SC condition, activations of the dorsal ACC (dACC, BA 32, Fig. 6) and lMFG (BA 6) in CCFS patients were higher than those in HCA. Correlation analyses revealed that although activation levels of the rMFG of all conditions were not correlated with fatigue scores in CCFS patients (Fig. 5B), those of all conditions were positively correlated with VAS scores for motivation (Fig. 5C). In addition, although correlation between the activation levels for the rMFG in the PV and PV + SC conditions and accuracies of these conditions in the PV and/or SC session was not observed (Fig. 5D), a positive correlation between activation levels for the rMFG in the SC and PV + SC conditions and accuracies of these conditions in the answer session was observed (Fig. 5E). In the PV + SC condition, activations of the dACC and lMFG were positively correlated with VAS scores for motivation and fatigue, respectively (Table 6).
Fig. 5.
Higher activities of patients with childhood chronic fatigue syndrome (CCFS) during divided attention and correlations.(A) Statistical parametric maps of greater activations of the brain regions in the CCFS group compared with the HCA group in each condition during the PV and/or SC session. The extent threshold was set at p = 0.05 with a correction for multiple comparisons at the cluster level. The height threshold was set at p = 0.005 (uncorrected) at the voxel level. Right (R) and left (L) sides are indicated. The extent of activations of the right middle frontal gyrus (rMFG; x = 38, y = 16, z = 36) among the PV, SC, and PV + SC conditions (PV minus CL, SC minus CL, and PV + SC minus CL) are shown. Values are the mean ± SD. In the patients, correlations are shown between (B) fatigue score or (C) score of visual analogue scale for motivation and activation levels of the rMFG in the PV, SC, and PV + SC conditions. (D) Correlations between accuracies of the PV and/or SC session in the PV and PV + SC conditions and activation levels of the rMFG in these conditions, and (E) correlations between accuracies of the answer session in the SC and PV + SC conditions and activation levels of the rMFG in these conditions are shown.
Fig. 6.
Higher activity of the dorsal anterior cingulate cortex in CCFS patients during divided attention.Statistical parametric maps of greater activations of the dorsal anterior cingulate cortex in the CCFS group compared with the HCA group in PV + SC condition during the PV and/or SC session. The extent threshold was set at p = 0.05 with a correction for multiple comparisons at the cluster level. The height threshold was set at p = 0.005 (uncorrected) at the voxel level. Right (R) and left (L) sides and y-axis (MNI coordinate) are indicated.
Table 6.
Correlations between activations of the dorsal anterior cingulate cortex (dACC) or left middle frontal gyrus (lMFG) and subjective scores or task performances of the dual task (PV + SC) condition during the PV and SC session in patients with childhood chronic fatigue syndrome.
| dACC (x = 4, y = 14, z = 40) |
lMFG (x = −34, y = −8, z = 50) |
|||
|---|---|---|---|---|
| r | p value | r | p value | |
| Fatigue score | −0.133 | 0.636 | 0.558 | 0.031 |
| VAS score for motivation | 0.653 | 0.008 | −0.207 | 0.458 |
| Accuracy (PV and/or SC session) | −0.279 | 0.314 | −0.063 | 0.824 |
| Accuracy (answer session) | 0.193 | 0.491 | −0.035 | 0.901 |
PV, picking out vowels; SC, story comprehension; PV + SC, concurrent processes of PV and SC; VAS, visual analogue scale; r, Pearson's correlation coefficient. Montréal Neurological Institute coordinates (x, y, z) are shown.
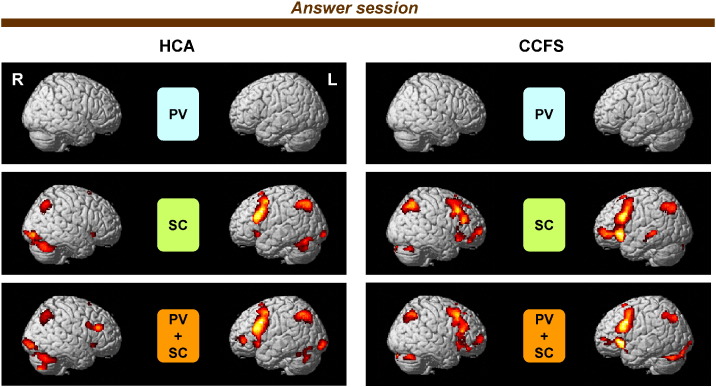
Imaging results for each condition in the answer session are shown in Fig. 7. In the SC condition, the lMFG, bilateral IFG, bilateral medial frontal gyri, bilateral IPL, bilateral SPL, and cerebellum were activated in HCA and CCFS patients. These brain regions were also activated in the PV + SC condition in HCA and CCFS patients. Unique or more strongly activated regions in the PV + SC condition in comparison with the SC condition were not observed in either HCA or CCFS patients. Activation levels of each condition between HCA and CCFS patients were not significantly different.
Fig. 7.
Statistical parametric maps (SPM) of activation for each condition in answer session.SPM of activation for each condition of picking out vowels (PV minus CL), story comprehension (SC minus CL) and concurrent processes both of PV and SC (PV + SC minus CL) in the answer session. The extent threshold was set at p = 0.05 with a correction for multiple comparisons at the cluster level for the entire brain. The height threshold was set at p = 0.005 (uncorrected) at the voxel level. Statistical parametric maps are superimposed on surface-rendered high-resolution MRIs. Right (R) and left (L) sides are indicated. HCA, healthy children and adolescents; CCFS, childhood chronic fatigue syndrome.
4. Discussion
4.1. Summary of results
Our principal findings were that: 1) activation in the lDIFG was greater in the dual task condition than in the two single task conditions in HCA and CCFS patients; 2) in HCA, this activation level was positively and negatively correlated with the fatigue score and the accuracy for story comprehension, respectively; 3) in CCFS patients, this activation level was positively and negatively correlated with accuracy for story comprehension and motivation score for the tasks, respectively; 4) the rDLPFC of CCFS patients was specifically activated in both the single and dual task conditions, and these activation levels were positively correlated with motivation scores for the tasks and accuracy for story comprehension; and 5) in CCFS patients, the dACC and lMFG were activated only in the dual task condition, and activation levels of the dACC and lMFG were positively associated with motivation and fatigue scores, respectively.
4.2. Activation patterns of KPT
The fronto-parietal areas, including the lDIFG and lSPL, are engaged by visual attentional processes (Kanwisher and Wojciulik, 2000). Bookheimer noted that increased activation of the lDIFG may reflect an increased need for attention to verbal memory processing (Bookheimer, 2002). In addition, greater activation in the left parietal lobule during tasks with a higher short-term memory load is associated with allocation of attentional resources (Magen et al., 2009). When two arithmetic tasks are performed concurrently, the tasks compete for limited resources (Wickens et al., 1983), especially when these tasks entail activation in the same parts of the cortex (Klingberg and Roland, 1997), such as the lDIFG and lSPL in the present study. Therefore, enhanced activations of the lDIFG and lSPL in the dual task condition engage more attentional processing than the single task conditions, due to greater and more complex demands on voluntary attentional resources. Consistent with the present findings, we previously found that in young adults, activations of the lDIFG and lSPL in the dual task condition were higher than in the two single task conditions of the KPT. In contrast, activations of the lFFG and lMTG, which are primary regions for processing of the PV and SC tasks, respectively, were lower in the dual task condition than in the two single task conditions (Mizuno et al., 2012). These cognitive compensation and decompensation patterns in the young adults were similar to those in children and adolescents in the present study, suggesting that the neural substrates of the KPT do not change dramatically from childhood to adulthood. However, in healthy children and adolescents, although the lDIFG was not strongly activated, the participants demonstrated greater dual task performance. This suggests differences in activity levels in the lDIFG in the KPT between children and adults, i.e., adults may perform the KPT through more energy-efficient neural processing in comparison with children and adolescents.
4.3. Excessive frontal activity
In HCA, activations of the lDIFG, which was more strongly activated in the dual task condition compared with the two single task conditions, were positively correlated with fatigue scores and negatively correlated with accurate comprehension. Higher performance of a high-effort dual task is associated with a decrease in activation of the prefrontal cortex related to attentional resources, including the IFG (Jaeggi et al., 2007), suggesting that minimal utilization of attentional resources results in better performance of the dual task. Because fatigue is related to an increase in brain activity during a high-effort cognitive task (Cook et al., 2007; Lange et al., 2005), fatigue may require utilization of more attentional resources during divided attention, which is reflected as greater activation of the lDIFG, resulting in lower performance. In contrast, there were positive correlations between activation of the lDIFG and accurate comprehension or mental effort level in the dual task condition in CCFS patients. This suggests that although severe fatigue consumes a considerable amount of attentional resources, severe fatigue forcibly recruits additional activation (cognitive compensation) and attentional resources, depending on the extent of mental effort, for better performance of the dual task. Namely, CCFS patients have an impetus to recruit additional attentional, working memory, and salience regions (MFG, dACC) (Menon and Uddin, 2010) in an attempt to complete the task despite their CFS disabilities.
In addition, a greater level of fatigue in CCFS patients induced additional activation of the rMFG (rDLPFC) in both single and dual task conditions. Our recent study revealed that increased activity of the right rMFG, which is included in the DLPFC, is involved in a facilitation system to increase the motor output caused by motivational input during physical fatigue conditions in healthy young adults (Tanaka et al., 2013). Moreover, consistent with the present findings, a previous study by Lange et al. showed that more extensive ACC and bilateral prefrontal regions of patients with adult CFS were activated during a verbal working memory task in comparison with healthy adults (Lange et al., 2005). It has been unclear whether these activations are caused by positive or negative emotion or whether they lead to better or poorer task performance. In the present study, we demonstrated that rDLPFC activation was associated with greater motivation and more accurate comprehension in CCFS patients, suggesting that the severely fatigued brain needs to recruit right frontal activation to perform the single and dual tasks appropriately, depending on the greater mental effort.
Only in the dual task condition were specifically activated regions (dACC and lMFG) observed. Because activation of the dACC was positively correlated with motivation level, to which we also referred previously (Holroyd and Yeung, 2012), activation may play a crucial role in motivation processing during the dual task trials in CCFS patients. In addition to the lDIFG, greater activation of the lMFG was observed, indicating that severe fatigue recruits activation of not only the primary brain region involved in the attentional resource, but also the adjacent region. In fact, a positive association between activation of the lMFG and the severity of fatigue was observed. Vasic et al. (2008) reported that decreased activity of the lMFG (BA 6) in adolescents and young adults with dyslexia was observed during a verbal working memory task. Lange et al. (2005) found that during a verbal working memory task, a wider area of the lMFG (BA 6/8) of CFS adults (270 voxels) was activated in comparison with healthy adults (7 voxels). These results suggest that this cognitive compensative activity, i.e., an increase in activity of the lMFG (BA 6), may be a common pathophysiological feature during an effortful executive verbal task in children and adults with CFS and may be a specific feature in CFS because this excessive activity of the lMFG (BA 6) has not been observed in other developmental disorders. Hence, patients with CCFS exhibit a wider area of activated frontal cortex during divided attention processing, and these excessive neural activations may contribute to a vicious cycle of further increases in fatigue.
Although previous imaging studies have reported that regional cerebral blood flow in the frontal regions of the brain is low during the resting state in both adult CFS patients (Kuratsune et al., 2002) and CCFS patients (Miike et al., 2004), more extensive frontal regions were activated in the present study, including bilateral MFG and dACC, particularly during the dual task condition. Therefore, CCFS may be characterized by overactivity of widespread frontal regions during divided attention processing. Other studies have demonstrated reduction of gray-matter volumes of the rDLPFC (BA 9/46) in adult CFS patients (de Lange et al., 2008; Okada et al., 2004) and an association between the severity of fatigue and volume reduction of the rDLPFC. In addition, in adult CFS patients, reductions in the biosynthesis of the neurotransmitters glutamate, aspartate, and gamma-aminobutyric acid through acetyl-carnitine in the bilateral MFG and ACC (Kuratsune et al., 2002) and reductions in serotonin transporters in the ACC (Yamamoto et al., 2004) were observed using positron emission tomography. In addition, our previous study using positron emission tomography revealed that although the regional cerebral flow and uptake of acetyl-carnitine in the lFFGs of adults with CFS were not changed, those in the lMTG were reduced compared with healthy adults (Kuratsune et al., 2002). This indicates that not only the cognitive compensation regions but also the cognitive decompensation regions such as the lMTG are related to the pathophysiology of CFS. In addition to cognitive compensation and cognitive decompensation during the verbal divided attention task, short bursts of activity may occur in a large number of brain regions in a state of illness that allow accurate task completion compared to HCA and changes in the default mode network in CCFS patients (Menon, 2011). A recent study reported the existence of cerebral oxidative stress and mitochondrial dysfunction in adult CFS patients (Shungu et al., 2012), suggesting that accumulation of free radicals by excessive neural activities in chronic fatigue may induce cerebral oxidative stress. Although further prospective study in fatigued children and adolescents and CCFS patients is necessary, it is possible that hyperactivity of the frontal regions in the brain during high-effort tasks is associated with pathophysiological alterations in the structures, neurotransmitter dynamics, and mitochondrial function in the frontal cortex from childhood to adulthood.
Ross et al. (2001) reported a decrease in performance of a dual task in adults with CFS. Unfortunately, no reports have been published about the neural substrates of reduced dual task processing in adults with CFS. Further study is necessary to identify the association between neural substrates of the KPT in adults with CFS and children and adolescents with CFS. Our previous studies demonstrated that performance in attention control tasks (divided attention and switching attention tasks) in patients with CCFS is reduced compared with that in healthy children and adolescents (Tomoda et al., 2007; Kawatani et al., 2011). In this study, we investigated the effects of combination therapy with cognitive behavioral therapy and antidepressant medication for 6 months on the severity of mental fatigue and switching attention performance in CCFS patients (Kawatani et al., 2011). This combination therapy decreased the severity of mental fatigue and improved the performance in the switching attention task. Correlations between these changes in fatigue severity and task performance by treatment were also observed. These results suggest that combined treatment with cognitive behavioral therapy and antidepressant medication is effective for improving poor attention characteristics associated with CCFS and may improve the cognitive compensation activity of the frontal cortex during the divided attention task. A review paper mentioned that the features of Japanese CCFS are a lower motivation to learn, lifestyle and social factors such as chronic shortage of sleep, highly competitive scholastic environment, hard training at sports clubs, serious trouble with human relations (e.g., bullying), changing living environments (e.g., moving to a new house), and exhaustion after viral infection (Miike et al., 2004). This suggests that further studies are necessary to evaluate the intervention effects of lifestyle choices and social factors in CCFS patients.
5. Conclusion
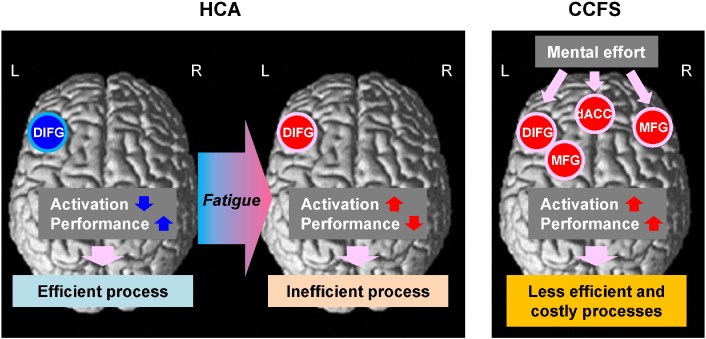
Fig. 8 provides a conceptual diagram of the conclusions from the findings of the present study. Better performance by HCA on the dual task was associated with activation of a limited area of the frontal cortex. This appears to be an energy-efficient process. Fatigue in HCA induces greater activation of the frontal cortex and lower performance, indicating an inefficient process. With the same task, CCFS patients exhibited a wider area of activated frontal regions related to attentional resources in order to obtain an increase in their poorer task performance by forcibly driving motivation. This is likely to be less efficient and costly in terms of energy requirements. This excessive frontal neural activation during a divided attention task is a characteristic feature of fatigued children and adolescents and CCFS patients. It seems to be related to the pathophysiology of CCFS patients and causes a vicious cycle of a further increase in fatigue. Previous studies have suggested that recovery or compensation in language function following development of a lesion in the left hemisphere may depend on mechanisms in the right hemisphere (Blasi et al., 2002; de Guibert et al., 2011). Specifically, greater activations of the right frontal regions during language and verbal working memory tasks are observed in patients with aphasia and dyslexia. In addition to recovery and compensation, we advocate a new concept of excessive brain activity caused by chronic fatigue.
Fig. 8.
Differences in the role of neural activation during divided attention in HCA with or without fatigue and CCFS patients.In healthy children and adolescents (HCA) without fatigue, although lDIFG was not strongly activated, the participants demonstrated greater dual task performance, indicating more efficient processing. Fatigue of HCA induced greater activation of the lDIFG and lower performance, indicating an inefficient process. In patients with childhood chronic fatigue syndrome (CCFS), greater mental effort strongly forced the bilateral frontal regions (dACC and MFG) into activation in order to obtain an increase in the patients' reduced performance, indicating energy-inefficiency and costly processing. These widespread activities of the frontal regions may induce a vicious cycle of further increase in fatigue.
Author contribution
K.M. took part in the planning and designing of the experiment, data analysis and interpretation and manuscript preparation. M.T. contributed to the design and planning of the experiment, data interpretation and manuscript preparation. H.C.T. contributed to the design of the fMRI tasks and data analysis and interpretation. T.J., J.K., A.T. and T.M. diagnosed and recruited the participants with CCFS and performed the experiments. Y.S. performed the experiments and data analysis. K.I. contributed to the planning of the experiment and recruited the participants who were healthy adolescents. N.S. contributed to the design of the fMRI tasks, and participated in data interpretation and manuscript preparation. Y.W. planned, designed and supervised the entire set of experiments, and participated in data interpretation and manuscript preparation. All the authors listed have seen and approved the final manuscript. Furthermore, we have taken due care to ensure the integrity of our work and our scientific reputation.
Conflicts of interest
The authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgments
This work was supported by the Japan Science and Technology Corporation (JST)/Research Institute of Science and Technology for Society (RISTEX) (07052628, Y.W.), the Cooperative Study Program of the National Institute for Physiological Sciences (604, Y.W.) and the Grant-in-Aid for Scientific Research (B) (25282211, K.M.) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan. We would like to thank Ms. Kaoru Yoshida and Ms. Kanako Tajima for their excellent technical assistance, and Forte Science Communications for editorial help with the manuscript. None of the authors have any conflict of interest.
References
- BERD (Benesse Educational Research and Development Center) Benesse Corporation; Tama: 2006. The 4th Survey of Attitudes Toward Study and Actual Learning in Elementary and Junior High School Students. [Google Scholar]
- Blasi V., Young A.C., Tansy A.P., Petersen S.E., Snyder A.Z., Corbetta M. Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron. 2002;36(1):159–170. doi: 10.1016/s0896-6273(02)00936-4. 12367514 [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu. Rev. Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. 12052907 [DOI] [PubMed] [Google Scholar]
- Chalder T., Berelowitz G., Pawlikowska T., Watts L., Wessely S., Wright D., Wallace E.P. Development of a fatigue scale. J. Psychosom. Res. 1993;37(2):147–153. doi: 10.1016/0022-3999(93)90081-p. 8463991 [DOI] [PubMed] [Google Scholar]
- Chaudhuri A., Behan P.O. Fatigue in neurological disorders. Lancet. 2004;363(9413):978–988. doi: 10.1016/S0140-6736(04)15794-2. 15043967 [DOI] [PubMed] [Google Scholar]
- Cook D.B., O'Connor P.J., Lange G., Steffener J. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. Neuroimage. 2007;36(1):108–122. doi: 10.1016/j.neuroimage.2007.02.033. 17408973 [DOI] [PubMed] [Google Scholar]
- de Guibert C., Maumet C., Jannin P., Ferré J.C., Tréguier C., Barillot C., Le Rumeur E., Allaire C., Biraben A. Abnormal functional lateralization and activity of language brain areas in typical specific language impairment (developmental dysphasia) Brain. 2011;134(10):3044–3058. doi: 10.1093/brain/awr141. 21719430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange F.P., Koers A., Kalkman J.S., Bleijenberg G., Hagoort P., van der Meer J.W., Toni I. Increase in prefrontal cortical volume following cognitive behavioural therapy in patients with chronic fatigue syndrome. Brain. 2008;131(8):2172–2180. doi: 10.1093/brain/awn140. 18587150 [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K., Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. 15850749 [DOI] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Worsley K.J. How many subjects constitute a study? Neuroimage. 1999;10(1):1–5. doi: 10.1006/nimg.1999.0439. 10385576 [DOI] [PubMed] [Google Scholar]
- Fukuda K., Straus S.E., Hickie I., Sharpe M.C., Dobbins J.G., Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994;121(12):953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. 7978722 [DOI] [PubMed] [Google Scholar]
- Garralda M.E., Rangel L. Annotation: chronic fatigue syndrome in children and adolescents. J. Child Psychol. Psychiatry. 2002;43(2):169–176. doi: 10.1111/1469-7610.00010. 11902596 [DOI] [PubMed] [Google Scholar]
- Grossman M., Cooke A., DeVita C., Chen W., Moore P., Detre J., Alsop D., Gee J. Sentence processing strategies in healthy seniors with poor comprehension: an fMRI study. Brain Lang. 2002;80(3):296–313. doi: 10.1006/brln.2001.2581. 11896643 [DOI] [PubMed] [Google Scholar]
- Haig-Ferguson A., Tucker P., Eaton N., Hunt L., Crawley E. Memory and attention problems in children with chronic fatigue syndrome or myalgic encephalopathy. Arch. Dis. Child. 2009;94(10):757–762. doi: 10.1136/adc.2008.143032. 19001478 [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Yeung N. Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn. Sci. (Regul. Ed.) 2012;16(2):122–128. doi: 10.1016/j.tics.2011.12.008. 22226543 [DOI] [PubMed] [Google Scholar]
- Jaeggi S.M., Buschkuehl M., Etienne A., Ozdoba C., Perrig W.J., Nirkko A.C. On how high performers keep cool brains in situations of cognitive overload. Cogn. Affect. Behav. Neurosci. 2007;7(2):75–89. doi: 10.3758/cabn.7.2.75. 17672380 [DOI] [PubMed] [Google Scholar]
- Kanwisher N., Wojciulik E. Visual attention: insights from brain imaging. Nat. Rev. Neurosci. 2000;1(2):91–100. doi: 10.1038/35039043. [DOI] [PubMed] [Google Scholar]
- Kawatani J., Mizuno K., Shiraishi S., Takao M., Joudoi T., Fukuda S., Watanabe Y., Tomoda A. Cognitive dysfunction and mental fatigue in childhood chronic fatigue syndrome − a 6-month follow-up study. Brain Dev. 2011;33(10):832–841. doi: 10.1016/j.braindev.2010.12.009. 21530119 [DOI] [PubMed] [Google Scholar]
- Klingberg T., Roland P.E. Interference between two concurrent tasks is associated with activation of overlapping fields in the cortex. Cogn. Brain Res. 1997;6(1):1–8. doi: 10.1016/s0926-6410(97)00010-4. [DOI] [PubMed] [Google Scholar]
- Kuratsune H., Yamaguti K., Lindh G., Evengård B., Hagberg G., Matsumura K., Iwase M., Onoe H., Takahashi M., Machii T., Kanakura Y., Kitani T., Långström B., Watanabe Y. Brain regions involved in fatigue sensation: reduced acetylcarnitine uptake into the brain. Neuroimage. 2002;17(3):1256–1265. doi: 10.1006/nimg.2002.1260. 12414265 [DOI] [PubMed] [Google Scholar]
- Lange G., Steffener J., Cook D.B., Bly B.M., Christodoulou C., Liu W.C., Deluca J., Natelson B.H. Objective evidence of cognitive complaints in chronic fatigue syndrome: a BOLD fMRI study of verbal working memory. Neuroimage. 2005;26(2):513–524. doi: 10.1016/j.neuroimage.2005.02.011. 15907308 [DOI] [PubMed] [Google Scholar]
- Magen H., Emmanouil T.A., McMains S.A., Kastner S., Treisman A. Attentional demands predict short-term memory load response in posterior parietal cortex. Neuropsychologia. 2009;47(8–9):1790–1798. doi: 10.1016/j.neuropsychologia.2009.02.015. 19428411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. 12880848 [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. (Regul. Ed.) 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. 21908230 [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. 20512370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miike T., Bell S.D. Chronic fatigue syndrome in childhood and adolescence. In: Watanabe Y., Evengård B., Natelson B.H., Jason L.A., Kuratsune H., editors. Fatigue Science for Human Health. Springer; New York: 2008. pp. 153–171. [Google Scholar]
- Miike T., Tomoda A., Jhodoi T., Iwatani N., Mabe H. Learning and memorization impairment in childhood chronic fatigue syndrome manifesting as school phobia in Japan. Brain Dev. 2004;26(7):442–447. doi: 10.1016/j.braindev.2003.10.004. 15351079 [DOI] [PubMed] [Google Scholar]
- Mizuno K., Tanaka M., Fukuda S., Imai-Matsumura K., Watanabe Y. Relationship between cognitive functions and prevalence of fatigue in elementary and junior high school students. Brain Dev. 2011;33(6):470–479. doi: 10.1016/j.braindev.2010.08.012. 20846803 [DOI] [PubMed] [Google Scholar]
- Mizuno K., Tanaka M., Fukuda S., Imai-Matsumura K., Watanabe Y. Relationship between cognitive function and prevalence of decrease in intrinsic academic motivation in adolescents. Behav. Brain Funct. 2011;7:4. doi: 10.1186/1744-9081-7-4. 21235802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K., Tanaka M., Fukuda S., Imai-Matsumura K., Watanabe Y. Divided attention of adolescents related to lifestyles and academic and family conditions. Brain Dev. 2013;35(5):435–440. doi: 10.1016/j.braindev.2012.07.007. 22877835 [DOI] [PubMed] [Google Scholar]
- Mizuno K., Tanaka M., Fukuda S., Sasabe T., Imai-Matsumura K., Watanabe Y. Changes in cognitive functions of students in the transitional period from elementary school to junior high school. Brain Dev. 2011;33(5):412–420. doi: 10.1016/j.braindev.2010.07.005. 20708862 [DOI] [PubMed] [Google Scholar]
- Mizuno K., Tanaka M., Ishii A., Tanabe H.C., Onoe H., Sadato N., Watanabe Y. The neural basis of academic achievement motivation. Neuroimage. 2008;42(1):369–378. doi: 10.1016/j.neuroimage.2008.04.253. 18550387 [DOI] [PubMed] [Google Scholar]
- Mizuno K., Tanaka M., Tanabe H.C., Sadato N., Watanabe Y. The neural substrates associated with attentional resources and difficulty of concurrent processing of the two verbal tasks. Neuropsychologia. 2012;50(8):1998–2009. doi: 10.1016/j.neuropsychologia.2012.04.025. 22571931 [DOI] [PubMed] [Google Scholar]
- Mizuno K., Watanabe Y. Neurocognitive impairment in childhood chronic fatigue syndrome. Front. Physiol. 2013;4:87. doi: 10.3389/fphys.2013.00087. 23626579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray S.O., He S. Contrast invariance in the human lateral occipital complex depends on attention. Curr. Biol. 2006;16(6):606–611. doi: 10.1016/j.cub.2006.02.019. 16546086 [DOI] [PubMed] [Google Scholar]
- Okada T., Tanaka M., Kuratsune H., Watanabe Y., Sadato N. Mechanisms underlying fatigue: a voxel-Based morphometric study of chronic fatigue syndrome. B.M.C. Neurol. 2004;4(1):14. doi: 10.1186/1471-2377-4-14. 15461817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. 5146491 [DOI] [PubMed] [Google Scholar]
- Ross S., Fantie B., Straus S.F., Grafman J. Divided attention deficits in patients with chronic fatigue syndrome. Appl. Neuropsychol. 2001;8(1):4–11. doi: 10.1207/S15324826AN0801_2. 11388122 [DOI] [PubMed] [Google Scholar]
- Shungu D.C., Weiduschat N., Murrough J.W., Mao X., Pillemer S., Dyke J.P., Medow M.S., Natelson B.H., Stewart J.M., Mathew S.J. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. N.M.R. Biomed. 2012;25(9):1073–1087. doi: 10.1002/nbm.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Fukuda S., Mizuno K., Imai-Matsumura K., Jodoi T., Kawatani J., Takano M., Miike T., Tomoda A., Watanabe Y. Reliability and validity of the Japanese version of the Chalder Fatigue Scale among youth in Japan. Psychol. Rep. 2008;103(3):682–690. doi: 10.2466/pr0.103.3.682-690. 19320199 [DOI] [PubMed] [Google Scholar]
- Tanaka M., Ishii A., Watanabe Y. Neural mechanism of facilitation system during physical fatigue. PLOS One. 2013;8(11):e80731. doi: 10.1371/journal.pone.0080731. 24278313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda A., Mizuno K., Murayama N., Joudoi T., Igasaki T., Miyazaki M., Miike T. Event-related potentials in Japanese childhood chronic fatigue syndrome. J. Pediatr. Neurol. 2007;5:199–208. [Google Scholar]
- Travis F. Cortical and cognitive development in 4th, 8th and 12th grade students. The contribution of speed of processing and executive functioning to cognitive development. Biol. Psychol. 1998;48(1):37–56. doi: 10.1016/s0301-0511(98)00005-2. 9676358 [DOI] [PubMed] [Google Scholar]
- Vasic N., Lohr C., Steinbrink C., Martin C., Wolf R.C. Neural correlates of working memory performance in adolescents and young adults with dyslexia. Neuropsychologia. 2008;46(2):640–648. doi: 10.1016/j.neuropsychologia.2007.09.002. 17950764 [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1991. Manual for the Wechsler Intelligence Scale for Children. [Google Scholar]
- Wickens C., Kramer A., Vanasse L., Donchin E. Performance of concurrent tasks: a psychophysiological analysis of the reciprocity of information-processing resources. Science. 1983;221(4615):1080–1082. doi: 10.1126/science.6879207. 6879207 [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Ouchi Y., Onoe H., Yoshikawa E., Tsukada H., Takahashi H., Iwase M., Yamaguti K., Kuratsune H., Watanabe Y. Reduction of serotonin transporters of patients with chronic fatigue syndrome. Neuroreport. 2004;15(17):2571–2574. doi: 10.1097/00001756-200412030-00002. 15570154 [DOI] [PubMed] [Google Scholar]