Abstract
It is well known that physical inactivity leads to loss of muscle mass, but it also causes bone loss. Mechanistically, osteoclastogenesis and bone resorption have recently been shown to be regulated by vibration. However, the underlying mechanism behind the inhibition of osteoclast formation is yet unknown. Therefore, we investigated whether mechanical vibration of osteoclast precursor cells affects osteoclast formation by the involvement of fusion-related molecules such as dendritic cell-specific transmembrane protein (DC-STAMP), and P2X7 receptor (P2X7R).
RAW264.7 (a murine osteoclastic-like cell line) cells were treated with 20 ng/ml receptor activator of NF-κB ligand (RANKL). For 3 consecutive days, the cells were subjected to 1 hour of mechanical vibration with 20 µm displacement at a frequency of 4 Hz and compared to the control cells that were treated under the same condition but without the vibration. After 5 days of culture, osteoclast formation was determined. Gene expression of DC-STAMP and P2X7R by RAW264.7 cells were determined after 1 hour mechanical vibration, while protein production of the DC-STAMP was determined after 6 hours of post incubation after vibration.
As a result, mechanical vibration of RAW264.7 cells inhibited the formation of osteoclasts. Vibration down-regulated DC-STAMP gene expression by 1.6-fold in the presence of RANKL and by 1.4-fold in the absence of RANKL. Additionally, DC-STAMP protein production was also down-regulated by 1.4-fold in the presence of RANKL and by 1.2-fold in the absence of RANKL in RAW264.7 cells in response to mechanical vibration. However, vibration did not affect P2X7R gene expression. Mouse anti-DC-STAMP antibody inhibited osteoclast formation in the absence of vibration.
Our results suggest that mechanical vibration of osteoclast precursor cells reduce DC-STAMP expression in osteoclast precursor cells leading to the inhibition of osteoclast formation.
Keywords: Mechanical vibration, Osteoclasts, DC-STAMP, P2X7R, RANKL
1. INTRODUCTION
Bones are subjected to a variety of mechanical loads during daily activities. Bone mass and architecture are continuously adapted to the daily mechanical loads. Osteocytes play an important role in adaptation of bone to mechanical loading by sensing the mechanical loads [1, 2]. Osteocytes are thought to regulate bone mass by orchestrating the balance between bone formation and resorption in response to mechanical cues, such as vibrations. Mechanistically, osteoclastogenesis and bone resorption have recently been shown to be regulated via osteocytes in response to vibration [3]; however, this result leaves an interesting question, whether osteoclastogenesis is directly regulated by vibration. Lately, Wu et al. has shown that low-magnitude high-frequency vibration (0.3 g, 45 Hz) of osteoclast precursor cells inhibits the formation of osteoclasts [4]. However, the underlying mechanism behind the vibration induced inhibition of osteoclast formation in osteoclast precursor cells is yet unknown.
Recent studies suggest that vibration can positively influence skeletal homeostasis. Animal studies have demonstrated that vibration (0.3 g, 30 Hz; 0.15 g, 90 Hz; 0.3 g, 45 Hz) stimulated an anabolic response in both weight-bearing [5, 6] and non-weight-bearing [7] bones. Moreover, vibration (3.0 g, 45 Hz; 0.3 g, 45 Hz) prevented mice from ovariectomy-induced osteoporosis [8] and decreased osteoclast activity in the adolescent mouse skeleton [9], providing evidence of vibration's anti-resorptive potential. Whole-body vibration (0.2 g, 30 Hz; 2.2–5.0 g, 35–40 HZ) of human subjects was found to be anabolic to the bone in vivo, as postmenopausal women treated with vibration stimulation gained higher bone mineral density (BMD) in hip and spine compared to the placebo group after 6–12 months [10, 11]. In dentistry, physiological vibrational (chewing) load is able to maintain alveolar bone in mice [12] and rehabilitate resorbed alveolar bone in rats [13]. Although the anabolic and anti-resorptive potential of vibration is becoming apparent, it is imperative to study the underlying mechanism behind the direct effect of vibration on osteoclast formation in osteoclast precursor cells.
Osteoclasts are multinucleated cells that arise from hematopoietic cells of the monocyte/macrophage lineage [14, 15]. The process of osteoclast formation is composed of several steps, including progenitor survival, differentiation to mononuclear pre-osteoclasts, fusion to multi-nuclear mature osteoclasts, and activation to the bone resorbing osteoclasts. The regulation of osteoclast formation has been extensively studied in which the receptor activator of NF-κB ligand (RANKL) - mediated signaling pathway, and downstream transcription factors play essential roles [16]. Several proteins that affect cell fusion have been identified. Among them, dendritic cell-specific transmembrane protein (DC-STAMP) is directly associated to osteoclast fusion in vivo. DC-STAMP has a seven-transmembrane domain structure similar to the members of the G protein–coupled receptor (GPCR) superfamily. Among the GPCR superfamily, the CCR5 chemokine receptor is known to function as direct cell adhesion molecules through interactions with their transmembrane-type ligands. Given the structural similarity between DC-STAMP and chemokine receptors, DC-STAMP may also function as a direct cell adhesion molecule by interacting with its ligand. The osteoclast fusion process may be initiated upon this adhesive interaction. The ligand for DC-STAMP may be membrane bound or soluble; a soluble ligand might be released by either of the fusion partners. DC-STAMP ligation may trigger fusion of the two cells directly or may trigger the expression of as yet unknown membrane-bound molecules (‘X’) that mediate fusion [17, 18]. DC-STAMP expression was rapidly up-regulated when mouse cells were cultured in the presence of osteoclast-promoting cytokines such as RANKL [17], and inhibition of murine DC-STAMP with a polyclonal antibody suppressed osteoclast formation [17]. Consistent with these observations, the phenotype of DC-STAMP knockout mice shows few multinucleated osteoclasts and increased bone mass [16]. In contrast, overexpression of DC-STAMP in mice resulted in a phenotype with accelerated cell-to-cell fusion during osteoclast precursors’ differentiation and enhanced bone resorption [19].
Moreover, the P2X7 receptor (P2X7R) has been implicated in the process of multinucleation and cell fusion. The P2X7R is a 595 amino acid plasma membrane receptor with approximately 40% sequence identity to other members of the P2X purinergic family [20]. It has been shown that blockade of P2X7R on osteoclast precursors using a blocking antibody inhibited multinucleated osteoclast formation in vitro [21]. Conversely, P2X7R deficient mice maintain the ability to form multinucleated osteoclasts [22].
In the present study, we studied the effect of vibration on osteoclast formation by subjecting osteoclast precursor RAW264.7 cells to vibration with magnitude of 20 µm displacement at frequency of 4 Hz. The aim of this study was to investigate whether mechanical vibration of RAW264.7 cells affects osteoclast formation by involvement of fusion-related molecules such as DC-STAMP and P2X7R.
2. MATERIAL AND METHODS
2.1. Cell culture
RAW264.7 (ATCC, Manassas, VA) cells between 10–14 passages were used for the osteoclast formation assay. RAW264.7 cells were cultured up to near-confluency in 75 cm2 culture flasks using α-MEM supplemented with 10% fetal bovine serum (ATCC, Manassas, VA), 100 IU/ml penicillin and 100 µg/ml streptomycin (Cellgro, Manassas, VA) at 37°C and 5% CO2 in air.
2.2. Mechanical vibration setup
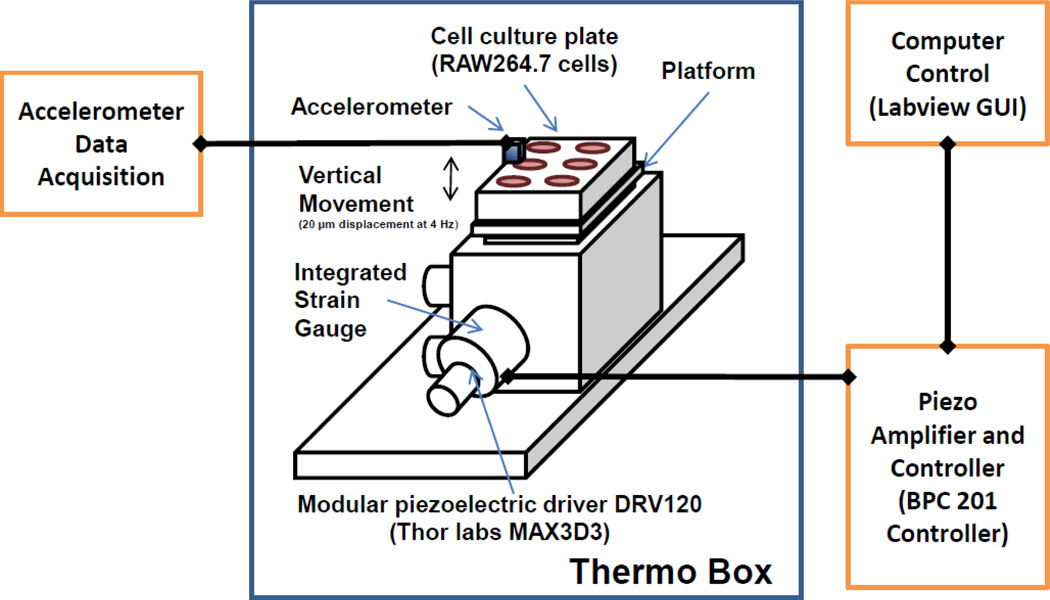
The experimental setup outlined in Fig. 1 contained both mechanical and electrical components that interface to form a complete mechatronic test system. The core of the mechanical component was a ThorLabs Max Series Modular Flexure Stage (MAX303) with a DRV120 actuator. The output of the stage was connected to an adapter plate which fits the regular cell culture plate. The core of the electrical system was a laptop running National Instruments (NI) Labview. The interface between the computer and all electrical components was done through an NI USB-6211 multifunction bus powered data-acquisition box (DAQ). The DAQ provided the input signal to a closed-loop piezo controller (Thorlabs BPC201) specifically designed for the DRV120 actuator. The controller provided the necessary control signal (including amplification) to the piezo. All high level control to the system was made through a custom graphical user interface (GUI) in Labview. After starting the GUI, the user chose the signal type (sinusoidal, square, trapezoidal), amplitude, and frequency as well as the total testing time. In this study, we specifically chose sinusoidal wave, magnitude of 20 µm displacement (peak to peak), frequency of 4 Hz and a total testing time of 1 hour. The entire mechanical component of this vibration system was housed in a thermo box at 37°C. A control was also done inside the thermo box. The cell cultures were placed on the flexure stage, but the vibration was not turned on.
Figure 1.
Experimental set-up. A rigid platform was custom-made to fit a standard multi-well tissue culture plate. A vertical vibration (20 µm displacement at 4Hz) was generated by a modular piezoelectric device and controlled by a piezo amplifier and controller connected to a computer equipped with a VibeLab user interface. An accelerometer was attached to the culture plate to confirm the vibration received by the cells. The mechanical component was housed in a thermo box at 37°C.
In previous study in which we found that physiological chewing (vibrational) load maintained alveolar bone in mice [12], which is supported by a recent study [13]. Our vibration parameters were determined on the following rationale: 20 µm displacement was the first phase of displacement in periodontal ligament of rat molar under functional loading [23], and 4 Hz was the chewing (vibration) frequency in mice [24]. As shown in Figure 1, the 20 µm displacement was generated and measured with an imbedded strain gauge provided in the Thorlabs peizoelectric actuator. The control of the actuator was provided by the off-the-shelf Thorlabs BPC controller. In order to confirm the vibration of the cell culture plate on which the cells were seeded, an external accelerometer was attached to the cell culture plate in a separate confirmation vibration experiment.
2.3. Mechanical vibration
RAW264.7 cells between passages 10–14 were harvested using cell scraper and seeded at 1.7×103 cells/well in 96-well tissue culture plates in α-MEM with 10% FBS and antibiotics. To induce osteoclast formation, RAW264.7 cells were incubated with or without 20 ng/ml mouse recombinant RANKL (R&D systems, Minneapolis, MN). After overnight seeding, the cells in the vibration group were subjected to mechanical vibration with 20 µm displacement at frequency of 4 Hz for 1 hour (Figure 1). The cells in the control group were treated under the same condition as the cells in the vibration group but without turning on the vibration. To avoid possible fluid perturbation within the wells when vibrations were applied, culture wells containing RAW264.7 cells of both the vibrated and non-vibrated groups were completely filled with 300 µl culture medium and tightly sealed with gas permeable sealing film (Excel Scientific, Victorville, CA) immediately prior to vibration. After 1 hour of vibration, the cells were either kept in culture for 5 days to assay osteoclast formation, or lysed immediately for total RNA isolation or post cultured for 6 hours for protein isolation and analysis as described below.
2.4. Osteoclast formation
RAW264.7 cells were seeded at a density of 1.7×103 cells/well in 96-well tissue culture plates. To induce osteoclast formation, RAW264.7 cells were incubated with 20 ng/ml RANKL. After overnight seeding, the cells in the vibration group were subjected to 1 hour of mechanical vibration per day for 3 consecutive days. The control group did not receive vibration. Culture medium containing 20 ng/ml RANKL was refreshed after 2 days. After 5 days of culture, the cells were fixed in 4% formaldehyde in PBS for 10 minimums. Fixed cells were washed with PBS, and stained for tartrate-resistant acid phosphatase (TRACP) according to the manufacturer's instructions (Sigma-Aldrich, St. Louis, MO). The number of TRACP-positive multinucleated (3 or more nuclei per cell) were counted using a Leica DM IL microscope (Leica, Wetzlar, Germany) equipped with a 10× objective.
To neutralize DC-STAMP protein produced, RAW264.7 cells were treated with 15 µg/ml of mouse anti-DC-STAMP (EMD Millipore Corporation. Billerica, CA). RAW264.7 cells were incubated with 20 ng/ml RANKL with or without mouse anti-DC-STAMP antibody. Culture medium containing 20 ng/ml RANKL and fresh anti-DCSTAMP antibody were refreshed after 2 days. Multinucleated TRACP-positive cells were counted after 5 days of culture.
2.5. Analysis of gene expression
Real-time polymerase chain reaction (PCR) was used to determine gene expression of DC-STAMP, P2X7R, and the housekeeping gene GAPDH (all primers from Applied Biosystems, Foster, CA). Total RNA was isolated using absolutely RNA miniprep kit® (Agilent Technologies, La Jolla, CA) according to the manufacturer’s instructions. cDNA synthesis was performed using 0.5–1 µg of total RNA in a 20 µl reaction mixture consisting of 5× VILO™ reaction mix and 10× SuperScript® enzyme mix (InVitrogen, Calrsbad, CA). Real time PCR reactions were performed using Taq-Man® Gene Expression assays (Applied Biosystems) in a StepOne™ real-time PCR system (Applied Biosystems). Gene expression values were normalized to that of the housekeeping gene GAPDH.
2.6. Western blot analysis
The cells were washed quickly with cold PBS (1×), lysed with 2× sample buffer and immediately boiled for 5 minutes. The lysis buffer contained 5 mM HEPES (pH 7.9), 150 mM NaCl, 26% glycerol (v/v), 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol and 0.5 mM phenylmethylsulfonyl fluoride. Before separation, the protein samples were centrifuged at 14,000× g for 10 minutes at room temperature to remove any cellular debris. Fifty micrograms of whole-cell lysate and a pre-stained molecular weight marker (Bio-RAD Laboratories, Hercules, CA) were boiled for 5 minutes, separated by 10% SDS-polyacrylamide gel electrophoresis and electrotransferred to a nitrocellulose membrane. Membranes were blocked in Tris-buffered saline containing 5% nonfat dry milk (Bio-RAD Laboratories) and 0.1% Tween-20 (TBST) and incubated with 2 µg/ml mouse anti-DC-STAMP (EMD Millipore Corporation, Billerica, CA) or mouse anti-GAPDH (1:1000 dilution, Cell signaling, Beverly, MA) antibodies overnight at 4°C. Following three washes in TBST, the membranes were incubated with the goat anti-mouse IgG hydroperoxidase conjugated secondary antibodies (1:5000) for 1 hour at room temperature. Immunodetection was performed using the enhanced chemiluminescence (ECL) method. Densitometry measurement was made by using Fuji Imaging software. For quantification, densitometries of DC-STAMP gel bands were normalized to that of GAPDH.
2.7. Statistics
In this study, each single experiment was repeated for at least 3 times on three different passages of RAW264.7 cells. Data were presented as mean ± SD in graphs. Differences between the means were statistically analyzed using one-way ANOVA with Tukey’s post hoc correction, and the significance was considered when P values were less than 0.05.
3. RESULTS
3.1. Mechanical vibration directly inhibits osteoclast formation from RAW264.7 cells
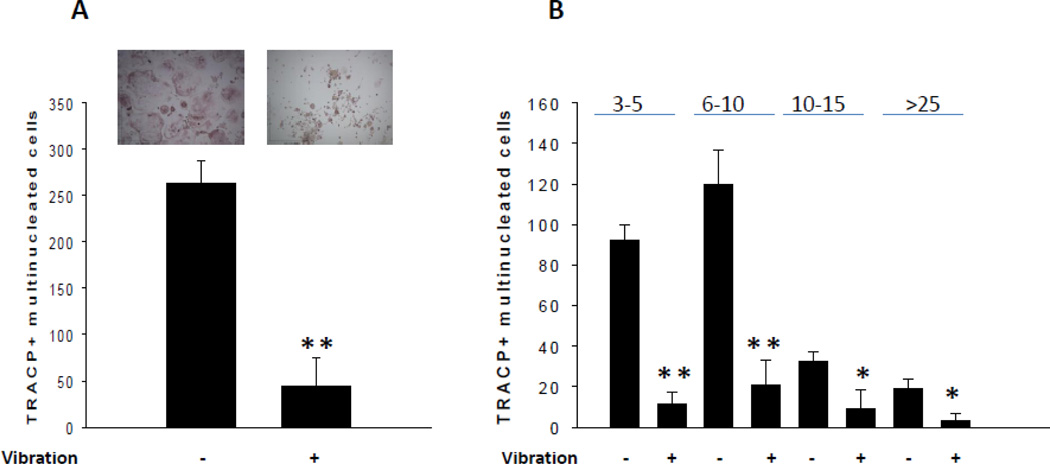
RAW264.7 cells cultured for 5 days in the presence of RANKL resulted in formation of TRACP-positive multinucleated cells (Fig. 2A). Mechanical vibration of RAW264.7 cells inhibited the formation of osteoclasts, as assessed by the number of TRACP-positive multinucleated cells after 5 days of culture (Fig. 2A). Mechanical vibration compared to static control conditions significantly inhibited the number of TRACP-positive multinucleated cells (Fig. 2B) in all groups representing the number of nuclei per cell.
Fig. 2. Effect of mechanical vibration on osteoclast formation.
Mechanical vibration significantly inhibited the total number of TRACP+ multinucleated cells (A) and in all subgroups representing the number of nuclei (3–5, 6–10, 10–15, >25) per cell (B) compared to the control (**P < 0.01, *P < 0.05, n = 7).
3.2. Mechanical vibration reduces DC-STAMP gene and protein expression
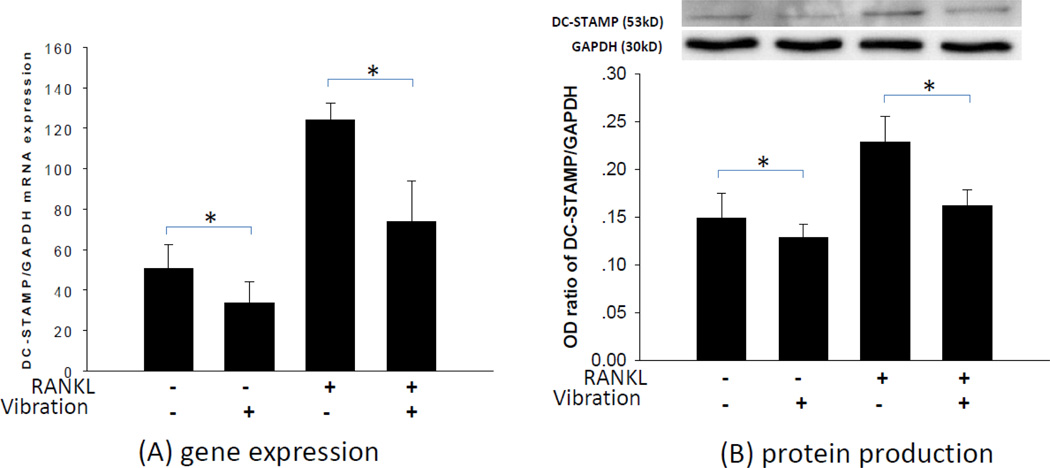
Since mechanical vibration inhibited the formation of osteoclasts, we then investigated whether mechanical vibration alters expression of cell fusion-related molecules, such as DC-STAMP and P2X7R by RAW264.7 cells. To assess whether mechanical vibration affects DC-STAMP expression, RAW264.7 cells were treated with or without 1 hour of vibration. Addition of RANKL to RAW264.7 cells up-regulated DC-STAMP gene expression by 2.5-fold and protein expression by 1.5-fold compared to the RANKL-untreated cells. Mechanical vibration significantly down-regulated DC-STAMP gene expression by 1.6-fold in the presence of RANKL and by 1.4-fold in the absence of RANKL (P < 0.05) (Fig. 3A). We also found that vibration decreased DC-STAMP protein production in RAW264.7 cells by 1.4-fold in the presence of RANKL and by 1.2-fold in the absence of RANKL (P < 0.05) (Fig. 3B).
Fig. 3. Effect of mechanical vibration on mRNA and protein expression of DC-STAMP in RAW 264.7 cells.
Mechanical vibration significantly down-regulated DC-STAMP gene expression by 1.6-fold in the presence of RANKL and by 1.4-fold in the absence of RANKL (A). Mechanical vibration significantly decreased DC-STAMP protein production by 1.4-fold in the presence of RANKL and by 1.2-fold in the absence of RANKL (B) (*P < 0.05, n = 3). A representative WB results was shown.
3.3. Mechanical vibration does not affect P2X7R gene expression
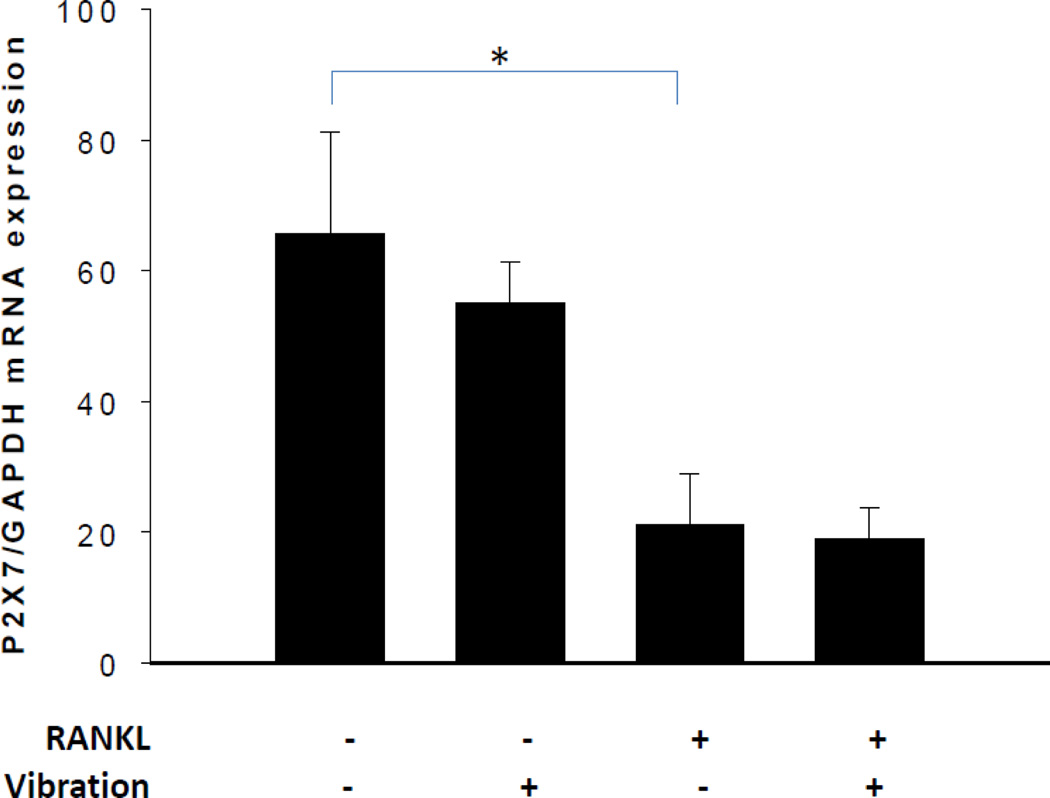
Next, we determined whether mechanical vibration affects P2X7R gene expression by RAW264.7 cells. As shown, mechanical vibration did not affect P2X7R gene expression in the presence and absence of RANKL (P > 0.05) (Fig. 4); however, we found that RANKL reduces P2X7R gene expression by 2.7-fold in RAW264.7 cells.
Fig. 4. Effect of mechanical vibration on mRNA expression of P2X7R in RAW 264.7 cells.
No difference was seen between vibration and non-vibration groups, either with or without RANKL. However, RANKL reduced P2X7R mRNA expression by 2.7-fold (*P < 0.05, n = 7).
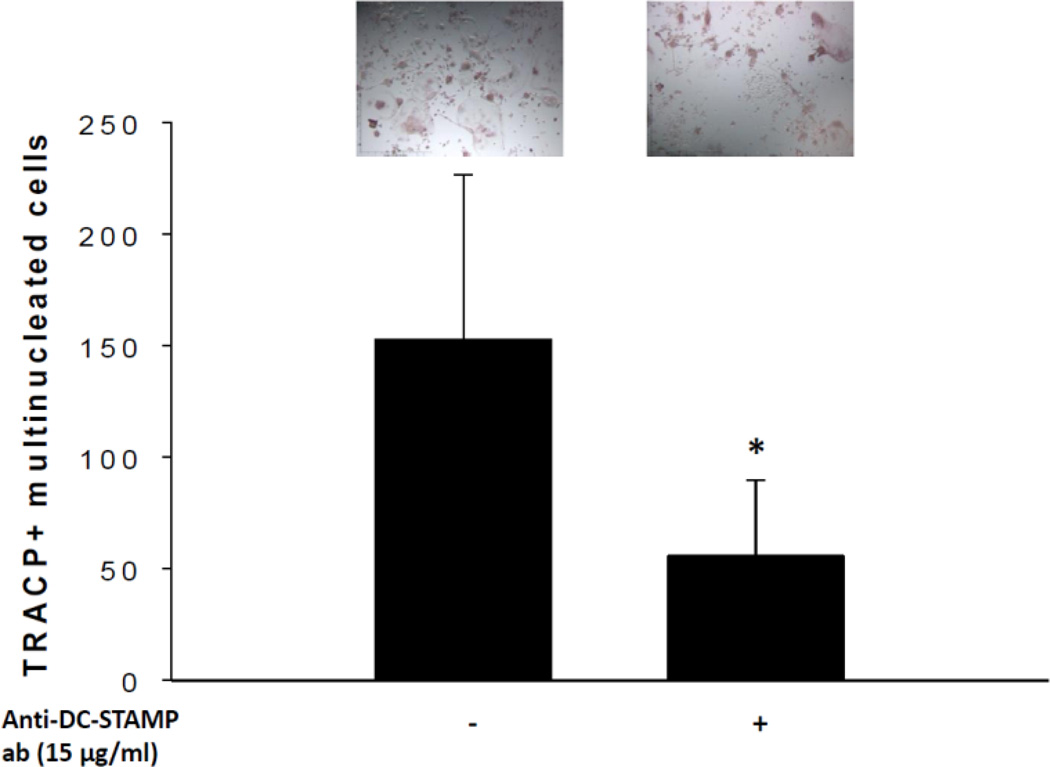
3.4. Inhibition of DC-STAMP reduces osteoclast formation from RAW264.7 cells
Since mechanical vibration significantly down-regulated DC-STAMP gene expression and protein production, we further studied whether inhibition of DC-STAMP in the absence of mechanical vibration could modulate the formation of osteoclasts. RAW264.7 cells were treated with or without mouse anti-DC-STAMP antibody in the presence of RANKL to neutralize DC-STAMP production. We found that 15 µg/ml concentration of mouse anti-DC-STAMP antibody inhibited the number of TRACP-positive multinucleated cells compared to control conditions (P<0.05) (Fig. 5).
Fig. 5. Effect of DC-STAMP inhibition on osteoclast formation in RAW cells.
15 µg/ml of mouse anti-DC-STAMP antibody inhibited the total number of TRACP+ multinucleated cells compared to control (*P < 0.05, n = 3).
4. DISCUSSION
In this study, we found that mechanical vibration inhibited osteoclast differentiation and fusion by suppressing DC-STAMP expression. These results are in agreement with a previous study showing that mechanical stress reduces osteoclast formation through DC-STAMP [25]. Furthermore, we found that gene expression of P2X7R, which is implicated in the process of multinucleation and cell fusion, did not change in response to vibration.
Osteoclast formation in vivo is thought to be induced by direct cell–cell contact of pre-osteoblastic/stromal cells with monocyte/macrophage osteoclast precursors [14]. Osteoblast lineage cells secrete key molecules, RANKL and macrophage colony stimulating factor (M-CSF), responsible for osteoclast differentiation. RANKL stimulates osteoclast precursors to commit to the osteoclastic phenotype by binding to its receptor, receptor activator of nuclear factor kappa B (RANK), on the surface of osteoclast precursors [16]. We found that mechanical vibration of osteoclast precursor cells inhibited RANKL-stimulated osteoclast formation. This is consistent with previous findings that low- magnitude high-frequency mechanical vibration of osteoclast precursor RAW264.7 cells inhibits osteoclast formation [4]. Unlike the study by Wu and colleagues [4], the culture wells containing RAW264.7 cells in this study were completely filled and tightly sealed with gas permeable sealing film immediately prior to vibration. This prevented fluid perturbation within the wells and thus possible fluid shear stimulation on the cells when the vibrations were applied, which made it possible to study the sole effect of vibration on osteoclast formation.
We then investigated the underlying mechanism of the vibration induced reduction of osteoclast formation. Since it has been shown that DC-STAMP and P2X7R molecules have an important role in the fusion of osteoclast precursors, we examined the role of DC-STAMP and P2X7R in the vibration induced inhibition of osteoclast formation. DC-STAMP is also found in macrophages and osteoclasts and is essential for the multinucleation of osteoclasts in the presence of RANKL and M-CSF [18]. Animal studies with DC-STAMP knockout mice suggest a critical role of DC-STAMP for osteoclast precursor fusion, since these mice have few multinucleated TRAP-positive osteoclasts and have increased bone mineral density [17, 18]. In contrast, experiments in DC-STAMP transgenic mice revealed accelerated bone resorption and a concomitant decrease in bone mass [19]. We found that mRNA and protein levels of DC-STAMP in RAW264.7 cells decreased in response to mechanical vibration along with a decrease in the number of osteoclasts. Moreover, inhibition of DC-STAMP with anti-DC-STAMP antibody reduced the number of osteoclasts. This suggests that mechanical vibration inhibits osteoclast formation by down-regulation of DC-STAMP expression in osteoclast precursor cells thereby inhibiting cell-cell fusion to form osteoclasts.
We then determined the effect of mechanical vibration on P2X7R gene expression. We found that mechanical vibration did not affect P2X7R gene expression in RAW264.7 cells. Therefore, P2X7R might not have a role in the vibration induced reduction in osteoclast formation. Interestingly, RANKL in the absence of vibration significantly reduced P2X7R gene expression in RAW264.7 cells. Moreover, the P2X7R deficient mice displayed higher number of osteoclasts and increased bone resorption compared to their wild-type littermates [22]. It is likely that P2X7R plays an intrinsic role in the process of osteoclast formation, however, this hypothesis needs further study.
It is widely accepted that mechanical loading of bone activates the osteocytes, which produce signaling molecules that have been shown to modulate the activity of osteoclasts [26–29]. Based on our results, it is possible that osteoclast precursor cells might also sense mechanical loading and modulate osteoclast formation. However, it is very challenging to use an in vivo model to confirm the direct effect of vibration on osteoclasts, which are regulated by many factors, including the osteocytes derived RANKL in response to mechanical vibration [3].
Osteoclast precursors are derived from hematopoietic stem cells residing in bone marrow confined by bony structures. Following the sequence of the differentiation of osteoclasts from early stage to mature, they migrate from the bone marrow to the surface of bone to be functional. In general, three mechanical factors exist in the bone marrow, namely hydrostatic pressure, viscosity and fluid shear stress [32]. A review of the current literature shows that to date there are no data showing how much of any of the three mechanical factors is sensed by osteoclast and its precursors in vivo. When the mononucleated precursors fuse to become multinucleated osteoclasts, the latter ones are closer or attached at the surface of the bone matrix, e.g. trabeculae. Although there is no direct evidence showing that cells attached to the bone matrix experience direct matrix deformation, computational models have shown that cells lying on the surface of bone would be sensitive to changes in the loading pattern, but less sensitive than the osteocytes embedded within the calcified matrix [39–40]. Further research examining the direct effect of loading on osteoclast precursor cells attached to the bone surface is needed to prove the direct regulation of osteoclastogenesis in response to load. According to some recent in vitro studies, however, neither mechanical strain [30] nor fluid shear stress [3, 31] seems to be the cause of the vibration induced response in bone cells. The effect of hydrostatic pressure due to the very limited amount of medium (300 µl/well) is also negligible in this study. This raises a larger question of what might be the mechanotransduction pathway, through which osteoclast formation is reduced by vibration. More studies will need to be done in the future.
In vivo, mesenchymal stromal cells and hematopoietic cells both reside in bone marrow, but they might respond to different type of mechanical load. Experimentally, mesenchymal stem or stromal cells (MSC) have been shown to be responsive to several forms of mechanical loading, e.g. mechanical strain [33–34] and fluid shear stress [35–37]. However, Lau et al (2011) failed to find the response of rat mesenchymal stromal cells to the low-magnitude (0.3 g) high-frequency (60 Hz) vibration [38]. Based on these previous studies and the findings in this study, it is speculated that mechanical vibration might particularly affect hematopoietic cells leading to osteoclast formation rather than mesenchymal cells, resulting in the ultimate anabolic effect of vibration on bone. Considering the uniqueness of osteoclast formation and the vibration regime (20 µm displacement and 4 Hz) used in this study, it may not be meaningful to compare our results to those from other vibration studies using totally different cell types and vibration regimes.
Although our study provides valuable insight into the role of DC-STAMP in the vibration induced inhibition of osteoclast formation, it has some limitations. Our experimental model does not exactly mimic the actual in vivo situation because osteoclast precursors in our study were seeded on a flat, stiff substrate while osteoclast precursors in vivo live in a different matrix environment, i.e. in bone marrow. In this study, we only applied vibration of 20 µm displacement at a frequency of 4 Hz. This low frequency and displacement provides very small acceleration and consequent forces acting on the cells. It is possible that other unknown factors may cause the observed response. Whether the vibrations of other magnitudes and frequencies (e.g. low-magnitude high-frequency vibration as typically used) produce a similar inhibitory effect on osteoclast formation through DC-STAMP is not known, and is the next question to be answered. Although our data show that DC-STAMP likely mediates the vibration induced reduction of osteoclast formation, there could be other signaling molecules involved in the vibration induced reduction of osteoclast formation, which would need to be further studied.
In conclusion, mechanical vibration of osteoclast precursor cells significantly inhibited osteoclast formation. Based on our results, mechanical vibration reduces DC-STAMP expression in osteoclast precursor cells leading to the inhibition of osteoclast formation. A better understanding of the underlying mechanism in the vibration induced inhibition of osteoclast formation might contribute to new treatment modalities to treat diseases such as osteopenia or osteoporosis. Mechanical vibration could be a non-pharmaceutical means for treatment of diseases like osteoporosis.
Highlights.
Mechanical vibration directly inhibits osteoclast formation from mouse macrophage cell line RAW264.7 cells.
Mechanical vibration down-regulates DC-STAMP gene expression and protein production but does not affect P2X7R gene expression in RAW264.7 cells.
The vibration induced reduction of osteoclast formation is mediated by DC-STAMP, but not by P2X7 purinergic receptor.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Toshihisa Kawai (The Forsyth Institute) for providing the RAW264.7 cells. We also like to thank Dr. Khadijah Makky (Marquette University) for her assistance in the use of the qPCR machine in her laboratory. This research is supported by the National Institute of Dental and Craniofacial Research (NIDCR) grant R03DE020867 (to D.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
REFERENCES
- 1.Cowin SC, Moss-Salentijn L, Moss ML. Candidates for the mechanosensory system in bone. J. Biomech. Eng. 1991;113:191–197. doi: 10.1115/1.2891234. [DOI] [PubMed] [Google Scholar]
- 2.Tatsumi S, Ishi K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell. Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Lau E, Al-Dujaili S, Guenther A, Liu D, Wang L, You L. Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone. 2010;46:1508–1515. doi: 10.1016/j.bone.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu SH, Zhong ZM, Chen JT. Low-magnitude high-frequency vibration inhibits RANKL-induced osteoclast differentiation of RAW264.7 cells. Int. J. Med. Sci. 2012;9:801–807. doi: 10.7150/ijms.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin C, Turner AS, Müller R, Mittra E, McLeod K, Lin W, Qin YX. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J. Bone Miner. Res. 2002;17:349–357. doi: 10.1359/jbmr.2002.17.2.349. [DOI] [PubMed] [Google Scholar]
- 6.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J. Biomech. 2007;40:1333–1339. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Garman R, Gaudette G, Donahue L, Rubin C, Judex S. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J. Orthop. Res. 2007;25:732–740. doi: 10.1002/jor.20354. [DOI] [PubMed] [Google Scholar]
- 8.Oxlund BS, Ørtoft G, Andreassen TT, Oxlund H. Low-intensity, high-frequency vibration appears to prevent the decrease in strength of the femur and tibia associated with ovariectomy of adult rats. Bone. 2003;32:69–77. doi: 10.1016/s8756-3282(02)00916-x. [DOI] [PubMed] [Google Scholar]
- 9.Xie L, Jacobson JM, Choi ES, Busa B, Donahue LR, Miller LM, Rubin CT, Judex S. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39:1059–1066. doi: 10.1016/j.bone.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J. Bone Miner. Res. 2004;19:343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 11.Verschueren SMP, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J. Bone Miner. Res. 2004;19:352–359. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- 12.Lai C, Liu D. Effect of Chewing (Occlusal Function) on Orthodontic Tooth Movement: A Pilot Radiological Study / By Christopher Lai [e-book] Ipswich, MA: 2009. Available from: MARQCATplus. [Google Scholar]
- 13.Mavropoulos A, Odman A, Ammann P, Kiliaridis S. Rehabilitation of masticatory function improves the alveolar bone architecture of the mandible in adult rats. Bone. 2010;47:687–692. doi: 10.1016/j.bone.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Lerner U. New molecules in the tumor necrosis factor ligand and receptor superfamilies with importance for physiological and pathological bone resorption. Crit. Rev. Oral. Biol. Med. 2004;15:64–81. doi: 10.1177/154411130401500202. [DOI] [PubMed] [Google Scholar]
- 15.de Vries TJ, Schoenmaker T, Hooibrink B, Leenen PJ, Everts V. Myeloid blasts are the mouse bone marrow cells prone to differentiate into osteoclasts. J. Leukoc. Biol. 2009;85:919–927. doi: 10.1189/jlb.0708402. [DOI] [PubMed] [Google Scholar]
- 16.Teitelbaum S. Osteoclasts: what do they do and how do they do it? Am. J. Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kukita T, Wada N, Kukita A, Kakimoto T, Sandra F, Toh K, Nagata K, Iijima T, Horiuchi M, Matsusaki H, Hieshima K, Yoshie O, Nomiyama H. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J. Exp. Med. 2004;200:941–946. doi: 10.1084/jem.20040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, Oike Y, Takeya M, Toyama Y, Suda T. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005;202:345–351. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasaki R, Ninomiya K, Miyamoto K, Suzuki T, Sato Y, Kawana H, Nakagawa T, Suda T, Miyamoto T. Cell fusion in osteoclasts plays a critical role in controlling bone mass and osteoblastic activity. Biochem. Biophys. Res. Commun. 2008;377:899–904. doi: 10.1016/j.bbrc.2008.10.076. [DOI] [PubMed] [Google Scholar]
- 20.Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J. Biol. Chem. 1997;272:5482–5486. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- 21.Di Virgilio F, Falzoni S, Chiozzi P, Sanz JM, Ferrari D, Buell GN. ATP receptors and giant cell formation. J. Leukoc. Biol. 1999;66:723–726. doi: 10.1002/jlb.66.5.723. [DOI] [PubMed] [Google Scholar]
- 22.Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, Grasser WA, Paralkar VM, Li M, Audoly LP, Gabel CA, Jee WS, Dixon SJ, Sims SM, Thompson DD. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol. Endocrinol. 2003;17:1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- 23.Naveh GR, Shahar R, Brumfeld V, Weiner S. Tooth movements are guided by specific contact areas between the tooth root and the jaw bone: A dynamic 3D microCT study of the rat molar. J Struct Biol. 2012;177:477–483. doi: 10.1016/j.jsb.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Byron CD, Hamrick MW, Wingard CJ. Alterations of temporalis muscle contractile force and histological content from the myostatin and Mdx deficient mouse. Arch Oral Biol. 2006;51:396–405. doi: 10.1016/j.archoralbio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Kameyama S, Yoshimura Y, Kameyama T, Kikuiri T, Matsuno M, Deyama Y, Suzuki K, Iida J. Short-term mechanical stress inhibits osteoclastogenesis via suppression of DC-STAMP in RAW264.7 cells. Int. J. Mol. Med. 2013;31:292–298. doi: 10.3892/ijmm.2012.1220. [DOI] [PubMed] [Google Scholar]
- 26.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 27.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, Kingery W, Malone AM, Kwon RY, Jacobs CR. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42:172–179. doi: 10.1016/j.bone.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulkarni RN, Bakker AD, Everts V, Klein-Nulend J. Inhibition of osteoclastogenesis by mechanically loaded osteocytes: involvement of MEPE. Calcif. Tissue Int. 2010;87:461–468. doi: 10.1007/s00223-010-9407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sen B, Xie Z, Case N, Styner M, Rubin CT, Rubin J. Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. J Biomech. 2011;44:593–599. doi: 10.1016/j.jbiomech.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uzer G, Manske SL, Chan ME, Chiang FP, Rubin CT, Frame MD, Judex S. Separating Fluid Shear Stress from Acceleration during Vibrations in Vitro: Identification of Mechanical Signals Modulating the Cellular Response. Cell Mol Bioeng. 2012;5:266–276. doi: 10.1007/s12195-012-0231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurkan UA, Akkus O. The mechanical environment of bone marrow: a review. Ann Biomed Eng. 2008;36:1978–1991. doi: 10.1007/s10439-008-9577-x. [DOI] [PubMed] [Google Scholar]
- 33.Rubin J, Murphy TC, Fan X, Goldschmidt M, Taylor WR. Activation of extracellular signal-regulated kinase is involved in mechanical strain inhibition of RANKL expression in bone stromal cells. J Bone Miner Res. 2002;17:1452–1460. doi: 10.1359/jbmr.2002.17.8.1452. [DOI] [PubMed] [Google Scholar]
- 34.Simmons CA, Matlis S, Thornton AJ, Chen S, Wang CY, Mooney DJ. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (ERK1/2) signaling pathway. J Biomech. 2003;36:1087–1096. doi: 10.1016/s0021-9290(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 35.Kreke MR, Sharp LA, Lee YW, Goldstein AS. Effect of intermittent shear stress on mechanotransductive signaling and osteoblastic differentiation of bone marrow stromal cells. Tissue Eng Part A. 2008;14:529–537. doi: 10.1089/tea.2007.0068. [DOI] [PubMed] [Google Scholar]
- 36.Sharp LA, Lee YW, Goldstein AS. Effect of low-frequency pulsatile flow on expression of osteoblastic genes by bone marrow stromal cells. Ann Biomed Eng. 2009;37:445–453. doi: 10.1007/s10439-008-9632-7. [DOI] [PubMed] [Google Scholar]
- 37.Kavlock KD, Goldstein AS. Effect of pulse frequency on the osteogenic differentiation of mesenchymal stem cells in a pulsatile perfusion bioreactor. J Biomech Eng. 2011;133:091005. doi: 10.1115/1.4004919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lau E, Lee WD, Li J, Xiao A, Davies JE, Wu Q, Wang L, You L. Effect of low-magnitude, high-frequency vibration on osteogenic differentiation of rat mesenchymal stromal cells. J Orthop Res. 2011;29:1075–1080. doi: 10.1002/jor.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullender MG, Huiskes R. Proposal for the regulatory mechanism of Wolff’s law. J. Orthop. Res. 1995;13:503–512. doi: 10.1002/jor.1100130405. [DOI] [PubMed] [Google Scholar]
- 40.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 1994;27:339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]