Abstract
Agmatine is a biogenic amine (l-arginine metabolite) of potential relevance to several central nervous system (CNS) conditions. The identities of transporters underlying agmatine and polyamine disposition in mammalian systems are not well defined. The SLC-family organic cation transporters (OCT) OCT1 and OCT2 and multidrug and toxin extrusion transporter-1 (MATE1) are transport systems that may be of importance for the cellular disposition of agmatine and putrescine. We investigated the transport of [3H]-agmatine and [3H]-putrescine in human embryonic kidney (HEK293) cells stably-transfected with hOCT1-, hOCT2-, and hMATE1. Agmatine transport by hOCT1 and hOCT2 was concentration-dependent, whereas only hOCT2 demonstrated pH-dependent transport. hOCT2 exhibited a greater affinity for agmatine (Km = 1.84 ± 0.38 mM) than did hOCT1 (Km = 18.73 ± 4.86 mM). Putrescine accumulation was pH- and concentration-dependent in hOCT2-HEK cells (Km = 11.29 ± 4.26 mM) but not hOCT1-HEK cells. Agmatine accumulation, in contrast to putrescine, was significantly enhanced by hMATE1 over-expression, and was saturable (Km = 240 ± 31 μM; Vmax = 192 ± 10 pmol/min/mg protein). Intracellular agmatine was also trans-stimulated (effluxed) from hMATE1-HEK cells in the presence of an inward proton-gradient. The hMATE1-mediated transport of agmatine was inhibited by polyamines, the prototypical substrates MPP+ and paraquat, as well as guanidine and arcaine, but not l-arginine. These results suggest that agmatine disposition may be influenced by hOCT2 and hMATE1, two transporters critical in the renal elimination of xenobiotic compounds.
Keywords: agmatine, organic cation transporter, multidrug and toxin extrusion transporter, OCT2, MATE1, agmatine transporter, polyamine, polyamine transporter, pH-dependent transport
Introduction
Agmatine and related polyamines (putrescine, spermidine, spermine) are naturally-occurring compounds implicated in cellular growth processes,1 and have been widely investigated for their role in the aberrant cellular proliferation of mammalian tissues (i.e., tumors). 2, 3–5 Agmatine (decarboxylated l-arginine) is an endogenous cation of potential relevance to several CNS conditions, including: 1) anxiety and depression,6, 7 2) neuropathic pain,8, 9 3) spinal cord and brain injury,10–13 4) drug addiction and tolerance,14, 15 5) tumor proliferation,2, 16 and 6) cellular toxicity.17, 18 In mammalian systems, the modulation of circulating agmatine and polyamine compounds occurs via three main mechanisms: 1) exogenous supply from food sources19–21 and gastrointestinal microbial production,22, 23 2) endogenous biosynthesis (degradation) via arginine and agmatine metabolism,22, 24–26 and 3) cellular sequestration (influx) and/or clearance (efflux) via membrane transporters. While the proteins that regulate agmatine and polyamine biosynthesis and metabolic degradation have been identified, little is known about the identity and function of agmatine and polyamine transporters.
Presently, the unidentified polyamine transport “system” (PTS) is the most commonly acknowledged (influx) transport system attributed to modulating intracellular polyamines in mammalian cells.27 As high cellular polyamine levels have been closely associated with carcinogenesis28 and tumor growth,29 the transport systems modulating intracellular polyamines have become an area of interest for the targeted delivery of polyamine-conjugated therapeutics in the treatment of cancer.30–32 Interestingly, agmatine, a polyamine-precursor and PTS substrate, has proven to be an effective inhibitor of polyamine-mediated tumor growth and proliferation both in vitro and in vivo,33 purportedly via the regulation of intracellular polyamines levels as consequence of antizyme induction and the correspondent inhibition of polyamine biosynthesis,5, 34 and the direct competition of polyamine influx transport.16, 33, 35 A more complete identification and characterization of agmatine and polyamine transport “systems” is needed in order to improve our understanding of the mechanisms regulating their concentrations in vivo.
As a consequence of their multivalent cationic nature at physiological pH, agmatine (1-amino-4-guanidobutane) and polyamine compounds exhibit limited passive permeability at cellular membranes and physiological barriers (e.g., the blood-brain barrier or renal tubules), and therefore likely rely on carrier transport systems for appreciable permeation at these structures. The human SLC-family transporters hOCT (1/2/3) and hMATE (1/2/2K) are potential carrier systems which may be involved in agmatine and polyamine transport. OCTs mediate the facilitated diffusion, or membrane-potential driven transport, of a wide variety of both toxic and therapeutic cations, and have predominant expression in the rodent and human liver (OCT1/OCT3), kidney (OCT2), and placenta/heart (OCT3).36 MATE transporters (MATE1/2/2K) are recently discovered H+/cation antiporters which mediate the electroneutral tubular and canalicular efflux exchange of intracellular cations in the kidney and liver.37 OCT and MATE transporters share overlapping substrate-specificity for several toxic and therapeutic cationic compounds, with the human orthologs of OCT2 and MATE1 both transporting the anti-cancer drug oxaliplatin,38 the anti-diabetic agent metformin,39, 40 and cytotoxins such as tetraethylammonium (TEA),41 paraquat,42, 43 and 1-methyl-4-phenylpyridinium (MPP+).37, 44
The identity of relevant agmatine and polyamine transport systems remains very limited. In particular, the nature of OCT-mediated agmatine transport remains unclear,45 as both agmatine influx46 and efflux47 has been demonstrated in cells over-expressing hOCT2. In addition, the interactions of agmatine and polyamine compounds/analogs with MATE transporters remains largely undefined, although a recent study demonstrated the transport of the PTS inhibitor48 paraquat by hMATE1, hOCT1, and hOCT2, but not hOCT3.42, 43
Taking into consideration both the localization and overlapping-substrate specificity of OCTs and MATE transporters, we propose that OCT1, OCT2, and MATE1 transporters are relevant to agmatine and polyamine disposition. In addition, the characterization of these systems may serve to elucidate and distinguish polyamine “transporters” from known transport systems, further defining the possible mechanisms that may regulate agmatine (polyamine) concentrations in vivo. We investigated the functional consequences of human OCT1, OCT2, and MATE1 over-expression for agmatine and putrescine transport. Our results suggest that agmatine disposition is influenced by hOCT2 and hMATE1, two closely coupled SLC-transporters classically involved in the uptake and elimination of organic cations in the renal proximal tubules.37
Material and Methods
Chemicals
[3H]-agmatine (60 Ci/mmol) and [3H]-putrescine dihydrochloride (60 Ci/mmol) were obtained from American Radiolabeled Chemicals (St. Louis, MO). [3H]-TEA chloride (88 Ci/mmol) was purchased from Moravek Biochemicals (Brea, CA). All unlabeled compounds were purchased from Sigma-Aldrich, Inc. (St. Louis, MO), except for arcaine sulfate which was purchased from Tocris Bioscience (Ellisville, MO).
Cell culture
Mock (vector-transfected) and human OCT1, OCT2, and MATE1-transfected HEK293 cells were kindly provided by Dr. Kathleen Giacomini, UCSF. Cells used for all experiments were between passages 5 and 25. HEK293 cells were cultured in Dulbecco’s Modified Eagle Medium (Mediatech, Inc., Herndon, VA) supplemented with 10% fetal bovine serum (SeraCare Life Sciences, Inc., Oceanside, CA), penicillin (100 U/mL) and streptomycin (100 μg/mL) (Sigma-Aldrich, Inc., St. Louis, MO), and hygromycin B (60 μg/mL) (EMD Chemicals, San Diego, CA).
Cellular accumulation experiments in HEK293 cells
Accumulation experiments were carried out in mock and stably-transfected hOCT1 and hOCT2 cells grown on BD BioCoat™ poly-D-lysine coated plates as previously described.43 Prior to the start of the experiment, confluent HEK293 cell monolayers were washed three times with 1 mL of blank assay buffer (122 mM NaCl, 25 mM NaHCO3, 10 mM glucose, 10 mM HEPES, 3 mM KCl, 1.2 mM MgSO4, 1.4 mM CaCl2, 0.4 mM K2HPO4, pH 7.4) and pre-incubated at 37 °C for 30 min. The blank assay buffer was aspirated and accumulation experiments were initiated with the addition of assay buffer containing tracer quantities (10 nM) of [3H]-agmatine, [3H]-putrescine, or [3H]-TEA (pH 7.4, unless noted otherwise). Accumulation experiments in hMATE1-HEK cells were conducted in assay buffer (125 mM NaCl, 4.8 mM KCl, 5.6 mM D-glucose, 1.2 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, and 25 mM Tricine) (pH 8.0, unless otherwise noted). Time-dependent accumulation experiments in hMATE1-HEK cells were done within the time-frame (15 min.) previously described.43 For concentration-dependent experiments, radiolabeled tracer compounds were supplemented with an appropriate amount of unlabeled compound to attain the appropriate total (radiolabeled plus unlabeled) concentrations. Tracer uptake values were then corrected to account for their dilution. Following accumulation experiments, cells were washed three times with ice-cold choline buffer solution (128 mM choline, 4.73 mM KCl, 1.25 mM CaCl2, 1.25 mM MgSO4, and 5 mM HEPES, pH 7.4). Cells were then lysed using a 500 μL of solution of 1% Triton-X, and incubated for 45 minutes on an orbital shaker (60 rpm) at 37 °C. Total protein concentration in each well was determined by the BCA™ protein assay (Pierce Biotechnology, Inc., Rockford, IL) and the corresponding radioactivity was determined by liquid scintillation counting (LS-6500, Beckman Coulter, Inc., Fullerton, CA).
Trans-stimulation of intracellular agmatine in hMATE1-HEK cells
Prior to the start of the trans-stimulation experiment, hMATE1-HEK cells were pre-incubated with radiolabeled agmatine (10 nM) for a period of 10 min. Following pre-incubation, cells were washed three times with ice-cold choline buffer, and a number of wells were lysed as controls. The remaining wells were incubated with fresh 37 °C blank assay buffer of pH 6.0 or pH 8.0 for 10 min. Blank assay buffer was then aspirated, and the cells were washed three times, lysed, and counted as described above. Intracellular agmatine levels following the trans-stimulation (TS) experiment were then normalized to control uptake ([(control-TS)/control]*100%).
Competition of agmatine accumulation in mock- and hMATE1-HEK cells
Agmatine accumulation (as described above) (10 min.) was investigated in mock- and hMATE1-HEK cells in the presence or absence (control) of structurally-related or prototypical substrate compounds (500 μM), including arginine, putrescine, spermidine, spermine, guanidine, arcaine, MPP+, and paraquat. Final values were normalized to agmatine accumulation in mock-control HEK cells.
Statistical Analysis
Unpaired Student’s t-test was used to compare differences between two groups (mock and transfected cells). Multiple comparisons were done using one-way ANOVA with Dunnett’s post-hoc analysis where appropriate. Data were analyzed using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). Data are expressed as the mean ± S.D. Differences were considered to be statistically significant at p < 0.05.
Results
Agmatine and putrescine transport in hOCT1- and hOCT2-HEK293 cells
We first investigated the influence of hOCT1 and hOCT2 for the intracellular accumulation of agmatine and putrescine. Steady-state (120 min) agmatine and putrescine accumulation was approximately 15- and 5-fold greater in hOCT2-HEK cells as compared to mock cells, respectively (Figs. 1a and 1b). Steady-state putrescine accumulation in mock-HEK cells was greater as compared to agmatine. Steady-state agmatine accumulation was approximately 11-fold greater in hOCT1-HEK cells as compared to mock-HEK cells (Fig. 1a), while putrescine accumulation in hOCT1-HEK cells was comparable to mock levels at 120 min (Fig. 1b). Investigation into the saturable transport of agmatine (pH 7.4) in hOCT1- and hOCT2-HEK cells revealed that agmatine was a higher-affinity substrate for hOCT2 (Fig. 2a) as compared to hOCT1 (Fig. 2b) (hOCT2-Km = 1.84 ± 0.38 mM; hOCT1-Km = 18.73 ± 4.86 mM). Putrescine accumulation in hOCT2-HEK cells was also saturable (Km = 11.29 ± 4.26 mM) (Fig. 2a), while putrescine uptake in hOCT1-HEK cells was largely linear within the concentration range examined (Fig. 2b).
Figure 1. Agmatine and Putrescine accumulation in mock-, hOCT1-, and hOCT2-HEK cells.
Agmatine (A) or Putrescine (B) accumulation was examined in HEK293 cells. Steady-state agmatine accumulation was significantly greater in hOCT1- and hOCT2-HEK cells as compared to mock cells. Steady-state putrescine accumulation was significantly greater in hOCT2-HEK cells as compared to mock- and hOCT1-HEK cells. Data are expressed as the mean ± SD (n = 3).
Figure 2. Concentration-dependence of agmatine and putrescine accumulation in hOCT2- and hOCT1-HEK cells.

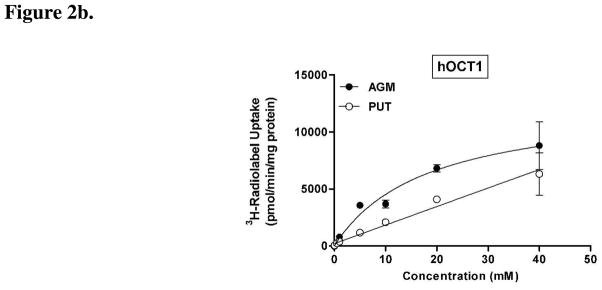
The concentration-dependent accumulation of agmatine (AGM) and putrescine (PUT) was examined in (a) hOCT2- and (b) hOCT1-HEK cells (pH 7.4). (a) Agmatine and putrescine accumulation (10 min) was saturable in hOCT2-HEK cells, with hOCT2 displaying a much higher affinity for agmatine (Km = 1.84 ± 0.38 mM) as compared to putrescine (Km = 11.29 ± 4.26 mM). (b) Agmatine, in contrast to putrescine, exhibited saturable uptake in hOCT1-HEK cells (Km = 18.73 ± 4.86 mM). Data are expressed as the mean ± SD and are representative data from two independent experiments done in triplicate (n = 6).
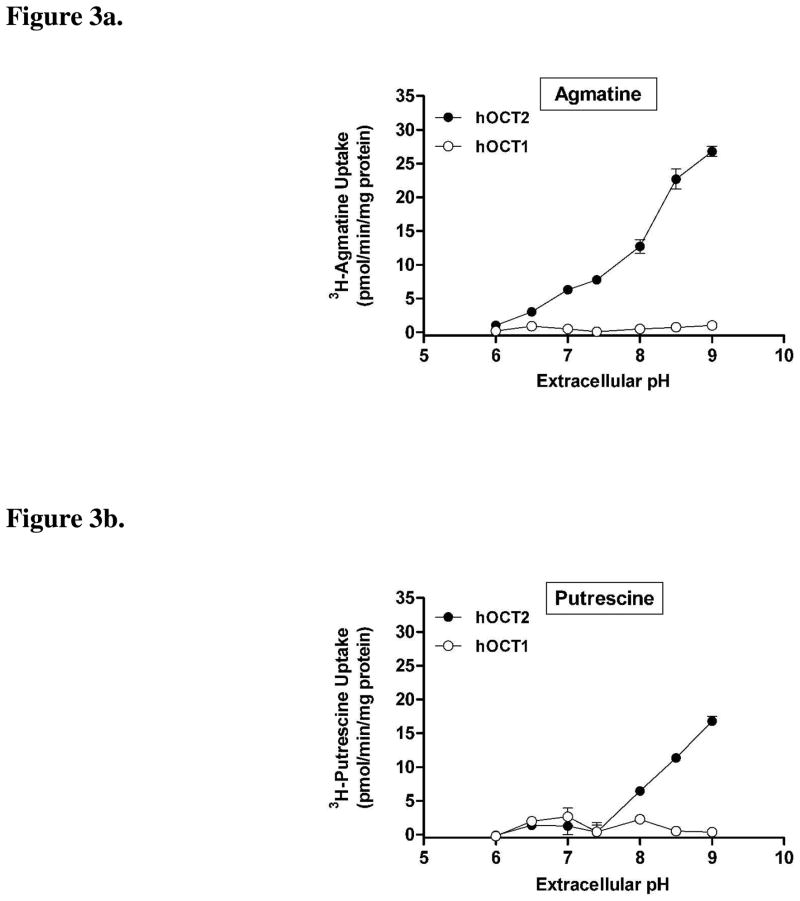
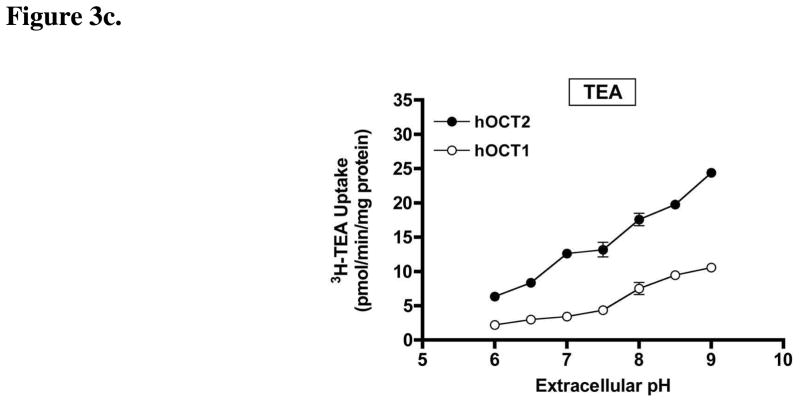
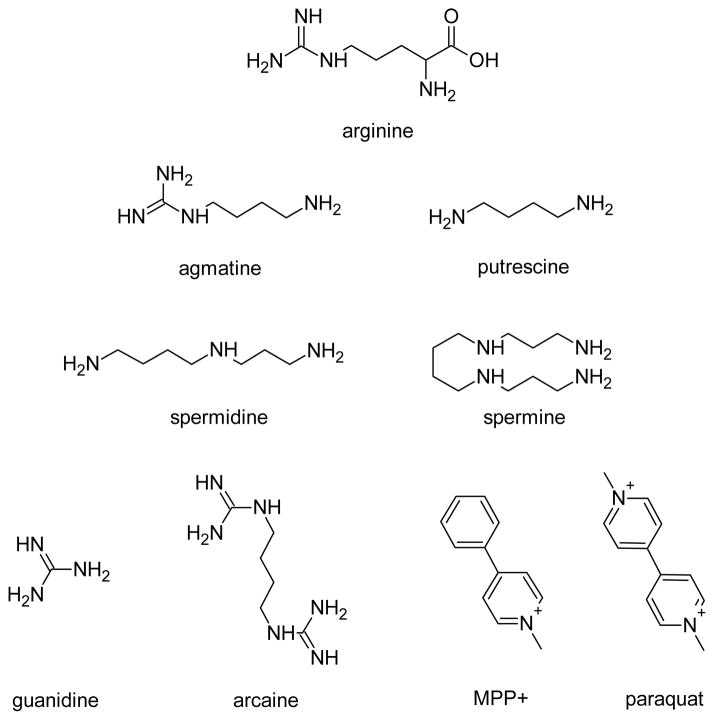
Since agmatine and putrescine have ionizable amine moieties (pKa = 9.3–10.7; ACD/pKa Database Version 11.01-Internal Reaction Centers Database) (see Structures), we investigated the influence of changes in extracellular pH on agmatine and putrescine transport by hOCT1 and hOCT2. The quaternary amine and prototypical OCT substrate TEA was also investigated for comparative purposes. Extracellular pH had little influence on the intracellular accumulation of agmatine (Fig. 3a) and putrescine (Fig. 3b) in hOCT1-HEK cells. By contrast, agmatine (Fig. 3a) and putrescine (Fig 3b) accumulation was markedly enhanced in hOCT2-HEK cells with increasing extracellular pH (Fig. 3b). Similar to agmatine (Fig. 3a), the accumulation of TEA in hOCT2-HEK cells was also influenced by extracellular pH (Fig. 3c), and exhibited enhanced accumulation under increasingly alkaline conditions. Unlike agmatine and putrescine, TEA accumulation was also influenced by changes in extracellular pH in hOCT1-HEK cells (Fig. 4). The accumulation of agmatine, putrescine, and TEA in mock-HEK cells was not influenced by extracellular pH (data not shown). Subsequent experiments demonstrated that changes in extracellular pH also had a prominent influence on agmatine and putrescine transport kinetics in hOCT2-HEK cells (Table 1), with both compounds exhibiting a higher affinity, and putrescine showing an increased transport rate (Vmax), at more alkaline extracellular pH.
Figure 3. pH-dependence of agmatine, putrescine, and TEA accumulation in hOCT1- and hOCT2-HEK cells.
The pH-dependent accumulation (10 min) of (a) agmatine, (b) putrescine, or (c) TEA was investigated in hOCT1- and hOCT2-HEK cells. Agmatine accumulation was markedly enhanced in hOCT2-HEK cells with increasing extracellular pH, exhibiting maximal uptake at pH 9.0, between pH 6.0 to 9.0. Putrescine accumulation was also enhanced in hOCT2-HEK cells at extracellular pH 8.0, 8.5, and 9.0 (compared to uptake at pH 7.4). Agmatine and putrescine accumulation was not influenced by changes in extracellular pH in hOCT1-HEK cells. TEA accumulation was increased in both hOCT1- and hOCT2-HEK cells with increasing extracellular pH. Values in all three studies are corrected for mock cellular uptake, which was not influenced by extracellular pH. Data are expressed as the mean ± SD and represent pooled data from two independent experiments done in triplicate (n = 6)
Figure 4.
Figure 4a. Agmatine accumulation in mock- and hMATE1-HEK cells
Agmatine (10 nM) accumulation was examined in mock- and hMATE1-HEK293 cells. Agmatine accumulation was significantly enhanced in hMATE1-HEK cells, as compared to mock cells. Data are expressed as the mean ± SD and represent pooled data from three independent experiments done in triplicate (n = 9) (*p < 0.05).
Figure 4b. Putrescine accumulation in mock- and hMATE1-HEK cells
Putrescine (10 nM) accumulation was examined in mock- and hMATE1-HEK293 cells. Putrescine accumulation was nearly identical in mock- and hMATE1-HEK cells. Interestingly, putrescine accumulation was more robust as compared to agmatine accumulation in either cell type. Data are expressed as the mean ± SD (n = 3).
Table 1.
Agmatine and putrescine transport kinetics in hOCT2-HEK cells
| pHe | Agmatine | Putrescine | ||
|---|---|---|---|---|
| Km (mM) | Vmax (nmol/min/mg) | Km (mM) | Vmax | |
| 6.0 | 19.01 ± 2.81 | 24.1 ± 2.1 | 33.45 ± 8.92 | 24.9 ± 4.6 |
| 7.4 | 1.84 ± 0.38 | 12.9 ± 0.7 | 11.29 ± 4.26 | 15.6 ± 2.8 |
| 8.5 | 0.74 ± 0.17 | 13.9 ± 0.7 | 3.60 ± 0.67 | 38.9 ± 2.2 |
| 9.5 | 0.39 ± 0.07 | 16.0 ± 0.5 | 1.41 ± 0.24 | 52.3 ± 2.1 |
Data are expressed as the mean ± S.D. and are representative data from two independent experiments (n = 6)
Structures.
Agmatine and putrescine transport in hMATE1-HEK293 cells
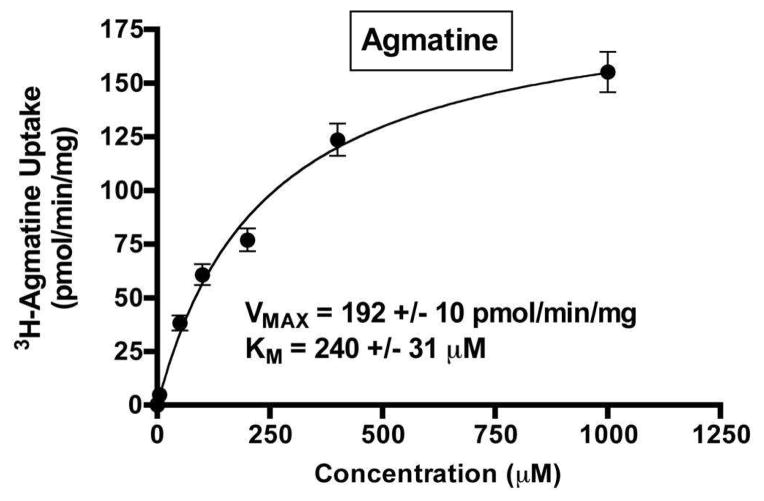
Since MATE1 shares similar localization and substrates with the liver and renal transporters OCT1 and OCT2, we investigated the functional significance of human MATE1 for agmatine and putrescine transport. The intracellular accumulation of agmatine was significantly enhanced by hMATE1 over-expression, relative to mock-HEK cells (Fig. 4a). By contrast, putrescine accumulation was nearly identical between mock and hMATE1-HEK cells (Fig. 4b), though both were substantially more robust than agmatine accumulation in hMATE1-HEK cells. Agmatine transport by hMATE1 was saturable (Fig. 5) (Km=240 ± 31 μM; Vmax=192 ± 10 pmol/min/mg protein), a result which was not observed for putrescine (data not shown).
Figure 5. Concentration-dependent agmatine accumulation in hMATE1-HEK cells.
The concentration-dependent accumulation of agmatine was examined in hMATE1-HEK cells (pH 8.0). Agmatine accumulation (10 min) was saturable, displaying high-affinity, low-capacity transport by hMATE1 (Km=240 ± 31 μM; Vmax=192 ± 10 pmol/min/mg protein). Final values are corrected for mock agmatine uptake at each corresponding concentration. Data are expressed as the mean ± SD and represent pooled data from three independent experiments done in triplicate (n = 9).
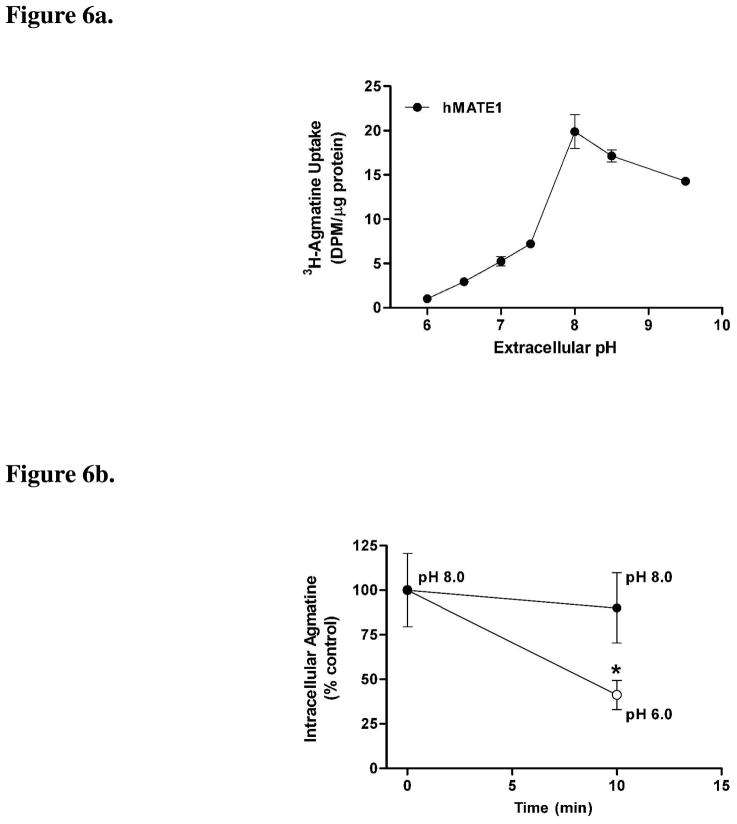
Since MATE1-mediated cation exchange is driven by the proton gradient established at cellular membranes we also investigated the influence of changes in extracellular pH for agmatine and putrescine transport by hMATE1. Agmatine accumulation was significantly influenced by the extracellular pH in hMATE1-HEK cells, displaying maximal uptake at pH 8.0 (Fig. 6a). Putrescine transport, by contrast, was not influenced by changes in extracellular pH in hMATE1-HEK cells (data not shown). A trans-stimulation experiment was used to verify the classical efflux nature of MATE1 for intracellular cation substrates under physiological conditions of an inwardly-directed proton gradient (pHe < pHi). As expected, intracellular agmatine was trans-stimulated from hMATE1-HEK cells into the extracellular buffer when the buffer pH was changed from pH 8.0 to 6.0 (Fig. 6b). In contrast, preloaded agmatine was not trans-stimulated from the intracellular compartment of hMATE1 cells when the extracellular buffer remained at pH 8.0 (Fig. 6b).
Figure 6.
Figure 6a. pH-dependent agmatine accumulation in hMATE1-HEK cells
The pH-dependent accumulation of agmatine (10 nM) was examined in hMATE1-HEK cells. Agmatine accumulation (10 min) was significantly influenced by extracellular pH in hMATE1-HEK cells, increasing from pH 6.0 to 8.0, after which uptake decreased. Data are expressed as the mean ± SD and represent pooled data from two independent experiments done in triplicate (n = 6).
Figure 6b. TRANS-stimulation of agmatine in hMATE1-HEK cells
Trans-stimulation of agmatine was examined in hMATE1-HEK cells. Intracellular agmatine was trans-stimulated in the presence of an inward-proton gradient (extracellular pH 6.0), but was unchanged when the extracellular pH was kept at 8.0. Data are expressed as the mean ± SD and represent pooled data from three independent experiments done in groups of eight (n = 24) (*p < 0.05 compared to control, time zero).
Competition studies were carried out to investigate the ability of structurally-related cations (arginine, putrescine, spermidine, spermine, guanidine, and arcaine) and prototypical MATE1 substrates (MPP+ and paraquat) to inhibit the intracellular accumulation of agmatine in mock- and hMATE1-HEK cells (Table 2). Agmatine accumulation in mock-HEK cells was significantly inhibited by the polyamines and arcaine, but not arginine, guanidine, MPP+, or paraquat. The addition of the unlabeled polyamines putrescine, spermidine, and spermine (500 μM) also partially inhibited agmatine accumulation in hMATE1-HEK cells, although to a lesser extent than in mock cells. The compounds arcaine, MPP+, and paraquat, and to a lesser extent guanidine, also significantly inhibited agmatine accumulation in hMATE1-HEK cells, while arginine had no significant influence toward agmatine accumulation in either cell type.
Table 2.
Competition of agmatine accumulation in mock- and hMATE1-HEK293 cells
| 3H-agmatine accumulation (% mock control) in the presence or absence (control) of competitive inhibitors (500 μM) | ||
|---|---|---|
| mock-HEK cells | hMATE1-HEK cells | |
| control | 100.0 ± 24.8 | 167.0 ± 21.0† |
| + arginine | 122.8 ± 33.5 | 158.5 ± 21.4 |
| + putrescine | 48.2 ± 21.3* | 129.2 ± 15.4* |
| + spermidine | 27.7 ± 8.5* | 92.2 ± 22.9* |
| + spermine | 32.5 ± 4.1* | 89.9 ± 13.6* |
| + guanidine | 121.0 ± 33.3 | 96.4 ± 23.4* |
| + arcaine | 11.2 ± 2.4* | 16.3 ± 4.1* |
| + MPP+ | 110.5 ± 37.9 | 30.3 ± 4.5* |
| + paraquat | 76.7 ± 31.4 | 31.0 ± 5.3* |
Data are expressed as the mean ± SD (n = 6 – 9)
p < 0.05 with respect to corresponding control uptake (one-way ANOVA with Dunnett’s post-hoc test))
p < 0.05 with respect to mock-control uptake (unpaired Student’s t-test)
Discussion
The identification of relevant agmatine and polyamine transporters and the characterization of agmatine transport are essential in order to more fully appreciate the physiological role of agmatine and the therapeutic potential of agmatine or novel agmatine-mimetic analogs. In the present study, OCT2 and MATE1 were identified as two human transporters mediating the bi-directional transport and cellular accumulation of agmatine.
The motivation to pursue this line of investigation arose from our interest in agmatine as an endogenous modulator of relevance to neuroadaptive phenomena including neurotoxicity,13, 49 opioid addiction15 and tolerance,15, 50 and neuropathic pain.10 While the neuromodulatory nature of agmatine has been proposed for some time,51 the identification of relevant agmatine transporters has remained limited. Therefore, the characterization of agmatine transport systems may provide additional support as to a transport mechanism regulating the disposition, and pharmacodynamic influence, of agmatine in the CNS and periphery.
Presently, the putative PTS remains the principal recognized transport “system” proposed for agmatine and polyamine uptake (influx) from the extracellular environment. While agmatine influx transport has been demonstrated by rodent and human OCT2 and OCT3,46 other reports have shown no differences in agmatine transport by hOCT1 and hOCT3, and an approximately 40% reduction in agmatine accumulation by hOCT2 over-expressing cells.47 Since further clarification is warranted, we investigated the involvement of the human transporters OCT1, OCT2, and MATE1 in agmatine and putrescine transport. The expression and localization of these transporters may determine agmatine cellular disposition, and the correspondent role of agmatine in physiological phenomenon related to the modulation of intracellular polyamines.
In the present study, the intracellular accumulation of agmatine was influenced by hOCT1 and hOCT2. Agmatine displayed low-millimolar affinity and high-capacity transport by hOCT2, and had an approximately 10-fold greater affinity for hOCT2 as compared to hOCT1. These findings may reflect differences in the interaction of positively charged agmatine moieties with negatively charged binding cleft residues, such as Glu448,52 which are highly conserved across OCT2- and OCT3-, but not OCT1-orthologs. Together, our results are in close agreement with those of Grundemann and colleagues., (2003)46 for agmatine transport by rat and human OCT1/OCT2,46 and are similar to the transport of agmatine in isolated rat kidney mitochondria53 (Km= 1.7 mM; Vmax= 7.9 nmol/min/mg-protein). The agmatine metabolite putrescine, on the other hand, exhibited an order of magnitude lower affinity for hOCT2 as compared to agmatine (Km= 11.29 ± 4.26 mM vs. Km=1.84 ± 0.38 mM), and appeared to be a low affinity substrate of hOCT1, a disparity which is likely attributable to the diamine structure of putrescine versus the monoamine/guanidinium nature of agmatine (see Structures).
Since the human ortholog of OCT2 recognizes the ionization of particular substrates,54 we investigated the influence of changes in extracellular pH for the interactions of agmatine and putrescine with hOCT1 and hOCT2. We observed a significant enhancement in agmatine accumulation in hOCT2 cells with increasing pH, a finding which was not seen in hOCT1-transfected cells. Putrescine accumulation was similarly enhanced in hOCT2 cell under alkaline conditions (pH > 7.4). Furthermore, investigation into hOCT2 agmatine and putrescine transport kinetics revealed a profound pH influence, with agmatine and putrescine displaying increased affinity at alkaline pH. In contrast to agmatine, putrescine exhibited increased transport capacity under more alkaline conditions, a finding that may be related to the interactions of putrescine with a constitutively expressed PTS. The fact that agmatine and putrescine possess ionizable amine moieties suggested that the pH-dependent enhancement in hOCT2 transport function was related to the relative concentration of the monovalent vs. the divalent form of agmatine and putrescine in the extracellular buffer.
In order to determine the influence of pH for hOCT2-mediated agmatine and putrescine transport, we investigated the transport of the quaternary amine and prototypical OCT substrate, TEA. In the present study, the transport of TEA by hOCT1 and hOCT2 was pH-dependent, consistent with previous observations for TEA uptake in rOCT1- and rOCT2-transfected cells.55, 56 Since TEA exists as a permanent monocation independent of extracellular pH, little or no significant alteration in hOCT2-mediated TEA accumulation would have been expected if the pH influence were caused by a change in the ionization state of the substrate compound. Surprisingly, we observed a similar behavior and magnitude of TEA accumulation in hOCT2-HEK cells to that of agmatine, suggesting that the ionization state (i.e., the relative concentrations of the monovalent and divalent forms) of agmatine and putrescine (primary amine groups) was not the likely contributing factor for their enhanced uptake by hOCT2. Although it is plausible that our observations are related to the influence of the excess protons on the cellular membrane potential, and consequent function of OCT2, previous evidence suggests that protons do not influence charge flow, or contribute to the inhibition of rOCT2-mediated transport function.53 While the mechanism responsible for the pH-dependent enhancement in OCT2-mediated cation transport has yet to be fully delineated, it is possible that the present findings are related to: 1) a reduction in the proton shielding of negatively-charged binding-cleft residues, such as Glu488,52 thereby enhancing the interactions of positively-charged substrates, such as agmatine, at higher pH, and/or 2) changes in the ionization state of negatively-charged binding-cleft residues, and the corresponding tertiary structure of hOCT2, ultimately influencing substrate interactions. In any case, it appears that the role of hOCT2 in the clearance of cations may be relevant to consider for diseases and conditions which influence body fluid pH (e.g., diabetic or alcoholic ketoacidosis).
In addition to the influx transport role of OCT1 and OCT2, the elimination of toxic and therapeutic cations from the liver and kidney involves the function of MATE1, with MATE1 representing the final excretory step.37, 57 Subsequently, we were also interested in the functional consequences of hMATE1 for agmatine and putrescine transport. At an extracellular buffer pH of 8.0, a condition which reverses the inwardly-directed proton gradient (causing inward cation flux) normally present under physiological conditions, agmatine accumulation was significantly enhanced in hMATE1 cells as compared to mock transfected cells. Agmatine transport by hMATE1 was also saturable (Km = 240 ± 31 μM; Vmax = 192 ± 10 pmol/min/mg protein), displaying an affinity and transport capacity comparable to the organic cations TEA37 (Km = 220 μM; Vmax = 226 pmol/min/mg protein) and paraquat43 (Km = 212 ± 19 μM; Vmax = 289 ± 46 pmol/min/mg protein) with hMATE1. Furthermore, agmatine influx and efflux transport by hMATE1 was pH-dependent, with pre-loaded agmatine undergoing trans-stimulated efflux from hMATE1 cells under conditions mimicking the acidic luminal environment of the renal proximal tubule cells. In contrast to agmatine, the polyamine putrescine did not appear to be a substrate of hMATE1, a finding which may reflect differences in the inherent specificity of hMATE1 for cations which possess guanidinium-moieties, as is the case for agmatine and metformin.40 While putrescine was not transported by hMATE1, putrescine did display a much more robust uptake in mock-HEK cells than agmatine in either cell type, suggesting the involvement of a constitutively expressed polyamine transport system in HEK cells with greater affinity for putrescine and perhaps distinct from that which transports agmatine.
In order to assess the influence of a constitutive polyamine transport system, and further explore the possible influence of structural cation moieties such as guanidinium in the hMATE1-mediated transport of agmatine, we investigated a number of structurally relevant molecules for their ability to competitively inhibit the accumulation of agmatine in mock- and hMATE1-HEK cells. Interestingly, only arcaine and polyamines significantly inhibited the accumulation of agmatine in mock-HEK cells. Although we did not observe any appreciable influence of hMATE1 for putrescine transport, putrescine and the higher order polyamines, spermidine and spermine, were able to partially inhibit the accumulation of agmatine in hMATE1-HEK cells, suggesting that polyamines may be non-transported competitive inhibitors of hMATE1 that may serve to regulate intracellular agmatine levels (e.g., polyamines may influence agmatine transport by hMATE1). While guanidine and the prototypical hMATE1 substrates MPP+ and paraquat did not influence agmatine accumulation in mock cells, these compounds, as well as arcaine, significantly inhibited agmatine accumulation by hMATE1. These results further supported the proposal that the difference in hMATE1 transport of agmatine, but not putrescine, is a consequence of hMATE1 recognition of agmatine’s guanidine moiety. Interestingly, the guanidine-containing agmatine precursor arginine did not influence agmatine accumulation in either mock- or hMATE1-HEK cells, a result which in hMATE1-transfected cells, is likely a result of less favorable steric interactions between negatively-charged hMATE1 active-site binding residues and the negatively-charged carboxylic acid group of arginine. Taken together, these results suggest that agmatine transport is influenced by a constitutively expressed transport system in mock-HEK cells not identical to hMATE1.
The present findings are consistent with the expression and transport function of OCT2 and MATE1 in the kidney (OCT1 in the liver) and may translate into an active renal (hepatic) secretion mechanism for agmatine in vivo. A model of such a mechanism is illustrated in Fig. 7. Although agmatine appears to be a substrate of hOCT2 and hMATE1, the overall significance of these systems under physiological conditions must be carefully considered.
Figure 7. Proposed mechanism of agmatine transport in tissues known to express OCT1, OCT2, and MATE1.
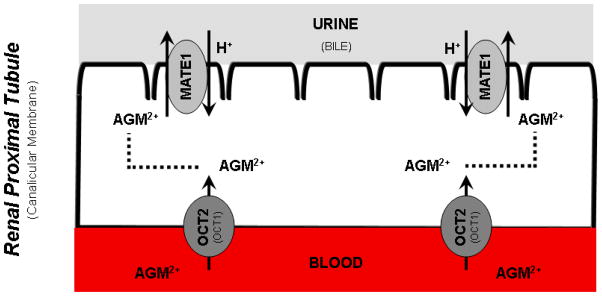
The following schematic is a proposed mechanism of agmatine transport in tissues (i.e., kidney and liver) widely-recognized to express OCT1, OCT2, and MATE1. Organic cation transporter (OCT) 1 and 2 mediate the facilitated influx transport of organic cations at the basolateral membrane of hepatocytes and renal proximal tubule cells, respectively. The multidrug and toxic compound extrusion (MATE) transporter 1, an H+/cation antiporter, is critical in the efflux elimination of various organic cations from the brush-border and canalicular membrane of the kidney and liver, respectively. The results of the present study suggest that the divalent cation agmatine (AGM) may be eliminated by OCT2-mediated uptake and MATE1-mediated secretion in the renal tubules. (Descriptions in parentheses on the graphic refer to equivalent structures in the liver).
While OCT1, OCT2, and MATE1 are typically considered renal and liver transporters, evidence suggests that these systems may also contribute to CNS drug disposition. In particular, OCT1 and OCT2 were recently shown to be present at the luminal membrane of brain microvessel endothelial cells isolated from mice, rats, and humans.58 Within this context, the CNS uptake of MPTP and its toxic metabolite, MPP+, were shown to be limited when co-administered with the OCT1/2 substrate, amantadine, suggesting that OCT1 and OCT2 participate in the blood-brain barrier (BBB) transfer of relevant substrate compounds.58 In addition to OCT1 and OCT2, MATE1 has been shown to be expressed in neuronal structures resembling astrocytic processes and brain capillaries.59 Thus considering the fact that agmatine rapidly enters the brain following systemic delivery,60 it is plausible that agmatine transport at the BBB may be mediated by OCT1, OCT2, and/or MATE1. Additional studies are needed in order to clarify the role these transport systems for the tissue specific disposition of agmatine in the brain and spinal cord, as well as in peripheral organ structures.
In summary, the present results illustrate a novel transport process, hMATE1, which in conjunction with hOCT2 contributes to agmatine transport and cellular accumulation. In addition, we have shown that human OCT2 and MATE1 could represent a bi-directional transport system for agmatine, a phenomenon that may translate into an effective renal clearance mechanism for agmatine in vivo. Further in vivo experiments are needed to elucidate the significance of OCT1, OCT2, and MATE1 transporters for agmatine pharmacokinetics and pharmacodynamics.
Acknowledgments
This work was supported in part through support from the National Institute on Drug Abuse supported Pharmaconeuroimmunology training program [T32DA007097] for T.N.W and through funding from the Academic Health Center of the University of Minnesota. We thank Dr. Kathleen Giacomini (UCSF) for kindly providing the stably-transfected HEK293 cells and Dr. Patrick Braun for constructive suggestions for this manuscript.
References
- 1.Katsuta H, Takaoka T, Nose K, Nagai Y. Effects of polyamines on the proliferation of mammalian cells in tissue culture. Jpn J Exp Med. 1975;45:345–354. [PubMed] [Google Scholar]
- 2.Wolf C, Bruss M, Hanisch B, Gothert M, von Kugelgen I, Molderings GJ. Molecular basis for the antiproliferative effect of agmatine in tumor cells of colonic, hepatic, and neuronal origin. Mol Pharmacol. 2007;71:276–283. doi: 10.1124/mol.106.028449. [DOI] [PubMed] [Google Scholar]
- 3.Dudkowska M, Lai J, Gardini G, Stachurska A, Grzelakowska-Sztabert B, Colombatto S, Manteuffel-Cymborowska M. Agmatine modulates the in vivo biosynthesis and interconversion of polyamines and cell proliferation. Biochim Biophys Acta. 2003;1619:159–166. doi: 10.1016/s0304-4165(02)00476-2. [DOI] [PubMed] [Google Scholar]
- 4.Mayeur C, Veuillet G, Michaud M, Raul F, Blottiere HM, Blachier F. Effects of agmatine accumulation in human colon carcinoma cells on polyamine metabolism, DNA synthesis and the cell cycle. Biochim Biophys Acta. 2005;1745:111–123. doi: 10.1016/j.bbamcr.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Satriano J, Matsufuji S, Murakami Y, Lortie MJ, Schwartz D, Kelly CJ, Hayashi S, Blantz RC. Agmatine suppresses proliferation by frameshift induction of antizyme and attenuation of cellular polyamine levels. J Biol Chem. 1998;273:15313–15316. doi: 10.1074/jbc.273.25.15313. [DOI] [PubMed] [Google Scholar]
- 6.Aricioglu F, Altunbas H. Is agmatine an endogenous anxiolytic/antidepressant agent? Ann N Y Acad Sci. 2003;1009:136–140. doi: 10.1196/annals.1304.014. [DOI] [PubMed] [Google Scholar]
- 7.Gong ZH, Li YF, Zhao N, Yang HJ, Su RB, Luo ZP, Li J. Anxiolytic effect of agmatine in rats and mice. Eur J Pharmacol. 2006;550:112–116. doi: 10.1016/j.ejphar.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 8.Karadag HC, Ulugol A, Tamer M, Ipci Y, Dokmeci I. Systemic agmatine attenuates tactile allodynia in two experimental neuropathic pain models in rats. Neurosci Lett. 2003;339:88–90. doi: 10.1016/s0304-3940(02)01456-8. [DOI] [PubMed] [Google Scholar]
- 9.Paszcuk AF, Gadotti VM, Tibola D, Quintao NL, Rodrigues AL, Calixto JB, Santos AR. Anti-hypernociceptive properties of agmatine in persistent inflammatory and neuropathic models of pain in mice. Brain Res. 2007;1159:124–133. doi: 10.1016/j.brainres.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 10.Fairbanks CA, Schreiber KL, Brewer KL, Yu CG, Stone LS, Kitto KF, Nguyen HO, Grocholski BM, Shoeman DW, Kehl LJ, Regunathan S, Reis DJ, Yezierski RP, Wilcox GL. Agmatine reverses pain induced by inflammation, neuropathy, and spinal cord injury. Proc Natl Acad Sci U S A. 2000;97:10584–10589. doi: 10.1073/pnas.97.19.10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilad GM, Salame K, Rabey JM, Gilad VH. Agmatine treatment is neuroprotective in rodent brain injury models. Life Sci. 1996;58:PL 41–46. doi: 10.1016/0024-3205(95)02274-0. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Lee YW, Park KA, Lee WT, Lee JE. Agmatine attenuates brain edema through reducing the expression of aquaporin-1 after cerebral ischemia. J Cereb Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu CG, Marcillo AE, Fairbanks CA, Wilcox GL, Yezierski RP. Agmatine improves locomotor function and reduces tissue damage following spinal cord injury. Neuroreport. 2000;11:3203–3207. doi: 10.1097/00001756-200009280-00031. [DOI] [PubMed] [Google Scholar]
- 14.Morgan AD, Campbell UC, Fons RD, Carroll ME. Effects of agmatine on the escalation of intravenous cocaine and fentanyl self-administration in rats. Pharmacol Biochem Behav. 2002;72:873–880. doi: 10.1016/s0091-3057(02)00774-8. [DOI] [PubMed] [Google Scholar]
- 15.Wade CL, Schuster DJ, Domingo KM, Kitto KF, Fairbanks CA. Supraspinally-administered agmatine attenuates the development of oral fentanyl self-administration. Eur J Pharmacol. 2008;587:135–140. doi: 10.1016/j.ejphar.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molderings GJ, Kribben B, Heinen A, Schroder D, Bruss M, Gothert M. Intestinal tumor and agmatine (decarboxylated arginine): low content in colon carcinoma tissue specimens and inhibitory effect on tumor cell proliferation in vitro. Cancer. 2004;101:858–868. doi: 10.1002/cncr.20407. [DOI] [PubMed] [Google Scholar]
- 17.Sugiura T, Tsutsui H, Takaoka M, Kobuchi S, Hayashi K, Fujii T, Matsumura Y. Protective effect of agmatine on ischemia/reperfusion-induced renal injury in rats. J Cardiovasc Pharmacol. 2008;51:223–230. doi: 10.1097/FJC.0b013e318161d758. [DOI] [PubMed] [Google Scholar]
- 18.Wang WP, Iyo AH, Miguel-Hidalgo J, Regunathan S, Zhu MY. Agmatine protects against cell damage induced by NMDA and glutamate in cultured hippocampal neurons. Brain Res. 2006;1084:210–216. doi: 10.1016/j.brainres.2006.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuell C, Elliott KA, Hanfrey CC, Franceschetti M, Michael AJ. Polyamine biosynthetic diversity in plants and algae. Plant Physiol Biochem. 2010 doi: 10.1016/j.plaphy.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Kawabata T, Ohshima H, Ino M. Occurrence of methylguanidine and agmatine in foods. IARC Sci Publ. 1978:415–423. [PubMed] [Google Scholar]
- 21.Kawabata T, Ohshima H, Ino M. Occurrence of methylguanidine and agmatine, nitrosatable guanidino compounds, in foods. J Agric Food Chem. 1978;26:334–338. doi: 10.1021/jf60216a038. [DOI] [PubMed] [Google Scholar]
- 22.Haenisch B, von Kugelgen I, Bonisch H, Gothert M, Sauerbruch T, Schepke M, Marklein G, Hofling K, Schroder D, Molderings GJ. Regulatory mechanisms underlying agmatine homeostasis in humans. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1104–1110. doi: 10.1152/ajpgi.90374.2008. [DOI] [PubMed] [Google Scholar]
- 23.Noack J, Dongowski G, Hartmann L, Blaut M. The human gut bacteria Bacteroides thetaiotaomicron and Fusobacterium varium produce putrescine and spermidine in cecum of pectin-fed gnotobiotic rats. J Nutr. 2000;130:1225–1231. doi: 10.1093/jn/130.5.1225. [DOI] [PubMed] [Google Scholar]
- 24.Coleman CS, Hu G, Pegg AE. Putrescine biosynthesis in mammalian tissues. Biochem J. 2004;379:849–855. doi: 10.1042/BJ20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grillo MA, Colombatto S. Metabolism and function in animal tissues of agmatine, a biogenic amine formed from arginine. Amino Acids. 2004;26:3–8. doi: 10.1007/s00726-003-0030-z. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Agmatine: an endogenous clonidine-displacing substance in the brain. Science. 1994;263:966–969. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- 27.Byers TL, Pegg AE. Properties and physiological function of the polyamine transport system. Am J Physiol. 1989;257:C545–553. doi: 10.1152/ajpcell.1989.257.3.C545. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien TG. The induction of ornithine decarboxylase as an early, possibly obligatory, event in mouse skin carcinogenesis. Cancer Res. 1976;36:2644–2653. [PubMed] [Google Scholar]
- 29.Russell DH. The roles of the polyamines, putrescine, spermidine, and spermine in normal and malignant tissues. Life Sci. 1973;13:1635–1647. doi: 10.1016/0024-3205(73)90111-2. [DOI] [PubMed] [Google Scholar]
- 30.Kruczynski A, Vandenberghe I, Pillon A, Pesnel S, Goetsch L, Barret JM, Guminski Y, Le Pape A, Imbert T, Bailly C, Guilbaud N. Preclinical activity of F14512, designed to target tumors expressing an active polyamine transport system. Invest New Drugs. 2009 doi: 10.1007/s10637-009-9328-3. [DOI] [PubMed] [Google Scholar]
- 31.Svensson KJ, Welch JE, Kucharzewska P, Bengtson P, Bjurberg M, Pahlman S, Ten Dam GB, Persson L, Belting M. Hypoxia-mediated induction of the polyamine system provides opportunities for tumor growth inhibition by combined targeting of vascular endothelial growth factor and ornithine decarboxylase. Cancer Res. 2008;68:9291–9301. doi: 10.1158/0008-5472.CAN-08-2340. [DOI] [PubMed] [Google Scholar]
- 32.Palmer AJ, Wallace HM. The polyamine transport system as a target for anticancer drug development. Amino Acids. 2010;38:415–422. doi: 10.1007/s00726-009-0400-2. [DOI] [PubMed] [Google Scholar]
- 33.Wang JF, Su RB, Wu N, Xu B, Lu XQ, Liu Y, Li J. Inhibitory effect of agmatine on proliferation of tumor cells by modulation of polyamine metabolism. Acta Pharmacol Sin. 2005;26:616–622. [PubMed] [Google Scholar]
- 34.Satriano J, Isome M, Casero RA, Jr, Thomson SC, Blantz RC. Polyamine transport system mediates agmatine transport in mammalian cells. Am J Physiol Cell Physiol. 2001;281:C329–334. doi: 10.1152/ajpcell.2001.281.1.C329. [DOI] [PubMed] [Google Scholar]
- 35.Isome M, Lortie MJ, Murakami Y, Parisi E, Matsufuji S, Satriano J. The antiproliferative effects of agmatine correlate with the rate of cellular proliferation. Am J Physiol Cell Physiol. 2007;293:C705–711. doi: 10.1152/ajpcell.00084.2007. [DOI] [PubMed] [Google Scholar]
- 36.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 37.Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, Moriyama Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci U S A. 2005;102:17923–17928. doi: 10.1073/pnas.0506483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoo S, Yonezawa A, Masuda S, Fukatsu A, Katsura T, Inui K. Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol. 2007;74:477–487. doi: 10.1016/j.bcp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Kimura N, Okuda M, Inui K. Metformin transport by renal basolateral organic cation transporter hOCT2. Pharm Res. 2005;22:255–259. doi: 10.1007/s11095-004-1193-3. [DOI] [PubMed] [Google Scholar]
- 40.Tanihara Y, Masuda S, Sato T, Katsura T, Ogawa O, Inui K. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol. 2007;74:359–371. doi: 10.1016/j.bcp.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Meyer zu Schwabedissen HE, Verstuyft C, Kroemer HK, Becquemont L, Kim RB. Human multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter: functional characterization, interaction with OCT2 (SLC22A2), and single nucleotide polymorphisms. Am J Physiol Renal Physiol. 2010;298:F997–F1005. doi: 10.1152/ajprenal.00431.2009. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Teranishi K, Li S, Yee SW, Hesselson S, Stryke D, Johns SJ, Ferrin TE, Kwok P, Giacomini KM. Genetic variants in multidrug and toxic compound extrusion-1, hMATE1, alter transport function. Pharmacogenomics J. 2009;9:127–136. doi: 10.1038/tpj.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Zhang S, Sorani M, Giacomini KM. Transport of paraquat by human organic cation transporters and multidrug and toxic compound extrusion family. J Pharmacol Exp Ther. 2007;322:695–700. doi: 10.1124/jpet.107.123554. [DOI] [PubMed] [Google Scholar]
- 44.Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch AE, Koepsell H. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997;16:871–881. doi: 10.1089/dna.1997.16.871. [DOI] [PubMed] [Google Scholar]
- 45.Molderings GJ, Bonisch H, Gothert M, Bruss M. Agmatine and putrescine uptake in the human glioma cell line SK-MG-1. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:671–679. doi: 10.1007/s002100100418. [DOI] [PubMed] [Google Scholar]
- 46.Grundemann D, Hahne C, Berkels R, Schomig E. Agmatine is efficiently transported by non-neuronal monoamine transporters extraneuronal monoamine transporter (EMT) and organic cation transporter 2 (OCT2) J Pharmacol Exp Ther. 2003;304:810–817. doi: 10.1124/jpet.102.044404. [DOI] [PubMed] [Google Scholar]
- 47.Heinen A, Bruss M, Bonisch H, Gothert M, Molderings GJ. Pharmacological characteristics of the specific transporter for the endogenous cell growth inhibitor agmatine in six tumor cell lines. Int J Colorectal Dis. 2003;18:314–319. doi: 10.1007/s00384-002-0466-8. [DOI] [PubMed] [Google Scholar]
- 48.Milovic V, Stein J, Piiper A, Gerhard R, Zeuzem S, Caspary WF. Characterization of putrescine transport across the intestinal epithelium: study using isolated brush border and basolateral membrane vesicles of the enterocyte. Eur J Clin Invest. 1995;25:97–105. doi: 10.1111/j.1365-2362.1995.tb01533.x. [DOI] [PubMed] [Google Scholar]
- 49.Yu CG, Fairbanks CA, Wilcox GL, Yezierski RP. Effects of agmatine, interleukin-10, and cyclosporin on spontaneous pain behavior after excitotoxic spinal cord injury in rats. J Pain. 2003;4:129–140. doi: 10.1054/jpai.2003.11. [DOI] [PubMed] [Google Scholar]
- 50.Wade CL, Eskridge LL, Nguyen HO, Kitto KF, Stone LS, Wilcox G, Fairbanks CA. Immunoneutralization of agmatine sensitizes mice to micro-opioid receptor tolerance. J Pharmacol Exp Ther. 2009;331:539–546. doi: 10.1124/jpet.109.155424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reis DJ, Regunathan S. Agmatine: an endogenous ligand at imidazoline receptors may be a novel neurotransmitter in brain. J Auton Nerv Syst. 1998;72:80–85. doi: 10.1016/s0165-1838(98)00091-5. [DOI] [PubMed] [Google Scholar]
- 52.Schmitt BM, Gorbunov D, Schlachtbauer P, Egenberger B, Gorboulev V, Wischmeyer E, Muller T, Koepsell H. Charge-to-substrate ratio during organic cation uptake by rat OCT2 is voltage dependent and altered by exchange of glutamate 448 with glutamine. Am J Physiol Renal Physiol. 2009;296:F709–722. doi: 10.1152/ajprenal.90323.2008. [DOI] [PubMed] [Google Scholar]
- 53.Arndt MA, Battaglia V, Parisi E, Lortie MJ, Isome M, Baskerville C, Pizzo DP, Ientile R, Colombatto S, Toninello A, Satriano J. The arginine metabolite agmatine protects mitochondrial function and confers resistance to cellular apoptosis. Am J Physiol Cell Physiol. 2009;296:C1411–1419. doi: 10.1152/ajpcell.00529.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barendt WM, Wright SH. The human organic cation transporter (hOCT2) recognizes the degree of substrate ionization. J Biol Chem. 2002;277:22491–22496. doi: 10.1074/jbc.M203114200. [DOI] [PubMed] [Google Scholar]
- 55.Sweet DH, Pritchard JB. rOCT2 is a basolateral potential-driven carrier, not an organic cation/proton exchanger. Am J Physiol. 1999;277:F890–898. doi: 10.1152/ajprenal.1999.277.6.F890. [DOI] [PubMed] [Google Scholar]
- 56.Urakami Y, Okuda M, Masuda S, Saito H, Inui KI. Functional characteristics and membrane localization of rat multispecific organic cation transporters, OCT1 and OCT2, mediating tubular secretion of cationic drugs. J Pharmacol Exp Ther. 1998;287:800–805. [PubMed] [Google Scholar]
- 57.Omote H, Hiasa M, Matsumoto T, Otsuka M, Moriyama Y. The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol Sci. 2006;27:587–593. doi: 10.1016/j.tips.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Lin CJ, Tai Y, Huang MT, Tsai YF, Hsu HJ, Tzen KY, Liou HH. Cellular localization of the organic cation transporters, OCT1 and OCT2, in brain microvessel endothelial cells and its implication for MPTP transport across the blood-brain barrier and MPTP-induced dopaminergic toxicity in rodents. J Neurochem. 2010;114(3):717–27. doi: 10.1111/j.1471-4159.2010.06801.x. [DOI] [PubMed] [Google Scholar]
- 59.Hiasa M, Matsumoto T, Komatsu T, Moriyama Y. Wide variety of locations for rodent MATE1, a transporter protein that mediates the final excretion step for toxic organic cations. Am J Physiol Cell Physiol. 2006;291:C678–686. doi: 10.1152/ajpcell.00090.2006. [DOI] [PubMed] [Google Scholar]
- 60.Piletz JE, May PJ, Wang G, Zhu H. Agmatine crosses the blood-brain barrier. Ann N Y Acad Sci. 2003;1009:64–74. doi: 10.1196/annals.1304.007. [DOI] [PubMed] [Google Scholar]