Abstract
Background:
Drug shortages have become all too common and affect all aspects of the health care delivery system. The increased number of drug shortages has had a negative impact on patient care as well as costly financial implications.
Objectives:
This article identifies the current problems and negative outcomes drug shortages have caused and provides a framework for how to best prepare for and combat future shortages. It highlights specific problems faced by health care system pharmacies in the Southeastern United States and the managerial responses to address these shortage situations.
Methods:
A 34-question, multiple-choice survey was distributed to pharmacy directors in North Carolina, South Carolina, Georgia, and Florida.
Results:
Of 549 surveys distributed, 219 (40%) responses were received. Respondents reported that drug shortages cause 1% to 5% error rates in hospitals and that 60% of the time drug shortages create unsafe conditions for patients and staff. Many of the respondents reported a 300% to 500% markup on medications on the shortage list. Seventy-six percent of institutions have autosubstitutions for drug shortages that have been preapproved by Pharmacy & Therapeutics Committees.
Conclusions:
The causes of drug shortages are multifaceted, and the safety and financial implications can be costly. In the short term, health care institutions can utilize pharmacists to assist in circumventing the drug shortage problem. The combined efforts of all health care professionals, the US government, manufacturers, and the lay public are necessary to bring awareness and plausible solutions to the drug shortage problems in the long term.
Keywords: drug shortage, medication error, price gauging
The increasing numbers of drug shortages in the United States and abroad have become far too commonplace. The number of medications on shortage lists tripled from 61 to 178 between 2005 and 2010, with over 200 reported in 2012 alone.1 As of March 2014, 754 drug products remain on shortage according to the US Food and Drug Administration (FDA).2 Shortages of critical drugs such as chemotherapy agents, analgesics, injectable nutritional supplements, anesthetics, anti-infectives, and cardiovascular agents are common.3–6 The primary causes for these shortages include inadequate raw materials, decreased number of manufacturers, and other factors that cause production to stall or be terminated.7,8 Delayed or lack of communication among the FDA, manufacturers, and health care providers may contribute to the inadequate preparation in managing drug shortages.1
The impact of drug shortages is multifaceted, with over 50% of health care practitioners believing that shortages have influenced practice and resulted in inferior patient care.9 A Canadian study from Hall et al relayed anesthesiologists’ opinions that drug shortages were responsible for prolonged recovery times, delayed surgical procedure scheduling, and increased recovery cost. Nearly half of them (49%) felt shortages were the impetus for the administration of inferior anesthetics.6 Drug shortages often impact vulnerable populations including cancer patients or neonates, for whom few, if any, equivalent alternatives exist; shortages may result in clinical complications, as exemplified by selenium shortages.10–13 Drug shortages may also force practitioners to prescribe infrequently used medications and concentrations, which can lead to medication errors as demonstrated with prior fentanyl shortages.6 Drug shortages have also impacted life outside of patient care; for example, a planned execution in Oklahoma was delayed due to a drug shortage.14
The financial impact of drug shortages in the United States is estimated at $99 million annually for acquisition costs alone.9 Many of the medications impacted by shortages are from generic manufacturers that hospital systems and third-party insurers depend on to provide cost-effective alternatives to branded products.1,7 Shortages not only impact drug costs but also productivity and efficiency. Pharmacist and nonpharmacist employees spend additional time procuring medications, identifying alternative therapies, and communicating with manufacturers about drug shortages. The problem has become so widespread that many hospital systems have hired pharmacy employees specifically dedicated to the task. 9,15
Rising costs and lack of availability may lead practitioners to reduce the waste of medications and to seek alternative ways to procure medications. Companies such as Pharma Tech recognize the time and financial impact of removing and discarding expired drugs from automated storage machines.16 With the help of software, institutions can safely and effectively remove expired drugs closer to their date to prevent wasting drugs on shortage. An alternative to procuring medications is to compound medications not readily available from raw materials. 8 Dependence on compounded forms of medications remains a high-risk practice, due to practitioners’ possible inexperience with specific compounds and serious complications resulting from contamination.17,18 Nine deaths occurred due to a break in the sterile process when alternative products were being compounded during an amino-acid shortage.19,20 Another high-risk method for obtaining limited medications is through the “grey market” in which middlemen obtain and sell scarce medications at highly inflated prices. The integrity of these drugs is unknown; some of them have been stolen or are counterfeit.21
Accreditation Council for Pharmacy Education (ACPE) standards and guidelines recommend that pharmacists in a hospital or health system pharmacy practice setting need to possess competencies in pharmacy systems, medication safety and quality, clinical applications, and professional practice. An example of performance competencies include the ability to describe the basic drug procurement process including drug selection, inventory management, back orders, recalls, drug waste, and handling of drug shortages and the relationship of shortages to safe, effective patient care.22 With drug shortages continuing at an alarming rate, the purpose of this study is to highlight the problems faced by health system pharmacies in the Southeastern United States, detail the response that is required to manage such shortages, and provide insight to circumvent future medication shortages.
Methods
A 34-question electronic survey was distributed via SurveyMonkey on behalf of the South Carolina Society of Health-System Pharmacists (SCSHP) to pharmacy directors in North Carolina, South Carolina, Georgia, and Florida. The board of pharmacy for each state was contacted in order to obtain the distribution list of pharmacy directors in that state. A total of 549 pharmacy directors, or their appointed designees, were given 2 weeks to respond to the survey. An additional reminder was sent to nonresponders after 1 week. The survey was not anonymous, as contact information was requested for any additional questions. All answers were kept confidential and were viewed by a single study investigator (B.S.).
Study investigators created the survey with input from members of SCSHP. Data collected from the survey included demographic information (title of person filling out survey and facility name). If drug shortage data were available, respondents answered specific drug shortages–related questions (Appendix). The majority of questions were multiple choice, allowing respondents to select a prespecified answer. The following number of questions were allotted for each topic related to drug shortages: 5 questions for alternative preparation of products, 6 questions on drug expiration, 2 questions about additional staff hours needed, 2 questions addressing hard to find companies, 4 questions on communication of drug shortages within the institution, 3 questions on medication errors, 1 question on purchase orders, and 1 question on pharmacy and therapeutics (P&T) committees. One question allowed respondents to rank the unsafe conditions presented by medication shortages on a 0 to 10 scale. All results were compiled into a Microsoft Excel database and descriptive analysis was conducted.
Results
A total of 549 surveys were administered to hospital pharmacy directors. Two hundred nineteen hospitals responded (40%); South Carolina had the highest response rate (81.8%). The response rate for the other 3 states surveyed was 28.6% for Florida, 41.9% for North Carolina, and 35.9% for Georgia. Fifty-one percent of the hospitals surveyed are tracking shortages (Figure 1). The majority of respondents reported that alternative suppliers are charging a 300% to 500% mark-up for hard-to-find drugs due to drug shortages (Figure 2). Examples of such drugs include furosemide, succinylcholine, metoclopramide, azithromycin injection, suxamethonium chloride, and ketorolac. Price gouging has occurred as a result of drug shortages, and 84% responders believe the government should control price gouging.
Figure 1.
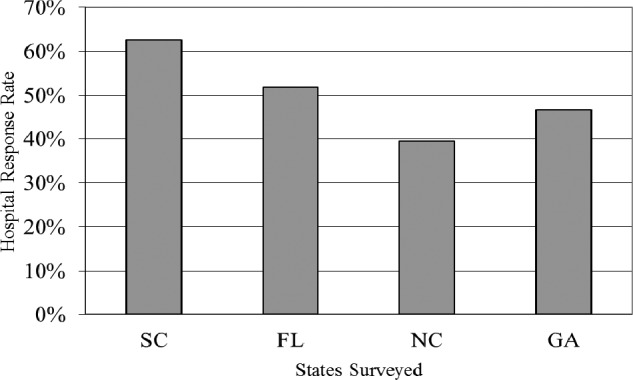
Percentage of hospitals tracking shortages by states surveyed.
Figure 2.
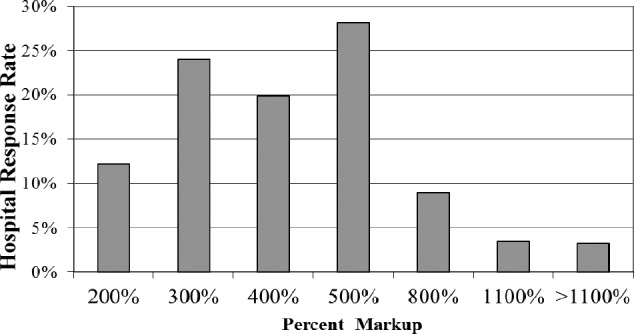
Mean mark-up charged by alternate pharmaceutical companies.
It is important that drug shortages are communicated effectively to physicians and hospital staff. The 2 most common modes of communicating such shortages in the hospitals surveyed are through P&T committee meetings and e-mail (Figure 3). Two to 5 staff hours per week in addition to regular committee work are needed to communicate the drug shortage information to physicians and hospital staff. An average of 10 additional staff hours per week are needed to add, change, and/or update hospital information systems with drug shortage information. We did not ascertain whether this additional time included nonpharmacy personnel hours.
Figure 3.
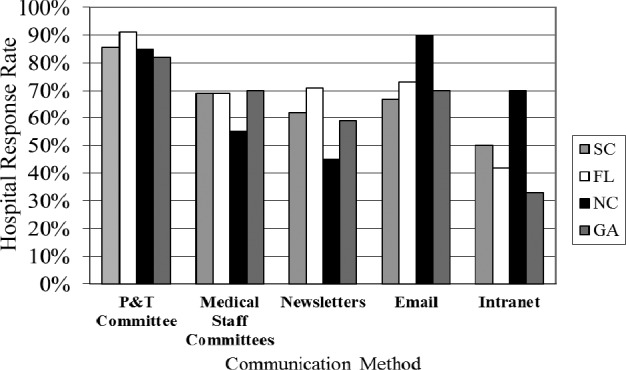
Methods hospital pharmacies use to communicate drug shortages.
Seventy-six percent of institutions surveyed have P&T preapproval for pharmacists’ autosubstitution for drug shortages. When surveyed, the majority of respondents stated that drug shortages cause a 1% to 5% error rate in hospitals, given all the manipulation that pharmacists are required to perform to deliver the drug in the most appropriate form to patients. Each state surveyed said that drug shortages create unsafe conditions for patients and staff 60% of the time. The majority of respondents ranked unsafe conditions presented by medication drug shortages as a 5 on a scale of 0 to 10.
When asked to estimate the additional staff hours (expressed as full-time equivalents [FTE]) needed to manage drug shortages, the most respondents stated that 0.5 FTE was required, followed by 1.0 FTE (Figure 4). The increase in FTE is needed to manage ordering and syringe preparation with an average of more than 5 different products being prepared in alternative dosage forms (eg, syringes). Over 400 additional syringes per month are being prepared as a result of drug shortages. The survey found at least a $2 extra cost per each prepackaged syringe for supplies (eg, labeling), not including drug costs. On average, 10 additional pharmacy personnel hours per week were needed to prepare these syringes; one state reported an average of 20 additional hours needed.
Figure 4.
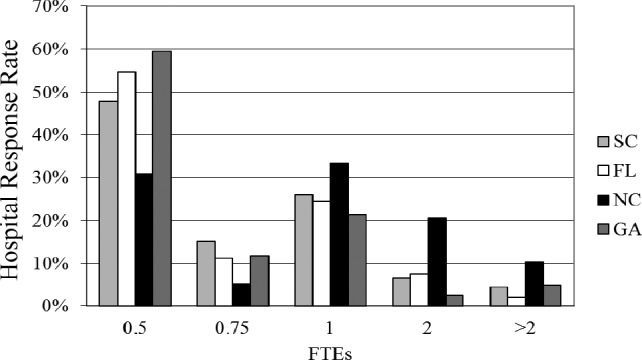
Additional hosptial pharmacy full-time equivalents (FTEs) required to manage drug shortages.
About 90% of respondents acknowledged that expiration dates are tracked in automated storage machines (ASM). Expired drugs are pulled from the ASM on a weekly basis (48%) followed closely by a monthly basis (46%).
Discussion
Drug shortages have become the new normal within the medical community, requiring the combined efforts of pharmacists, manufacturers, the US government, and the lay public to manage this national crisis. Results of the current survey corroborate those of past assessments, demonstrating how shortages have impacted hospital pharmacies and the delivery of optimal patient care.9,23
When a health care institution is faced with a drug shortage, it must follow a systematic process to address the shortage. Contacting the manufacturer for direct orders and centralizing stock, which according to our survey takes at least an additional 0.5 to 1 FTE, are useful tactics that can bridge the gap until supplies are available from regular channels. When simple solutions are not a possibility, investigation of therapeutic alternatives becomes necessary. Alternatives must be identified through literature in a timely manner and shared with health care colleagues to prevent any patient care delays and ensure appropriate therapy. An example of this process occurred at our institution when etomidate injection was unavailable. Through collaborative discussion prior to the depletion of product, alternative medications were identified to reduce adverse effects during intubation. Pharmacy staff and key physicians were educated on the changes to ensure a smooth transition to the alternatives during emergent intubation procedures. This process involved personnel from other departments including information technology and communications.
Health systems can play a vital role in preventing shortages by reducing the time between the medication’s expiration date and its removal from inventory and by developing a critical medication list that allows less stringent control on inventory turns. Per our survey, institutions are pulling soonto-expire medications on a weekly basis followed closely by a monthly basis. Pulling medications on a weekly basis assists in a multitude of facets – decreased numbers of expired medications having to be returned and allowing more time to utilize them, less inventory to order, and decreased waste during a shortage. In addition, a list of critical medications that are prone to shortages should be maintained, and these medications should not be pulled until they are closer to the listed expiration. Tight inventory turnovers have become the new business model, and reduced “overstock” can make all the difference between a true shortage and managed inventory shortage. Creating a critical medication list and having a buffer for “must have” medications can be the difference between optimal patient care and a devastating shortage.
Drug shortages have led to numerous medication errors and negative clinical outcomes, often due to the use of compounded products or alternative preparations.15 Several examples include the compounding of sodium bicarbonate solutions with potassium acetate instead of the intended sodium acetate and heparin drip preparation errors during the heparin premix shortage. Critical shortages in chemotherapeutic agents may have contributed to unnecessary morbidity and mortality.24 Over 60% of respondents in our survey indicated that drug shortages created an unsafe environment, and an additional 10 hours per week was required to update hospital information systems due to drug shortages. Sound-alike/look-alike drugs (SALAD), unfamiliarity with alternative medications, inappropriate dilution of concentrated alternatives, expired medications in automated storage cabinets, and altered expiration dating for USP <797> conformity are some of the issues found in the literature.25 Education, enhanced communication, and implementation of system checks and balances are the key components to preventing medication errors. Negative clinical outcomes have included nutrient deficiencies among neonates that were seen during the selenium shortage and respiratory arrest due to larger vial size of fentanyl being used during a shortage of the smaller ampoules.7,15,26–29 Most recently, a critical shortage of intravenous fluids, including normal saline, has greatly impacted health systems.2
The Institute for Safe Medication Practices (ISMP) has described the importance of determining how the use of alternative drugs could affect current prescribing practices, storage of the drug, final product preparation (including directions for admixing), drug administration procedures, SALAD, and the use of technology (eg, electronic prescribing, bar-coding systems, automated dispensing cabinets, smart pump libraries).25,30 It is evident from our survey that addressing these shortage-related safety concerns equates to more FTE hours: 0.5 to 1 FTE for ordering, 10 hours per week for syringe preparation, 10 hours per week for hospital information system updates, and 2 to 5 hours per week for communication to physicians and hospital staff. Our survey findings regarding FTE results are similar to those found in other surveys.31 Several examples of our systematic process and action steps to addresses drug shortages are included in Table 1.
Table 1. Examples of action steps at Palmetto Health to prevent medication errors resulting from drug shortages.
| Drug/agent impacted by shortage | Action taken |
| Dobutamine vials | Dobutamine premix bag use during a vial shortage and education on its SALAD potential with dopamine premix bags |
| TPN software template changes and staff education regarding any parenteral nutrition preparation changes | |
| Multitrace-4 for parenteral nutrition | Addamel N substitution for multitrace-4 adult concentrate with highlighted education on the dosing difference of 10 mL vs 1 mL per adult parenteral nutrition preparation |
| Epinephrine abboject | Preparation of “epinephrine kits” for emergency boxes that contained appropriate supplies and preparation instructions during the epinephrine Abboject shortage |
Note: SALAD = sound-alike/look-alike drugs; TPN = total parenteral nutrition.
Over 80% of respondents in our survey believe the government should control price gouging. It is of utmost importance for health care providers to notify the Federal Trade Commission of any price gouging concerns, the FDA about identified drug shortages, and the FDA Office of Criminal Investigations regarding grey market issues. These offices are in place to assist with these issues; however, they are counting on health care providers to notify them about these concerns. In addition, it is the professional duty of pharmacists to complete shortage surveys when requested to help solidify numbers and provide data to lobbyists and legislators for action steps.
Two bills were introduced to the Senate and House of Representatives in 2011 and 2013 to address the drug shortages problem.32 The proposed legislation would require manufacturers to inform the FDA of any “discontinuance, interruption, or adjustment in manufacturing of a drug product that might result in a shortage.”1 Another proposed solution is for the FDA to initiate a grading system for plant inspections.1 The current pass/fail model does not allow manufacturers to remain open and fix less offensive problems; this change would prevent factory closings that contribute to drug shortages.1 The grading system, similar to the ones used for food service industry, would give companies incentive to maintain a high standard because this information will be shared with consumers.1 Although FDA regulatory activity is necessary to ensure the safe delivery of medications to the supply chain, the US House of Representatives Oversight Committee recommends that it be done in a manner in which the implications of these actions on the supply of the nation’s most critical medications are taken into account.33
Some questions remain unanswered. Have group purchasing organizations contributed to the shortage crisis by contracting single entity manufacturers for particular products? Have these contracts put an extra burden on a single manufacturing source to supply a large US population? Would dual contracting be a smarter choice, allowing multiple manufacturers to produce and supply medications, and prevent a potential shortage? Questions that were not addressed in the current survey but should be explored in follow-up drug shortage research include the following: Do automatic substitutions in the setting of drug shortages have any effect on costs to the pharmacy and/or patient? Do the automatic substitutions potentially cause a delayed outcome or extended admission for the patient?
There are several limitations to our survey. The response rate for this survey was 40%, and lack of response was attributed to lack of time, lack of interest, length of the survey, and/or simple disregard of the survey request. Although we are unable to verify this with assurance, we believe that only one survey was completed per institution as there was only one line of communication to each pharmacy director/ designee. The highest response rate was from South Carolina, most likely due to the fact that the investigative team was from South Carolina. Responses from each institution may have varied due to differences in the make up of the hospitals’ patients and drug suppliers. Respondents were not able to provide open-ended answers to the questions, which may have restricted their responses; however, we did not receive any direct comments from respondents related to this perceived restriction. Additionally, we did not evaluate staff hours required outside of hospital pharmacy personnel. Survey respondents were from 4 states in the southeast United States, however we believe the results can be generalized to other areas of the country.
Conclusion
Medication shortages have become common and require significant pharmacy personnel hours to manage in hospitals and health systems. Shortages also impact the clinical outcomes of many vulnerable patient populations. Pharmacists are uniquely qualified to support health care organizations by leading efforts to minimize the impact of drug shortages on patient care. Educating hospital staff, physicians, pharmacists, and other clinical support service personnel is important. Based on the results of the survey, other institutions should consider implementing some of the drug shortage management strategies that were identified. These include allowing autosubstitutions, expiring medications closer to their expiration date, ordering directly from the manufacturers, and being prepared to authorize additional FTE hours to help manage severe shortages. Development of a critical institution-specific medication list for which par levels are elevated can also be valuable in preventing an internal shortage. The profession should consider formally educating pharmacy students about drug shortages and global management strategies through their didactic curriculum. Beyond health professionals, manufacturers, and government agencies, patients remain a key advocate for addressing the drug shortage crisis.
Acknowledgments
At the time of the writing of this article, LaVetra Sims was a pharmacy student at the South Carolina College of Pharmacy, University of South Carolina.
The authors declare no conflicts of interest. Data were presented in part at the 2013 South Carolina Society of Health-System Pharmacists Annual Meeting; March 27, 2013; Charleston, South Carolina.
Appendix
Drug Shortage Survey Questions
- What is the average percent markup that hard to find companies are charging due to drug shortages?
- 200% 300% 400% 500% 800% 1100% >1100%
- Do you think the government should control hard to find companies price gouging?
- Yes No
- Please estimate additional annual staff hours needed to manage ordering during drug shortages (#FTE).
- 1,040 hours (0.5 FTE)
- 1,560 hours (0.75 FTE)
- 2,080 hours (1.0 FTE)
- 4,160 hours (2.0 FTE)
- >4,160 hours (2.0 FTE)
- Are you preparing doses in syringes for your patients due to drug shortages?
- Yes No
- How many different products are you preparing syringes for due to drug shortages?
- ≤2 ≤3 ≤4 ≤5 >5
- How many syringes do you prepare per month (estimate)?
- 100 200 300 400 >400
- What do you estimate the cost of each prepackaged syringe to be including labeling, excluding the drug?
- ≤$2 ≤$3 ≤$5 >$7
- If preparing syringes, what additional increase in hours do you estimate per week in preparing syringes?
- ≤5 ≤10 ≤15 ≤20 ≤30
- Would a temporary or permanent extension of the repackaging expiration date (28 days) help you with current drug shortages?
- Yes No
- Should the expiration date be changed from 28 days if preparing in a sterile environment/hood?
- Yes No
- What would be a reasonable time period for an expiration date that would not jeopardize stability or sterility?
- 45 days 60 days 90 days 120 days
- Do you track expiration dates in automated dispensing machines?
- Yes No
- How often are expired drugs pulled from you automated dispensing machine cabinets?
- Weekly Bimonthly Monthly Quarterly
- How many days in advance are they pulled?
- ≤3 ≤7 ≤14 ≤30 ≤60 >60
- Do you communicate shortages to physicians and hospital staff?
- Yes No
- How is the drug shortage information communicated (check all that apply)?
- P&T committee Medical staff committee Newsletters E-mail Internet
- Over and above your regular committees/newsletters, can you estimate the increase in number of hours that is required to communicate drug shortage information to hospital staff and physicians per week?
- ≤2 ≤5 ≤10 ≤20 >20
- Please estimate the increase number of hours per week required by your staff to add, change, and update hospital systems with information due to drug shortages?
- ≤10 ≤20 ≤30 ≤40 ≤50 >50
- Have you informed your hospital administration of your problems with drug shortages?
- Yes No
- Do you feel that standardizing concentrations in hospitals as recommended by The Joint Commission is possible given the current situation?
- Yes No
- Do you have P&T-approved autosubstitutions for drug shortages that pharmacists can do?
- Yes No
- Please estimate what percentage increase in errors that might occur in your hospital given all the drug manipulations that pharmacists must do to deliver the drug in the most appropriate form to patients because of drug shortages (include all staff that administer, prepare, and order mediations)?
- <1% <2% <5% <10% >10%
- With so many recalls, what percent of the drugs do you believe might be given to a patient before you can remove from your inventory?
- <1% <3% <5% >5%
- How many purchase orders do you have at your facility from drug shortages?
- <10 <20 <30 <40 <50 >50
-
How frequently do drug shortages create unsafe conditions for your patients and your staff?
On a scale of 0-10 with 0 being never and 10 being always, please select one.
References
- 1.Schweitz SO. How the US Food and Drug Administration can solve the prescription drug shortage problem. Am J Public Health. 2012;103:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Current Drug Shortage Index. US Food and Drug Administration Web site. http://www.fda.gov/Drugs/DrugSafety/Drug-Shortages/ucm050792.htm#2. [Accessed Mar 20;, 2014. ]; [Google Scholar]
- 3.McBride A, Holle LM, Westendorf C, et al. National survey on the effect of oncology drug shortages on cancer care. Am J Health Syst Pharm. 2013;70:609–617. [DOI] [PubMed] [Google Scholar]
- 4.Mendez MN, Gibbs L, Jacobs RA, et al. Impact of a pipercillin-tazobactam shortage on antimicrobial prescribing and the rate of vancomycin resistant enterococci and Clostridium difficile infections. Pharmacotherapy. 2006;26:61–67. [DOI] [PubMed] [Google Scholar]
- 5.Pluss-Suard C, Pannatier A, Ruffieux C, et al. Changes in the use of broad-spectrum antibiotics after cefepime shortage: A time series analysis. Antimicrob Agents Chemother. 2012;56:989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall R, Bryson GL, Flowerdew G, et al. Drug shortages in Canadian anesthesia: A national survey. Can J Anaesth. 2013;60:539–551. [DOI] [PubMed] [Google Scholar]
- 7.Rochon PA, Gurwitz JH. Drug shortages and clinicians: Comment on coping with critical drug shortages. Arch Intern Med. 2012;172(19):1499–1500. [DOI] [PubMed] [Google Scholar]
- 8.Rosoff PM, Kuldip MA, Patel R, et al. Coping with critical drug shortages: An ethical approach for allocating scarce resources in hospitals. Arch Intern Med. 2012;172:1494–1499. [DOI] [PubMed] [Google Scholar]
- 9.Baumer AM, Clark AM, Witmer DR, et al. National survey of the impact of drug shortages in acute care hospitals. Am J Health Syst Pharm. 2004;61:2015–2022. [DOI] [PubMed] [Google Scholar]
- 10.Havrilesky LJ, Garfield CF, Barnett JC, et al. Economic impact of paclitaxel shortage in patients with newly diagnosed ovarian cancer. Gynecol Oncol. 2012;125:631–634. [DOI] [PubMed] [Google Scholar]
- 11.Strausbaugh LJ, Jernigan DB, Liedtke LA, et al. National shortages of antimicrobial agents: Results of 2 surveys from Infectious Diseases Society of America Emerging Infections Network. Clin Infect Dis. 2001;33:1495–1501. [DOI] [PubMed] [Google Scholar]
- 12.Davis C, David PJ, Horslen S. Selenium deficiency in pediatric patients with intestinal failure as a consequence of drug shortage. J Parenter Enteral Nutr. 2014;38:115–118. [DOI] [PubMed] [Google Scholar]
- 13.Hanson C, Thoene M, Wagner J, et al. Parenteral nutrition additive shortages: The short-term, long-term and potential epigenetic implications in premature and hospitalized infants. Nutrients. 2012;4:1977–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McBride B. Oklahoma lawyers: Drugs not available for execution. MSN News. http://news.msn.com/crime-justice/okla-lawyers-drugs-not-available-for-execution#tscptme Accessed March19, 2014. [Google Scholar]
- 15.Traynor K. Drug shortages mount in 2010. Am J Health Syst Pharm. 2010;67:1492–1494. [DOI] [PubMed] [Google Scholar]
- 16.Expiration and recall management. Pharma Tech Medical Equipment Trading LLC. http://www.pharmauae.com/products/expiration-and-recall-management/ [Accessed Mar 19;, 2014 .];
- 17.Guharoy R, Noviasky J, Haydar Z. Compounding pharmacy conundrum: “We cannot live without them but we cannot live with them” according to the present paradigm. Chest. 2013;143:896–900. [DOI] [PubMed] [Google Scholar]
- 18.Mahaguna V, Dermott JM, Zhang F, et al. Investigation of product quality between extemporaneously compounded progesterone vaginal suppositories and an approved progesterone vaginal gel. Drug Dev Ind Pharm. 2004;30:1069–1078. [DOI] [PubMed] [Google Scholar]
- 19.Kuwahara T, Kaneda S, Shimono K, et al. Effects of lipid emulsion and multivitamins on the growth of microorgranisms in peripheral parenteral nutrition solutions. Int J Med Sci. 2013;10:1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holcomobe B. Parenteral nutrition product shortages: Impact on safety. J Parenter Enteral Nutr. 2012;36:44S–47S. [DOI] [PubMed] [Google Scholar]
- 21.Woodward C. Prices gone wild: Grey market “scalpers” scoring windfall in American drug market. CMAJ. 2012;84:E119–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Accreditation Council for Pharmacy Education Web site. https://www.acpe-accredit.org/pdf/GuidanceStandards-2016DRAFTv60FIRSTRELEASEVERSION.pdf Accessed August12, 2014.
- 23.Kaakeh R, Sweet B, Reilly C, et al. Impact of drug shortages on U.S. health systems. Am J Health Syst Pharm. 2011;68:1811–1819. [DOI] [PubMed] [Google Scholar]
- 24.Mcbride A, Holle L, Westendorf C, et al. National survey on the effect of oncology drug shortages on cancer care. Am J Health Syst Pharm. 2013;70:609–17. [DOI] [PubMed] [Google Scholar]
- 25.Institute for Safe Medication Practices; A shortage of everything except errors: Harm associated with drug shortages. ISMP Medication Safety Alert. 2012;17(8):1–3. [Google Scholar]
- 26.Institute for Safe Medication Practices; Survey links PN component shortage to adverse outcomes. ISMP Medication Safety Alert. 2014;19:1–2. [Google Scholar]
- 27.Institute for Safe Medication Practices; Results of our survey on drug storage, stability, compatibility, and beyond use dating. ISMP Medication Safety Alert. 2012;17(6):1–4. [Google Scholar]
- 28.Oliveira G, Thielken L, McCarthy R. Shortage of perioperative drugs: Implications for anesthesia practice and patient safety. Anesth Analg. 2011;113:1429–1435. [DOI] [PubMed] [Google Scholar]
- 29.Hanson C, Thoene M, Wagner J, et al. Parenteral nutrition additive shortages: The short-term, long-term and potential epigenetic implications in premature and hospitalized infants. Nutrients. 2012;4:1977–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Institute for Safe Medication Practices. Weathering the storm: Managing the drug shortage crisis. ISMP Medication Safety Alert. 2010;15(20):1–4. [Google Scholar]
- 31.McLaughlin M, Kotis D, Thomson K, et al. Effects on patient care caused by drug shortages: A survey. J Manag Care Pharm. 2013;19:783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Life saving drug shortage act. Govtrack.us. https://www.govtrack.us/ Accessed March19, 2014.
- 33.Issa D. US House of Representatives Committee on Oversight and Government Reform: FDA’s contribution to the drug shortage crisis. 2012. http://oversight.house.gov/wp-content/uploads/2012/06/6-15-2012-Report-FDAs-Contributionto-the-Drug-Shortage-Crisis.pdf Published June 15, 2012. Accessed March19, 2014.


