Abstract
Primary cardiac involvement, an under-recognised manifestation of the idiopathic inflammatory myopathies (IIM) and systemic sclerosis (SSc)-spectrum disorders, is associated with significant mortality. Within these two conditions, traditional skeletal muscle enzyme testing may not effectively distinguish between skeletal and cardiac muscle involvement, especially in patients with subclinical cardiac disease. Accurate biomarkers are thus required to screen for cardiac disease, to better inform both therapeutic decision-making and treatment response. The widespread uptake of cardiac troponin testing has revolutionised the management of acute coronary syndromes. Whereas cardiac troponin I (cTnI) appears specific to the myocardium, cardiac troponin T (cTnT) is also expressed by skeletal muscle, including regenerating skeletal muscle tissue. There is increasing interest about the role of cardiac troponins as a putative biomarker of primary cardiac involvement in IIM and SSc-spectrum disorders. Herewith we discuss subclinical cardiac disease in IIM and SSc-spectrum disorders, the respective roles of cTnI and cTnT testing, and the re-expression of cTnT within regenerating skeletal muscle tissue. There remains wide variation in access to cardiac troponin testing nationally and internationally. We propose two pragmatic clinical pathways using cardiac troponins, preferably measuring concomitant cTnT followed by confirmatory (cardiac) cTnI to screen patients for subclinical cardiac disease and/or low-grade skeletal muscle disease activity, and also an agenda for future research, and also an agenda for future research.
Keywords: idiopathic inflammatory myopathy, systemic sclerosis, cardiac, troponin, biomarker
INTRODUCTION
It is increasingly recognised that primary cardiac involvement in the idiopathic inflammatory myopathies (IIM) and systemic sclerosis (SSc)-spectrum disorders is common, often subclinical and associated with a poor prognosis[1-6]. Careful clinical assessment and investigation are required to identify any ‘disease-related’ (e.g. pulmonary arterial hypertension [PAH] in patients with SSc) or ‘other’ (e.g. ischaemic heart disease) secondary causes of cardiac involvement. It is critical to differentiate primary cardiac involvement from these secondary causes, as the prognosis and management may differ significantly, and there are now effective treatments available for several of these conditions (e.g. PAH). The pathological processes driving terminal myocardial fibrosis, thought to be the main pathology underpinning ‘primary’ cardiac involvement in IIM and SSc-spectrum disorders[3,4,6], include myocardial inflammation[3,4,6] and microvascular ischaemia (including recurrent, focal injury)[7,8], Furthermore, an increased risk of cardiovascular disease (including coronary artery disease) has also been described in patients with both IIM[9] and SSc-spectrum disorders[10].
Case identification in current clinical practice is often heavily reliant upon the patient reporting suggestive cardiac symptoms (e.g. chest pain, palpitations) and physicians arranging subsequent investigations including cardiac enzymes (e.g. pro-BNP), electrocardiogram (ECG) and imaging (echocardiogram/cardiac magnetic resonance [CMR]). Effective, non-invasive, biomarkers could potentially allow the identification (through screening) of patients with subclinical primary cardiac involvement, with a view to early intervention. To this end, cardiac troponins could represent such a possible biomarker.
TROPONIN
The troponin complex comprises the regulatory proteins (troponins C, I and T) that regulate the thin filament system in cardiac and striated muscle, but not smooth muscle[11-13]. Troponin C binds to calcium ions (exposing myosin binding sites), troponin I binds to actin (inhibiting the interaction between actin and myosin) and troponin T binds to tropomyosin (transferring calcium-induced conformational change to the thin filament system)[11-13]. Both troponin I and T exist in three isoforms: fast- and slow-twitch skeletal muscle, and cardiac muscle forms[14,15]; each coded for by different genes, whereas the amino acid sequences of cardiac and skeletal troponin C are identical[12].
Cardiac troponin I (cTnI) is considered to be exclusive to myocardial tissue[11-15]. In contrast, cardiac troponin T (cTnT) has been detected (including by immunohistochemistry) in developing foetal[15], healthy adult and regenerating adult skeletal muscle tissue, including in patients with IIM[11,12,16].
RAISED CARDIAC TROPONINS IN OTHER CONDITIONS
The measurement of cardiac troponins (cTnI/cTnT) has revolutionised the management of acute coronary syndromes (ACS), providing a method to risk stratify patients through the detection of (even minimal) myocardial injury[12,14,17-18], with comparable sensitivities and specificities in ACS[18]. Cardiac troponins may be elevated due to a number of other conditions (Table 1)[19-21].
Table 1.
The cardiac and non-cardiac causes of raised troponin (note this is not an exhaustive list).
| Cardiac | Direct myocardial damage (e.g. myocardial contusion) |
| Infiltrative (e.g. amyloidosis and sarcoidosis) | |
| Inflammatory (e.g. myocarditis and/or pericarditis, including infective causes) | |
| Ischaemic heart disease (e.g. myocardial infarction) | |
| Myocardial demand (e.g. tachyarrhythmia) | |
| Myocardial strain (e.g. heart failure) | |
| Non-cardiac | Acute aortic dissection |
| Acute respiratory distress syndrome | |
| Chemotherapy | |
| Hypotension/shock | |
| Muscle trauma | |
| Pulmonary embolism | |
| Renal failure | |
| Subarachnoid haemorrhage/stroke | |
| Strenuous exercise | |
| Sympathomimetic drugs |
CARDIAC TROPONIN ASSAYS
Several generations of cardiac troponin assays have now been developed. First generation cTnT assays could cross-react with skeletal muscle troponin because cTnT only differs from skeletal muscle troponin T by 6-11 amino acids. In contrast, cTnI assays do not exhibit cross-reactivity because cTnI has an 31 additional amino acids compared to skeletal troponin I[16]. The currently used “high sensitivity” troponin (cTnI/second generation onward cTnT) assays show no cross-reactivity to skeletal muscle troponin[16].
CARDIAC TROPONINS IN PATIENTS WITH IIM & SSC-SPECTRUM DISORDERS
In a number of case reports and studies, a raised cTnT has been reported to be detected (including by later generation assays) in patients with IIM in the absence of cardiac involvement[22-25], and without an increase in cTnI[22,24,25]. In a study of 39 patients with IIM (none of whom had clinically apparent myocardial involvement), cTnT was elevated in 19 patients (41%); whereas only one patient (2.5%) had an elevated cTnI[16]. Several studies have reported a close correlation between an elevated cTnT and CK[22-24], and in particular, with CK-MB[22,23,25]. It is thought that regenerating skeletal muscle tissue undergoes a phenotype switch, in which previously repressed cTnT (along with CK-MB) is re-expressed in adult, regenerating skeletal muscle tissue, as has been demonstrated in skeletal muscle biopsies from patients with polymyositis[11].
Elevated cTnT levels have been reported in patients with SSc-spectrum disorders (including both early diffuse and established limited cutaneous SSc), often with florid, clinically apparent, and often severe cardiac disease[26,27]. Evidence of inflammatory cardiac disease (myocarditis/endocarditis) by CMR, with improvement in cTnT, and cardiac function with immunosuppression has been reported[26,27].
SKELETAL MUSCLE TROPONIN
Skeletal muscle troponin measurement may help to investigate for a skeletal source of an elevated (total) CK, and as a biomarker of skeletal muscle disease activity. Skeletal enzymes (particularly CK) are commonly used to assess skeletal muscle disease activity in IIM and SSc-spectrum disorders. The International Myositis Assessment and Clinical Studies (IMACS) Group recommends measuring at least 2 of four muscle-associated enzymes (CK, the transaminases [alanine/aspartate transaminase], lactate dehydrogenase and aldolase) to assess disease activity[28]. In the presence of regenerating skeletal muscle tissue, an elevated total CK may not represent inflammatory muscle disease and therefore not require intensification of immunomodulation. An increased CK (reflected in an increased CK-MB/total CK ratio) could be falsely interpreted as representing myocardial injury. In one study (which included 16 patients with IIM), skeletal muscle troponin I was found to correlate well (including with treatment response) with total CK and CK-MB, but not cTnI[29].
PROPOSED PATHWAYS USING CARDIAC TROPONINS TO SCREEN PATIENTS FOR SUBCLINICAL CARDIAC DISEASE AND/OR LOW-GRADE SKELETAL MUSCLE DISEASE ACTIVITY
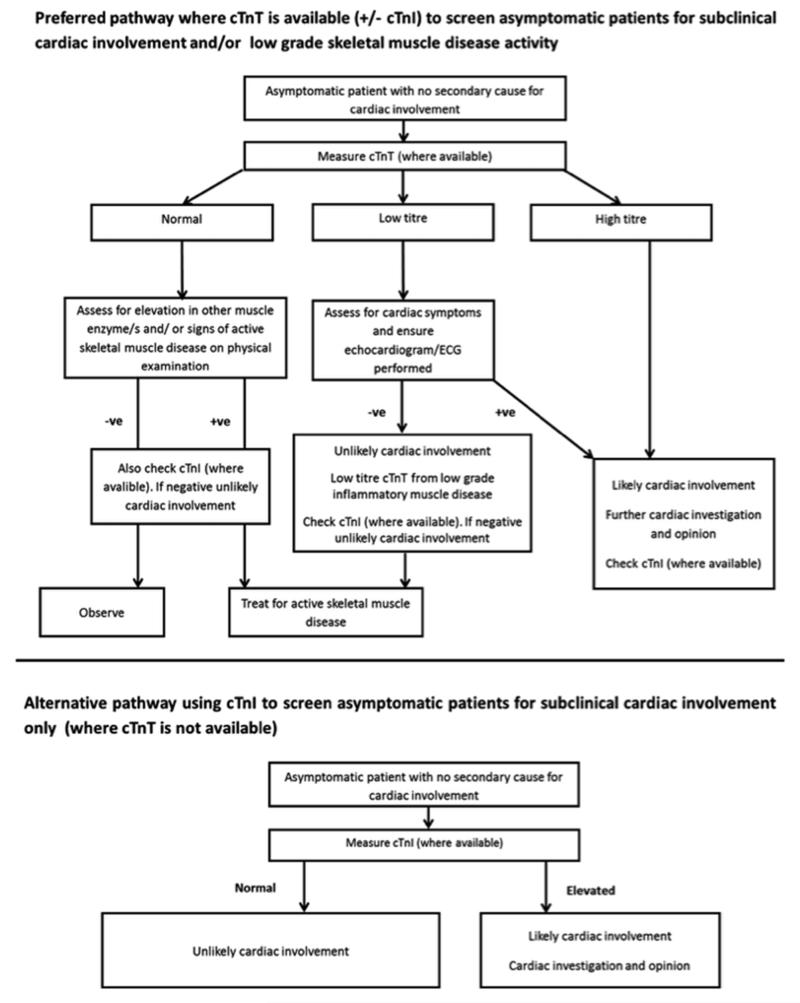
Access to cardiac troponin testing varies both nationally and internationally. We propose two pragmatic pathways to screen asymptomatic patients without known cardiac disease at presentation for subclinical cardiac involvement and/or low-grade skeletal muscle disease activity, preferably utilising cTnT, followed by confirmatory cTnI measurement (where cTnI is positive, then the patient likely has cardiac involvement). Where cTnT is unavailable, then cTnI should be measured (screening patients for subclinical cardiac involvement only) [Figure 1].
Figure 1.
Two proposed pathways using cardiac troponin testing (T and/or I) to screen asymptomatic patients at presentation with IIM and SSc-spectrum disorders for subclinical cardiac disease and/or low-grade skeletal muscle inflammatory disease activity. cTnI, cardiac troponin I; cTnT, cardiac troponin T; ECG, electrocardiogram.
Where available, cTnT should be initially measured at presentation (along with preferred skeletal muscle enzyme testing), the rationale being that cTnT can be used to screen for subclinical cardiac involvement/ or low-grade skeletal muscle disease activity (if cTnT is more sensitive than CK), thereby informing management. If the cTnT titre is relatively low, especially in the absence of cardiac symptoms/signs, then it may be of skeletal muscle (rather than cardiac) origin. Nevertheless at low cTnT titres, cardiac symptoms should be assessed and investigations (echocardiogram/ECG) arranged; if cardiac involvement is then suspected, further cardiac investigation/opinion is then warranted. If symptoms/investigations are negative, the patient should be treated as for skeletal muscle inflammatory disease. Where available, cTnI should be measured (in the absence of an elevated cTnI titre, then the patient is unlikely to have cardiac involvement). If cTnT is elevated (in the absence of cardiac symptoms/signs) in the presence of either another elevated muscle enzyme/s and/or clinical features of active skeletal disease (potentially masking a cardiac source of cTnT), where available, cTnI should be checked (a raised titre likely reflecting cardiac involvement).
Where cTnT is not available, cTnI should be measured to assess for cardiac involvement (as the generally accepted view is that cTnI is specific to cardiac muscle tissue). If cTnI is elevated, then the patient likely has cardiac involvement, requiring further cardiac investigation/opinion. Concomitant cTnT measurement is potentially problematic, as an elevated cTnT titre could be falsely attributed to cardiac, and therefore missing active skeletal muscle involvement in the context of an elevated (cardiac) cTnI
RESEARCH AGENDA
There are a number of key questions that require future research to address the relevance of a raised cTnT/cTnI in a patient with IIM or SSc-spectrum disorder, and whether cTnT is a more sensitive marker of skeletal muscle disease activity than CK. Firstly, carefully designed prospective pilot studies are required to examine the levels of cTnT and cTnI and to correlate these with other markers of cardiac disease (e.g. CMR) and skeletal muscle disease (e.g. serial muscle biopsies and magnetic resonance imaging), including to confirm that cTnT is solely a biomarker of skeletal regenerating skeletal muscle tissue, and if not, then whether it is more sensitive than CK for active inflammatory muscle disease activity. Secondly, does the level of cardiac troponin (cTnI/cTnT) relate to the amount of cardiac muscle tissue involvement? If so, then clinically useful levels of cardiac troponin (cTnI/cTnT) will need to be defined in order to risk stratify patients, and to confirm an association with primary cardiac disease (e.g. by CMR/endomyocardial biopsy) as these will both inform therapeutic decision-making. Thirdly, is the contribution of myocardial inflammation (which could affect troponin release) compared to fibrosis similar both within and between IIMs and SSc-spectrum disorders? Fourthly, is there a ‘cut-off’, which a raised cTnT cannot be solely attributed to skeletal muscle inflammation? Lastly, in asymptomatic patients with a negative cardiac troponin (including cTnI), does this completely exclude cardiac disease? Do they still require further cardiac investigation? Or should patients with a negative troponin be further risk stratified? For example (and not limited to) would unexplained tachycardia, ECG abnormalities and/or overlap with myositis either clinically or serologically (signal recognition particle/PM-Scl) warrant cardiac opinion/investigations?
CONCLUSION
In conclusion, cardiac troponins are a widely available, relatively inexpensive investigation with high sensitivity and specificity to detect myocardial injury. Whereas cTnI is considered to be specific to cardiac muscle tissue, cTnT is also re-expressed within regenerating adult skeletal muscle tissue. We propose that cardiac troponins are a potential screening tool to detect asymptomatic cardiac involvement and furthermore that cTnT may represent a more sensitive biomarker for low-grade skeletal muscle inflammation (e.g. than CK). Future cross-sectional and prospective studies are required to further validate the use of cardiac troponins in patients with IIM and SSc-spectrum disorders, correlation with other biomarkers (e.g. CMR) and the response to intervention.
Acknowledgments
Funding: All authors none to declare.
Footnotes
Competing Interests: All authors none to declare.
REFERENCES
- 1.Denbow CE, Lie JT, Tancredi RG, et al. Cardiac involvement in polymyositis: a clinicopathologic study of 20 autopsied patients. Arthritis Rheum. 1979;22:1088–92. doi: 10.1002/art.1780221007. [DOI] [PubMed] [Google Scholar]
- 2.Steen VD, Medsger TA., Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43:2437–44. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.Lundberg IE. The heart in dermatomyositis and polymyositis. Rheumatology (Oxford) 2006;45:iv18–21. doi: 10.1093/rheumatology/kel311. [DOI] [PubMed] [Google Scholar]
- 4.Kahan A, Coghlan G, McLaughlin V. Cardiac complications of systemic sclerosis. Rheumatology (Oxford) 2009;48:iii45–8. doi: 10.1093/rheumatology/kep110. [DOI] [PubMed] [Google Scholar]
- 5.Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69:1809–15. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Wang GC, Ma L, et al. Cardiac involvement in adult polymyositis or dermatomyositis: a systematic review. Clin Cardiol. 2012;35:686–91. doi: 10.1002/clc.22026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulkley BH, Ridolfi RL, Salyer WR, Hutchins GM. Myocardial lesions of progressive systemic sclerosis. A cause of cardiac dysfunction. Circulation. 1976;53(3):483–90. doi: 10.1161/01.cir.53.3.483. [DOI] [PubMed] [Google Scholar]
- 8.Meune C, Vignaux O, Kahan A, et al. Heart involvement in systemic sclerosis: Evolving concept and diagnostic methodologies. Arch Cardiovasc Dis. 2010;103:46–52. doi: 10.1016/j.acvd.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Lai YT, Dai YS, Yen MF, et al. Dermatomyositis is associated with an increased risk of cardiovascular and cerebrovascular events: a Taiwanese population-based longitudinal follow-up study. Br J Dermatol. 2013;168:1054–9. doi: 10.1111/bjd.12245. [DOI] [PubMed] [Google Scholar]
- 10.Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis. 2013;72:1188–93. doi: 10.1136/annrheumdis-2012-202007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodor GS, Survant L, Voss EM, et al. Cardiac troponin T composition in normal and regenerating human skeletal muscle. Clin Chem. 1997;43:476–84. [PubMed] [Google Scholar]
- 12.Skeik N, Patel DC. A review of troponins in ischemic heart disease and other conditions. Int J Angiol. 2007;16:53–8. doi: 10.1055/s-0031-1278248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clause KC, Tchao J, Powell MC, et al. Developing cardiac and skeletal muscle share fast-skeletal myosin heavy chain and cardiac troponin-I expression. PLoS One. 2012;7:e40725. doi: 10.1371/journal.pone.0040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams JE, 3rd, Bodor GS, Dávila-Román VG, et al. Cardiac troponin I. A marker with high specificity for cardiac injury. Circulation. 1993;88:101–6. doi: 10.1161/01.cir.88.1.101. [DOI] [PubMed] [Google Scholar]
- 15.Anderson PAW, Malouf NN, Oakeley AE, et al. Troponin T isoform expression in humans: a comparison among normal and failing adult heart, fetal heart and adult and fetal skeletal muscle. Circ Res. 1991;69:1226–33. doi: 10.1161/01.res.69.5.1226. [DOI] [PubMed] [Google Scholar]
- 16.Erlacher P, Lercher A, Falkensammer J, et al. Cardiac troponin and beta-type myosin heavy chain concentrations in patients with polymyositis or dermatomyositis. Clin Chim Acta. 2001;306:27–33. doi: 10.1016/s0009-8981(01)00392-8. [DOI] [PubMed] [Google Scholar]
- 17.Hamm CW, Goldmann BU, Heeschen C, et al. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N Engl J Med. 1997;337:1648–53. doi: 10.1056/NEJM199712043372302. [DOI] [PubMed] [Google Scholar]
- 18.Maynard S, Menown I, Adgey A. Troponin T or troponin I as cardiac markers in ischaemic heart disease. Heart. 2000;83:371–3. doi: 10.1136/heart.83.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeremias A, Gibson CM. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med. 2005;142:786–91. doi: 10.7326/0003-4819-142-9-200505030-00015. [DOI] [PubMed] [Google Scholar]
- 20.Agewall S, Giannitsis E, Jernberg T, et al. Troponin elevation in coronary vs. non-coronary disease. Eur Heart J. 2011;32:404–11. doi: 10.1093/eurheartj/ehq456. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton JS, Sharpe PC. Two cases of inflammatory muscle disease presenting with raised serum concentrations of troponin T. J Clin Pathol. 2005;58:1323–4. doi: 10.1136/jcp.2005.025734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarzmeier JD, Hamwi A, Preisel M, et al. Positive troponin T without cardiac involvement in inclusion body myositis. Hum Pathol. 2005;36:917–21. doi: 10.1016/j.humpath.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Lindberg C, Klintberg L, Oldfors A. Raised troponin T in inclusion body myositis is common and serum levels are persistent over time. Neuromuscul Disord. 2006;16:495–7. doi: 10.1016/j.nmd.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Aggarwal R, Lebiedz-Odrobina D, Sinha A, et al. Serum cardiac troponin T, but not troponin I, is elevated in idiopathic inflammatory myopathies. J Rheumatol. 2009;36:2711–4. doi: 10.3899/jrheum.090562. [DOI] [PubMed] [Google Scholar]
- 25.Cox FM, Delgado V, Verschuuren JJ, et al. The heart in sporadic inclusion body myositis: a study in 51 patients. J Neurol. 2010;257:447–51. doi: 10.1007/s00415-009-5350-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinser R, Frerix M, Meier FMP, et al. Endocardial and myocardial involvement in systemic sclerosis-is there a relevant inflammatory component? Joint Bone Spine. 2013;80:320–3. doi: 10.1016/j.jbspin.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Vasta B, Flower V, Bucciarelli-Ducci C, et al. Abnormal cardiac enzymes in systemic sclerosis: a report of four patients and review of the literature. Clin Rheumatol. 2014;33:435–8. doi: 10.1007/s10067-013-2405-1. [DOI] [PubMed] [Google Scholar]
- 28.Miller FW, Rider LG, Chung YL, et al. Proposed preliminary core set measures for disease outcome assessment in adult and juvenile idiopathic inflammatory myopathies. Rheumatology (Oxford) 2001;40:1262–73. doi: 10.1093/rheumatology/40.11.1262. [DOI] [PubMed] [Google Scholar]
- 29.Kiely PDW, Bruckner FE, Nisbet JA, et al. Serum skeletal troponin I in inflammatory muscle disease: relation to creatine kinase, CKMB and cardiac troponin I. Ann Rheum Dis. 2000;59:750–1. doi: 10.1136/ard.59.9.750. [DOI] [PMC free article] [PubMed] [Google Scholar]