Abstract
Opioids can be classified according to their mode of synthesis into alkaloids, semi-synthetic and synthetic compounds.
There are three classical receptors (DOP, KOP and MOP). The novel NOP receptor is considered to be a non-opioid branch of the opioid receptor family.
Opioids can either act as agonists, antagonists or partial agonists at these receptors.
Opioid agonists bind to G-protein coupled receptors to cause cellular hyperpolarisation.
MOP receptor agonists act in the central and peripheral nervous system to elicit analgesia.
Introduction
Morphine is commonly considered to be the archetypal opioid analgesic and the agent to which all other painkillers are compared. For several thousand years, opioid-based analgesic agents have been used, in one form or another, to control pain There is evidence to suggest that as long ago as 3000BC the opium poppy, Papaver somniferum, was cultivated for its active ingredients in what is now modern day Iraq. More recently, from the sixteenth to nineteenth centuries, laudanum (an alcoholic mix of opium and various ingredients) was frequently used as a popular remedy in European medicine. It was, however, not until morphine was isolated from opium in 1806 by Sertürner that modern opioid pharmacology was truly born. In 1847 the chemical formula for morphine was deduced and this, coupled with the invention of the hypodermic needle in 1853, led to the more precise and widespread clinical use of morphine1,2.
Classification
Though morphine is the most widely known extract of Papaver somniferum, four naturally occurring alkaloids (plant derived amines) can be isolated from it: morphine, codeine, papaverine and thebaine. Of these alkaloids, morphine, codeine (a prodrug of morphine) and papaverine have found a place in clinical practice; morphine and codeine as analgesic agents and papaverine, a compound devoid of any pain killing properties, as a smooth muscle relaxant. Following Sertürner's isolation of morphine as the active component of the opium poppy, simple chemical manipulations of these basic opiate alkaloids began to yield a range of semi-synthetic opioids useful in clinical medicine (agents such as diamorphine, dihydrocodeine, buprenorphine, nalbuphine, naloxone and oxycodone). During the 20th century a number of synthetic opioids were also produced either by design or serendipitously. These synthetic compounds can be divided into four chemical groupings, the morphinan derivatives (levorphanol, butorphanol), the diphenylheptane derivatives (methadone, propoxyphene), the benzomorphan derivatives (pentazocine, phenazocine) and the phenylpiperidine derivatives (pethidine, alfentanil, fentanyl, sufentanil and remifentanil) (Table 1)2,3.
Table 1.
Classification of opioids by synthetic process.
| Naturally occuring | Semi-synthetic compounds | Synthetic compounds |
|---|---|---|
| Morphine | Heroin | Pethidine |
| Codeine | Dihydromorphone | Fentanyl |
| Thebaine | Buprenorphine | Methadone |
| Papaverine | Oxycodone | Alfentanil |
| Remifentanil | ||
Opioids can also be classified according to their effect at opioid receptors. In this manner opioids can be considered as agonists, partial agonists and antagonists. Agonists interact with a receptor to produce a maximal response from that receptor (analgesia following morphine administration is an example). Conversely antagonists bind to receptors but produce no functional response, while at the same time preventing an agonist from binding to that receptor (naloxone). Partial agonists bind to receptors but only elicit a partial functional response no matter the amount of drug administered (buprenorphine).
Opioid receptors
In addition opioids can be categorized according to the type of opioid receptor at which they produce their effects. Classically there are considered to be three opioid receptors. These receptors are all G-protein coupled receptors, and were originally named mu (after morphine, its most commonly recognised exogenous ligand), delta (after vas deferens, the tissue within which it was first isolated) and kappa (after ketocyclazocine, the first ligand to act at this receptor). In 1996 the International Union of Pharmacology (IUPHAR) renamed the receptors OP1 (the delta receptor), OP2 (the kappa receptor) and OP3 (the mu receptor). In 2000 this nomenclature was again changed to DOP, KOP and MOP; this remains the current classification. (Table 2)1,4. Some authorities describe the existence of multiple subtypes of the three classical opioid receptors, but this is not a belief held by all researchers within the field. The classical opioid receptors are distributed widely within the central nervous system and to a lesser extent throughout the periphery, occupying sites within the vas deferens, knee joint, gastrointestinal tract, heart and immune system amongst others5.
Table 2.
Changes in the classification of the classical opioid receptors over time.
| Pre-cloning | Post-cloning | IUPHAR 1996 | IUPHAR 2000 |
|---|---|---|---|
| δ | DOR | OP1 | DOP |
| κ | KOR | OP2 | KOP |
| μ | MOR | OP3 | MOP |
Soon after the discovery of the opioid receptors a series of endogenous ligands active at the receptors were discovered in brain extracts. Three pro-hormone precursors provide the parent compounds from which these endogenous ligands are derived. Proenkephalin is cleaved to form met-enkephalin and leu-enkephalin which bind to the DOP receptor. Dynorphin A and B are derived from prodynorphin and are agonists at the KOP receptor. Pro-opiomelanocortin (POMC) is the parent compound for β-endorphin, an agonist at the MOP receptor, though it is capable of displaying agonist activity at all three classical opioid receptors1,3,6. Two further endogenous peptides act as agonists at the MOP receptor, endomorphin 1 and 2, but no precursor has yet been identified (Table 3). There is significant cross talk between the endogenous agonists and the three classical receptors.
Table 3.
Opioid receptors and their endogenous ligands and precursors.
| Receptor | Precursor | Peptide |
|---|---|---|
| DOP | Pro-enkephalin | [Met]-enkephalin [Leu]-enkephalin |
| KOP | Pro-dynorphin | Dynorphin-A Dynorphin-B |
| MOP | POMC | β-Endorphin |
| Unknown | Endomorphin-1 Endomorphin-2 |
|
| NOP | Pre-pro-nociceptin | N/OFQ |
In 1994 a fourth G-protein coupled endogenous opioid like receptor was found and subsequently named the nociceptin receptor (NOP). Rapidly after this discovery came the isolation from brain extracts of its endogenous ligand nociceptin/orphanin FQ (N/OFQ). This endogenous ligand is similarly derived from a precursor compound, in this instance from the polypeptide precursor pre-pro-nociceptin. Though the N/OFQ/NOP system does not bind naloxone, nor are its effects reversed by naloxone, it is a G-protein coupled receptor system that shares a marked similarity to the known amino acid sequences of the classical opioid receptors (the NOP receptor shows 80% homology to the KOP receptor)1,7. At a cellular level N/OFQ acts to produce similar actions to those described for the classical opioid receptors above. For these reasons it has been classified as the fourth opioid receptor; however, due to its lack of response to the classical opioid antagonist (naloxone) some pharmacologists have questioned the wisdom of this classification. IUPHAR considers the NOP receptor to be a non-opioid branch of the opioid receptor family4.
In clinical practice the stimulation of the differing opioid receptors produces a range of effects along with analgesia, which are often dependent upon the location of the receptor. Agonists binding to MOP receptors may cause analgesia but also sedation, respiratory depression, bradycardia, nausea and vomiting and a reduction in gastric motility. Similar activation of DOP receptors can cause spinal and supraspinal analgesia and reduce gastric motility, while KOP receptor stimulation may produce spinal analgesia, a diuresis and dysphoria. Spinally N/OFQ has been shown to produce analgesia and hyperalgesia, dependent upon the administered concentration, and allodynia. Supraspinally when administered intracerebrovascularly it is thought to produce a pro-nociceptive anti-analgesic effect, due to an inhibition of endogenous opioid tone1,8.
Intracellular events
Though producing subtly different functional effects all of these receptors display similar cellular responses following receptor activation. Binding of an opioid agonist to a G-protein coupled opioid receptor on the transmembrane portion of the receptor causes the α subunit of the G-protein to exchange its bound guanosine diphosphate (GDP) molecule with intracellular guanosine triphosphate (GTP). This then allows the α-GTP complex to dissociate away from the βγ complex. Both of these complexes (α-GTP and βγ) are then free to interact with target proteins. In the case of a classical opioid agonist binding to its G-protein receptor, this results in the inhibition of adenylate cyclase. This in turn causes a reduction in intracellular cyclic adenosine monophosphate (cAMP) levels. Additionally these complexes interact with a number of ion channels, producing activation of potassium conductance and an inhibition of calcium conductance. The net effect of these changes is of a reduced intracellular cAMP, a hyperpolarisation of the cell in question and, for neuronal cells, reduced neurotransmitter release (Figure 1). In some cell types activation of opioid receptors can also paradoxically cause a release of calcium from intracellular stores, leading to an increase in the intracellular calcium concentration6,9.
Figure 1.
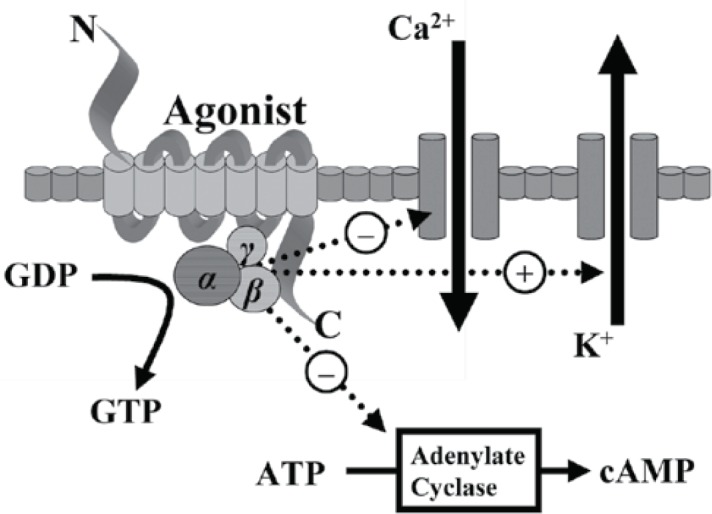
Intracellular changes occurring following the binding of an opioid agonist to a G-protein coupled opioid receptor.
Opioid mediated analgesia
Opioid receptors are distributed throughout the central nervous system and within peripheral tissue of neural and non-neural origin. Centrally, the periaqueductal grey (PAG), locus cereleus and rostral ventral medulla show high concentrations of opioid receptors, while opioid receptors are also present in the substantia gelatinosa of the dorsal horn.
Within the central nervous system activation of MOP receptors in the midbrain is thought to be a major mechanism of opioid induced analgesia. Here MOP agonists act by indirectly stimulating descending inhibitory pathways which act upon the periaqueductal grey (PAG) and nucleus reticularis paragigantocellularis (NRPG) with the net effect of an activation of descending inhibitory neurones. This leads to greater neuronal traffic through the nucleus raphe magnus (NRM), increasing stimulation of 5-HT and enkephalin-containing neurons which connect directly with the substantia gelatinosa of the dorsal horn. This in turn results in a reduction of nociceptive transmission from the periphery to the thalamus. Exogenous and endogenous opioids can also exert a direct inhibitory effect upon the substantia gelatinosa (in the dorsal horn) and peripheral nociceptive afferent neurones, reducing nociceptive transmission from the periphery (Figure 2). This series of cellular events and mechanisms produces much of the analgesic effect commonly seen following the administration of MOP agonists.
Figure 2.
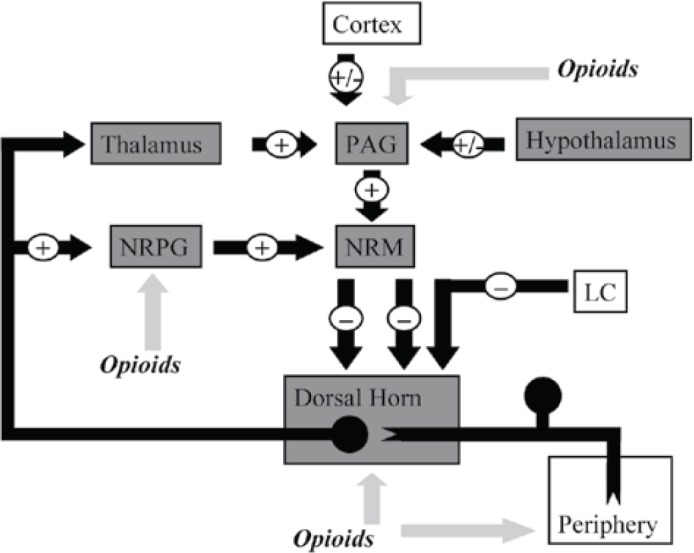
This figure shows schematically the descending inhibitory pathways. Areas shaded grey display a high expression of opioid receptors and their endogenous ligands. MOP agonists produce analgesia either by indirectly increasing neuronal traffic through the descending pathway at the NRPG and PAG, or by directly inhibiting nociceptive afferents in the periphery.
PAG = Periaqueductal grey
NRPG = Nucleus reticularis paragigantocellularis
NRM = Nucleus raphe magnus
LC = Locus cereleus
Clinical opioids
All opioids used in clinical practice today work at the MOP receptor, though some have additional activity at one or more of the other opioid receptors. There are presently no agents available for medical use which are specific to just DOP, KOP or NOP receptors (agonists, antagonists or partial agonists).
Both codeine and morphine, two of the naturally occurring alkaloids present in the opium poppy, are MOP agonists commonly used as analgesic agents within the pain clinic. Codeine first needs to be metabolised by the body to morphine for it to display any activity. A percentage of the population lack the ability to perform this conversion and so derive limited pain relief from it. Morphine also has some action at both DOP and KOP receptors. Oxycodone, another commonly used analgesic, is a semi-synthetic derivative of thebaine. It has been used clinically for almost ninety years and mediates its analgesic properties through both MOP and KOP receptors. In contrast, buprenorphine is one of the few opioid partial agonists available for medical administration, working as a partial agonist at both the MOP and NOP receptors (it also has activity at DOP and KOP receptors)1,10. In practice this means that it produces analgesic effects at lower plasma concentrations via its interaction with the MOP receptor, but anti-analgesic effects at high doses through interactions with the NOP receptor. It is less sedating and produces less euphoria than a full MOP agonist. These properties make it a useful drug within the pain clinic as the potential for respiratory depression and overdose is reduced compared to MOP agonists.
Naloxone and naltrexone are antagonists at all three classical opioid receptors, attenuating or reversing the effects of agonists at all these sites. They do not however bind to the NOP receptor and therefore would not modulate the effects of any future NOP agonists or partial agonists.
References
- 1.McDonald J, Lambert DG. Opioid Receptors. Contin Educ Anaesth Crit Care Pain 2005; 5: 22–25. [Google Scholar]
- 2.Blakemore PR, White JD. Morphine, the Proteus of organic molecules Chem. Commun 2002: 1159–1168. [DOI] [PubMed]
- 3.Charlton JE. (Ed.) Opioids. In: Core Curriculum for Professional Education in Pain. IASP Press; 2005. [Google Scholar]
- 4.Dhawan BN, Cesselin F, Raghubir R, et al. International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol Rev 1996; 48: 567–592. [PubMed] [Google Scholar]
- 5.Stein C, Schafer M, Machelska H. Attacking pain at its source: new perspectives on opioids. Nat Med 2003; 9: 1003–1008. [DOI] [PubMed] [Google Scholar]
- 6.Corbett AD, Henderson G, McKnight AT, et al. 75 years of opioid research: the exciting but vain quest for the Holy Grail. British J Pharmacology 2006; 147: S153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calo' G, Guerrini R, Rizzi A, et al. Pharmacology of nociceptin and its receptor: a novel therapeutic target. British Journal of Pharmacology 2000; 129: 1261–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan Z, Hirakawa N, Fields HL. A cellular mechanism for the bidirectional pain-modulating actions of orphanin FQ/nociceptin. Neuron 2000; 26: 515–522. [DOI] [PubMed] [Google Scholar]
- 9.Harrison C, Smart D, Lambert DG. Stimulatory effects of opioids. British J Anaesthesia 1998; 81: 20–28. [DOI] [PubMed] [Google Scholar]
- 10.Soichiro I, Minami M, Satoh M, et al. Burenorphine antinociception is abolished, but naloxone-sensitive reward retained, in mu-opioid receptor knockout mice Neuropsychopharmacology 2004; 29: 1656–1663. [DOI] [PubMed] [Google Scholar]
Further Reading
- Bailey PL, Egan TD, Stanley TH. In: Miller RD. (Ed). Anesthesia, 5th ed. Churchill Livingstone; 2000. [Google Scholar]
- Eguchi M. Recent advances in selective opioid receptor agonists and antagonists. Med Res Rev 2004; 24: 182–212. [DOI] [PubMed] [Google Scholar]
- Collett BJ. Chronic opioid therapy for non-cancer pain. Br J Anaesth 2001; 87: 133–143. [DOI] [PubMed] [Google Scholar]
- British Pain Society. Recommendations for the appropriate use of opioids for persistent non-cancer pain. A consensus statement prepared on behalf of the Pain Society, the Royal College of Anaesthetists, the Royal College of General Practitioners and the Royal College of Psychiatrists. British Pain Society March 2004.


