Abstract
Insomnia is a major source of distress to people with chronic pain; many of whom presenting for treatment want tangible help with sleep.
Compared to chronic pain patients who do not have trouble sleeping, those who do, report more severe pain, longer pain duration, greater levels of anxiety, depression and health anxiety, and worse impairment in physical and psychosocial functioning.
Sleep disturbance experienced by patients with chronic pain can be characterised by longer sleep onset, more frequent and longer awakenings after sleep onset, shorter total sleep time, lower sleep efficiency and poorer sleep quality. Such pattern of disturbance is analogous to that of primary insomnia.
The relationship between pain and sleep is likely to be bi-directional, although exactly how the two problems interact is little understood.
The offer of sleep advice and the use of pharmacotherapy for pain-related insomnia have their respective limitations. Psychological and behavioural treatments demonstrated to be effective for both primary and comorbid insomnia may be a viable treatment alternative.
Introduction
Considering that pain infliction and sleep deprivation are two common methods of torture, it is not difficult to imagine the misery of a person who suffers from not just chronic pain but also insomnia. Having trouble sleeping is a major source of distress to many people living with chronic pain (1). Amongst the people attending pain clinics, those who report trouble sleeping are usually those who are more disabled (2). In addition to better pain control, these patients presenting for treatment frequently name sleep as one specific area with which they want tangible help (3). It is therefore of medical importance to understand the interaction between pain and sleep and to be aware of the various assessment and treatment options for pain patients with persistent insomnia.
The last three decades saw a welcomed influx of scholarly literature investigating the impact of sleep disturbance on chronic pain, alongside their interrelationship. The current review aims to provide a concise summary of the psychophysiological aspects of these topics. The Sleep and Pain book published by the International Association for the Study of Pain provides a state-of-the-art overview of the whole spectrum of this fascinating research (4).
Prevalence of sleep disturbance in chronic pain
A consistently high prevalence of sleep complaints has been documented for a range of chronic pain conditions, including arthritis, back/neck pain, fibromyalgia, headaches and orofacial pain. Compared to pain-free individuals, a number of studies have indicated that people reporting chronic pain are at elevated risk of experiencing problems sleeping. In the general community, approximately 20% of the people living with chronic pain report at least one symptom of insomnia compared to only 7.4% in those without chronic pain (5). Within the healthcare system, as many as 90% of the patients attending a pain management centre for treatment also report at least one sleep complaint (2,6) and more than 65% of these patients would identify themselves as “poor sleepers” (7). The rates of insomnia tend to be lower when stricter assessment criteria are employed. In a recent study that used a standardised insomnia assessment questionnaire, the prevalence of moderate to severe clinical insomnia was estimated to be 53% amongst patients seeking treatment from a pain specialist clinic, compared to only 4% in age- and sex-matched pain-free controls (8, Fig. 1). These findings reveal the scale of the problem and highlight the necessity to study insomnia in the chronic pain population.
Figure 1.
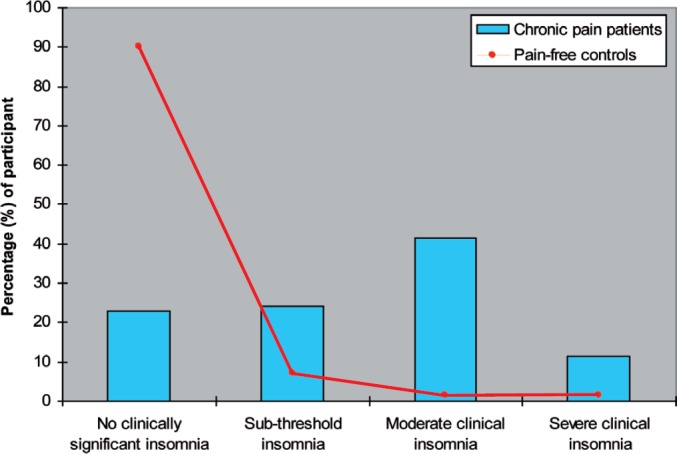
Prevalence of clinical insomnia among chronic pain patients and age- and sex-matched pain-free controls. The presence of clinical insomnia was determined by the validated scores of the Insomnia Severity Index (ISI), (17); ISI score 0–7 = no clinically significant insomnia, ISI score 8–14 = sub-threshold insomnia, ISI score 15–21 = moderate clinical insomnia, ISI score 22–28 = severe clinical insomnia.
Clinical features
Clinical interviews suggest that many chronic pain patients seek respite from pain by going to sleep (9). Ironically, falling asleep in itself is a struggle for most of these patients, and more often than not the sleep obtained is light and disrupted with awakenings, leaving the patients feeling tired and unrefreshed the next morning.
Sleep abnormalities (see Table 1 for a glossary of sleep terms*)
Table 1.
Glossary of Commonly-Used Sleep Terms.
| Equipment | |
| Polysomnography (PSG) | Sleep-assessment technology based on electrophysiological recordings of brain activity that radiates from the scalp (EEG; electroencephalogram) along with muscle activity (EMG; electromyogram) and eye movement (EOG; electrooculogram). The electrical output is scored and categorised into different stages of sleep/wakefulness to provide information about the pattern and architecture of sleep. |
| Actigraphy | Sleep-assessment technology that provides an estimate of sleep/wakefulness by monitoring the amount of physical motion. Aided by scoring algorithms, actigraphy provides information about the sleep pattern and diurnal variations of physical activity. Continuous use of actigraphy over successive 24 hour periods can provide reliable information about a person's sleep-wake cycle. |
| Measure parameters | |
| Sleep onset latency (SOL) | The total amount of time taken to fall asleep. |
| Wake after sleep onset (WASO) | The total amount of time awake after sleep onset. |
| Total sleep time (TST) | The total amount of sleep obtained. |
| Sleep efficiency (SE) | The percentage of time asleep throughout the entire sleep period, which can be calculated by: (TST/total time in bed) x 100%. |
| Sleep quality (SQ) | A subjective rating of the quality of the sleep obtained and the restful/restored feeling upon waking. |
| NREM sleep | Non rapid eye movement sleep; one of the two main states of sleep alternating with REM sleep in cycles. NREM sleep is conventionally subdivided into 4 stages (Stages 1, 2, 3 and 4). Stages 1 and 2 are regarded as lighter and Stages 3 and 4 are regarded as deeper stages of sleep. NREM sleep is characterised by synchronous EEG activity and is usually associated with minimal mental and physical activity. |
| REM sleep | Rapid eye movement sleep; the second main state of sleep. REM sleep is defined by EEG activation, muscle atonia and episodic burst of rapid eye movements. It occurs in 4–6 discrete episodes, making up between 20% to 25% of a normal night of sleep. This state of sleep is associated with dreaming. |
| Spindle activity | Bursts of EEG activity (12–14 cps) of at least 0.5 seconds that occur in and define Stage 2 sleep. |
Research data from a handful of studies using daily sleep diaries or retrospective sleep questionnaires present a similar picture. Compared to pain patients who do not have problems sleeping, those who do, report a significantly longer sleep onset latency*, more frequent and lengthy awakenings after sleep onset*, shorter total sleep time* and poorer sleep quality*. Whilst there may be discrepancies, this subjective data is largely consistent with the objective sleep findings reported in some other studies using actigraphy* and polysomnography*. In these studies, sleep fragmentation and poorer sleep quality are indicated by (i) the presence of more body movements during sleep, (ii) more sleep-stage shifts, (iii) more frequent awakenings, (iv) lower sleep efficiency*, (v) an increase in Stage 1 or 2 sleep with less spindle activity*, (vi) a reduction in Stage 3 or 4 sleep and (vii) an intrusion of electroencephalographic (EEG) activity in the alpha range (8–13 cps) during non rapid eye movement (NREM*) sleep. Although it has been suggested that the alpha intrusion may be an EEG signature of sleep disturbance specific to chronic pain (fibromyalgia in particular); the balance of evidence so far indicates otherwise.
There is a dearth of literature directly looking at differences in sleep abnormalities across various individual pain conditions. From several previous reviews (10,11,12), it appears that different subgroups of pain patients may exhibit characteristically different objective sleep findings (e.g., increased REM sleep abnormalities in Irritable Bowel Syndrome, increased nocturnal bruxism in Temporomandibular Disorder/chronic orofacial pain, excessive percentage of slow wave sleep and REM sleep in Migraine). More systematic profiling of sleep disturbance in subgroups of chronic pain patients is required to extend our understanding of the subtle differences. At the broadest level, it seems reasonable to assume that the general pattern of sleep disturbance across painful conditions is similar to that of primary insomnia.
Clinical and psychological correlates
In addition to greater pain severity (7,13) and longer pain duration (14,15), pain patients with sleep disturbance also tend to report higher levels of anxiety, depression and health anxiety than those without any sleep issues (16). In some studies, these general psychological factors have been identified as independent predictors of the severity of sleep complaints, over and above the effects of demographics and pain. A small number of specific cognitive-behavioural processes have also been linked to the degree of insomnia severity in chronic pain; these include pain attention (17), affective interpretation of pain (8), pre sleep pain related thoughts (13), dysfunctional beliefs and attitude about sleep (18) and the idea of using sleep as an escape from pain (9).
Although the relative role of and interaction between these factors await further theoretical refinement and empirical investigation, further research of this kind is likely to be clinically informative. It would point to the processes (changeable) rather than the traits (stable) that are potentially involved in the development/maintenance of insomnia in this population, suggesting possible targets for intervention.
Pain-sleep interaction
There has been a growing awareness that untreated insomnia could serve to aggravate pain perception and contribute to poorer physical and psychosocial functioning. Such recognition has generated a widely accepted theory that the relationship between pain and sleep is reciprocal.
Pain affects sleep
Consistent with the hypothesis that chronic pain contributes to worse sleep, 60% of pain clinic patients attribute the genesis of insomnia to pain (6) with 53–90% reporting the onset of insomnia to be around or following the onset of chronic pain (6,7). Though not always, pain intensity has been identified in various cross-sectional studies as a significant predictor of sleep disruption. Further evidence suggesting a causal link between pain and sleep comes from laboratory studies, in which the induction of acute experimental painful stimuli has been shown to induce arousal/wakefulness in otherwise normal-sleeping healthy volunteers. It is not certain to what extent these findings could be applied to the clinical population to whom pain is an almost constant experience.
Sleep affects pain
Whilst less intuitive, evidence is also accumulating in support of the hypothesis that insomnia contributes to worse pain. At the community level, a few large-scale population surveys have now shown that insomnia is a significant risk factor for the development/maintenance of pain symptoms. People reporting insomnia symptoms are at least 3 times more likely to have a chronic painful physical condition (5). Poor sleep quality has been found to predict not only pain but also fatigue, daytime inactivity and poorer physical and psychosocial functioning (2). Indeed, several well-designed experimental studies have demonstrated that sleep deprivation and selective sleep disruption (slow wave sleep in particular) for no less than 3 consecutive nights can decrease pain threshold, amplify negative mood and produce somatic symptoms mimicking those of fibromyalgia in healthy volunteers (19). These findings dovetail with those derived from an often-cited prospective sleep diary study of fibromyalgia patients, showing that increased day time pain is sequentially linked with poorer night time sleep and poorer night time sleep is in turn associated with worse pain the following day (17).
Is there more to the reciprocal link?
Although evidence from various studies so far has converged to suggest a bi-directional link between sleep and pain, the relationship apparently is more complex than it seems. More recently, research has begun to delineate and to identify the mechanisms underpinning the pain-sleep relationship. Findings from the latest studies have hinted at a possibility that (i) it is the disruption of sleep continuity rather than simple sleep deprivation that impairs pain tolerance and increases pain perception (20) and (ii) the association between sleep and pain may be mediated by depression (21) and/or cognitive-behavioural factors similar to those involved in the maintenance of primary insomnia (8,18,20). It is also helpful to bear in mind that sleep disturbance in some pain patients may have been triggered/aggravated by drugs they have taken for pain relief (e.g., opioids and non-steroid anti-inflammatory drugs that contain caffeine). These findings and observations open up exciting avenues for further investigation and will have direct relevance to the development of treatment for pain-related insomnia.
Current treatment approach
Conventionally, sleep disturbance occurring in the context of chronic pain is considered within the framework of “secondary insomnia”, with pain regarded as the primary problem and insomnia largely as a symptom. An unfortunate legacy of this conceptualisation is that sleep complaints in chronic pain patients are overlooked at worst, and at best symptomatically controlled in mainstream treatments. Patients may be offered some sleep tips, although in the context of primary insomnia sleep hygiene as a stand-alone/add-on treatment offers minimal help to people with chronic sleep disturbance (22). Patients may be prescribed benzodiazepines or antidepressants with sedative qualities. These drugs can alleviate sleep onset problems and lengthen total sleep time in the short term but they alter the sleep architecture. Safety and common side effects aside, the effectiveness associated with the long-term consumption of these drugs are yet to be established. Clinical observations suggest that a large number of hypnotic-dependent insomniacs continue to report poor quality sleep.
Consistently, there is some evidence suggesting that most pain patients (86%) taking these medications to help with their sleep continue to report problems sleeping (23).
More recently, the validity and clinical utility of the secondary insomnia concept have been challenged. In the 2005 National Institutes of Health States of the Science Conference Statement, the term “comorbid insomnia” is recommended to replace “secondary insomnia” for concerns that the use of the latter term may promote (i) under-treatment and (ii) under-recognition of the dynamic interaction between sleep and the co-occurring medical/psychiatric disorders. This recommendation bolsters earlier calls for more proactive treatments for insomnia in chronic pain advocated by colleagues in the field of behavioural sleep medicine.
Clinical assessment
Brief standardised insomnia questionnaires can easily be administered within the context of a pain clinic to acquire information about the form, pattern, severity and duration of the sleep disturbance. Two example questionnaires widely used in the sleep field are the Insomnia Severity Index (24) and the Pittsburgh Sleep Quality Index (25). These questionnaires are short and easy to administer. More importantly, they are extensively validated and far more reliable than a single item rating scale to index the severity of the sleep complaint. The information obtained from these questionnaires would help the clinician formulate a better treatment plan and facilitate the referral process if specialist assessment and treatment of sleep disorders are warranted.
Treatment alternatives
As mentioned earlier, the use of sleep advice and pharmacotherapy for persistent insomnia amongst pain patients have their respective limitations. Presenting as a treatment alternative, psychological and behavioural interventions for insomnia are now recognised as an effective treatment for not only primary insomnia but also insomnia associated with other medical and psychiatric conditions (22). This type of intervention adopts a multi-component approach, targeting various psychological mechanisms contributing to the persistence of the problem. Treatment components may include (i) stimulus control therapy, (ii) sleep restriction, (iii) relaxation training, (iv) paradoxical intention, (v) biofeedback, (vi) cognitive therapy, (vii) sleep hygiene education and (viii) imagery training (see Table 2 for description). The utility of such treatment has been tested in chronic non-malignant pain patients meeting diagnostic criteria for insomnia. There are at least 2 published randomised controlled trials (26,27) demonstrating effectiveness in alleviating pain patients' difficulty sleeping with some parallel improvements observed in mood and daytime functioning. Although interestingly no significant reduction in pain is noted in either of these trials, these findings challenge the myth of secondary insomnia and show promise in offering sleep intervention as an adjunct/integrated treatment for pain patients with clinical insomnia.
Table 2.
Description of Individual Treatment Components Commonly Included as part of the Multi-component Cognitive Behaviour Therapy for Insomnia
| Therapy | Content | Objective(s) | Level of recommendation* |
|---|---|---|---|
| Stimulus control therapy | Stimulus control therapy involves 5 main instructional procedures: (i) go to bed only when sleepy, (ii) use the bed/bedroom for only sleep and sex, (iii) get out of bed when fails to fall asleep within 15–20 minutes, (iv) maintain a regular sleep-wake schedule, and (v) avoid daytime naps | To train the patient to re-associate the bed and bedroom with rapid sleep onset | Standard† |
| Sleep restriction | Sleep restriction involves cutting the amount of time in bed down to the actual amount of time asleep | To increase sleep pressure and consolidate sleep by introducing a mild form of sleep deprivation | Guideline‡ |
| Relaxation training | Relaxation training involves techniques (e.g., progressive muscle relaxation) that aim at reducing sleep-interfering somatic or cognitive tension at or around bedtime | To deactivate the arousal system and facilitate sleep onset | Standard |
| Paradoxical intention | Paradoxical intention involves instructing the patient to remain awake and avoid any effort/intention to fall asleep | To reduce sleep effort and performance anxiety that inhibits sleep onset | Guideline |
| Biofeedback | Biofeedback involves providing visual or auditory feedback to patients to help increase their control over some biological responses (e.g., blood pressure, muscle tension, heart rate) | To reduce somatic arousal and self-efficacy | Guideline |
| Cognitive therapy | Cognitive therapy involves identifying and challenging patient's unhelpful cognitions about sleep and replacing them with more helpful substitutes, through the flexible use of a range of discussion techniques (e.g., reappraisal of threat, attention shifting, hypothesis testing) | To alter unhelpful beliefs and attitude about sleep and to reduce patients' emotional distress associated with sleep. | No recommendation level |
| Sleep hygiene education | Sleep hygiene education involves teaching the patients the potential beneficial or detrimental impact of certain environmental (e.g., noise, lighting, ventilation, temperature and level of comfort of the bedroom), dietary (e.g., meal times, consumption of stimulants, alcohol and sleep aid) and behavioural (e.g., exercise, daytime napping) factors on sleep | To increase awareness of environmental factors and health practices that may either promote or interfere with sleep | No recommendation level |
| Imagery training | Imagery training involves the use of visualisation techniques to focus patients' attention on pleasant or neutral images. | To reduce pre sleep cognitive arousal or shift the focus of attention away from distressing sleep-interfering thoughts | No recommendation level |
Notes.
Level of recommendation in the American Academy of Sleep Medicine Report; Morgenthaler, T. et al. (2006). Practice parameters for the psychological and behavioural treatment of insomnia: An update. An American Academy of Sleep Medicine Report. Sleep, 29 (11), 1415–9.
“Standard” is defined as “this is a generally accepted patient-care strategy, which reflects a high degree of clinical certainty”
“Guideline” is defined as “this is a patient-care strategy, which reflects a moderate degree of clinical certainty”
Future research
The recent surge of interest in sleep and pain has highlighted many gaps in our knowledge. On the basis of the review above, it seems important for future research to further delineate the clinical, physiological and psychological profiles of sleep disturbance associated with chronic pain. Currently, the transplant of psychological and behavioural understanding and treatment of primary insomnia for use with pain-related insomnia is largely results-driven. It would be beneficial for the long-term growth of this field to develop conceptual frameworks to help synthesise existing evidence and guide future research. Rigorous experimental and longitudinal studies in both healthy volunteers and chronic pain patients would be needed to identify and evaluate mechanisms that may underpin the co-occurrence of chronic pain and insomnia.
Apparently, the pain-sleep relationship is not as straightforward as it first appears. Whilst research has successfully demonstrated a temporal link between worse pain and worse sleep, progressive improvement in sleep (introduced by treatment) does not necessarily bring about reduction in pain. This underscores the importance to develop a better understanding of the pathways through which sleep and pain interact. Finally, from a pragmatic point of view, if we now know that the pure form of cognitive behaviour therapy for insomnia has limited effect on pain, it may be useful to consider developing hybrid treatments that target the maintaining mechanisms shared by insomnia and chronic pain. This novel model of intervention may be a way forward in the future.
Author Note
Nicole Tang is funded by a Post Doctoral Award from the National Institute of Health Research (Department of Health), United Kingdom
Reference List
- 1.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. European Journal of Pain 2006; 10:287–333. [DOI] [PubMed] [Google Scholar]
- 2.McCracken LM, Iverson GL. Disrupted sleep patterns and daily functioning in patients with chronic pain. Pain Research and Management 2002;7(2):75–9. [DOI] [PubMed] [Google Scholar]
- 3.Casarett D, Karlawish J, Sankar P, Hirschman K, Asch DA. Designing pain research from the patient's perspective: What trial end points are important to patients with chronic pain. Pain Medicine 2001;2(4):309–16. [DOI] [PubMed] [Google Scholar]
- 4.Lavigne G, Sessle BJ, Choinière M, Soja PJ, (Eds). Sleep and Pain. Seattle, US: International Association for the Study of Pain; 2007. [Google Scholar]
- 5.Ohayon MM. Relationship between chronic painful physical condition and insomnia. Journal of Psychiatric Research 2005;39:151–9. [DOI] [PubMed] [Google Scholar]
- 6.Smith MT, Perlis ML, Smith MS, Giles DE, Carmody TP. Sleep quality and presleep arousal in chronic pain. Journal of Behavioral Medicine 2000;23(1):1–13. [DOI] [PubMed] [Google Scholar]
- 7.Morin CM, Gibson D, Wade J. Self-reported sleep and mood disturbance in chronic pain patients. Clinical Journal of Pain 1998;14(4):311–4. [DOI] [PubMed] [Google Scholar]
- 8.Tang NKY, Wright KJ, Salkovskis PM. Prevalence and correlates of clinical insomnia co-occurring with chronic back pain. Journal of Sleep Research 2007;16:85–95. [DOI] [PubMed] [Google Scholar]
- 9.Tang NKY, Salkovskis PM, Hodges A, Soong E, Hanna M, Hester J. Chronic pain syndrome associated with health anxiety: A qualitative thematic comparison between pain patients with high and low health anxiety. British Journal of Clinical Psychology; in press. [DOI] [PubMed]
- 10.Drewes AM, Arendt-Nielsen L. Pain and sleep in medical diseases: Interactions and treatment possibilities. Sleep Research Online 2001;4(2):67–76. [Google Scholar]
- 11.Menefee LA, Cohen MJM, Anderson WR, Doghramji K, Frank ED, Lee H. Sleep disturbance and non-malignant chronic pain: A comprehensive review of the literature. Pain Medicine 2000;1(2):156–172. [DOI] [PubMed] [Google Scholar]
- 12.Moldofsky H. Sleep and pain. Sleep Medicine Reviews 2001;5(5):387–398. [DOI] [PubMed] [Google Scholar]
- 13.Smith MT, Perlis ML, Carmody TP, Smith MS, Giles DE. Pre sleep cognitions in patients with insomnia secondary to chronic pain. Journal of Behavioral Medicine 2001;24(1):93–113. [DOI] [PubMed] [Google Scholar]
- 14.Saatcioglu O, Cam-Celikel F. Sleep disturbance in female chronic pain patients. The Pain Clinic 2006;18(2):137–145. [Google Scholar]
- 15.Atkinson JH, Ancoli-Israel S, Slater MA, Garfin SR, Gillin JC. Subjective sleep disturbance in chronic back pain. The Clinical Journal of Pain 1988;4:225–232. [Google Scholar]
- 16.Pilowsky I, Crettenden I, Townley M. Sleep disturbance in pain clinic patients. Pain 1985;23:27–33. [DOI] [PubMed] [Google Scholar]
- 17.Affleck G, Urrow S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain 1996;68:363–8. [DOI] [PubMed] [Google Scholar]
- 18.Theadom A, Cropley M. Dysfunctional beliefs, stress and sleep disturbance in fibromyalgia. Sleep Medicine; in press. [DOI] [PubMed]
- 19.Hakkionen S, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. Journal of Sleep Research 2001;10:35–42. [DOI] [PubMed] [Google Scholar]
- 20.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep 2007;30(4):494–505. [DOI] [PubMed] [Google Scholar]
- 21.Naughton F, Ashworth P, Skevington SM. Does sleep quality predict pain-related disability in chronic pain patients? The mediating roles of depression and pain severity. Pain 2007;127:243–52. [DOI] [PubMed] [Google Scholar]
- 22.Morgenthaler T, Kramer M, Alessi C, Friedman L, Boehlecke B, Brown T, et al. Practice parameters for the psychological and behavioral treatment of insomnia: An update. An American Academy of Sleep Medicine Report. Sleep 2006;29(11):1415–9. [PubMed] [Google Scholar]
- 23.King SA, Strain JJ. Benzodiazepine use by chronic pain patients. Clinical Journal of Pain 1990;6(2):143–7. [DOI] [PubMed] [Google Scholar]
- 24.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine 2001;2:297–307. [DOI] [PubMed] [Google Scholar]
- 25.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 26.Currie SR, Wilson KG, Pontefract AJ, de Laplante L. Cognitive-behavioral treatment of insomnia secondary to chronic pain. Journal of Consulting and Clinical Psychology 2000;68(3):407–16. [DOI] [PubMed] [Google Scholar]
- 27.Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR. Behavioral insomnia therapy for fibromyalgia patients: A randomized clinical trial. Archives of Internal Medicine 2005;165:2527–35. [DOI] [PubMed] [Google Scholar]


