Abstract
Sex differences in the prevalence of painful conditions appear after puberty
Variation in symptom severity across the menstrual cycle occurs in a number of clinical pain conditions
Sex steroid hormones act at a number of sites in both the peripheral and central nervous systems and in both reproductive and non-reproductive tissues
Sex steroid hormones have traditionally been thought to alter transcription; however, there is evidence that there are also non-genomic effects
Sex steroid hormones can have organisational effects from as early as in utero
The relationship between sex hormones and pain is complex
Introduction
One of the most striking physiological differences between men and women is in sex steroid hormones, both the absolute levels and the occurrence of cyclical fluctuations in women (Fig.1). These hormones are known to be responsible for the embryological development of a male or female phenotype and for successful reproductive function after puberty. More recently, observations such as the marked differences in pain symptoms between males and females in the period between puberty and the menopause, and the cyclical variations in many clinical pain symptoms in women have suggested that they may also have a role in altering the pain experience. The aim of this review is to examine the available evidence that sex steroid hormones have a role in pain and to identify possible mechanisms of action for these effects.
Figure 1:
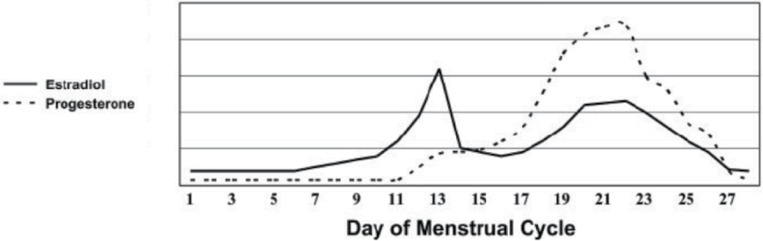
Schematic illustrating how Estradiol and Progesterone vary over a 28 day menstrual cycle (adapted from (11)).
Clinical Pain
As well as the differences in hormonal status between males and females, the different phases of female life (puberty, reproductive maturity, pregnancy and the post-menopausal period) are also accompanied by marked variation in hormone levels. Furthermore, many women choose to alter their own hormonal status by the use of hormonal contraception and HRT. Here we consider the evidence that clinical pain conditions vary with hormonal state.
Dramatic changes in sex hormones occur around puberty and it is at this point that sex differences in clinical pain conditions also begin to be observed. Initial studies did not show correlations between age and the development of painful conditions in girls or boys. However, the timing of puberty is very variable between individuals and more recent studies that control for the stage of pubertal development rather than chronological age have shown an association with pain. For both sexes, the probability of experiencing a painful condition increases with increasing pubertal development1.
With the onset of regular ovulation and menstruation, it can be seen that a number of clinical pain conditions show variation in symptom severity across the menstrual cycle. Clearly the pain of dysmenorrhoea is, by definition, associated with the menstrual cycle, however, the symptoms of temperomandibular joint (TMJ) dysfunction, fibromyalgia, Irritable Bowel Syndrome (IBS), Interstitial Cystitis (IC) and migraine can also show cyclical variation2. The greatest reports of pain symptoms appear to occur at times of low or rapidly falling estrogen levels and the use of the combined oral contraceptive pill (COCP) to give a more constant hormonal level can improve these symptoms. Furthermore, complete abolition of hormonal fluctuation with gonadotrophin releasing hormone agonists (GnRHa) (effectively causing a reversible medical menopause) can improve the symptoms of both IBS3 and IC4. This hypo-estrogenic state can worsen headaches and migraine, although achieving a steady hormonal state with a GnRHa and additional low-dose estradiol has been shown to improve migraine5.
During pregnancy, the cyclical fluctuations in hormones cease and instead a steady increase in the levels of both progesterone and estrogen is seen towards term which fall rapidly after delivery. The concentrations of a number of other steroid hormones also vary from the non-pregnant state and may have an effect on painful conditions, including prolactin and relaxin. Many clinical pain conditions improve during pregnancy including arthritis, migraine and often pelvic pain and there is an associated reduction in pain sensitivity a phenomenon known as pregnancy-induced analgesia6. However, pregnancy itself can be associated with the development of pain especially mechanical back pain and symphysis pubis dysfunction (SPD). The painful symptoms of systemic lupus erythematosus (SLE) usually worsen with pregnancy7.
After the menopause, when levels of estrogen and progesterone are very low, the sex differences in pain become much less marked. However, the use of hormone replacement therapy (HRT) in postmenopausal women has been associated with the development of pain conditions including back and TMJ pain2.
From puberty onwards, men have significantly higher levels of testosterone and its metabolites than women. Testosterone appears to have an analgesic effect protecting against the development of painful conditions such as TMJ pain8. Rheumatoid arthritis patients (both male and female) have been shown to have lower androgen levels than sex-matched controls, and androgen administration improves their symptoms, whilst female workers with lower testosterone levels have more work-related neck and shoulder injuries9. However, investigation of the specific effects of testosterone are complicated by the fact that much is metabolised in vivo to estradiol by aromatase, and this is therefore an issue which needs to be addressed in future studies.
Perhaps one of the more intriguing studies to be published recently explored the effect of systemic hormone administration to both male to female (MtF) and female to male (FtM) transsexuals (n=73) during the process of sex reassignment10. They observed that approximately one third of the MtF subjects developed chronic pain during their treatment with estrogen and androgens, and even those that did not, reported a decreased tolerance to painful events and an enhanced sensitivity to thermal stimuli (both warm and cold). Of those FtM subjects who had chronic pain before the start of treatment, more than half improved after commencing testosterone treatment, reporting reduced numbers of painful episodes and shorter lengths of those that did occur. Clearly, psychological effects cannot be ignored in this group of subjects, however, this is the only situation where the hormonal milieu in humans can be ethically altered to that of the opposite gender and therefore gives us interesting insights.
Experimental Pain
It is possible that the effects of hormones on clinical pain are due to a hormonal effect on pain sensitivity. If this were the case then we would expect to see alterations in sensitivity to experimental pain across the menstrual cycle. In fact, in healthy women, the results of the many studies addressing this question have frequently been contradictory, with some showing no change and others showing changes in differing directions. Different methods of applying a painful stimulus (heat, electrical, cold pressor etc.) have been used at different body sites and tissue depths (skin, subcutis, muscle and viscera) and this may be one of the reasons for the differences found. However, the largest methodological problem is with the definition of cycle phase, which differs between studies, is often too wide and does not account for different cycle lengths and anovulatory cycles. A recent meta-analysis11 found that there is no evidence to conclude that a difference in sensitivity to experimental pain across the menstrual cycle exists in healthy women, except perhaps for electrical stimuli to subcutaneous tissues.
Two brain imaging studies have looked at whether differences in pain sensitivity in different hormonal states can be visualised. In one painful heat was applied to the skin overlying the left masseter muscle during a period of low estrogen and a period of high estrogen (no significant difference in progesterone levels)12. There was no significant difference between pain ratings at these two time points, however, different activation patterns were seen. In the other study13 a finger was immersed in painfully hot water during the follicular (low estrogen/progesterone) and the luteal (higher estrogen/progesterone) phases. They found significantly different pain and pain-related unpleasantness ratings and again differences in brain activation patterns between the two phases. These studies suggest that although pain sensitivity may not vary with hormonal status in healthy women, the pain experience, particularly the emotional-affective component, may well do.
In women with clinical pain conditions, however, cyclical variations in pain sensitivity can be demonstrated. For example, although no difference in rectal sensitivity to balloon distension across the cycle is seen in healthy women, in those with IBS an increased sensitivity is seen during the menstrual phase14. Similarly, variations in pain sensitivity have also been demonstrated in women with IC, dysmenorrhoea and fibromyalgia.
Although many studies have attempted to establish the effects of hormones on experimental pain perception in women, few have looked at their effects in men. One small study showed testosterone to be associated with a reduced sensitivity to tactile stimulation, both on the finger and the penis15, whilst in rats testosterone treatment is associated with a reduction in pain thresholds16.
Emotion
In addition to its sensory aspect, pain is an emotional experience. It is therefore of interest that the life time patterns in pain symptoms in men and women are closely mirrored by those of mood disorders17, though with the addition of a perimenopausal peak in mood disorders. Comparing post-puberty with pre-puberty, rates of significant depression increased two-fold for boys but more than four-fold for girls1. In Premenstrual Dysphoric Disorder (PMD), there is no evidence that abnormal levels of hormones occur (unlike in depression associated with thyroid or pituitary dysfunction), rather, it appears that some women are more sensitive to the mood destabilising effects of these hormones18. It is not inconceivable therefore, that a similar situation may exist for pain.
Possible sites and mechanisms of action
The traditional view of sex steroid hormones was that they act on specific membrane receptors along the hypothalamic-pituitary-gonadal axis to alter downstream transcription. These effects would therefore take hours to days to appear. More recent work has challenged this view. Rapid, reversible changes in neuronal excitability in the brain and spinal cord have been demonstrated secondary to steroid hormone administration which cannot be genomic effects19. Both estrogen and androgen receptors have been identified throughout the body, including in both the peripheral and central nervous systems, supporting the idea that they have a role outside of reproductive function. To further complicate matters these steroids can also be synthesised within the central nervous system itself from endogenous cholesterol9 and activation of different receptor sub-types (e.g. estrogen receptor (ER) α and β) can exert different effects.
It is now known that the actions of sex steroid hormones on the brain can be both organisational (during in utero development and early neonatal life) and activational. Exposure to steroid hormones during brain development has been shown to effect a variety of sexually dimorphic behaviours in a number of species, including play patterns, sexual behaviour, spatial learning, maternal behaviour and bird song20. These hormones can originate from the maternal circulation (either endogenous or exogenous), the fetus itself or a twin/litter sibling. Animal studies suggest that neonatal exposure to testosterone is necessary to see a male response to pain7 and to morphine analgesia21 whilst early exposure to estrogens alters both the anatomy and physiology of the hippocampus9. In the developed nervous system, steroid hormones can modulate neurotransmission in the brain, spinal cord and peripheral nerves, alter the excitability of specific brain areas and influence the availability of receptors for themselves and other ligands including opiates and serotonin7,9,22. Furthermore, progesterone is well known to have GABAergic actions and thus is likely to have an effect on pain7. We believe that these effects on the CNS could have a substantial influence on pain perception.
Peripheral structures outside of the reproductive and nervous system can also be affected by steroid hormones, including the immune system, bone, joint surfaces, ligaments and blood vessels7. Thus alterations in the structure or function of these “end-organs” secondary to variations in sex steroid hormone levels could also increase or decrease the sensation of pain and/or could be involved in the disease process itself. It is likely, therefore, that hormones exert their effect on pain at a number of sites (Table 1).
Table 1:
Some non-reproductive actions of steroid hormones
| Estrogen | Progesterone | Testosterone | |
| Brain | ↑Mu-opioid receptor availability | Anxiolytic | Analgesic |
| ↑Hippocampal excitability | Sedative | ↑↓seizure threshold | |
| ↑5-HT | Analgesic | ↑Noradrenaline | |
| ↓Noradrenaline | Anticonvulsant | Mediate aggressive behaviour | |
| ↑↓anxiety/stress | Modulate GABA | Organisational effects on sexually dimorphic behaviours | |
| Promote myelination | |||
| Mediate male aggression towards infants | Modulate endogenous opioids | ||
| Regulate aromatase activity | |||
| Spinal Cord | Modulate dorsal horn response to pain | Mediate hypersensitivity after nerve root damage | Modulate dorsal horn response in neuropathic pain |
| Neuroprotective | |||
| Peripheral Nerve | Sensitise uterine and cervical afferents ↑glutamatergic nociceptor activity | Neuroprotective | Facilitate release of ACh |
| Immune System | Δ T and B cell proliferation and phenotype | Anti-inflammatory | ↓cellular immune response |
| Δ cytokine and immunoglobulin balance | Modulate immune response | ||
| Musculoskeletal System | ↑bone deposition | ↑bone deposition | ↑bone density and strength |
| ↑muscle mass recovery following disuse | Smooth muscle relaxant | ↑muscle mass | |
| Cardiovascular System | Δ NO synthesis | ↑vasodilation | ↑vasoconstriction |
| Δ vasodilation |
Abbreviations: 5-HT, 5- hydroxytryptamine; ACh, acetylcholine; NO, nitric oxide
Conclusions
Thus it can be seen that there is copious evidence that sex steroid hormones affect pain and that this may be, at least in part, responsible for the differences in pain experience between men and women. However, it is also clear that the relationship is not a simple one. It is likely to involve dose-dependant organisational and activational effects and actions at a number of sites outside the reproductive system, including a wide variety in the nervous system, as well as effects on disease processes themselves. Furthermore, there may be interactions between the different hormones which also need to be taken into account. More research is necessary to improve both our understanding of this complex area and our management of painful conditions. It is therefore important in future pain studies that both the sex and hormonal status of the subjects are taken into account.
Contributor Information
Katy Vincent, Research Fellow, Functional Magnetic Resonance Imaging of the Brain Centre, John Radcliffe Hospital, Oxford.
Irene Tracey, Professor, Functional Magnetic Resonance Imaging of the Brain Centre, John Radcliffe Hospital, Oxford.
References
- 1.LeResche L, Mancl LA, Drangsholt MT, Saunders K, Korff MV. Relationship of pain and symptoms to pubertal development in adolescents. Pain 2005; 118(1–2): 201–9. [DOI] [PubMed] [Google Scholar]
- 2.LeResche L. Epidemiologic perspectives on sex differences in pain. In: Fillingim RB, ed. Sex, gender and pain. Seattle: IASP Press; 2000: 233–49. [Google Scholar]
- 3.Mathias JR, Clench MH, Reeves-Darby VG, Fox LM, Hsu PH, Roberts PH, Smith LL, Stiglich NJ. Effect of leuprolide acetate in patients with moderate to severe functional bowel disease. Double-blind, placebo-controlled study. DigDis Sci 1994; 39(6): 1155–62. [DOI] [PubMed] [Google Scholar]
- 4.Lentz GM, Bavendam T, Stenchever MA, Miller JL, Smalldridge J. Hormonal manipulation in women with chronic, cyclic irritable bladder symptoms and pelvic pain. AJOG 2002; 186(6): 1268–71. [DOI] [PubMed] [Google Scholar]
- 5.Martin V, Wernke S, Mandell K, Zoma W, Bean J, Pinney S, Liu J, Ramadan N. Medical Oophorectomy With and Without Estrogen Add-Back Therapy in the Prevention of Migraine Headache. Headache 2003; 43(4): 309–21. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho B, Angst MS, Fuller AJ, Lin E, Mathusamy AD, Riley ET. Experimental Heat Pain for Detecting Pregnancy-Induced Analgesia in Humans. Anesth Analg 2006; 103(5): 1283–7. [DOI] [PubMed] [Google Scholar]
- 7.Craft RM. Modulation of pain by estrogens. Pain 2007; 132 (Supplement 1):S3–S12. [DOI] [PubMed] [Google Scholar]
- 8.Fischer L, Clemente JT, Tambeli CH. The Protective Role of Testosterone in the Development of Temporomandibular Joint Pain. J Pain 2007; 8(5): 437–42. [DOI] [PubMed] [Google Scholar]
- 9.Aloisi AM, Bonifazi M. Sex hormones, central nervous system and pain. Horm Behav 2006; 50(1): 1–7. [DOI] [PubMed] [Google Scholar]
- 10.Aloisi AM, Bachiocco V, Costantino A, Stefani R, Ceccarelli I, Bertaccini A, Meriggiola MC. Cross-sex hormone administration changes pain in transsexual women and men. Pain 2007; 132(Supplement 1): S60–S7. [DOI] [PubMed] [Google Scholar]
- 11.Sherman JJ, LeResche L. Does experimental pain response vary across the menstrual cycle? A methodological review. Am J Physiol Regul Integr Comp Physiol 2006; 291(2): R245–56. [DOI] [PubMed] [Google Scholar]
- 12.de Leeuw R, Albuquerque RJ, Andersen AH, Carlson CR. Influence of estrogen on brain activation during stimulation with painful heat. J Oral Maxillofac Surg. 2006; 64(2): 158–66. [DOI] [PubMed] [Google Scholar]
- 13.Choi JC, Park SK, Kim YH, Shin YW, Kwon JS, Kim JS, Ji-Woong M.D, Kim, Soon Yul M.D, Sang Gyu M.D, Moo Sam L. Different brain activation patterns to pain and pain-related unpleasantness during the menstrual cycle. Anesthesiology 2006; 105(1): 120–7. [DOI] [PubMed] [Google Scholar]
- 14.Houghton LA, Lea R, Jackson N, Whorwell PJ. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut 2002; 50: 471–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burris AS, Gracely RH, Carter CS, Sherins RJ, Davidson JM. Testosterone therapy is associated with reduced tactile sensitivity in human males. Horm Behav 1991; 25(2): 195–205. [DOI] [PubMed] [Google Scholar]
- 16.Rao SSS, Saifi AAQ. Effect of testosterone on threshold of pain. Indian J Physiol Pharmacol 1981; 25(4): 387–8. [PubMed] [Google Scholar]
- 17.Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J Affect Disord 2003; 74(1): 67–83. [DOI] [PubMed] [Google Scholar]
- 18.Rubinow DR, Schmidt PJ. Gonadal steroid regulation of mood: The lessons of premenstrual syndrome. Front Neuroendocrinol 2006; 27(2): 210–6. [DOI] [PubMed] [Google Scholar]
- 19.Evrard HC, Balthazart J. Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. J Neurosci 2004; 24(33): 7225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual Differentiation of the Vertebrate Brain: Principles and Mechanisms. Front Neuroendocrino. 1998; 19(4): 323–62. [DOI] [PubMed] [Google Scholar]
- 21.Cicero TJ, Nock B, O'Connor L, Meyer ER. Role of Steroids in Sex Differences in Morphine-Induced Analgesia: Activational and Organizational Effects. J Pharmacol Exp Ther 2002; 300(2): 695–701. [DOI] [PubMed] [Google Scholar]
- 22.Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta J-K. Pronociceptive and Antinociceptive Effects of Estradiol through Endogenous Opioid Neurotransmission in Women. J Neurosci 2006; 26(21): 5777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]


