Abstract
Mice remain the most studied animal model in pancreas research. Since the findings of this research are typically extrapolated to humans, it is important to understand both similarities and differences between the 2 species. Beside the apparent difference in size and macroscopic organization of the organ in the 2 species, there are a number of less evident and only recently described differences in organization of the acinar and ductal exocrine tissue, as well as in the distribution, composition, and architecture of the endocrine islets of Langerhans. Furthermore, the differences in arterial, venous, and lymphatic vessels, as well as innervation are potentially important. In this article, the structure of the human and the mouse pancreas, together with the similarities and differences between them are reviewed in detail in the light of conceivable repercussions for basic research and clinical application.
Keywords: anatomy, exocrine, endocrine, human pancreas, islet of Langerhans, innervation, mouse pancreas, structure, vasculature
Introduction
Due to the clinical importance of diabetes, pancreatitis, endocrine tumors and pancreatic cancer, the structure of the exocrine and endocrine pancreas has been studied extensively. However, since the studies in humans are inherently limited to scarce samples at intermittent and often unpredictable intervals, animal models have largely subdued the research field, among which the mouse model is used most often. While the gross anatomical similarities and differences between the 2 species are well established, a large part of our knowledge on cellular architecture in humans is rooted in studies conducted on rodents, especially on mice. Our knowledge of pancreas structure is growing constantly and with it the translation of this knowledge into research and clinical practice. Thus, it is becoming increasingly important to conceive differences between the human and the mouse pancreas and understand the limitations stemming from them.
With the exception of a few articles1-5 and book chapters6,7 that cover some aspects of the human and the mouse pancreas in a comparative way, there is, to the best of our knowledge, no single article or book chapter that would systematically and exhaustively deal with this important topic. Thus, the aim of our review is to survey the similarities and differences between the mouse and the human pancreas by appreciating latest discoveries in the field and pointing out issues of special importance for the clinical and research use.
First, the gross anatomy of the human and the mouse pancreas will be outlined. This section could be particularly valuable to researchers with a background in human medicine, starting to use mice as a model organism for pancreas research, and vice versa, to researchers trained in animal biology and beginning to work with human specimens. Next, we describe in detail the exocrine and endocrine part of the human and the mouse pancreas at the tissue level. This section shall help dismiss some commonly encountered textbook misconceptions about pancreas histology, for example the one regarding microarchitecture of the human islet of Langerhans (islet). The final sections on blood perfusion, lymphatics, and innervation are intended for surgeons and researchers dealing with the pancreas in situ and studying the role of neurotransmitters and neuropeptides in pancreas biology.
Macroscopic Anatomy of the Pancreas
The human pancreas1 is a well-defined solitary organ. Macroscopically, it can be divided into 3 major parts: the head, the body, and the tail.8 There are no clear-cut borders between these parts. Generally, the left border of the superior mesenteric artery (SMA) is considered the border between the head and the body, whereas the midpoint of the body and tail combined is considered the border between the body and the tail.9 Some authors distinguish a fourth and a fifth part, both of which are more generally parts of the head; the uncinate process located beneath the SMA, and the neck or isthmus, a thinned part situated over the SMA.8,9 The head of the pancreas is a C-shaped part aligned with the upper curvature of duodenum. The flat narrow body of the pancreas is located underneath the stomach extending almost horizontally in the medial plane. It crosses with the superior mesenteric artery and vein, abdominal aorta, inferior vena cava, and portal vein. The tail of the pancreas touches the hilum of the spleen. The gland is 14–18 cm long, 2–9 cm wide and 2–3 cm thick, weighing 50–100g.8,10-12 The pancreas is surrounded by a fibrous capsule from which connective tissue septa extend into the gland dividing its parenchyma into distinct lobes and lobules (small lobes).13,14 Mesenchyme accounts for approximately 15–25% of total pancreas volume (TPV) and contains numerous fat cells.10,15
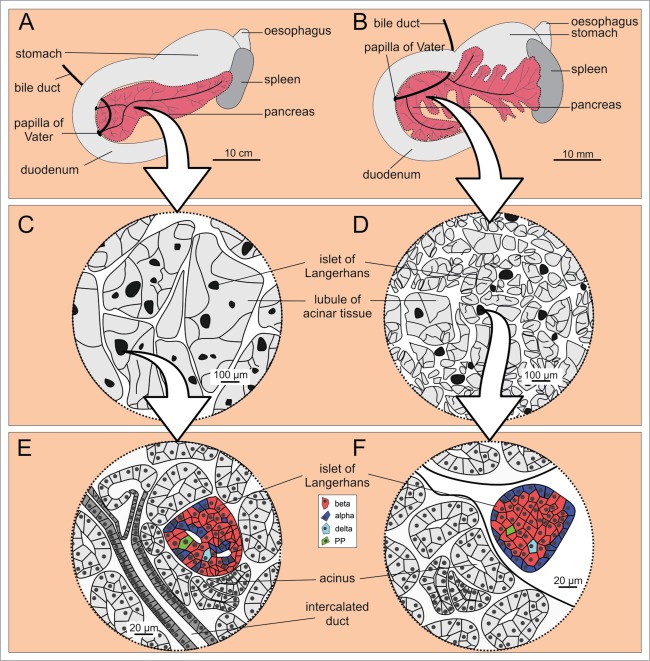
In mice, on the other hand, the pancreas is not as well defined an organ as in humans but is rather diffusely distributed within the mesentery of the proximal small intestine in a dendritic manner.16 Macroscopically, 3 major parts can be distinguished: the duodenal, the splenic, and the gastric lobe (Fig. 1).17 The largest lobe that makes up over a half of TPV is the splenic lobe (SL). It extends horizontally between the duodenum and the spleen and is homologous to the body and the tail of the human pancreas.18 The duodenal lobe is invested in the mesentery surrounding the duodenum and is homologous to the head of the human pancreas.19,20 The third and the smallest macroscopically distinguishable lobe is the gastric lobe (GL). It can be viewed as a large part of the splenic lobe from which it develops during ontogeny21 and it was suggested that the GL is homologous to the pyramidal process also named the ear or auricle of the human pancreas. This is a highly variable part of pancreas existing in approximately half of adult humans at the inferior margin of the transition of the head to the body and protruding toward the stomach (Fig. 1).21-23
Figure 1.
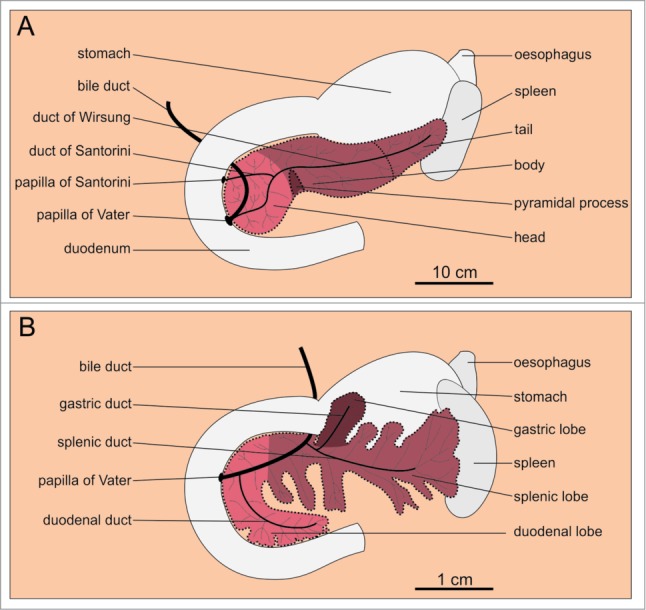
Macroscopic anatomy of the human and the mouse pancreas. (A) Human pancreas consists of the head, the body, and the tail. (B) Mouse pancreas has 3 lobes that are less well defined: the duodenal, the gastric, and the splenic lobe. Note the 10-fold difference in linear dimension (which accounts for the 1000-fold difference in the size of the organ as well as the organism). The color coding indicates homologous parts.
The main lobes of the mouse pancreas are often separated by patches of adipose, connective, and lymphatic tissue. Therefore, special attention needs to be paid to entirely remove the pancreas and precisely determine its mass.
Microscopic Anatomy of the Exocrine Pancreas
Together with the aforementioned mesenchyme, the exocrine pancreas amounts to 96–99% of TPV.10,24 Each lobe of pancreas consists of several smaller lobes called lobules. Their size is proportional to the size of the organism. In humans, lobules measure 1–10 mm in diameter,13,14 whereas in mice they are 0,5–1,5 mm in diameter.25,26
In humans, the demarcations between lobules are incomplete and the whole parenchyma is a continuous unit.14,27 Each anatomical lobule is a single glandular lobule receiving one major duct. Arteries, however, do not run in parallel with ducts and the number of arteries per one glandular lobule is 2–9. In other words, each glandular lobule consists of a few vascular or primary lobules, each of which receives a single artery.27 Each pancreatic lobule is composed of structures called acini. An acinus is a cluster of pyramidal acinar cells forming a dome-like structure that funnels secretions from apical poles of acinar cells into the lumen of what is called an intercalate duct. The intercalated ducts drain into intralobular ducts (located within lobules) and these, while exiting lobules, drain into larger diameter interlobular ducts (located between lobules). The latter in turn converge into the main pancreatic duct (also called the duct of Wirsung) that extends along the whole pancreatic gland in humans. The ducts entering the lobules are mostly second- or third-order branches of the main pancreatic duct. The main pancreatic duct then empties into the duodenum adjacent to the entrance of the common bile duct. The most distal parts of both ducts form the so called hepatopancreatic ampulla (ampulla of Vater). The ampulla opens into the duodenum via the major duodenal papilla (papilla of Vater). As a variety, the human pancreas may have one accessory duct also called the duct of Santorini that is a remnant of the distal part of the main duct of dorsal pancreas present during the development of the gland and leads into the duodenum independently from the main duct and proximally to it.27-29
In up to 3% of people undergoing endoscopic retrograde cholangiopancreatography, the so called anomalous pancreatobiliary junction can be found, where the pancreatic duct joins the bile duct a few centimeters outside the duodenal wall.28 In these people, the incidence of gallbladder and bile duct carcinoma is increased, possibly due to reflux and stasis of a mixture of bile and pancreatic juice in the bile duct and gallbladder.28,30
The mouse pancreas, on the other hand, displays a quite different ductal anatomy. A large interlobular duct drains each of the 3 lobes. The splenic and the gastric duct merge and join with the common bile duct well before the common pancreatic/bile duct enters the wall of the duodenum. In 70% of animals, the point where the common splenic/gastric duct joins the bile duct is proximal to the point where the duodenal duct joins with the common pancreatic/bile duct (Fig. 1).18 Accessory small ducts may individually empty into the bile duct or directly into the duodenum.19
When injecting enzymes into the ductal tree for isolation of islets31 or agarose for preparation of tissue slices,32–34 it should be kept in mind that – depending on the point of injection, possible clamping and anatomical variations – a variable permeation of the excretory system with the injected substance might be expected. Due to anatomical differences, the techniques developed for preparation of rodent isolated islets and tissue slices are not directly applicable on human pancreas.35,36
In humans, impaction of a gall stone at the level of ampulla is a specific cause of pancreatitis. More than a century ago, Opie proposed that due to the impaction, a common channel between the pancreatic and the common bile duct is created and that the entry of bile into the pancreatic excretory system triggers the inflammation.37,38 Duct ligation and injection of bile into the ductal system of mice are used in studying the pathophysiology of pancreatitis, however this approach is questionable in mice where a common pancreatic/bile duct is present normally.1
Microscopic Anatomy of the Endocrine Pancreas
The remaining 1–4% of TPV comprise endocrine microorgans called islets of Langerhans.10,13,24,39 They are of circular to oval to highly irregular cross-sections and are composed of a few to several thousand endocrine cells. In addition to islets, single endocrine cells can be found scattered throughout the acinar and ductal tissue.40-42 The islet size distributions are similar in humans and mice.13,24,43-46 At least 5 types of polypeptide-hormone-secreting endocrine cells can be found in islets. Most numerous are the beta cells that synthesize and secrete insulin. Beta cells make up 50–70% and 60–80% of cells in islets in humans and mice, respectively. Alpha cells, contributing 20–40% and 10–20% of the total number of cells in human and mice, respectively, secrete glucagon. Thus, in mice the ratio between beta and alpha cells is higher than in humans. Delta cells and PP cells releasing somatostatin and pancreatic polypeptide, respectively, are the least frequent cell type and present less than 10% of cells in humans and less than 5% of cells in mice.10,44,47-49 However, in the posterior head of the human pancreas which is a vestige of the ventral pancreas anlage, the proportion of PP cells among endocrine cells is around 70%.10,50,51 Finally, the hormone ghrelin is released from epsilon cells which present less than 1 % of cells.52
Beside a relative lack of insulin and a relative surplus of glucagon, changes in concentrations of other islet hormones have been described in type 2 diabetes mellitus.2
Until recently, the spatial organization of cells within the islet, i.e. their microarchitecture, was believed to be markedly different in the 2 species. In mice, islets were reported to be composed of a mantle containing mainly non-beta cells and a core containing practically exclusively beta cells.48,49,53-55 In humans, on the other hand, in addition to the mantle-core pattern,53 other patterns were reported. Insulin positive cells were shown to arrange in clusters,56 in a ribbon-like pattern,57 or to be dispersed throughout the islet in a rather unorganized manner.48,49
More recently, it was proposed that the human islet cells form trilaminar plates composed of 2 layers of alpha cells surrounding a single layer of beta cells. This sandwich is supposed to contain pits surrounded by alpha cells and protruding along the vessels into the beta cell middle plate.10,11,58,59 Finally, the whole sandwich is believed to be folded into a U- or O-shaped islet. This organization conceivably promotes heterologous contacts between alpha and beta cells but still allows for a functional continuum between beta cells in the middle layer.58,60,61 In mice, on the other hand, homologous contacts between alpha cells in the mantle and between beta cells in the core predominate.49 There is evidence that alpha cells stimulate beta cell function62,63 and it is tempting to speculate that the specialized architecture of human islets could at least partially explain their greater sensitivity to glucose compared with mouse islets.64,65
The above idea finds further support in recent studies demonstrating that human-type islets can be found in mice in states characterized by an increased demand for insulin, such as inflammation, obesity, diabetes, and pregnancy.44,66 This suggests that the pattern of cell distribution in larger mammals, for example humans, may in addition to the increased number of islets (see below) provide a part of the functional compensation for the increased demand for insulin due to larger body mass.44 However, only a small number of alpha cells are found in the islet core in the domestic pig with a body mass similar to humans.44,67 Moreover, in some mammals considerably larger than humans, for example the white whale68 and the african elephant,69 the mantle-type islets seem to prevail. On the other hand, in some mammals considerably smaller than humans, for example the fruit bat 70and the guinea pig,71 the human-type islets were described, as reviewed recently.47 Thus, it seems difficult to relate islet architecture to increased body size only and other factors that might influence islet architecture, such as the diet or circadian patterns, are worth exploring with regard to their effect on islet architecture in the future.
Finally, it was shown that in both mice44,47 and humans,44,45,58,72 the architecture of islets is size-dependent, with smaller islets (<100 μm in diameter) being of the mantle-type and larger islets displaying the more complex organization.59,73
Noteworthy, at present, different types of islets are defined based on distribution of different types of cells within islets. Including other morphological features, such as innervation and vascularization, might reveal additional similarities and differences between islets and help us get a clearer picture. To sum up, some human islets may possess architectural characteristics of typical mouse islets and vice versa.
The relationship with the size of the organism observed for the size of the exocrine lobules does not hold true in the case of the islets.25,26 Namely, the range of observed islet sizes is similar in mice and humans (and also other species), with the upper boundary at around 500–700 μm in diameter.44,47,72,73 It seems that there is an upper limit of size that is optimal for function.25,26,44,74-78 There is a proportionately larger number of islets in species with larger body mass, such that despite the fairly constant islet size, a virtually linear dependence exists between the total islet number and body mass across species.79,80 The mouse pancreas contains approximately 1000–500021,81 and the human pancreas approximately 1.000.000–15.000.000 islets,13,24,82 a ratio that equals the ratio between masses of the 2 organisms. The total mass of islets in a normal human pancreas is estimated at 0,5–1,5 g, and the cumulative number of cells at an average of one billion (109).10,13,83,84 The total mass of islets in a normal mouse pancreas is estimated at 1–8 mg.85,86 Within species, a linear relationship between beta cell mass and body mass was described in humans younger than 20 y of age,86 as well as a linear relationship between beta cell mass and body mass index in adults.83,84 In mice, there is a linear relationship between beta cell mass and body mass.87-90
In mice, the distribution of islets and the beta cell mass in different lobes seems to be rather heterogeneous. For the most frequently used strain of mice C57BL/6, the number of islets per unit of volume is the highest in the gastric lobe, followed by the duodenal and the splenic lobe, with volume densities in the first 2 being 75% and 20% higher than in the latter, respectively. However, the islets in the GL are the smallest and the proportion of beta cells they contribute to the total beta cell volume (12%) is the same as is the proportion of GL volume in the TPV (12%). On the other hand, islets in the DL together contain a disproportionately high volume of beta cells (40%) with regard to the volume that the DL contributes to TPV (≈33%). From this, it can be easily deduced that with regard to its volume (55% of TPV), the SL contributes the least part of beta cell volume (48%).21 Other distributions were reported for other strains.82
In experimental models of diabetes and in investigations of gene effects on islet development, the differences between lobes must be kept in mind.
In the human pancreas, the density of islets per unit volume was recently reported to be similar in the head and the body, but approximately 2-fold higher in the tail.73 Earlier studies, however, detected a more continuous increase in the density of islet volume per volume of total pancreatic tissue from the head to the tail, with 2%, 3%, and 4% endocrine cells per volume of total pancreatic tissue in the head, the body, and the tail, respectively.10,24 Although it was reported that there are a larger number of smaller islets in the head and a smaller number of larger islets in the tail,24 the cellular composition and microarchitecture of the islets were shown to be more constant throughout the pancreas in humans than in mice, with the exception of the posterior head containing islets rich in PP-secreting gamma cells and poor in alpha and beta cells.50,91
In human isolated islets, no regional differences in glucose stimulated insulin secretion could be detected.73 In patients with type 2 diabetes, the head region of the pancreas and in particular larger islets within the head seem to suffer the most pronounced beta cell loss.73 Type 2 diabetes mellitus is a major risk factor for pancreatic cancer, together with smoking and obesity,92 and interestingly, it most often arises in the head of the pancreas where it also displays lower malignancy in comparison with the body and tail.73,93 It remains to be investigated whether there is a common factor underlying both susceptibilities of the head region.
In mice, the islets are mostly interlobular in position, and less likely intralobular than in humans1,26,41 (Fig. 2). In the latter case, they are usually located on the edge of lobules.25,26 A possible explanation for this difference is that in larger animals, such as humans, the larger lobules ontogenetically emerge by fusion of originally smaller lobules and that this way, originally interlobularly positioned islets become enveloped with acinar tissue, thereby changing their position from inter- to intralobular.25 Merkwitz et al. have recently proposed an alternative explanation. Islets originate from progenitor cells within the epithelium of the developing ductal system and appear very early during the development of the ductal system. The first islets to develop remain close to the most proximal ducts, i.e., interlobular. Islets that develop later, i.e. from more distal branches of the ductal system, acquire an intralobular position. Within each species, there is probably a centrifugal temporal pattern of an increasing proportion of intralobular islets. Intraspecies differences between islets may arise from the fact that the shift is not complete, and the interspecies differences may result from different time points during ontogeny at which this shift comes to a halt.41
Figure 2.
Microscopic anatomy of the human (A, C, and E) and the mouse (B, D, and F) pancreas. (A and B) Macroscopic anatomy of the human and the mouse pancreas, respectively. (C and D) Magnifying a portion of pancreas reveals larger lobules in humans when compared to mouse, whereas the islets of Langerhans are of fairly comparable size in humans and mice. (E and F) Cell composition and location of the islets of Langerhans within the pancreas are markedly different in the 2 species. Note the more diffusely distributed endocrine cells in humans (E) and the mantle-core pattern in mice (F).
Changes in Pancreas Anatomy During Ontogenesis
The process of ontogenesis is a source of differences in the structure of pancreas within a single species. To critically evaluate similarities and differences between 2 different species, it is desirable to compare specimens at equivalent ontogenetic stages. An important caveat in the foregoing discussion about differences in islet architecture is the fact that unfortunately, in some seminal studies the age of mice49,58 or human donors56 or both48 was not specified, while in the studies that specified the age, considering the expected lifespan for humans94 and mice,95 human donors10,11,44,49,57-59,72,73 (n=9 studies; average age=53 years, i.e., >60% of lifespan) were relatively older than mice44,54,55,66 (n=4 studies; average age=16 weeks, i.e., <20% of lifespan). Thus, could some of the differences presented above as interspecies differences be due to our biased review of samples at different ontogenetic stages? Additionally, how does the microscopic structure of pancreas change in the course of a lifetime? A few recent studies involving younger human donors and older mice may provide some starting points to address these questions.
Studies on human islets from the prenatal period to late adulthood provide evidence that human islets probably attain an adult architecture by 2 y after birth.86,96 This is accompanied by an increase in beta cell area and mass, as well as beta to alpha cell ratio, predominantly due to a burst of beta cell proliferation,86,96,97 and by an increase in beta to delta cell ratio, due to both beta cell proliferation and a decrease in delta cell mass.96 The beta cell replication rate is greatest during the first 2 y of age and declines toward adolescence,86,96 a pattern similar to the postnatal expansion of beta cells in mice.98 An increase in islet diameter rather than islet number from the neonatal period is the main mechanism of beta cell expansion,86,96 since roughly 10% of beta cell mass increase from birth to adolescence can be ascribed to new islet formation and 90% to an increase in islet size.86 Importantly, no major quantitative or architectural changes have been described in humans during adolescence.86,96 In addition, exocrine pancreas volume increases roughly linearly throughout childhood and adolescence (to 20 y of age). This increase exceeds beta cell expansion, such that a decrease in fractional beta cell area and islet density are observed during this period.86,99 During 20 to 60 y of age the exocrine pancreas volume remains stable and then decreases beyond 60 y of age.99 In contrast, beta cell mass as well as islet structure seem to remain largely constant between 20 and 100 y of age.83,84 Thus, fractional beta cell area remains stable between 20 and 60 y of age and increases beyond 60 y of age.84 Despite the shift toward the adult human-type architecture, it should be stressed that (i) single cells, (ii) pure and mixed clusters, (iii) mantle-type islets, and (iv) human-type islets can be found in humans beyond 2 y of age.86,100 The above 4 patterns overlap with the scheme for prenatal development of islets by Ferner101 and Robb.102,103 Therefore, from the appearance of first endocrine cells (8 weeks prenatally) to 2 y of age, there is a shift to a preponderance of human-type islets, but this shift is not complete.96,100-105
Studies utilizing older mice suggest that the mantle-type islet probably remains the predominant architectural type into old age.85,106-108 In addition, beta cell area, mass, as well as total pancreas mass increase with age, as does the body mass of ad libitum fed mice.85,106,107 The number of islets increases during the first 3–4 weeks and remains stable thereafter.78,109 The stability of islets is further supported by the finding that there is a low replication rate in all types of islet cells in 1-year-old mice and that the islet architecture in these mice is of the mantle type.110,111 The exocrine tissue expands more slowly than beta cell mass. Consequently, the fractional beta cell area increases with age.85,107
In sum, we believe that the described differences between human and mouse pancreas tissue, especially in islet architecture, are due to true biological interspecies differences. In the following chapters, vascularization and innervation of the pancreas will be covered, for which interspecies differences and ontogenetic changes are less well described. It should be acknowledged that interstrain112,113 and interindividual114 differences might further complicate the situation, but these points are beyond the scope of this paper.
Major Blood Vessels of the Pancreas
The pancreas receives approximately 1% of the cardiac output.115 The arterial blood to the pancreas is derived from the first 2 of the 3 major branches of the abdominal aorta: the celiac and the superior mesenteric artery (Fig. 3).8,116-119 The head of the pancreas is supplied by 2 arcades: an anterior and a posterior.120-123 The anterior arcade is formed by the anterior superior pancreaticoduodenal artery (PDA), an indirect branch (via the gastroduodenal artery) of the common hepatic artery, which is the rightmost branch of the celiac artery.120 The anterior superior PDA anastomoses with pancreatic branches of the splenic artery.117,120 The posterior arcade is formed by the posterior superior PDA that is the most proximal branch of the gastroduodenal artery.121 The anterior and posterior superior PDA anastomose with the anterior and posterior inferior PDA that both stem from the superior mesenteric artery.122,123 The body and the tail of the pancreas (i.e., the dorsal pancreas) are supplied by pancreatic branches of the splenic artery which is the leftmost branch of the celiac artery, and by the dorsal pancreatic artery that branches off near the origin of either celiac, hepatic, or splenic artery. Its right branch anastomoses with the anterior superior PDA whereas its left branch forms the transverse pancreatic artery (also termed the inferior pancreatic artery). The latter runs along the inferior border of the pancreas and usually anastomoses with the pancreatica magna artery, the largest pancreatic branch of the splenic artery in the middle or in the left third of the gland119,124 (Fig. 3A).
Figure 3.
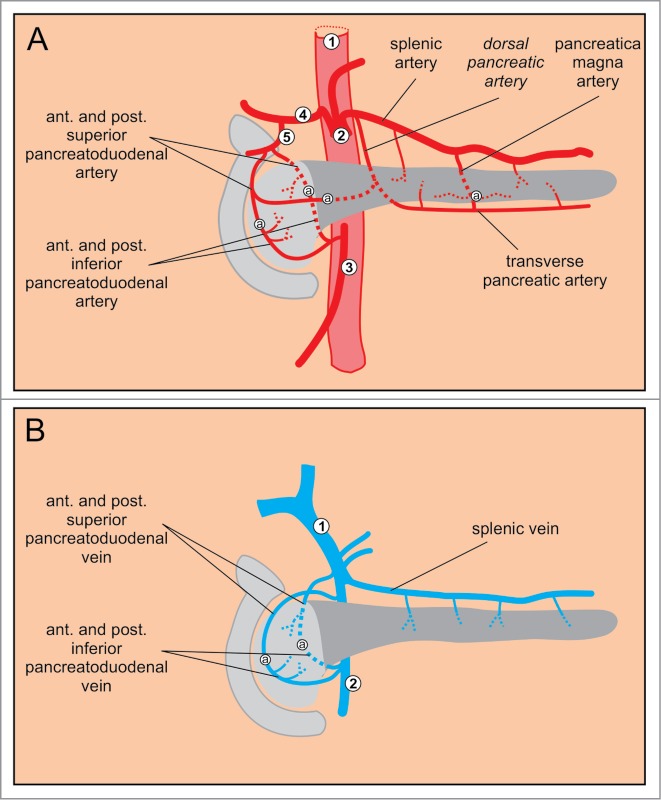
Major blood vessels of the pancreas. (A) Arteries. 1 abdominal aorta, 2 celiac artery, 3 superior mesenteric artery, 4 common hepatic artery, 5 gastroduodenal artery. (B) Veins. 1 portal vein, 2 superior mesenteric vein.
The venous system of the pancreas drains blood into the portal vein.116 The venous drainage from the body and the tail of the pancreas is less constant than their arterial supply. In general, the splenic vein collects blood via multiple small braches.118 The blood from the head of the pancreas is drained in a pattern similar to the arterial system. The anterior venous arcade is formed by the anterior superior and inferior pancreaticoduodenal veins (PDV) that both drain into the superior mesenteric vein. Similarly, the posterior arcade is composed of the posterior superior and inferior PDV. The posterior inferior PDV drains blood into the superior mesenteric vein, whereas the posterior superior PDV drains directly into the portal vein (Fig. 3B).8,117,118 Numerous anastomoses exist in the venous system that are typically more irregular than those in the arterial system.8 In mice, the anatomy of the major arteries and veins supplying and draining the pancreas is largely homologous to the one in humans.16,125
Microvasculature of the Pancreas
The smallest intralobular arteries, arterioles, and capillaries are collectively termed the microvasculature of the pancreas.126 At the level of the microvasculature, the perfusion of the gland is rather different in humans and mice.
In humans, due to their predominant location within the lobules, the islet capillaries lead blood to capillaries surrounding acini, which is named the insulo-acinar portal system.25,41,77 Therefore, the blood from the islets is collected in efferent capillaries that exit islets to form a secondary capillary network supplying the acinar cells. Islet vasculature was reported to contain approximately 3-times more smooth muscle cells in humans where smooth muscle cells can be found deep within the islets than in mice where smooth muscle cells are associated only with the feeding arterioles at the islet periphery.127
In mice, due to the mostly interlobular location of islets, their venous blood is collected by interlobular veins forming the so called insulo-venous system. The less frequent intralobular islets pass blood either to the acinar capillary network or to interlobular veins.7,25,26,41 Importantly, via both the insulo-acinar and insulo-venous system, the blood is ultimately passed to the systemic circulation only indirectly after passing the liver.116,128
The insulo-acinar portal system is the anatomical substrate for the influences that the endocrine pancreas may exert upon the exocrine pancreas.129
The islets are highly vascularized and receive approximately 10-times more blood than the exocrine part when expressed per unit weight of tissue (i.e., approximately 15% vs. 85% of total pancreas blood flow).130 In both mice and humans, 1–5 arterioles enter each islet (depending on the size of the islet) and divide into fenestrated capillaries that form a dense capillary network resembling the kidney glomeruli.82,131 They are 5-times more numerous and bear 10-times more fenestrae than the capillaries in the surrounding acinar tissue. The capillaries possess a basement membrane.7 Capillaries have been reported to constitute 7–8% of islet volume.132
Interestingly, in mice it has been shown that the blood may follow a specific microcirculatory pattern with regard to the cell type.128,131,133 Bearing in mind that according to the classical picture of islet microarchitecture, in mice the non-beta cells form a mantle around the core of beta cells, the blood perfusion was suggested to be organized in 3 possible patterns: (i) from periphery to center (blood reaches the non-beta cells first),26,134 (ii) from center to periphery (blood reaches beta cells first)133 or (iii) from one pole of the islet to the other (no hierarchical perfusion with regard to the cell type)133,135 (Fig. 4). The evidence for the described patterns of perfusion was based on structural vascular cast studies. It was recently shown that in mice, 66% of islets have the center-to-periphery pattern and the remaining islets the pole-to-pole flow across the islet.133
Figure 4.
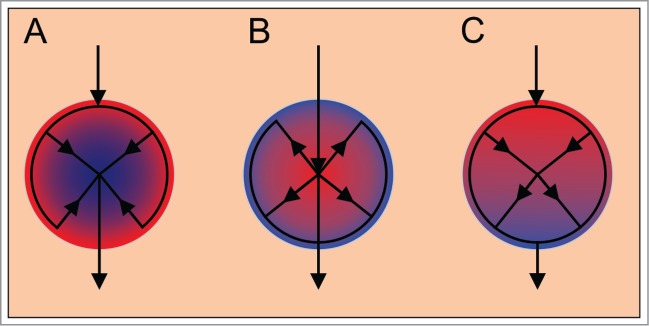
Three possible patterns of islet microcirculation. (A) From periphery to center. (B) From center to periphery, blood reaches the β cells first. (C) From one pole to the other. Red color indicates arterial blood and blue color venous blood.
There are some indications that the center-to-periphery pattern also prevails in humans, as revealed by functional studies of perfusion.136 However, since in humans the cell type distribution seems to be much less orderly, it was suggested that in humans the pattern of perfusion does not follow a clear cell type pattern.25,49
Considering the fact that in human islets, the alpha cell distribution was shown not to be random but that alpha cells form 2 outermost layers flanking a beta cell rich middle layer of a folded trilaminar plate and that endothelial cells were never found in the middle layer, it is possible that in human islets, a hierarchy of perfusion exists, with arterial blood coming in contact with alpha cells first.58
The Lymphatic System
The internal lymphatic system
The fine organization of the internal lymphatic system of the pancreas has been described practically exclusively in rodents. The internal lymphatic system of the mouse pancreas starts with blind-beginning intralobular lymphatic vessels that run in the larger intralobular connective tissue septa alongside smallest blood vessels and ducts and at a certain distance from acinar cells.137,138 The evidence suggests that in mice, every lobule has many intralobular vessels.137 They empty into interlobular vessels running in the connective tissue that separates lobules and also contains the interlobular blood vessels and ducts. Only about 20% of endothelial cells have been found close to acinar cells, 10-times less to ductal cells, and only exceptionally were they found close to endocrine cells of islets.138 They have never been observed within the islets but always at their periphery. Among rodents, contacts were more numerous in mice than in rats, due to the topographical arrangement of mouse islets at the periphery of lobules or within the interlobular connective tissue septa, alongside lymphatic vessels.137 Extrapolating this conclusion to humans, one could assume that the contact between the internal lymphatic system and islets is more pronounced in mice, due to a higher proportion of interlobular islets. The largest interlobular vessels, sometimes referred to as collecting vessels, reach the surface of the gland and drain into the external lymphatic system of the pancreas.138 There are no major structural differences between the intra- and interlobular lymphatic vessels. Both are lined by a continuous non-fenestrated endothelium supported by a discontinuous basal lamina, the latter being most prominent at sites where valves protrude into the lumen.138,139
Both insulin and enzymes of the exocrine pancreas are found in the lymph leaving pancreas through the thoracic duct. Insulin enters the lymph by means of leakage from islets to interstitial fluid or directly through lymphatic vessels in close contact with an interlobular islet. The flow of lymph from the pancreas is low compared to blood flow and the concentration of insulin in the lymph is lower than in plasma. Thus, the mass flow of insulin to the systemic circulation via lymph is much less than via portal venous blood (probably less than 1% of total delivery).137,138,140 It should be kept in mind, however, that the insulin entering the bloodstream via lymph does not undergo the first pass metabolism in the liver. Thus, a role for this route cannot be excluded, particularly in hyperinsulinemic states, such as insulinoma.137 Evidence suggests that inadequate removal of extracellular fluid and pancreatic enzymes from interstitium by the lymphatic overflow system might play an etiological role in pancreatitis, which can in turn further damage the interstitium and lymphatic vessels, initiating a vicious circle.137,138
The external lymphatic system
When the collecting vessels emerge on the surface of the pancreas they drain into the surface network of lymphatic vessels. From here, larger lymphatic vessels transport the lymph to the regional lymph nodes. There are 7 main groups of lymphatic vessels, each associated with a corresponding group of blood vessels: superior vessels (along the splenic blood vessels), inferior vessels (along transverse pancreatic artery), anterosuperior, anteroinferior, posterosuperior, posteroinferior pancreaticoduodenal vessels (along the corresponding arteries), and the gastroduodenal vessels. The first 2 drain the left part of the body and the tail, the latter 5 the right part of the body and the head of the pancreas. Due to its clinical importance in carcinoma, the external system has been studied extensively in humans. However, there exists controversy regarding nomenclature of lymph nodes, with a descriptive and an alternative numerical system.138 Here we briefly review the most important lymph node groups according to the descriptive system with their corresponding numerical notations in parentheses (Fig. 5). The lymph nodes of the pancreas are organized in 2 major groups.8,138 The first group of nodes lie approximately along the outline of the gland. Left to the aorta are the splenic and gastrosplenic nodes situated within and superior to the splenic hilus (10), as well as suprapancreatic (11) and infrapancreatic (18) nodes lying along the splenic and inferior pancreatic artery, respectively. These nodes receive lymph from the body and the tail of the pancreas. To the right of aorta, there are 5 main groups of nodes that drain the head. First, as an extension of the suprapancreatic nodes to the right side there are the hepatic (8) and hepatoduodenal (12) nodes. Along the superior and inferior anterior and posterior pancreaticoduodenal arteries lie the next 4 groups of nodes named the superior anterior (17a), superior posterior (13a), inferior anterior (17b), and inferior posterior (13b) pancreaticoduodenal nodes. The second major group of nodes are associated with abdominal aorta and its trunks, the paraaortic (16), celiac (9), superior mesenteric (14), and the middle colic nodes (15).138,141 Nodes 1–7 belong to the perigastric lymph nodes and are believed not to drain the pancreas.141
Figure 5.
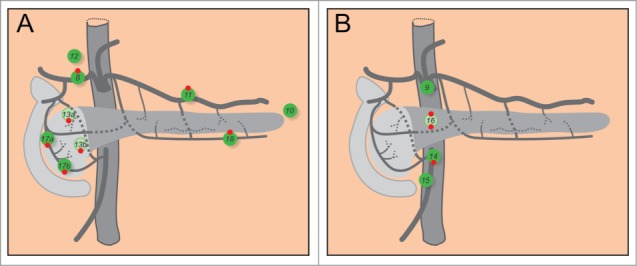
Lymph nodes of the pancreas. (A) Groups of lymph nodes that outline the gland. (B) Groups of nodes at the aorta. 8: hepatic nodes. 9: celiac nodes. 10: splenic and gastrosplenic nodes. 11: suprapancreatic nodes. 12: hepatoduodenal nodes. 13a: superior posterior nodes. 13b: inferior posterior nodes. 14: superior mesenteric nodes. 15: middle colic nodes. 16: paraaortic nodes. 17a: superior anterior nodes. 17b: inferior anterior nodes. 18: infrapancreatic nodes. Red dots on the top and at the bottom of lymph nodes indicate the nodes most frequently involved in carcinoma of the body or the tail, and the head, respectively.
Four out of 5 patients with pancreatic carcinoma display lymph node involvement.141 Rich anastomoses between lymphatic vessels that promote fluid drainage make predictions about the exact routes of spreading of pancreatic cancer practically impossible.138,142 In carcinoma of the body and tail of the pancreas, nodes 8, 11, 16 and 18 are most frequently involved by metastatic spread and only nodes 17 have been spared in all cases.141,143 In carcinoma of the head, nodes 13, 17, 14, and 16 are involved most often, and only nodes 10 and 15 have been spared and nodes 11 and 18 have been involved only in cases of cancers extending to the neck or body.141,142,144 It has been demonstrated that the embryological origin of the tissue influences the spread of cancer of the head of the pancreas. Tumors confined to the part of the head stemming from the ventral pancreas spread to nodes around the superior mesenteric artery (14), whereas tumors confined to the part of the head stemming from the dorsal pancreas spread to nodes around the common hepatic artery (8) and in the hepatoduodenal ligament (12).145
All of the lymph is eventually drained into the left subclavian vein via cisterna chyli and the thoracic duct.8
Data on the internal lymphatic system in humans and the external lymphatic system in mice are lacking. Thus, differences between the lymphatic systems of mice and men are largely unexplored.137,138 Given the practical importance of the lymphatic system in pancreatitis and cancer, further research in this field is called for.
Innervation
The pancreas is richly innervated by sympathetic, parasympathetic, and afferent fibers.126,146-149 Nerve fibers enter or exit pancreas as neurovascular stalks, follow the blood vessels also within the pancreatic tissue and end or begin close to capillary walls and endocrine cells.150 They do not form classical synapses with target cells, but have release sites from which they presumably release neurotransmitters into the extracellular space and influence more than one target at a time.148
Autonomic nerves are believed to mediate the insulin response during the cephalic phase of feeding as well as to contribute to the increase in glucagon and the decrease in insulin secretion during sympathetic stimulation in animals and humans.146,151-154
Due to recent advancements in experimental approaches enabling studies of many cells at a time in situ in isolated islets and thick tissue slices as well as in in vivo, a clearer picture of the structure and function of the nervous system within pancreas is beginning to emerge, together with differences between mice and humans.32,33,127,155-161
Sympathetic efferent fibers
Cell bodies of preganglionic sympathetic nerves are located in the lateral horn of the thoracic and the upper lumbar segments of the spinal cord (C8-L3) (Fig. 6).126,146 The myelinated axons of these cells project to ganglion cells in the paravertebral sympathetic ganglia. However, some neurons pass the paravertebral ganglia without forming a synapse. These neurons travel via the splanchnic nerves to form synapses within the prevertebral sympathetic ganglia, i.e., the celiac ganglia and the superior mesenteric ganglion.126,146,147 Additionally, intrapancreatic sympathetic ganglia have been reported.146,147
Figure 6.
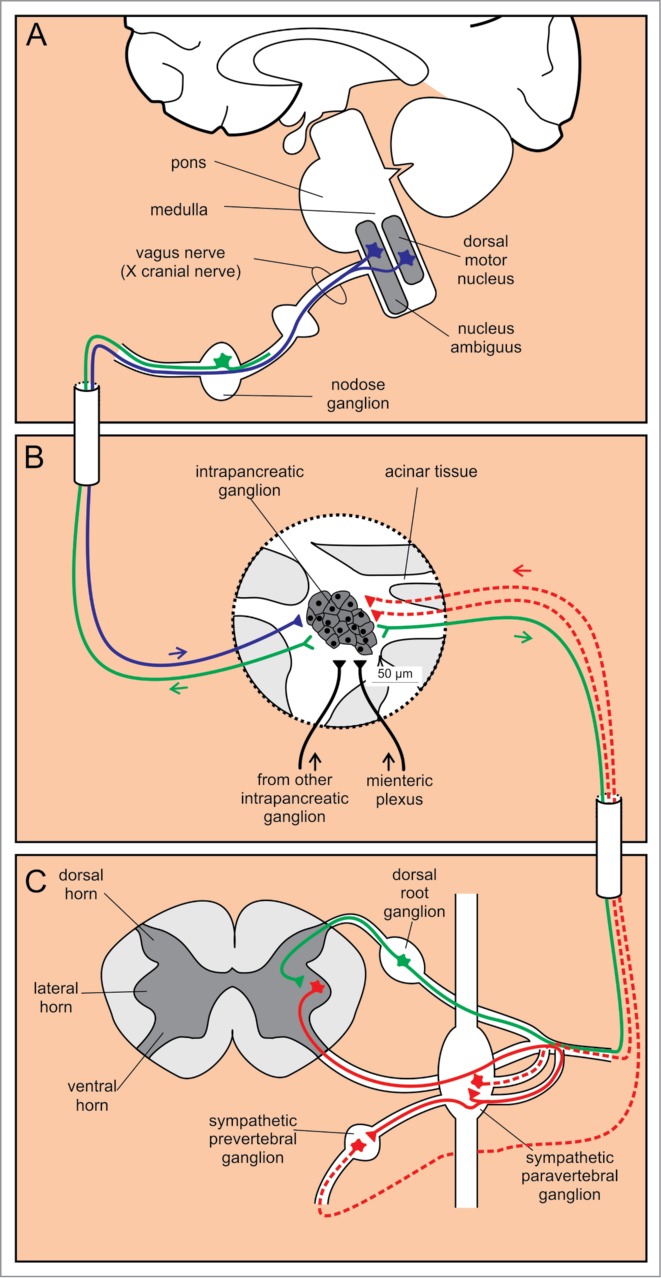
Autonomic innervation of intrapancreatic ganglia. (A) Cell bodies of efferent parasympathetic fibers are located in 2 nuclei in the medulla, whereas the afferent sensory cell bodies are positioned in the nodose ganglion. (B) Intrapancreatic ganglia receive input from sympathetic (red) and parasympathetic (blue) fibers, from other intrapancreatic ganglia and from the myenteric plexus (black). Additionally, sensory fibers (green) project from intrapancreatic ganglia. (C) Preganglionic efferent sympathetic fibers (red solid line) project from cell bodies in the lateral horn of the spinal cord to paravertebral and prevertebral sympathetic ganglia. From here, the postganglionic sympathetic fibers (red dashed lines) project to the intrapancreatic ganglia. Sensory afferent fibers (green) have their cell bodies in the dorsal root ganglia.
The fibers projecting from the prevertebral ganglia reach the pancreas either within the mixed autonomic nerves or directly.146,162 In humans, the body and the tail of the pancreas are innervated from nerve fibers originating in the celiac plexus and accompanying 2 arteries: the splenic artery around which they form the so called splenic plexus, and the transverse pancreatic artery (Fig. 7). More recently, a nerve that enters the pancreas independently of the celiac ganglion/plexus was described. This nerve winds around the main pancreatic duct rather than following blood vessels.163 The head of the pancreas receives the majority of nerve fibers.164 It is innervated from the anterior hepatic plexus distributed along the common hepatic artery, and from the posterior hepatic plexus at the dorsal aspect of the portal vein, both of which originate from the celiac plexus. Nerves derived from the superior mesenteric ganglion follow the inferior PDA and enter the uncinate process.163 In mice, distribution of autonomic nerves is largely homologous to the one in humans,165,166 but with nerve densities more equally distributed among different lobes.149
Figure 7.
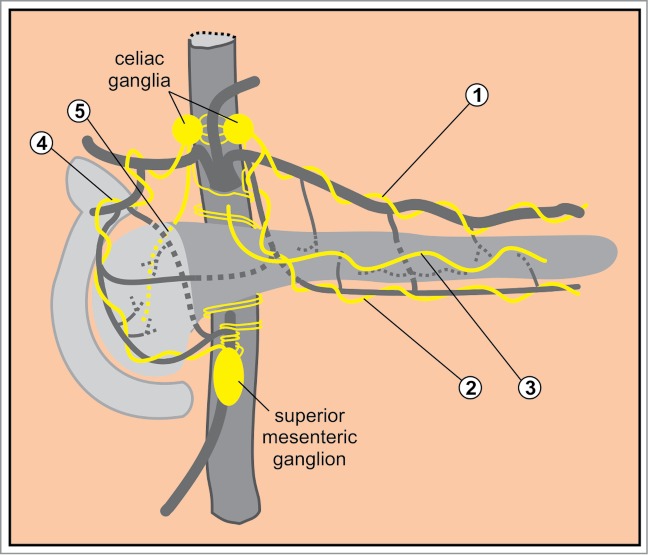
Macroscopic autonomic innervation of the pancreas. 1: the splenic plexus accompanying the splenic artery; 2: the plexus accompanying the transverse pancreatic artery; 3: the plexus around the main pancreatic duct; 4: the anterior hepatic plexus; 5: the posterior hepatic plexus.
As already mentioned, lymph node involvement is one of the most important prognostic factors in pancreatobiliary tract carcinomas. In general, lymph node metastasis is established by lymphatic invasion, however, tumor cells were shown to be able to spread into the hilum of lymph nodes via neural invasion. The knowledge of patterns of neural architecture may improve curative procedures.167 Moreover, embryological development of the pancreas served as a useful template for patterns of extrapancreatic nerve plexus invasion of pancreatic head carcinoma.168
In the exocrine pancreas, the sympathetic axons contact predominantly the intrapancreatic ganglia, blood vessels, and exocrine ducts and inhibit the exocrine secretion indirectly via inhibiting stimulatory influences from ganglia and via vasoconstriction diminishing the blood flow.126 In mice, however, innervation of the acinar tissue is rather poor.150
In the islets in mice, major nerves running along interlobular arteries branch to form nerve plexuses toward the islets.150 Sympathetic axons are in contact with alpha cells, but not beta cells (Fig. 8). Additionally, they innervate vessel smooth muscle cells and the perivascular space, forming the so called sympathetic neurovascular complex.158
Figure 8.
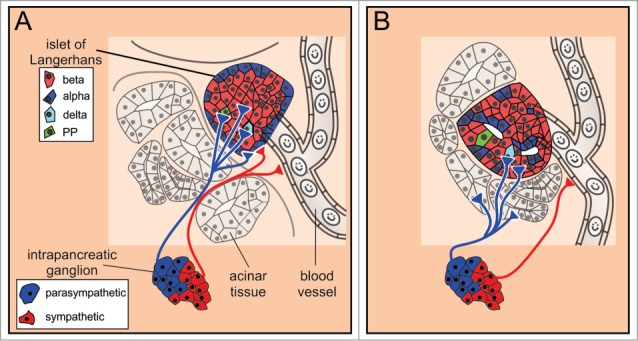
The autonomic innervation of islets of Langerhans. (A) In mice, the postganglionic sympathetic fibers contact α cells and smooth muscle cells of the blood vessels, whereas the parasympathetic fibers contact all endocrine cell types. (B) In humans, the islets are sparsely innervated. The sympathetic and parasympathetic postganglionic fibers preferentially contact vascular smooth muscle cells and exocrine tissue, respectively.
In humans, the sympathetic fibers only sparsely contact the endocrine cells directly. Rather, they preferentially innervate vascular smooth muscle cells. The above findings suggest that in addition to the direct effect on endocrine cells in mice, the sympathetic nervous control of the endocrine pancreas is indirect, via controlling blood flow to the islet (similar to the sympathetic influence in the exocrine pancreas) or by acting on targets distant from the release site (similar to the communication pathway in the pituitary gland).127
Splanchnic nerve stimulation increases the release of glucagon and inhibits the release of insulin and somatostatin from the pancreas. The effects of splanchnic nerve stimulation are rather contradictory for the pancreatic polypeptide.147,148 Supporting the idea that the effects of catecholamines are at least partially mediated via release into islet blood vessels is the finding that sympathetic stimulation increases the concentration of noradrenaline in the pancreatic vein.169 Since the pancreatic veins drain into the portal system, this spillover could contribute to the metabolic effects of catecholamines in the liver170,171
Parasympathetic efferent fibers
The preganglionic fibers of the parasympathetic limb originate in 2 nuclei of the medulla oblongata: the dorsal motor nucleus of vagus and the nucleus ambiguus (Fig. 6).126,146 The efferent fibers exit medulla as the bulbar outflow tract, travel mainly along the vagus nerve and to a smaller extent within the splanchnic nerves. The efferent fibers enter the pancreas either directly or indirectly after traversing the celiac ganglion without forming a synapse (Fig. 7). In both cases these fibers join the neural plexuses along arteries and intermingle with the sympathetic fibers.164 Macroscopic innervation in mice is similar to the one in humans.165 The vagal fibers enter the pancreas along the vessels reaching intrapancreatic ganglia that contain 2–30 neurons and are positioned within the interlobular connective tissue, lobules, and islets.126,146 Importantly, in addition to the input from parasympathetic preganglionic fibers, neurons within the intrapancreatic ganglia receive input from other pancreatic ganglia, sympathetic fibers, the myenteric plexus, as well as the sensory fibers (see below).126
In the exocrine part of the pancreas, short unmyelinated postganglionic fibers extend from multiple poles of the intrapancreatic ganglia to the acinar and ductal epithelial cells, ductal smooth muscle cells, to vascular plexuses, as well as to other ganglia. Activation of these fibers stimulates secretion from acinar and ductal cells, constriction of ducts, as well as vasodilation.126,164 As mentioned above, the scarce innervation of the acinar tissue in mice may imply a major role for hormonal control of exocrine secretion in rodents.150
In the islets, postganglionic axons reach to all types of cells in the mouse (Fig. 8).127,146 Recently, it was suggested that the pattern of parasympathetic innervation in humans is different from the one in mice. First, in contrast to mice, only a few parasympathetic axons penetrate the islet in humans and most of the axons terminate in the exocrine tissue.127 Second, it was demonstrated recently that human beta and delta cells respond to stimulation with acetylcholine, whereas alpha cells are poorly responsive to acetylcholine.172 However, this does not rule out the possibility that human alpha cells might respond to neuropeptides released from parasympathetic fibers.146,173 Interestingly, alpha cells themselves are supposed to be the main source of acetylcholine in human islets.174 Here, this classical neurotransmitter probably assumes the role of a paracrine signal controlling endocrine, vascular, and immune cells.175
Generally, parasympathetic activation is believed to increase the release of insulin, glucagon, somatostatin, as well as the pancreatic polypeptide in various species, although the exact contributions of acetylcholine and neuropeptides to these effects remain to be determined.146,148 The pancreatic polypeptide is often used as a specific marker of parasympathetic stimulation of islets, since it is released from the islets only by vagal stimulation. However, as mentioned previously, it is released from a subpopulation of gamma cell-rich islets in the head of the pancreas and thus cannot be regarded as representative of all islets.148
Afferent fibers
In humans, the islets also contain sympathetic afferent fibers that are sensitive to capsaicin and contain substance P (SP) or calcitonin gene-related product (CGRP) as neurotransmitters. They exit the pancreas within the sympathetic splanchnic nerves and transmit noci- and mechano-receptive sensory information to cell bodies within the dorsal root ganglia and further on to preganglionic sympathetic neurons in the spinal medulla (lateral horn) (Fig. 6).126 These fibers innervate the exocrine as well as the endocrine tissue and may play a role in pancreatitis and diabetes mellitus,126,148 as well as in pain accompanying pancreatic cancer or pancreatitis.149 In mice, similar to humans, these fibers also arise from DRG and exit the pancreas via the splanchnic nerve.149,166
Cell bodies of vagal afferent neurons are located within the nodose ganglia in humans (Fig. 6). Similarly to the sympathetic afferents, these cells are sensitive to capsaicin and contain SP and/or CGRP. They innervate the blood vessels, ducts, acini, and islets. At present, centripetal pathways of these neurons are not known.126 In mice, this route is most likely of less importance.149,166
Dorsal root ganglion sympathetic afferent neurons send collaterals to efferent ganglia, representing a neuroanatomical substrate for monosynaptic vegetative reflexes. For example, SP and CGRP released at intrapancreatic ganglia inhibit exocrine secretion. Intrapancreatic ganglia are also contacted by vagal afferents.126
The functional studies showing similar systemic effects of the autonomic nerves in mice and humans imply that if structural differences do exist between islets of mice and humans, they are either not great enough to significantly impact the function or are coupled with differences at the level of signaling that, together with the morphological differences, produce a similar physiological response at the systemic level.
Other cells
The islet is sheathed with a capsule that is composed of collagen and glia.13 Other cells found throughout islets are the pericytes, macrophages, and dendritic cells.13,175-177
A short summary of the most important similarities and differences
Table 1 summarizes the major hallmark differences and similarities between the mouse and human pancreas tissue.
Table 1.
Summary of differences between the mouse and the human pancreas.
| Scale | Property | Mouse | Human |
|---|---|---|---|
| Organ | Anatomical type | Diffuse/dendritic, lobular, soft | Solitary, compact, firm |
| Ducts | Main duct joins with the bile duct proximal to the entry into duodenum | Main duct joins the bile duct at the point of entry into the duodenum | |
| Numerous accessory ducts | A single accessory duct | ||
| Tissue | Diameter of lobules | 0.5–1.5 mm | 1–10 mm |
| Diameter of islets | Single cells to 500–700 μm | Single cells to 500–700 μm | |
| Number of islets | 1,000–5,000 | 1,000,000–15,000,000 | |
| Location of islets | More random, interlobular | Uniform, intralobular | |
| Microvascular pattern | Insulo-venous system prevails, insulo-acinar portal system also present | Insulo-acinar portal system prevails | |
| Order of perfusion | Center-to-periphery (66%), and polar | Most likely polar | |
| Cells | % of β and α cells | Beta cells: 60–80 % | Beta cells: 50–70 % |
| Alpha cells: 10–20 % | Alpha cells: 20–40 % | ||
| Microarchitecture of islets | Mantle islets predominate | Trilaminar islets predominate | |
| Sympathetic fibers | Scarce innervation of exocrine tissue | Rich innervation of exocrine tissue | |
| Contact with α cells and vascular smooth muscle cells | Contact with vascular smooth muscle cells | ||
| Parasympathetic fibers | Scarce innervation of exocrine tissue | Rich innervation of exocrine tissue | |
| Contact all types of endocrine cells | Contact β and delta cells, possibly α cells |
Conclusions
The morphological differences between human and mouse pancreatic tissue seem to be numerous and call for inclusion of human tissue into basic and applied research. We suggest that in analyzing and reporting interspecies differences, the intra- and interspecies variability both be considered and accounted for whenever this is practically achievable. Future research is also expected to shed new light on interstrain and interindividual differences. A detailed presentation of islet development and structure in the prenatal period is beyond the scope of this paper. Thus, we mainly focused on data relevant for explaining differences in the postnatal period. Additionally, we limited ourselves to structural aspects. The emerging developmental, physiological, and pathopysiological intra- and interspecies differences, such as in gene expression,2,106,178-181 in functional phenotypes,4,5,42,64,161,182,183 and in disease progression84,184-187 deserve separate reviews.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The work presented in this paper was supported through the Slovenian Research Agency Programmes (P3–0310–2334 and P3–0396) and the Center of Excellence for Integrated Approaches in Chemistry and Biology of Proteins. The paper Structural Similarities and Differences between the Human and the Mouse Pancreas was produced within the framework of the operation entitled "Center for Open Innovation and Research of the University of Maribor. The operation is co-funded by the European Regional Development Fund and conducted within the framework of the Operational Program for Strengthening Regional development Potentials for the period 2007 –2013, development priority 1: "Competitiveness of companies and research excellence, priority axis 1.1:"Encouraging competitive potential of enterprises and research excellence." The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Case RM. Is the rat pancreas an appropriate model of the human pancreas? Pancreatology 2006; 6:180-90; PMID:16534243; http://dx.doi.org/ 10.1159/000091849 [DOI] [PubMed] [Google Scholar]
- 2. Levetan CS, Pierce SM. Distinctions between the islets of mice and men: implications for new therapies for type 1 and 2 diabetes. Endocr Pract 2013; 19:301-12; PMID:23186955; http://dx.doi.org/ 10.4158/EP12138.RA [DOI] [PubMed] [Google Scholar]
- 3. Grouwels G, Vasylovska S, Olerud J, Leuckx G, Ngamjariyawat A, Yuchi Y, Jansson L, Van de Casteele M, Kozlova EN, Heimberg H. Differentiating neural crest stem cells induce proliferation of cultured rodent islet beta cells. Diabetologia 2012; 55:2016-25; PMID:22618811; http://dx.doi.org/ 10.1007/s00125-012-2542-0 [DOI] [PubMed] [Google Scholar]
- 4. Rutter GA, Hodson DJ. Minireview: intraislet regulation of insulin secretion in humans. Mol Endocrinol 2013; 27:1984-95; PMID:24243488; http://dx.doi.org/ 10.1210/me.2013-1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farnsworth NL, Benninger RKP. New insights into the role of connexins in pancreatic islet function and diabetes. FEBS Letters 2014; 588:1278-87; PMID:24583073; http://dx.doi.org/ 10.1016/j.febslet.2014.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heller RS. The Comparative Anatomy of Islets. In: Islam MS, ed. Islets of Langerhans. Berlin: Springer-Verlag; Berlin, 2010:21-37 [DOI] [PubMed] [Google Scholar]
- 7. Treuting PM, Dintzis S, Liggitt D, Frevert CW. Comparative Anatomy and Histology: A Mouse and Human Atlas. Elsevier Science, 2011 [Google Scholar]
- 8. Bockman DE. Anatomy of the Pancreas. In: Go VLW, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA, eds. The Pancreas: Biology, Pathobiology, and Disease New York: Raven Press, 1993:1-8 [Google Scholar]
- 9. Suda K, Nobukawa B, Takase M, Hayashi T. Pancreatic segmentation on an embryological and anatomical basis. J Hepatobiliary Pancreat Surg 2006; 13:146-8; PMID:16547676; http://dx.doi.org/ 10.1007/s00534-005-1039-3 [DOI] [PubMed] [Google Scholar]
- 10. Rahier J, Wallon J, Henquin JC. Cell populations in the endocrine pancreas of human neonates and infants. Diabetologia 1981; 20:540-6; PMID:6116638; http://dx.doi.org/ 10.1007/BF00252762 [DOI] [PubMed] [Google Scholar]
- 11. Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia 1983; 24:366-71; PMID:6347784; http://dx.doi.org/ 10.1007/BF00251826 [DOI] [PubMed] [Google Scholar]
- 12. Ogilvie RF. A quantitative estimation of the pancreatic islet tissue. QJM 1937; 6:287-300 [DOI] [PubMed] [Google Scholar]
- 13. In't Veld P, Marichal M. Microscopic Anatomy of the Human Islet of Langerhans. The Islets of Langerhans. In: Islam MS, ed.: Springer; Netherlands, 2010:1-19 [DOI] [PubMed] [Google Scholar]
- 14. Yaginuma N, Takahashi T, Saito K, Kyoguku M. The microvasculature of the human pancreas and its relation to Langerhans islets and lobules. Pathol Res Pract 1986; 181:77-84; PMID:3517840; http://dx.doi.org/ 10.1016/S0344-0338(86)80191-1 [DOI] [PubMed] [Google Scholar]
- 15. Rahier J, Wallon J, Henquin JC. Abundance of somatostatin cells in the human neonatal pancreas. Diabetologia 1980; 18:251-4; PMID:6154624; http://dx.doi.org/ 10.1007/BF00251925 [DOI] [PubMed] [Google Scholar]
- 16. Bunnag SC, Bunnag S, Warner NE. Microcirculation in the islets of Langerhans of the mouse. Anat Rec 1963; 146:117-23; PMID:14016852; http://dx.doi.org/ 10.1002/ar.1091460205 [DOI] [PubMed] [Google Scholar]
- 17. Liu XY, Xue LA, Zheng XA, Yan S, Zheng SS. Pancreas transplantation in the mouse. Hepatob Pancreatic Dis Int 2010; 9:254-8 [PubMed] [Google Scholar]
- 18. Watanabe S, Abe K, Anbo Y, Katoh H. Changes in the mouse exocrine pancreas after pancreatic duct ligation - a qualitative and quantitative histological study. Arch Histol Cytol 1995; 58:365-74; PMID:8527243; http://dx.doi.org/ 10.1679/aohc.58.365 [DOI] [PubMed] [Google Scholar]
- 19. Böck P, Abdel-Moneim M, Egerbacher M. Development of pancreas. Microsc Res Tech 1997; 37:374-83; PMID:9220417; http://dx.doi.org/10.1002/(SICI)1097-0029(19970601)37:5/6%3c374::AID-JEMT2%3e3.3.CO; 2-R [DOI] [PubMed] [Google Scholar]
- 20. Villasenor A, Chong DC, Henkemeyer M, Cleaver O. Epithelial dynamics of pancreatic branching morphogenesis. Development 2010; 137:4295-305; PMID:21098570; http://dx.doi.org/ 10.1242/dev.052993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hörnblad A, Cheddad A, Ahlgren U. An improved protocol for optical projection tomography imaging reveals lobular heterogeneities in pancreatic islet and beta-cell mass distribution. Islets 2011; 3:204-8; PMID:21633198; http://dx.doi.org/ 10.4161/isl.3.4.16417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nagai H. Configurational anatomy of the pancreas: its surgical relevance from ontogenetic and comparative-anatomical viewpoints. J Hepatobiliary Pancreat Surg 2003; 10:48-56; PMID:12918457 [PubMed] [Google Scholar]
- 23. Hörnblad A, Eriksson AU, Sock E, Hill RE, Ahlgren U. Impaired spleen formation perturbs morphogenesis of the gastric lobe of the pancreas. PLoS ONE 2011; 6:e21753; PMID:21738788; http://dx.doi.org/ 10.1371/journal.pone.0021753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saito K, Iwama N, Takahashi T. Morphometrical analysis on topographical difference in size distribution, number and volume of islets in the human pancreas. Tohoku J Exp Med 1978; 124:177-86; PMID:347635; http://dx.doi.org/ 10.1620/tjem.124.177 [DOI] [PubMed] [Google Scholar]
- 25. Murakami T, Hitomi S, Ohtsuka A, Taguchi T, Fujita T. Pancreatic insulo-acinar portal systems in humans, rats, and some other mammals: Scanning electron microscopy of vascular casts. Microsc Res Tech 1997; 37:478-88; PMID:9220425; http://dx.doi.org/10.1002/(SICI)1097-0029(19970601)37:5/6%3c478::AID-JEMT10%3e3.0.CO; 2-N [DOI] [PubMed] [Google Scholar]
- 26. Murakami T, Fujita T, Miyake T, Ohtsuka A, Taguchi T, Kikuta A. The insulo-acinar portal and insulo-venous drainage systems in the pancreas of the mouse, dog, monkey, and certain other animals - a scanning electron-microscopic study of corrosion casts. Arch Histol Cytol 1993; 56:127-47; PMID:8373657; http://dx.doi.org/ 10.1679/aohc.56.127 [DOI] [PubMed] [Google Scholar]
- 27. Watanabe T, Yaegashi H, Koizumi M, Toyota T, Takahashi T. The lobular architecture of the normal human pancreas: a computer-assisted three-dimensional reconstruction study. Pancreas 1997; 15:48-52; PMID:9211492; http://dx.doi.org/ 10.1097/00006676-199707000-00007 [DOI] [PubMed] [Google Scholar]
- 28. Tadokoro H, Takase M, Nobukawa B. Development and congenital anomalies of the pancreas. Anatomy Res Int 2011; 2011: 1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reichert M, Rustgi AK. Pancreatic ductal cells in development, regeneration, and neoplasia. J Clin Invest 2011; 121:4572-8; PMID:22133881; http://dx.doi.org/ 10.1172/JCI57131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tadokoro H, Takase M, Nobukawa B. Unusual fusion between ventral and dorsal primordia causes anomalous pancreaticobiliary junction. Pathol Int 2008; 58:498-502; PMID:18705770; http://dx.doi.org/ 10.1111/j.1440-1827.2008.02263.x [DOI] [PubMed] [Google Scholar]
- 31. Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 1967; 16:35-9; PMID:5333500; http://dx.doi.org/ 10.2337/diab.16.1.35 [DOI] [PubMed] [Google Scholar]
- 32. Speier S, Rupnik M. A novel approach to in situ characterization of pancreatic ß-cells. Pflügers Archiv 2003; 446:553-8; PMID:12774232; http://dx.doi.org/ 10.1007/s00424-003-1097-9 [DOI] [PubMed] [Google Scholar]
- 33. Stožer A, Dolenšek J, Rupnik MS. Glucose-Stimulated Calcium Dynamics in Islets of Langerhans in Acute Mouse Pancreas Tissue Slices. PLoS One 2013; 8:e54638; http://dx.doi.org/ 10.1371/journal.pone.0054638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skelin Klemen M, Dolenšek J, Stožer A, Rupnik M. Measuring Exocytosis in Endocrine Tissue Slices. In: Thorn P, ed. Exocytosis Methods: Humana Press, 2014:127-46 [Google Scholar]
- 35. Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes 1988; 37:413-20; PMID:3288530; http://dx.doi.org/ 10.2337/diab.37.4.413 [DOI] [PubMed] [Google Scholar]
- 36. Marciniak A, Cohrs CM, Tsata V, Chouinard JA, Selck C, Stertmann J, Reichelt S, Rose T, Ehehalt F, Weitz J, et al. Using pancreas tissue slices for in situ studies of islet of Langerhans and acinar cell biology. Nat Protoc 2014; 9:2809-22; PMID:25393778; http://dx.doi.org/ 10.1038/nprot.2014.195 [DOI] [PubMed] [Google Scholar]
- 37. Opie EL, Meakins JC. Data concerning the etiology and pathology of hemorrhagic necrosis of the pancreas (acute hemorrhagic pancreatitis). J Exp Med 1909; 11:561-78; PMID:19867267; http://dx.doi.org/ 10.1084/jem.11.4.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Löhr JM, Schneider A, Diehl SJ, Witt H. Opie's hypothesis revisited: Acute pancreatitis due to bile reflux into the pancreas. Pancreatology 2012; 12:39-40; PMID:22487472; http://dx.doi.org/ 10.1016/j.pan.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 39. Langerhans P. Beiträge zur mikroskopischen Anatomie der Bauchspeicheldrüse: Inaugural-Dissertation. Berlin: Lange, 1869 [Google Scholar]
- 40. Bouwens L, Pipeleers DG. Extra-insular beta cells associated with ductules are frequent in adult human pancreas. Diabetologia 1998; 41:629-33; PMID:9662042; http://dx.doi.org/ 10.1007/s001250050960 [DOI] [PubMed] [Google Scholar]
- 41. Merkwitz C, Blaschuk OW, Schulz A, Lochhead P, Meister J, Ehrlich A, Ricken AM. The ductal origin of structural and functional heterogeneity between pancreatic islets. Prog Histochem Cytochem 2013; 48:103-40; PMID:24100070; http://dx.doi.org/ 10.1016/j.proghi.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 42. Rupnik M. The physiology of rodent beta-cells in pancreas slices. Acta Physiologica 2009; 195:123-38; PMID:18983446; http://dx.doi.org/ 10.1111/j.1748-1716.2008.01927.x [DOI] [PubMed] [Google Scholar]
- 43. Hellman B. Actual distribution of the number and volume of the islets of langerhans in different size classes in non-diabetic humans of varying ages. Nature 1959; 184:1498-9; PMID:14400897; http://dx.doi.org/ 10.1038/1841498a0 [DOI] [PubMed] [Google Scholar]
- 44. Kim A, Miller K, Jo J, Kilimnik G, Wojcik P, Hara M. Islet architecture: A comparative study. Islets 2009; 1:129-36; PMID:20606719; http://dx.doi.org/ 10.4161/isl.1.2.9480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kilimnik G, Jo J, Periwal V, Zielinski MC, Hara M. Quantification of islet size and architecture. Islets 2012; 4:167-72; PMID:22653677; http://dx.doi.org/ 10.4161/isl.19256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jo J, Choi MY, Koh D-S. Size distribution of mouse langerhans islets. Biophys J 2007; 93:2655-66; PMID:17586568; http://dx.doi.org/ 10.1529/biophysj.107.104125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Steiner DJ, Kim A, Miller K, Hara M. Pancreatic islet plasticity Interspecies comparison of islet architecture and composition. Islets 2010; 2:135-45; PMID:20657742; http://dx.doi.org/ 10.4161/isl.2.3.11815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 2005; 53:1087-97; PMID:15923354; http://dx.doi.org/ 10.1369/jhc.5C6684.2005 [DOI] [PubMed] [Google Scholar]
- 49. Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren P-O, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 2006; 103:2334-9; PMID:16461897; http://dx.doi.org/ 10.1073/pnas.0510790103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rahier J, Wallon J, Gepts W, Haot J. Localization of pancreatic polypeptide cells in a limited lobe of the human neonate pancreas: remnant of the ventral primordium? Cell Tissue Res 1979; 200:359-66; PMID:487404; http://dx.doi.org/ 10.1007/BF00234848 [DOI] [PubMed] [Google Scholar]
- 51. Stefan Y, Grasso S, Perelet A, Orci L. The pancreatic polypeptide-rich lobe of the human pancreas: Definitive identification of its derivation from the ventral pancreatic primordium. Diabetologia 1982; 23:141-2; PMID:7128966; http://dx.doi.org/ 10.1007/BF01271177 [DOI] [PubMed] [Google Scholar]
- 52. Andralojc KM, Mercalli A, Nowak KW, Albarello L, Calcagno R, Luzi L, Bonifacio E, Doglioni C, Piemonti L. Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia 2009; 52:486-93; PMID:19096824; http://dx.doi.org/ 10.1007/s00125-008-1238-y [DOI] [PubMed] [Google Scholar]
- 53. Orci L, Unger RH. Functional Subdivision of Islets of Langerhans and Possible Role of D Cells. Lancet 1975; 2:1243-4; PMID:53729; http://dx.doi.org/ 10.1016/S0140-6736(75)92078-4 [DOI] [PubMed] [Google Scholar]
- 54. Ku SK, Lee HS, Lee JH. An immunohistochemical study on the pancreatic endocrine cells of the C57BL/6 mouse. J Vet Sci 2002; 3:327-33; PMID:12819383 [PubMed] [Google Scholar]
- 55. Pfeifer CR, Shomorony A, Aronova MA, Zhang G, Cai T, Xu H, Notkins AL, Leapman RD. Quantitative analysis of mouse pancreatic islet architecture by serial block-face SEM. J Struct Biol 2015; 189:44-52; PMID:25448885; http://dx.doi.org/ 10.1016/j.jsb.2014.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Erlandsen SL, Hegre OD, Parsons JA, McEvoy RC, Elde RP. Pancreatic islet cell hormones distribution of cell types in the islet and evidence for the presence of somatostatin and gastrin within the D cell. J Histochem Cytochem 1976; 24:883-97; PMID:60437; http://dx.doi.org/ 10.1177/24.7.60437 [DOI] [PubMed] [Google Scholar]
- 57. Grube D, Bohn R. The microanatomy of human islets of Langerhans, with special reference to somatostatin (D-) cells. Arch Histol Jpn 1983; 46:327-53; PMID:6139102 [DOI] [PubMed] [Google Scholar]
- 58. Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller YD, Giovannoni L, Parnaud G, Berney T. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes 2010; 59:1202-10; PMID:20185817; http://dx.doi.org/ 10.2337/db09-1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Henquin J, Rahier J. Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia 2011; 54:1720-5; PMID:21465328; http://dx.doi.org/ 10.1007/s00125-011-2118-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Martin F, Soria B. Glucose-induced [Ca2+]i oscillations in single human pancreatic islets. Cell Calcium 1996; 20:409-14; PMID:8955555; http://dx.doi.org/ 10.1016/S0143-4160(96)90003-2 [DOI] [PubMed] [Google Scholar]
- 61. Quesada I, Todorova MG, Alonso-Magdalena P, Beltrá M, Carneiro EM, Martin F, Nadal A, Soria B. Glucose induces opposite intracellular ca2+ concentration oscillatory patterns in identified alpha- and beta-cells within intact human islets of Langerhans. Diabetes 2006; 55:2463-9; PMID:16936194; http://dx.doi.org/ 10.2337/db06-0272 [DOI] [PubMed] [Google Scholar]
- 62. Huypens P, Ling Z, Pipeleers D, Schuit F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia 2000; 43:1012-9; PMID:10990079; http://dx.doi.org/ 10.1007/s001250051484 [DOI] [PubMed] [Google Scholar]
- 63. Wojtusciszyn A, Armanet M, Morel P, Berney T, Bosco D. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia 2008; 51:1843-52; PMID:18665347; http://dx.doi.org/ 10.1007/s00125-008-1103-z [DOI] [PubMed] [Google Scholar]
- 64. Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol 2013; 75:155-79; PMID:22974438; http://dx.doi.org/ 10.1146/annurev-physiol-030212-183754 [DOI] [PubMed] [Google Scholar]
- 65. Henquin JC, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes 2006; 55:3470-7; PMID:17130494; http://dx.doi.org/ 10.2337/db06-0868 [DOI] [PubMed] [Google Scholar]
- 66. Kharouta M, Miller K, Kim A, Wojcik P, Kilimnik G, Dey A, Steiner DF, Hara M. No mantle formation in rodent islets—The prototype of islet revisited. Diabetes Res Clin Pract 2009; 85:252-7; PMID:19595468; http://dx.doi.org/ 10.1016/j.diabres.2009.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jay TR, Heald KA, Carless NJ, Topham DE, Downing R. The distribution of porcine pancreatic beta-cells at ages 5, 12 and 24 weeks. Xenotransplantation 1999; 6:131-40; PMID:10431790; http://dx.doi.org/ 10.1034/j.1399-3089.1999.00009.x [DOI] [PubMed] [Google Scholar]
- 68. Quay WB. Pancreatic Weight and Histology in the White Whale. J Mammal 1957; 38:185-92; http://dx.doi.org/ 10.2307/1376308 [DOI] [Google Scholar]
- 69. Van Aswegen G, Van Noorden S, Kotze SH, De Vos V, Schoeman JH. The intestine and endocrine pancreas of the African elephant: a histological immunocytochemical and immunofluorescence study. Onderstepoort J Vet Res 1996; 63:335-40; PMID:9173365 [PubMed] [Google Scholar]
- 70. Michelmore AJ, Keegan DJ, Kramer B. Immunocytochemical identification of endocrine cells in the pancreas of the fruit bat,rousettus aegyptiacus. Gen Comp Endocrinol 1998; 110:319-25; PMID:9593652; http://dx.doi.org/ 10.1006/gcen.1998.7077 [DOI] [PubMed] [Google Scholar]
- 71. Reddy SN, Bibby NJ, Elliott RB. Cellular distribution of insulin, glucagon, pancreatic polypeptide hormone and somatostatin in the fetal and adult pancreas of the guinea pig: a comparative immunohistochemical study. Eur J Cell Biol 1985; 38:301-5; PMID:2864246 [PubMed] [Google Scholar]
- 72. Kilimnik G, Zhao B, Jo J, Periwal V, Witkowski P, Misawa R, Hara M. Altered islet composition and disproportionate loss of large islets in patients with type 2 diabetes. PLoS One 2011; 6:e27445; PMID:22102895; http://dx.doi.org/ 10.1371/journal.pone.0027445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang X, Misawa R, Zielinski MC, Cowen P, Jo J, Periwal V, Ricordi C, Khan A, Szust J, Shen J, et al. Regional differences in islet distribution in the human pancreas - preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS One 2013; 8:e67454; PMID:23826303; http://dx.doi.org/ 10.1371/journal.pone.0067454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Takei S, Teruya M, Grunewald A, Garcia R, Chan EK, Charles MA. Isolation and function of human and pig islets. Pancreas 1994; 9:150-6; PMID:8190716; http://dx.doi.org/ 10.1097/00006676-199403000-00002 [DOI] [PubMed] [Google Scholar]
- 75. Kenmochi T, Miyamoto M, Une S, Nakagawa Y, Moldovan S, Navarro RA, Benhamou PY, Brunicardi FC, Mullen Y. Improved quality and yield of islets isolated from human pancreata using a two-step digestion method. Pancreas 2000; 20:184-90; PMID:10707935; http://dx.doi.org/ 10.1097/00006676-200003000-00012 [DOI] [PubMed] [Google Scholar]
- 76. Schmidt-Nielsen K. Scaling, why is Animal Size So Important? Cambridge: Cambridge University Press, 1984 [Google Scholar]
- 77. Murakami T, Fujita T, Taguchi T, Nonaka Y, Orita K. The blood vascular bed of the human pancreas, with special reference to the insulacinar portal system - scanning electron-microscopy of corrosion casts. Arch Histol Cytol 1992; 55:381-95; PMID:1482603; http://dx.doi.org/ 10.1679/aohc.55.381 [DOI] [PubMed] [Google Scholar]
- 78. Jo J, Kilimnik G, Kim A, Guo C, Periwal V, Hara M. Formation of pancreatic islets involves coordinated expansion of small islets and fission of large interconnected islet-like structures. Biophys J 2011; 101:565-74; PMID:21806924; http://dx.doi.org/ 10.1016/j.bpj.2011.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bouwens L, Rooman I. Regulation of pancreatic beta-cell mass. Physiol Rev 2005; 85:1255-70; PMID:16183912; http://dx.doi.org/ 10.1152/physrev.00025.2004 [DOI] [PubMed] [Google Scholar]
- 80. Montanya E, Nacher V, Biarnés M, Soler J. Linear correlation between beta-cell mass and body weight throughout the lifespan in Lewis rats: role of beta-cell hyperplasia and hypertrophy. Diabetes 2000; 49:1341-6; PMID:10923635; http://dx.doi.org/ 10.2337/diabetes.49.8.1341 [DOI] [PubMed] [Google Scholar]
- 81. Bock T, Pakkenberg B, Buschard K. Increased islet volume but unchanged islet number in ob/ob mice. Diabetes 2003; 52:1716-22; PMID:12829638; http://dx.doi.org/ 10.2337/diabetes.52.7.1716 [DOI] [PubMed] [Google Scholar]
- 82. El-Gohary Y, Sims-Lucas S, Lath N, Tulachan S, Guo P, Xiao X, Welsh C, Paredes J, Wiersch J, Prasadan K, et al. Three-dimensional analysis of the islet vasculature. Anat Rec (Hoboken) 2012; 295:1473-81; http://dx.doi.org/ 10.1002/ar.22530 [DOI] [PubMed] [Google Scholar]
- 83. Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008; 10:32-42; http://dx.doi.org/ 10.1111/j.1463-1326.2008.00969.x [DOI] [PubMed] [Google Scholar]
- 84. Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. beta-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care 2013; 36:111-7; PMID:22875233; http://dx.doi.org/ 10.2337/dc12-0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes 2009; 58:1365-72; PMID:19265026; http://dx.doi.org/ 10.2337/db08-1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008; 57:1584-94; PMID:18334605; http://dx.doi.org/ 10.2337/db07-1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bonner-Weir S. Islet growth and development in the adult. J Mol Endocrinol 2000; 24:297-302; PMID:10828822; http://dx.doi.org/ 10.1677/jme.0.0240297 [DOI] [PubMed] [Google Scholar]
- 88. Jiang Y, Nishimura W, Devor-Henneman D, Kusewitt D, Wang H, Holloway MP, Dohi T, Sabo E, Robinson ML, Altieri DC, et al. Postnatal expansion of the pancreatic beta-cell mass is dependent on survivin. Diabetes 2008; 57:2718-27; PMID:18599523; http://dx.doi.org/ 10.2337/db08-0170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Liu J, Yu P, Qian W, Li Y, Zhao J, Huan F, Wang J, Xiao H. Perinatal bisphenol A exposure and adult glucose homeostasis: identifying critical windows of exposure. PLoS One 2013; 8:e64143; PMID:23675523; http://dx.doi.org/ 10.1371/journal.pone.0064143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wu X, Wang L, Schroer S, Choi D, Chen P, Okada H, Woo M. Perinatal survivin is essential for the establishment of pancreatic beta cell mass in mice. Diabetologia 2009; 52:2130-41; PMID:19644667; http://dx.doi.org/ 10.1007/s00125-009-1469-6 [DOI] [PubMed] [Google Scholar]
- 91. Wang X, Zielinski MC, Misawa R, Wen P, Wang T-Y, Wang C-Z, Witkowski P, Hara M. Quantitative analysis of pancreatic polypeptide cell distribution in the human pancreas. PLoS One 2013; 8:e55501; PMID:23383206; http://dx.doi.org/ 10.1371/journal.pone.0055501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Li D. Diabetes and pancreatic cancer. Mol Carcinog 2012; 51:64-74; PMID:22162232; http://dx.doi.org/ 10.1002/mc.20771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Artinyan A, Soriano PA, Prendergast C, Low T, Ellenhorn JDI, Kim J. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB 2008; 10:371-6; PMID:18982154; http://dx.doi.org/ 10.1080/13651820802291233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Raftery A, Chunn J, Gerland P, Ševčíková H. Bayesian probabilistic projections of life expectancy for all countries. Demography 2013; 50:777-801; PMID:23494599; http://dx.doi.org/ 10.1007/s13524-012-0193-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Harrison DE, Archer JR. Genetic differences in effects of food restriction on aging in mice. J Nutr 1987; 117:376-82; PMID:3559752 [DOI] [PubMed] [Google Scholar]
- 96. Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, Wright AJ, Atkinson MA, Rhodes CJ. Formation of a human beta-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab 2012; 97:3197-206; PMID:22745242; http://dx.doi.org/ 10.1210/jc.2012-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 2000; 49:1325-33; PMID:10923633; http://dx.doi.org/ 10.2337/diabetes.49.8.1325 [DOI] [PubMed] [Google Scholar]
- 98. Georgia S, Bhushan A. beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest 2004; 114:963-8; PMID:15467835; http://dx.doi.org/ 10.1172/JCI200422098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Saisho Y, Butler AE, Meier JJ, Monchamp T, Allen-Auerbach M, Rizza RA, Butler PC. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat 2007; 20:933-42; PMID:17879305; http://dx.doi.org/ 10.1002/ca.20543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Proshchina AE, Savel'yev SV. Immunohistochemical Study of alpha- and beta-Cell Distribution in Human Pancreatic Langerhans Islets of Various Types. Bull Exp Biol Med 2013; 155:798-801; PMID:24288769; http://dx.doi.org/ 10.1007/s10517-013-2255-5 [DOI] [PubMed] [Google Scholar]
- 101. Ferner H. Das Inselsystem des pankreas: Entwicklung, Histobiologie und Pathophysiologie mit besonderer Berücksichtigung des Diabetes mellitus. Thieme 1952 [Google Scholar]
- 102. Robb PM. Development of the islets of Langerhans in man. Nature 1961; 190:1018; PMID:13742049; http://dx.doi.org/ 10.1038/1901018a0 [DOI] [PubMed] [Google Scholar]
- 103. Robb P. The development of the islets of Langerhans in the human foetus. Exp Physiol 1961; 46:335-43; http://dx.doi.org/ 10.1113/expphysiol.1961.sp001551 [DOI] [PubMed] [Google Scholar]
- 104. Jeon J, Correa-Medina M, Ricordi C, Edlund H, Diez JA. Endocrine cell clustering during human pancreas development. J Histochem Cytochem 2009; 57:811-24; PMID:19365093; http://dx.doi.org/ 10.1369/jhc.2009.953307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Meier JJ, Köhler CU, Alkhatib B, Sergi C, Junker T, Klein HH, Schmidt WE, Fritsch H. beta-cell development and turnover during prenatal life in humans. Eur J Endocrinol 2010; 162:559-68; PMID:20022941; http://dx.doi.org/ 10.1530/EJE-09-1053 [DOI] [PubMed] [Google Scholar]
- 106. Tschen S-I, Dhawan S, Gurlo T, Bhushan A. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes 2009; 58:1312-20; PMID:19228811; http://dx.doi.org/ 10.2337/db08-1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sone H, Kagawa Y. Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia 2005; 48:58-67; PMID:15624098; http://dx.doi.org/ 10.1007/s00125-004-1605-2 [DOI] [PubMed] [Google Scholar]
- 108. Stolovich-Rain M, Hija A, Grimsby J, Glaser B, Dor Y. Pancreatic beta cells in very old mice retain capacity for compensatory proliferation. J Biol Chem 2012; 287:27407-14; PMID:22740691; http://dx.doi.org/ 10.1074/jbc.M112.350736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Miller K, Kim A, Kilimnik G, Jo J, Moka U, Periwal V, Hara M. Islet formation during the neonatal development in mice. PLoS One 2009; 4:e7739; PMID:19893748; http://dx.doi.org/ 10.1371/journal.pone.0007739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes 2005; 54:2557-67; PMID:16123343; http://dx.doi.org/ 10.2337/diabetes.54.9.2557 [DOI] [PubMed] [Google Scholar]
- 111. Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 2007; 12:817-26; PMID:17488631; http://dx.doi.org/ 10.1016/j.devcel.2007.04.011 [DOI] [PubMed] [Google Scholar]
- 112. Berglund ED, Li CY, Poffenberger G, Ayala JE, Fueger PT, Willis SE, Jewell MM, Powers AC, Wasserman DH. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes 2008; 57:1790-9; PMID:18398139; http://dx.doi.org/ 10.2337/db07-1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ku SK, Lee HS, Lee JH. Immunohistochemical study of the pancreatic endocrine cells in the BALB/c mice: an unique distributional pattern of glucagon. J Vet Sci 2002; 3:167-73; PMID:12514327 [PubMed] [Google Scholar]
- 114. Nunemaker CS, Dishinger JF, Dula SB, Wu R, Merrins MJ, Reid KR, Sherman A, Kennedy RT, Satin LS. Glucose Metabolism, Islet Architecture, and Genetic Homogeneity in Imprinting of Ca2+i and Insulin Rhythms in Mouse Islets. PLoS One 2009; 4:e8428; PMID:20037650; http://dx.doi.org/ 10.1371/journal.pone.0008428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lewis MP, Reber HA, Ashley SW. Pancreatic blood flow and its role in the pathophysiology of pancreatitis. J Surg Res 1998; 75:81-9; PMID:9614861; http://dx.doi.org/ 10.1006/jsre.1998.5268 [DOI] [PubMed] [Google Scholar]
- 116. Wharton GK. The blood supply of the pancreas, with special reference to that of the islands of Langerhans. Anatomical Record 1932; 53:55-81; http://dx.doi.org/ 10.1002/ar.1090530108 [DOI] [Google Scholar]
- 117. Mikami Y, Otsuka A, Unno M. Surgical Vascular Anatomy and Histology. In: Beger H, Matsuno S, Cameron J, Rau B, Sunamura M, Schulick R, eds. Diseases of the Pancreas: Springer; Berlin Heidelberg, 2008:19-28 [Google Scholar]
- 118. Meyers M, Charnsangavej C, Oliphant M. Patterns of spread of disease from the pancreas. Meyers' Dynamic Radiology of the Abdomen: Springer; New York, 2011:259-74 [Google Scholar]
- 119. Woodburne RT, Olsen LL. The arteries of the pancreas. Anat Rec 1951; 111:255-70; PMID:14894836; http://dx.doi.org/ 10.1002/ar.1091110209 [DOI] [PubMed] [Google Scholar]
- 120. Bertelli E, Di Gregorio F, Bertelli L, Mosca S. The arterial blood supply of the pancreas: a review. I. The superior pancreaticoduodenal and the anterior superior pancreaticoduodenal arteries. An anatomical and radiological study. Surg Radiol Anat 1995; 17:97-106, 1-3; PMID:7597559; http://dx.doi.org/ 10.1007/BF01627566 [DOI] [PubMed] [Google Scholar]
- 121. Bertelli E, Di Gregorio F, Bertelli L, Civeli L, Mosca S. The arterial blood supply of the pancreas: a review. II. The posterior superior pancreaticoduodenal artery. An anatomical and radiological study. Surg Radiol Anat 1996; 18:1-9; PMID:8685804; http://dx.doi.org/ 10.1007/BF03207753 [DOI] [PubMed] [Google Scholar]
- 122. Bertelli E, Di Gregorio F, Bertelli L, Civeli L, Mosca S. The arterial blood supply of the pancreas: a review. III. The inferior pancreaticoduodenal artery. An anatomical review and a radiological study. Surg Radiol Anat 1996; 18:67-74; PMID:8782310; http://dx.doi.org/ 10.1007/BF01795221 [DOI] [PubMed] [Google Scholar]
- 123. Bertelli E, Di Gregorio F, Bertelli L, Orazioli D, Bastianini A. The arterial blood supply of the pancreas: a review: IV. The anterior inferior and posterior pancreaticoduodenal aa., and minor sources of blood supply for the head of the pancreas. An anatomical review and radiologic study. Surg Radiol Anat 1997; 19:203-12; PMID:9381324 [PubMed] [Google Scholar]
- 124. Bertelli E, Di Gregorio F, Mosca S, Bastianini A. The arterial blood supply of the pancreas: a review. V. The dorsal pancreatic artery. An anatomic review and a radiologic study. Surg Radiol Anat 1999; 20:445-52; http://dx.doi.org/ 10.1007/s00276-998-0445-z [DOI] [PubMed] [Google Scholar]
- 125. Cook MJ. The anatomy of the laboratory mouse. Academic Press, 1965 [Google Scholar]
- 126. Love JA, Yi E, Smith TG. Autonomic pathways regulating pancreatic exocrine secretion. Auton Neurosci 2007; 133:19-34; PMID:17113358; http://dx.doi.org/ 10.1016/j.autneu.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 127. Rodriguez-Diaz R, Abdulreda Midhat H, Formoso Alexander L, Gans I, Ricordi C, Berggren P-O, Caicedo A. Innervation Patterns of Autonomic Axons in the Human Endocrine Pancreas. Cell Metab 2011; 14:45-54; PMID:21723503; http://dx.doi.org/ 10.1016/j.cmet.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Brunicardi FC, Stagner J, BonnerWeir S, Wayland H, Kleinman R, Livingston E, Guth P, Menger M, McCuskey R, Intaglietta M, et al. Microcirculation of the islets of langerhans - long beach veterans administration regional medical education center symposium. Diabetes 1996; 45:385-92; PMID:8603757; http://dx.doi.org/ 10.2337/diab.45.4.385 [DOI] [PubMed] [Google Scholar]
- 129. Henderson JR, Daniel PM, Fraser PA. The pancreas as a single organ: the influence of the endocrine upon the exocrine part of the gland. Gut 1981; 22:158-67; PMID:6111521; http://dx.doi.org/ 10.1136/gut.22.2.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Henderson JR, Moss MC. A morphometric study of the endocrine and exocrine capillaries of the pancreas. Q J Exp Physiol 1985; 70:347-56; http://dx.doi.org/ 10.1113/expphysiol.1985.sp002920 [DOI] [PubMed] [Google Scholar]
- 131. Konstantinova I, Lammert E. Microvascular development: learning from pancreatic islets. BioEssays 2004; 26:1069-75; PMID:15382139; http://dx.doi.org/ 10.1002/bies.20105 [DOI] [PubMed] [Google Scholar]
- 132. Saito KEN, Yaginuma N, Takahashi T. Differential volumetry of A, B and D Cells in the pancreatic islets of diabetic and nondiabetic subjects. Tohoku J Exp Med 1979; 129:273-83; PMID:392812; http://dx.doi.org/ 10.1620/tjem.129.273 [DOI] [PubMed] [Google Scholar]
- 133. Nyman LR, Wells KS, Head WS, McCaughey M, Ford E, Brissova M, Piston DW, Powers AC. Real-time, multidimensional in vivo imaging used to investigate blood flow in mouse pancreatic islets. J Clin Invest 2008; 118:3790-7; PMID:18846254; http://dx.doi.org/ 10.1172/JCI36209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wayland H. Microcirculation in pancreatic function. Microsc Res Tech 1997; 37:418-33; PMID:9220421; http://dx.doi.org/10.1002/(SICI)1097-0029(19970601)37:5/6%3c418::AID-JEMT6%3e3.0.CO; 2-9 [DOI] [PubMed] [Google Scholar]
- 135. Nyman LR, Ford E, Powers AC, Piston DW. Glucose-dependent blood flow dynamics in murine pancreatic islets in vivo. Am J Physiol Endocrinol Metab 2010; 298:E807-E14; PMID:20071562; http://dx.doi.org/ 10.1152/ajpendo.00715.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Stagner JI, Samols E. The vascular order of islet cellular perfusion in the human pancreas. Diabetes 1992; 41:93-7; PMID:1345782; http://dx.doi.org/ 10.2337/diab.41.1.93 [DOI] [PubMed] [Google Scholar]
- 137. Regoli M, Bertelli E, Orazioli D, Fonzi L, Bastianini A. Pancreatic lymphatic system in rodents. Anat Rec 2001; 263:155-60; PMID:11360232; http://dx.doi.org/ 10.1002/ar.1090 [DOI] [PubMed] [Google Scholar]
- 138. O'Morchoe CCC. Lymphatic system of the pancreas. Microsc Res Tech 1997; 37:456-77; PMID:9220424; http://dx.doi.org/10.1002/(SICI)1097-0029(19970601)37:5/6%3c456::AID-JEMT9%3e3.0.CO; 2-B [DOI] [PubMed] [Google Scholar]
- 139. Navas V, O'Morchoe PJ, O'Morchoe CC. Lymphatic valves of the rat pancreas. Lymphology 1991; 24:146-54; PMID:1791725 [PubMed] [Google Scholar]
- 140. Henderson JR. Insulin in body fluids other than blood. Physiol Rev 1974; 54:1-22; PMID:4587246 [DOI] [PubMed] [Google Scholar]
- 141. Kayahara M, Nagakawa T, Ohta T, Kitagawa H, Ueno K, Tajima H, Elnemr A, Miwa K. Analysis of paraaortic lymph node involvement in pancreatic carcinoma. Cancer 1999; 85:583-90; PMID:10091731; http://dx.doi.org/10.1002/(SICI)1097-0142(19990201)85:3%3c583::AID-CNCR8%3e3.0.CO; 2-J [DOI] [PubMed] [Google Scholar]
- 142. Kayahara M, Nagakawa T, Kobayashi H, Mori K, Nakano T, Kadoya N, Ohta T, Ueno K, Miyazaki I. Lymphatic flow in carcinoma of the head of the pancreas. Cancer 1992; 70:2061-6; PMID:1327485; http://dx.doi.org/10.1002/1097-0142(19921015)70:8%3c2061::AID-CNCR2820700808%3e3.0.CO; 2-V [DOI] [PubMed] [Google Scholar]
- 143. Kayahara M, Nagakawa T, Futagami F, Kitagawa H, Ohta T, Miyazaki I. Lymphatic flow and neural plexus invasion associated with carcinoma of the body and tail of the pancreas. Cancer 1996; 78:2485-91; PMID:8952555; http://dx.doi.org/10.1002/(SICI)1097-0142(19961215)78:12%3c2485::AID-CNCR6%3e3.0.CO; 2-J [DOI] [PubMed] [Google Scholar]
- 144. Nagakawa T, Kobayashi H, Ueno K, Ohta T, Kayahara M, Miyazaki I. Clinical study of lymphatic flow to the paraaortic lymph nodes in carcinoma of the head of the pancreas. Cancer 1994; 73:1155-62; PMID:8313317; http://dx.doi.org/10.1002/1097-0142(19940215)73:4%3c1155::AID-CNCR2820730406%3e3.0.CO; 2-H [DOI] [PubMed] [Google Scholar]
- 145. Kitagawa H, Ohta T, Makino I, Tani T, Tajima H, Nakagawara H, Ohnishi I, Takamura H, Kayahara M, Watanabe H, et al. Carcinomas of the ventral and dorsal pancreas exhibit different patterns of lymphatic spread. Front Biosci 2008; 13:2728-35; PMID:17981748; http://dx.doi.org/ 10.2741/2880 [DOI] [PubMed] [Google Scholar]
- 146. Ahrén B. Autonomic regulation of islet hormone secretion – Implications for health and disease. Diabetologia 2000; 43:393-410; http://dx.doi.org/ 10.1007/s001250051322 [DOI] [PubMed] [Google Scholar]
- 147. Gilon P, Henquin J-C. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev 2001; 22:565-604; PMID:11588141 [DOI] [PubMed] [Google Scholar]
- 148. Rodriguez-Diaz R, Caicedo A. Novel approaches to studying the role of innervation in the biology of pancreatic islets. Endocrinol Metab Clin North Am 2013; 42:39-56; PMID:23391238; http://dx.doi.org/ 10.1016/j.ecl.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lindsay TH, Halvorson KG, Peters CM, Ghilardi JR, Kuskowski MA, Wong GY, Mantyh PW. A quantitative analysis of the sensory and sympathetic innervation of the mouse pancreas. Neuroscience 2006; 137:1417-26; PMID:16388907; http://dx.doi.org/ 10.1016/j.neuroscience.2005.10.055 [DOI] [PubMed] [Google Scholar]
- 150. Ushiki T, Watanabe S. Distribution and ultrastructure of the autonomic nerves in the mouse pancreas. Microsc Res Tech 1997; 37:399-406; PMID:9220419; http://dx.doi.org/10.1002/(SICI)1097-0029(19970601)37:5/6%3c399::AID-JEMT4%3e3.0.CO; 2-9 [DOI] [PubMed] [Google Scholar]
- 151. Havel PJ, Ahren B. Activation of autonomic nerves and the adrenal medulla contributes to increased glucagon secretion during moderate insulin-induced hypoglycemia in women. Diabetes 1997; 46:801-7; PMID:9133547; http://dx.doi.org/ 10.2337/diab.46.5.801 [DOI] [PubMed] [Google Scholar]
- 152. Teff KL, Mattes RD, Engelman K. Cephalic phase insulin release in normal weight males: verification and reliability. Am J Physiol 1991; 261:E430-6; PMID:1928335 [DOI] [PubMed] [Google Scholar]
- 153. Taborsky GJ, Jr., Ahren B, Havel PJ. Autonomic mediation of glucagon secretion during hypoglycemia: implications for impaired alpha-cell responses in type 1 diabetes. Diabetes 1998; 47:995-1005; PMID:9648820; http://dx.doi.org/ 10.2337/diabetes.47.7.995 [DOI] [PubMed] [Google Scholar]
- 154. Gilliam LK, Palmer JP, Taborsky GJ, Jr. Tyramine-mediated activation of sympathetic nerves inhibits insulin secretion in humans. J Clin Endocrinol Metab 2007; 92:4035-8; PMID:17684049; http://dx.doi.org/ 10.1210/jc.2007-0536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Dolenšek J, Stožer A, Skelin Klemen M, Miller EW, Slak Rupnik M. The relationship between membrane potential and calcium dynamics in glucose-stimulated beta cell syncytium in acute mouse pancreas tissue slices. PLoS One 2013; 8:e82374; http://dx.doi.org/ 10.1371/journal.pone.0082374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Takahashi N, Kishimoto T, Nemoto T, Kadowaki T, Kasai H. Fusion pore dynamics and insulin granule exocytosis in the pancreatic islet. Science 2002; 297:1349-52; PMID:12193788; http://dx.doi.org/ 10.1126/science.1073806 [DOI] [PubMed] [Google Scholar]
- 157. Low J, Mitchell J, Do O, Bax J, Rawlings A, Zavortink M, Morgan G, Parton R, Gaisano H, Thorn P. Glucose principally regulates insulin secretion in mouse islets by controlling the numbers of granule fusion events per cell. Diabetologia 2013; 56:2629-37; PMID:23995471; http://dx.doi.org/ 10.1007/s00125-013-3019-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Chiu YC, Hua TE, Fu YY, Pasricha PJ, Tang SC. Three-D imaging and illustration of the perfusive mouse islet sympathetic innervation and its remodelling in injury. Diabetologia 2012; 55:3252-61; PMID:22930160; http://dx.doi.org/ 10.1007/s00125-012-2699-6 [DOI] [PubMed] [Google Scholar]
- 159. Rodriguez-Diaz R, Speier S, Molano RD, Formoso A, Gans I, Abdulreda MH, Cabrera O, Molina J, Fachado A, Ricordi C, et al. Noninvasive in vivo model demonstrating the effects of autonomic innervation on pancreatic islet function. Proc Natl Acad Sci 2012; 109:21456-61; http://dx.doi.org/ 10.1073/pnas.1211659110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Speier S, Nyqvist D, Cabrera O, Yu J, Molano RD, Pileggi A, Moede T, Kohler M, Wilbertz J, Leibiger B, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med 2008; 14:574-8; PMID:18327249; http://dx.doi.org/ 10.1038/nm1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Meneghel-Rozzo T, Rozzo A, Poppi L, Rupnik M. In vivo and in vitro development of mouse pancreatic ß-cells in organotypic slices. Cell Tissue Res 2004; 316:295-303; PMID:15085425; http://dx.doi.org/ 10.1007/s00441-004-0886-6 [DOI] [PubMed] [Google Scholar]
- 162. Richins CA. The innervation of the pancreas. J Comp Neurol 1945; 83:223-36; PMID:21009099; http://dx.doi.org/ 10.1002/cne.900830303 [DOI] [PubMed] [Google Scholar]
- 163. Yi SQ, Miwa K, Ohta T, Kayahara M, Kitagawa H, Tanaka A, Shimokawa T, Akita K, Tanaka S. Innervation of the pancreas from the perspective of perineural invasion of pancreatic cancer. Pancreas 2003; 27:225-9; PMID:14508126; http://dx.doi.org/ 10.1097/00006676-200310000-00005 [DOI] [PubMed] [Google Scholar]
- 164. Tiscornia OM. The neural control of exocrine and endocrine pancreas. Am J Gastroenterol 1977; 67:541-60; PMID:20775 [PubMed] [Google Scholar]
- 165. Honjin R. The innervation of the pancreas of the mouse, with special reference to the structure of the peripheral extension of the vegetative nervous system. J Comp Neurol 1956; 104:331-71; PMID:13357632; http://dx.doi.org/ 10.1002/cne.901040302 [DOI] [PubMed] [Google Scholar]
- 166. Su HC, Bishop AE, Power RF, Hamada Y, Polak JM. Dual intrinsic and extrinsic origins of CGRP- and NPY-immunoreactive nerves of rat gut and pancreas. J Neurosci 1987; 7:2674-87; PMID:2442325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Kayahara M, Nakagawara H, Kitagawa H, Ohta T. The nature of neural invasion by pancreatic cancer. Pancreas 2007; 35:218-23; PMID:17895841; http://dx.doi.org/ 10.1097/mpa.0b013e3180619677 [DOI] [PubMed] [Google Scholar]
- 168. Makino I, Kitagawa H, Ohta T, Nakagawara H, Tajima H, Ohnishi I, Takamura H, Tani T, Kayahara M. Nerve plexus invasion in pancreatic cancer: spread patterns on histopathologic and embryological analyses. Pancreas 2008; 37:358-65; PMID:18972625; http://dx.doi.org/ 10.1097/MPA.0b013e31818166e6 [DOI] [PubMed] [Google Scholar]
- 169. Ahren B, Veith RC, Taborsky GJ, Jr. Sympathetic nerve stimulation versus pancreatic norepinephrine infusion in the dog: 1). Effects on basal release of insulin and glucagon. Endocrinology 1987; 121:323-31; PMID:3297643; http://dx.doi.org/ 10.1210/endo-121-1-323 [DOI] [PubMed] [Google Scholar]
- 170. Dunning BE, Scott MF, Neal DW, Cherrington AD. Direct quantification of norepinephrine spillover and hormone output from the pancreas of the conscious dog. Am J Physiol 1997; 272:E746-55; PMID:9176171 [DOI] [PubMed] [Google Scholar]
- 171. Mundinger TO, Verchere CB, Baskin DG, Boyle MR, Kowalyk S, Taborsky GJ, Jr. Galanin is localized in sympathetic neurons of the dog liver. Am J Physiol 1997; 273:E1194-202; PMID:9435536 [DOI] [PubMed] [Google Scholar]
- 172. Molina J, Rodriguez-Diaz R, Fachado A, Jacques-Silva MC, Berggren PO, Caicedo A. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes 2014; 63:2714-26; PMID:24658304; http://dx.doi.org/ 10.2337/db13-1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Ahren B, Taborsky GJ, Jr. The mechanism of vagal nerve stimulation of glucagon and insulin secretion in the dog. Endocrinology 1986; 118:1551-7; PMID:3512257; http://dx.doi.org/ 10.1210/endo-118-4-1551 [DOI] [PubMed] [Google Scholar]
- 174. Rodriguez-Diaz R, Dando R, Jacques-Silva MC, Fachado A, Molina J, Abdulreda MH, Ricordi C, Roper SD, Berggren PO, Caicedo A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat Med 2011; 17:888-92; PMID:21685896; http://dx.doi.org/ 10.1038/nm.2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Rodriguez-Diaz R, Menegaz D, Caicedo A. Neurotransmitters act as paracrine signals to regulate insulin secretion from the human pancreatic islet. J Physiol 2014; 592:3413-3417 PMID:24591573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Eberhard D, Kragl M, Lammert E. 'Giving and taking': endothelial and beta-cells in the islets of Langerhans. Trends Endocrinol Metab 2010; 21:457-63; PMID:20359908; http://dx.doi.org/ 10.1016/j.tem.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 177. Eberhard D, Lammert E. The pancreatic beta-cell in the islet and organ community. Curr Opin Genet Dev 2009; 19:469-75; PMID:19713099; http://dx.doi.org/ 10.1016/j.gde.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 178. Kohler CU, Olewinski M, Tannapfel A, Schmidt WE, Fritsch H, Meier JJ. Cell cycle control of beta-cell replication in the prenatal and postnatal human pancreas. Am J Physiol Endocrinol Metab 2011; 300:E221-30; PMID:20978233; http://dx.doi.org/ 10.1152/ajpendo.00496.2010 [DOI] [PubMed] [Google Scholar]
- 179. Shu L, Zien K, Gutjahr G, Oberholzer J, Pattou F, Kerr-Conte J, Maedler K. TCF7L2 promotes beta cell regeneration in human and mouse pancreas. Diabetologia 2012; 55:3296-307; PMID:22945304; http://dx.doi.org/ 10.1007/s00125-012-2693-z [DOI] [PubMed] [Google Scholar]
- 180. Rankin MM, Kushner JA. Aging induces a distinct gene expression program in mouse islets. Islets 2010; 2:345-52; PMID:21099336; http://dx.doi.org/ 10.4161/isl.2.6.13376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Szabat M, Lynn FC, Hoffman BG, Kieffer TJ, Allan DW, Johnson JD. Maintenance of beta-cell maturity and plasticity in the adult pancreas: developmental biology concepts in adult physiology. Diabetes 2012; 61:1365-71; PMID:22618775; http://dx.doi.org/ 10.2337/db11-1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. MacDonald MJ, Longacre MJ, Stoker SW, Kendrick M, Thonpho A, Brown LJ, Hasan NM, Jitrapakdee S, Fukao T, Hanson MS, et al. Differences between Human and Rodent Pancreatic Islets: low pyruvate carboxylase, atp citrate lyase, and pyruvate carboxylation and high glucose-stimulated acetoacetate in human pancreatic islets. J Biol Chem 2011; 286:18383-96; PMID:21454710; http://dx.doi.org/ 10.1074/jbc.M111.241182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Fox JE, Seeberger K, Dai XQ, Lyon J, Spigelman AF, Kolic J, Hajmrle C, Joseph JW, Kin T, Shapiro AM, et al. Functional plasticity of the human infant beta-cell exocytotic phenotype. Endocrinology 2013; 154:1392-9; PMID:23449893; http://dx.doi.org/ 10.1210/en.2012-1934 [DOI] [PubMed] [Google Scholar]
- 184. Menge BA, Tannapfel A, Belyaev O, Drescher R, Müller C, Uhl W, Schmidt WE, Meier JJ. Partial Pancreatectomy in Adult Humans Does Not Provoke beta-Cell Regeneration. Diabetes 2008; 57:142-9; PMID:17959931; http://dx.doi.org/ 10.2337/db07-1294 [DOI] [PubMed] [Google Scholar]
- 185. Fellmann L, Nascimento AR, Tibirica E, Bousquet P. Murine models for pharmacological studies of the metabolic syndrome. Pharmacol Ther 2013; 137:331-40; PMID:23178510; http://dx.doi.org/ 10.1016/j.pharmthera.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 186. Yip L, Fathman CG. Type 1 diabetes in mice and men: gene expression profiling to investigate disease pathogenesis. Immunol Res 2014; 58:340-50; PMID:24682832; http://dx.doi.org/ 10.1007/s12026-014-8501-8 [DOI] [PubMed] [Google Scholar]
- 187. Chandrasekera PC, Pippin JJ. Of rodents and men: species-specific glucose regulation and type 2 diabetes research. Altex 2014; 31:157-76; PMID:24270692; http://dx.doi.org/ 10.14573/1309231 [DOI] [PubMed] [Google Scholar]