Abstract
We have previously reported that human endogenous retrovirus-K (HERV-K) envelope (env) protein is a tumor-associated antigen (TAA) for cancer vaccines, and that its antibodies (mAbs) possess antitumor activity against cancer. In this study, a chimeric antigen receptor (CAR) specific for HERV-K env protein (K-CAR) was generated using anti-HERV-K mAb. K-CAR T cells from peripheral blood mononuclear cells (PBMCs) of 9 breast cancer (BC) patients and 12 normal donors were able to inhibit growth of, and to exhibit significant cytotoxicity toward, BC cells but not MCF-10A normal breast cells. The antitumor effects in cancer cells were significantly reduced when control T cells were used, or the expression of HERV-K was knocked down by an shRNA. Secretion of multiple cytokines, including IFNγ, TNF-α, and IL-2, was significantly enhanced in culture media of BC cells treated with K-CARs. Significantly reduced tumor growth and tumor weight was observed in xenograft models bearing MDA-MB-231 or MDA-MB-435.eB1 BC cells. Importantly, the K-CAR prevented tumor metastasis to other organs. Furthermore, downregulation of HERV-K expression in tumors of mice treated with K-CAR correlated with upregulation of p53 and downregulation of MDM2 and p-ERK. Importantly, the expression of HERV-K env protein in metastatic tumor tissues treated with K-CAR T cells correlated with the expression of Ras. Our results indicate that HERV-K env protein is an oncoprotein and may play an important role in tumorigenesis related to p53 and Ras signaling pathways. Anti-HERV-K treatment, including K-CAR treatment, shows potential for immunotherapy of BC.
Keywords: breast cancer, chimeric antigen receptor, HERV-K, metastasis, and Ras
Introduction
Clinical trials for a variety of malignant diseases have shown that T-cell therapy may be effective and even curative for some patients.1 Many TAAs have been identified, and their ability to induce antitumor T-cell immunity has been documented in clinical studies.2 A promising approach in cancer treatment is adoptive immunotherapy using CAR-engineered T cells to redirect specificity toward a particular TAA3-5 in a manner independent of the major histocompatibility complex. Targeting solid tumors is more challenging because of rare target antigens, poor T cell trafficking to the tumor site, and less cytotoxicity in the local tumor immunosuppressive microenvironment.6 CARs have been used in only a few cases for treatment of solid tumors, and efficacy has not been great.6 CAR-based therapy of solid tumors has included the use of CARs targeting colorectal cancer,7 ovarian cancer,8 BC,9 prostate cancer,10 and metastatic renal cell carcinoma.11 One of main challenges in the field of T-cell engineering aimed at optimizing T lymphocyte function is receptor specificity, as engineered T cells endowed with high-affinity receptors proved significantly toxic when TAAs were targeted that are also expressed, even at a low level, on normal tissue: so-called ‘on-target’ toxicity.
Genome sequencing reveals that 8% of the human genome consists of human endogenous retroviruses (HERVs) and roughly half of our DNA is made up of transposable elements that include HERVs.12-14 HERV type K of the HML2 subtype is the most recently integrated and most intact retrovirus in the human genome.15 These most recently acquired proviruses of the HERV-K family can express viral proteins and produce viral particles.16,17 In previous studies we reported that expression of HERV-K env protein in malignant BC cells was substantially higher than in normal or non-malignant breast cells,18-20 suggesting that HERV-K might be reactivated and implicated in carcinogenesis. Monoclonal and single-chain antibodies against the HERV-K env protein recently proved capable of blocking the proliferation of human BC cells in vitro and inhibiting tumor growth in mice bearing xenograft tumors.21 In addition, we found that HERV-K env protein is capable of acting as a TAA, activating both T-cell and B-cell responses in BC patients22 and ovarian cancer patients.23 These data indicate that HERV-K env protein may be a good candidate for CAR development.
Here we genetically modified T cells using the Sleeping Beauty (SB) system to stably introduce a single-chain variable fragment (scFv: G11D10)21 generated from an anti-HERV-K monoclonal antibody (mAb: 6H5)22 to produce an HERV-K specific CAR (K-CAR). Antitumor effects of K-CAR were demonstrated in vitro and in vivo.
Results
Expression of HERV-K env protein in breast cancer cells and human cancer tissues
The expression of HERV-K env protein on the BC cell membrane and cytoplasm was observed in three BC cell lines to a greater extent than in two non-malignant breast cell lines by flow cytometry ((fluorescence-activated cell sorting (FACS); Fig. S1A), immunofluorescence (Fig. S1B), immunohistochemistry (IHC; Fig. S1C), and immunoblot (Fig. S1D) using anti-HERV-K mAb 6H5,22 confirming our findings from previous studies.18-22,24 Expression of HERV-K env protein was increased 4–6-fold in BC cells, compared to the level of expression in non-malignant breast cell lines, with normalization to β-actin. MDA-MB-231 and MDA-MB-435.eB1 BC cells were used as targets for adoptive T therapy in subsequent studies. IHC was used to detect the expression of HERV-K in various human cancer tissues and normal tissues in a single tissue array (n = 214; Table 1). These data provide strong evidence that HERV-K env protein is expressed in only cancer cells and tissues, but not in normal cells and tissues. HERV-K expression in normal tissues, which we did not observe, could be associated with off-target toxicity and compromise its use in immunotherapy.
Table 1.
The expression profiles of HERV-K proteins
| Tissues | Negative | Positive | N |
|---|---|---|---|
| Brain tumor | 21 (100%) | 0 | 21 |
| Breast AdCa* | 3 (7.14%) | 39 (92.86%) | 42 |
| Colonic AdCa | 0 | 12 (100%) | 12 |
| Lung cancer | 4 (9.76%) | 37 (90.24%) | 41 |
| Lymphoma | 33 (82.5%) | 7 (17.5%) | 40 |
| Melanoma | 4 (23.53%) | 13 (76.47%) | 17 |
| Prostate AdCa | 0 | 3 (100%) | 3 |
| Normal tissues (33 organs) | 38 (100%) | 38 | |
| Total cases | 214 |
AdCa: Adenocarcinoma.
HERV-K specific CAR construction and propagation
6H5 mAb and its scFv were previously shown to exhibit specificity and sensitivity in detecting and binding HERV-K env protein on BC cell lines,21 and scFv were used for generation of K-CAR (Fig. S2A), as described previously.25 K562 cells expressing HERV-K env gene in a lentiviral vector (Fig. S2B) were used as artificial antigen-presenting cells (aAPCs) to propagate CD3+ T cells expressing K-CAR. The expression of HERV-K env in K562 cells was demonstrated by reverse transcription-polymerase chain reaction (RT-PCR) (Fig. S2C, left panel), immunoblot (Fig. 2C, right panel), and FACS using 6H5 mAb (Fig. S2D).
In vitro expansion of K-CAR T cells originating from BC patients and normal female donors
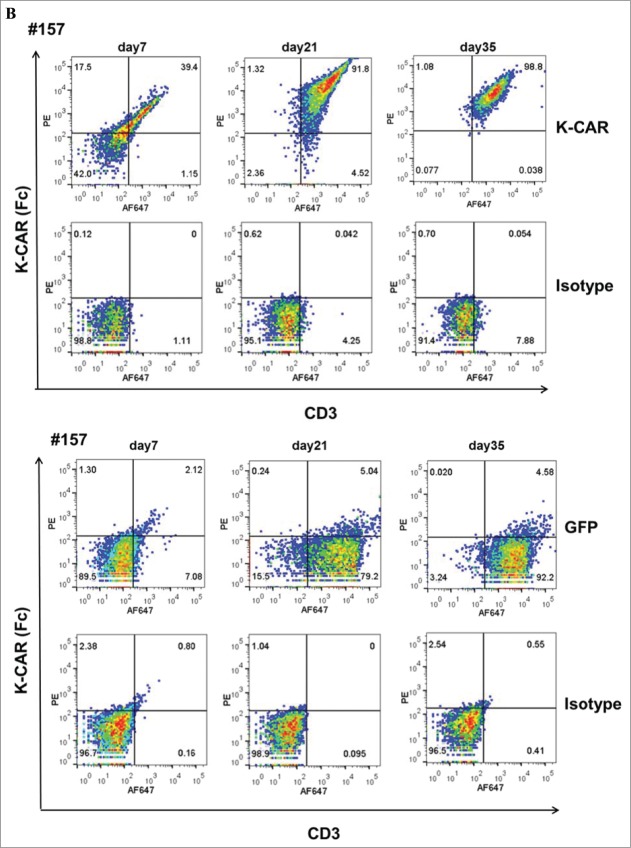
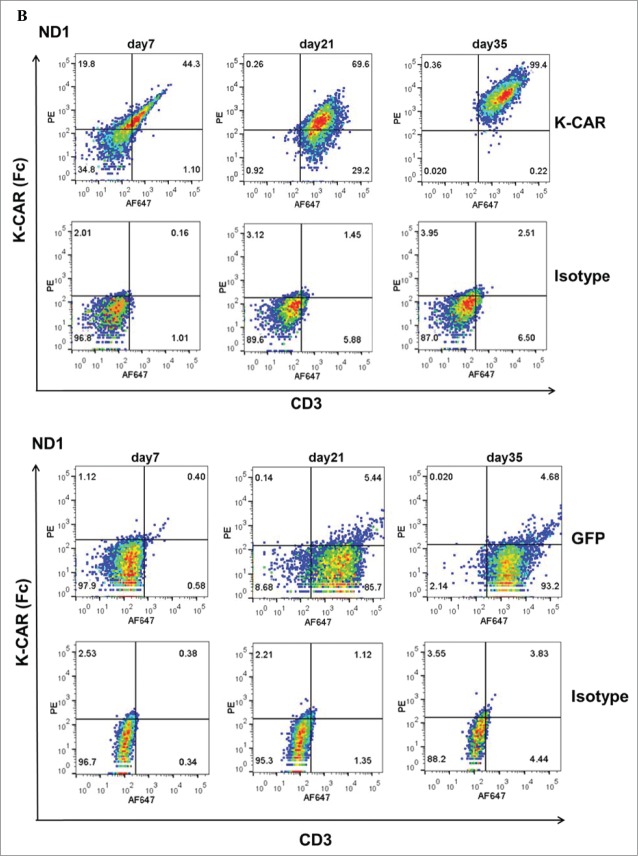
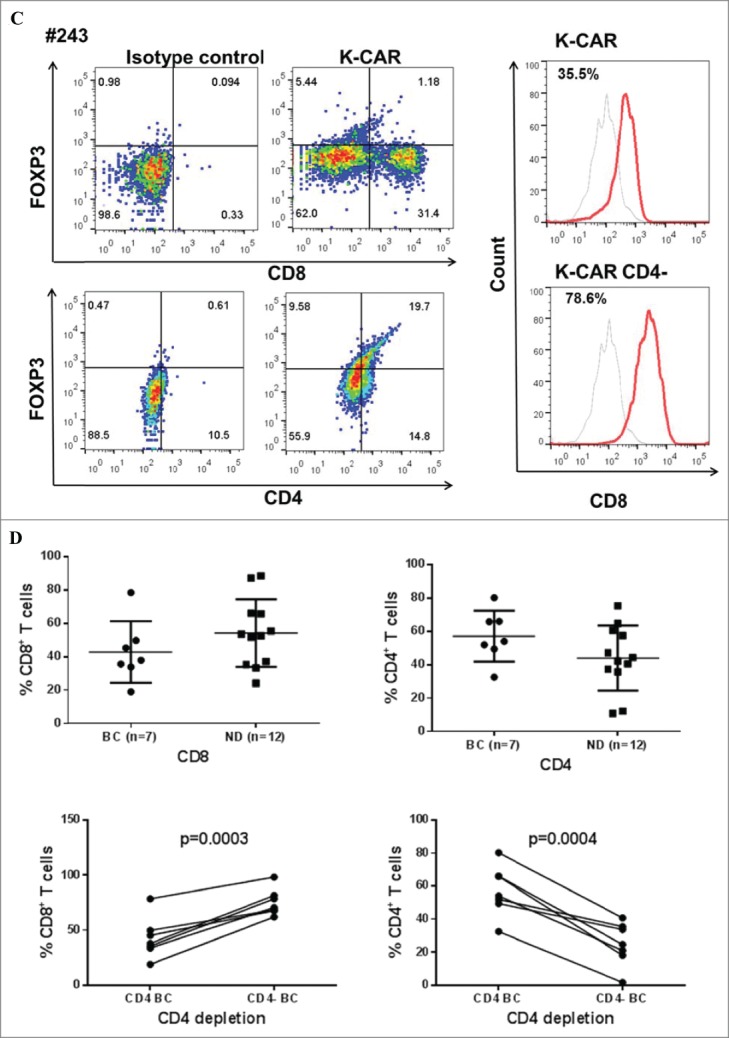
PBMCs from 9 BC patients (Table 2) and 12 normal female donors (NDs) were electroporated with HERV-KCD28MZ SB transposon (pSBSO) along with pCMV-SB11 transposase and cultured with IL-2 to expand CD3+ T cells expressing K-CAR. scFv expression in K-CAR T cells from various donors (BC: n = 9 and ND: n = 12) was further confirmed by RT-PCR using primers specific to the 6H5 scFv (Fig. 1A), and growth of T cells was monitored over time by microscopy (Fig. S3A, top panel). The percentage of K-CAR T cells generated from PBMCs was determined post-electroporation by FACS using anti-CD3 and anti-Fc antibodies. Sample FACS results are shown in Fig. 1B. K-CAR T cells from patient #157 had a significantly lower proliferation rate than cells from ND1 (Fig. S3A). Nearly all T cells from BC patients as well as NDs expressed the K-CAR on day 28 post-electroporation, as measured by expression of the Fc backbone (Fig. S3B). Specific lysis of K562-HERV-K cells by K-CAR T cells obtained from two normal donors was observed, as determined by a cytotoxic T lymphocyte (CTL) assay (Fig. S3C). Percentages of CD4+ and CD8+ T cells as well as T regulatory cells (Tregs: FOXP3 and CD25 positive) were determined. A sample from a BC patient (#243) revealed a higher percentage of FOXP3 in CD4+ T cells than in CD8+ T cells (Fig. 1C, left panel), and increased percentages of CD8+ T cells were observed after CD4+ depletion (Fig. 1C, right panel). Higher percentages of both FOXP3+ and CD25+ T cells were demonstrated in K-CAR obtained from the BC patient than from ND1 control (Fig. S3D). Higher percentages of CD4+ than CD8+ T cells were observed in BC patients (n = 7) compared with NDs (n = 12), but the differences were not significant (Fig. 1D, top panel). CD4+ cell T depletion resulted in significantly enhanced percentages of CD8+ and significantly decreased percentages of CD4+ T cells in K-CAR T cells from BC patients (Fig. 1D, bottom panel).
Table 2.
Demographic, medical, and independent variables measured at baseline of breast cancer
| Acc.* | Age/Race | Diagnosis | Stage | ER§ | PR§ | Her2 | NG | Mastectomy | FAC‖ | Neoadjuvant | Taxol | Radiation | Endocrinotherapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 37 | 37bf† | IDC‡ | III | pos§ | pos | pos | 3 | Yes | |||||
| 78 | 61hf† | IDC | IIIA | Pos | pos | neg | ? | Yes | |||||
| 108 | 55wf† | Mets IDC‡ | IIIB | Pos | pos | neg | 3 | Yes | Yes | Yes | Yes | ||
| 157 | 59wf | DCIS‡ | I | Pos | pos | N/A | 2 | Yes | |||||
| 229 | 54hf | IDC | I | neg§ | neg | neg | 3 | Yes | Yes | Yes | Yes | ||
| 233 | 37hf | IDC | IIA | Neg | neg | neg | 3 | Yes | Yes | Yes | Yes | ||
| 237 | 58af† | DCIS | I | Pos | pos | N/A | ? | Yes | |||||
| 243 | 56bf | DCIS | I | Neg | neg | neg | 3 | Yes | |||||
| 257 | 69wf | ILC‡ | IIA | Pos | pos | pos | 3 | Yes | Yes | ||||
| 261 | 41wf | DCIS+IDC | ? | Pos | pos | neg | 2 | Yes | Yes | Yes | Yes | ||
| 267 | 57wf | ILC | IIA | Pos | pos | neg | 2 | Yes | Yes | Yes | Yes |
Acc: accession number.
wf: white female; hf: hispanic female; af: asian female; bf: black female.
IDC: invasive ductal carcinoma; Mets IDC: metastatic IDC; DCIS: ductal carcinoma in situ; ILC: invasive lobular carcinoma.
ER: estrogen receptor; PR: progesterone receptor; pos: positive; neg: negative.
FAC: fluorouracil 500 mg/m2, doxorubicin 50 mg/m2, and cyclophosphamide 500 mg/m2.
Figure 1 .
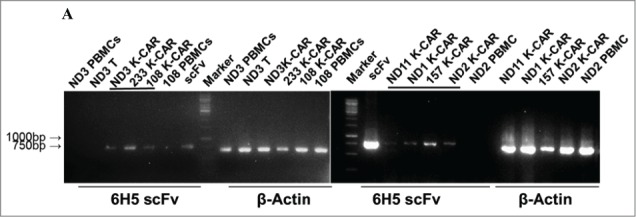
Characterization of K-CAR in various donors. (A) RT-PCR was employed to detect the expression of 6H5 scFv (700 bp) using scFv specific primers. Amplified β-actin was used as a loading control, and scFv plasmid was used as a positive control. Expression of 6H5 scFv was demonstrated in K-CAR T cells obtained from BC patients and NDs. No scFv expression was detected in control T cells or PBMCs. (B) Both Fc+ and CD3+ T cell populations were determined in T cells transfected with K-CAR or GFP from patient 157 (top panel) and ND1 (bottom panel) by FACS using anti-Fc and CD3 antibodies on days 7, 21 and 35 post-transfection. The isotype alone was used as control. (C) Both FOXP3+ and CD8+ or CD4+ T cells from BC patient 243 were determined by FACS (left panel). The percentage of CD8+ T cells was increased in K-CAR T cells after CD4+ depletion (right panel). (D) Lower percentages of CD8+ and higher percentages of CD4+ T cells were demonstrated in K-CAR T cells obtained from BC patients than from NDs (top panel). Significantly enhanced CD8+ (P = 0.0003) and reduced CD4+ (P = 0.0004) T cell populations were demonstrated in T cells from BC patients (n = 7) after CD4 depletion.
Figure 1.
(Continued)
Figure 1.
(Continued)
Figure 1.
(Continued)
Antitumor effects of K-CAR in vitro
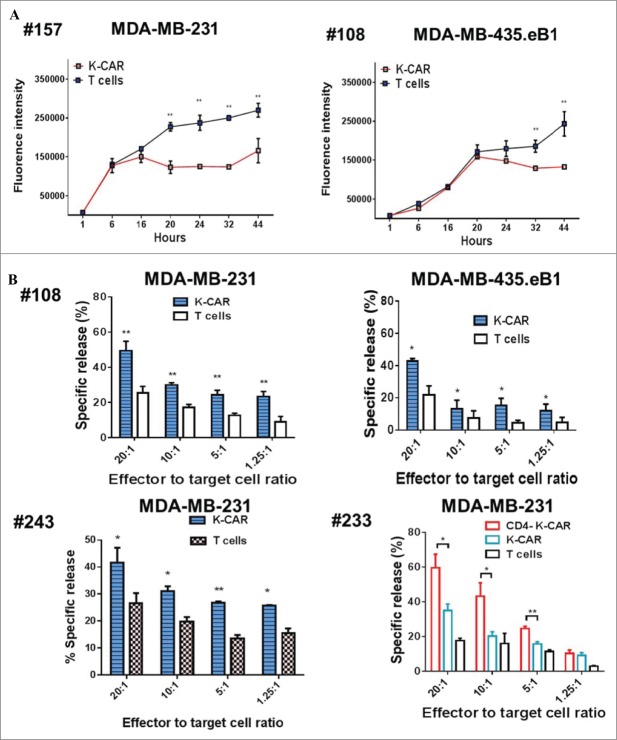
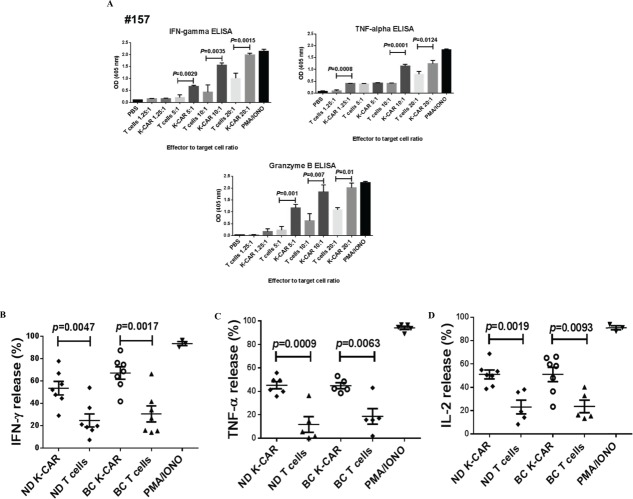
Antitumor effects were evaluated in two BC lines treated in vitro with K-CAR+ T cells from BC patients #157 or #108. Significantly reduced growth was observed in two BC cell lines treated with K-CAR T cells from both BC patients (Fig. 2A).
Figure 2 .
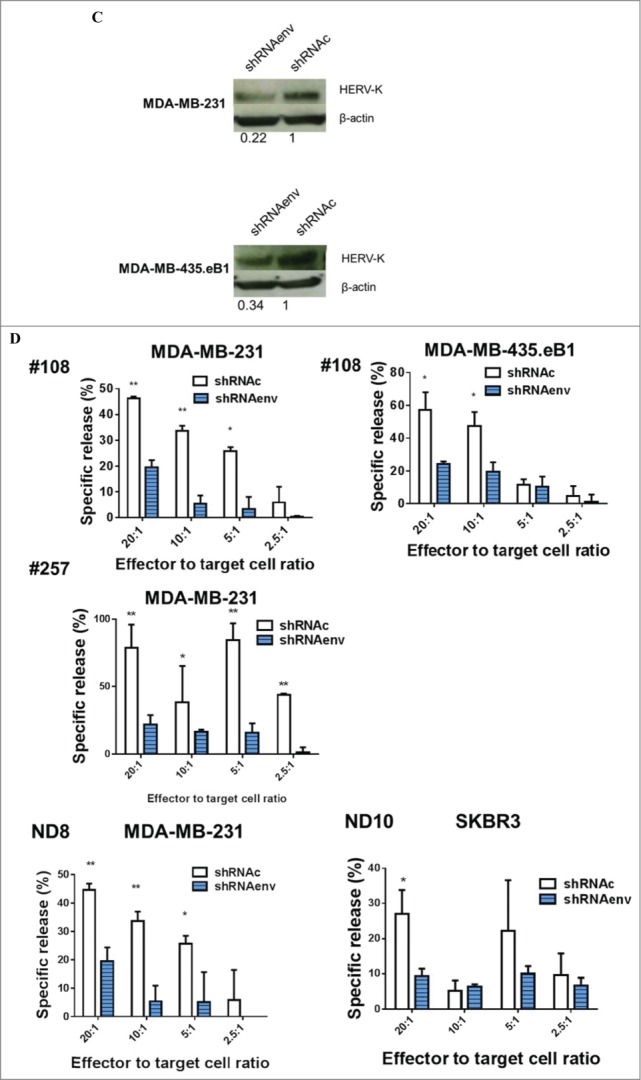
Detection of antitumor effects in vitro. (A) Inhibition of BC cell growth was observed when these cells were treated with K-CARs from patients (157 and 108), using the Alamar Blue assay. (B) CTL assays were employed to determine the cytotoxicity of K-CAR toward BC cells at various ratios of effector to target. Significantly greater lysis was demonstrated in both cell lines using K-CAR from patient 108 (top panel) and 243 (bottom-left panel). Enhanced specific lysis was observed in MDA-MB-231 cells using K-CAR from patient 233 with CD4+ T depletion (CD4-K-CAR), compared to no depletion of CD4+ T cells (K-CAR; bottom-right panel). (C) The expression of HERV-K was determined in BC cell lines transduced with a shRNAenv or control shRNAc by immunoblot assays using 6H5 mAb. Expression of HERV-K protein was downregulated by approximately 70–80%, compared to the expression level of shRNAc transduced cells. (D) The specific lysis of K-CAR T cells obtained from patients 108 and 257 or normal female donors was reduced in BC target cells stably transduced with shRNAenv compared with shRNAc. This result indicates that specific lysis is antigen-dependent.
Figure 2.
(Continued)
CTL assays were further employed to analyze the cytotoxicity of K-CAR T cells. Specific lysis was significantly greater in BC cell lines treated with K-CAR T cells when compared to treatment with control (wild-type) T cells generated from patient #108 (metastasis IDC) and #243 (a triple negative DCIS; Fig. 2B). Enhanced specific lysis was observed in BC cell lines treated with K-CAR T cells with depletion of CD4+ T cells when compared to treatment without depletion from cells derived from a triple negative invasive ductal carcinoma (IDC) patient #233. To further test the specificity for HERV-K, the HERV-K env RNA was knocked down in both cell lines using an shRNA targeted to HERV-K env (shRNAenv) using a pGreenPuro vector (Fig. S4A). An immunoblot analysis showed about 70–80% knockdown in HERV-K protein levels of shRNAenv treated cells compared to cells stably transduced with scrambled shRNAc (Fig. 2C). The specific lysis of BC cells by K-CAR T cells from BC patients (#108 and #257) was significantly reduced after knockdown of HERV-K env RNA (Fig. 2D, top panel). Reduced cytotoxicity of K-CAR toward BC cell lines was also observed after shRNA knockdown in K-CARs generated from NDs (ND8 and ND10 Fig. 2D, bottom panel). These results suggest that the potency of K-CAR T cells in eliminating tumors in vitro depends on antigen being specifically expressed on the surface of BC cells.
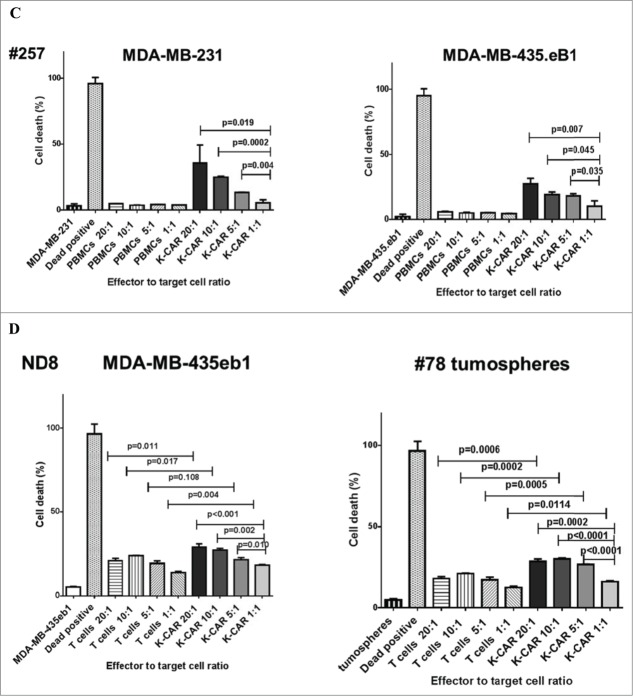
The specific killing capability of K-CAR was further tested on various breast cell lines using the co-stained Live/Dead Viability Assay, using 5:1 or 20:1 ratios of K-CAR T cells to target cells that included BC cell lines and MCF-10A breast cells (Fig. 3A, quantitated in Fig. 3B). A significantly larger percentage of dead cells (red fluorescence) was observed for BC cell lines than for MCF-10A cells (Fig. S4B). Significantly increased killing of BC cells was observed for K-CAR from a BC patient (#257) in comparison to the patient's autologous PBMC cells, with a higher ratio of K-CAR cells to target cells leading to a higher percentage of target cell death (Fig. 3C) by FACS using the cell-impermeant viability indicator ETHD1. Significantly increased killing of BC cells was also observed for K-CAR from a normal donor (ND8) in comparison to her autologous T cells, with a higher ratio of K-CAR cells to target cells leading to a higher percentage of target cell death (Fig. 3D). HERV-K-CAR redirected T cells mediated potent effector activity against HERV-K+ BC cell lines as well as a primary patient sample cultured as tumorspheres from a biopsy (patient 78).
Figure 3 .
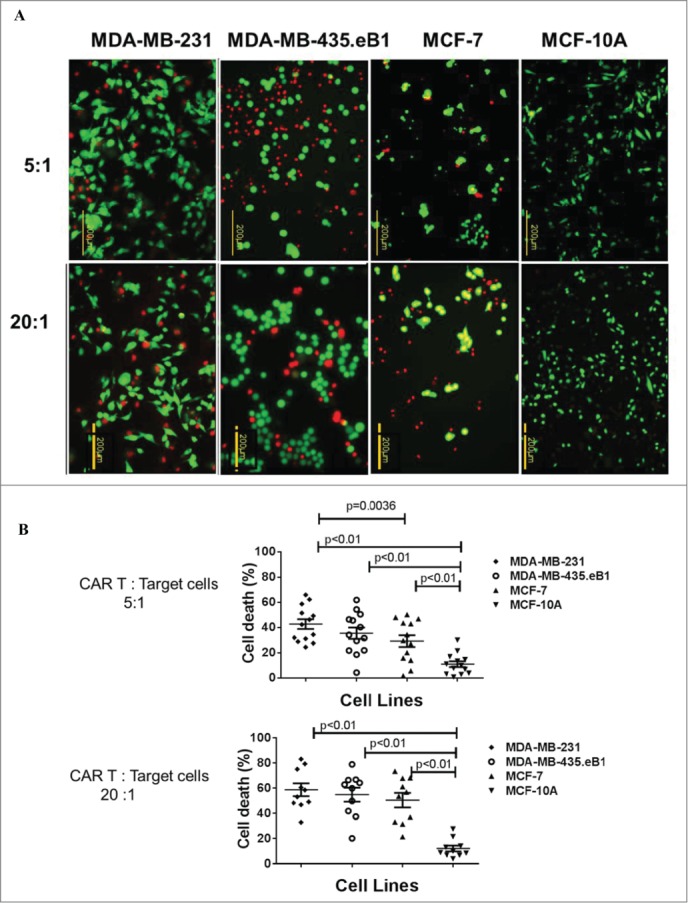
Specificity of cell death assay of K-CAR. (A) Target cells were loaded with K-CAR (5:1 ratio of K-CAR T cells to target cells, top panel; or 20:1 ratio, bottom panel) for 4 h. Live cells (green color) and putative dead cells (red color) were identified using the co-stained Live/Dead Viability Assay. EthD-1 penetrates cells with membrane damage and binds to nucleic acids to produce red fluorescence in dead cells. (B) Significantly greater numbers of dead cells were found in K-CAR-treated BC cells compared with MCF-10A after random counts of eight fields per well. Cell death was greatest in BC cells that had higher expression of HERV-K env protein (MDA-MB-231>MDA-MB-435.eB1>MCF-7>MCF-10A). (C) Cell viability was also analyzed by FACS. The percentage of dead target cells increased with the ratio of K-CAR T cells to target cells for patient 257 (Fig. 3C) or ND8 (Fig. 3D), but not with the ratio of PBMC (Fig. 3C) or control T cells (Fig. 3D) to target cells. Primary tumorspheres grown from a tumor biopsy of patient 78 were used as target cells in Fig. 3D (right panel). Target cells treated with 0.3% Triton X-100 detergent were used as positive controls for cell death.
Figure 3.
(Continued)
Quantification of cytokine release
Release of IFNγ, TNF-α, and Granzyme B, assessed by ELISA (Fig. 4A), was greater from BC target cells mixed with K-CAR T cells from a BC patient (#157) than when mixed with control T cells obtained from the same patient. Released intracellular cytokine levels, determined using flow cytometry, were greater after 4-h stimulation of MDA-MB-231 target cells at a 20:1 ratio with K-CAR T cells from BC patient 243 or from a ND1 than after stimulation with the corresponding T cells from these subjects (Fig. S4C). Significantly increased levels of IFNγ (Fig. 4B), TNF-α (Fig. 4C) and IL-2 (Fig. 4D) were released by target cells after K-CAR T cell stimulation compared with control T cells, but there was not a significant difference in cytokine stimulation when K-CAR T cells obtained from BC patients were compared with K-CAR T cells from NDs. These data strengthen the previous observation that K-CAR T cells stimulate Th1 cytokine secretion, which may promote killing of target cells.
Figure 4.
Cytokine release. (A) An ELISA assay was used to detect cytokine release from BC cells treated with K-CAR or control T cells. Significantly enhanced IFNγ, TNF-α, and Granzyme B release into the culture media was demonstrated in target cells treated with K-CAR compared to treatment with control T cells generated from BC patient 157. A greater release of cytokine was detected as the ratio of target to effector increased. PMA/IONO was used as a positive control to stimulate maximal cytokine release. The percentage of cytokine release in target cells treated with K-CAR from BC patients or from normal female donors was determined by FACS assays using antibodies for various cytokines. Significantly enhanced release of IFNγ (B), TNF-α (C), and IL-2 (D) was observed from target cells treated with K-CAR T cells compared with control T cells. No significant difference was found between target cells treated with K-CAR T cells from BC patients or normal female donors.
Antitumor effects of K-CAR T cells in vivo
Antitumor effects of K-CAR+ T cells were evaluated using human tumor xenograft mouse models. Significantly reduced tumor growth (Fig. 5A), tumor sizes (Fig. 5B, top panel) and tumor weights (Fig. 5B, bottom panel) were observed in mice bearing BC cells treated with K-CAR T cells compared to control T or no treatment (blank). Tumor sizes and weights were determined. Significantly reduced tumor sizes and tumor weights were observed in mice bearing MDA-MB-435.eB1 cells treated with K-CAR, compared to treatment with PBMCs with or without IL-2, or IL-2 treatment only (Fig. S4D).
Figure 5 .
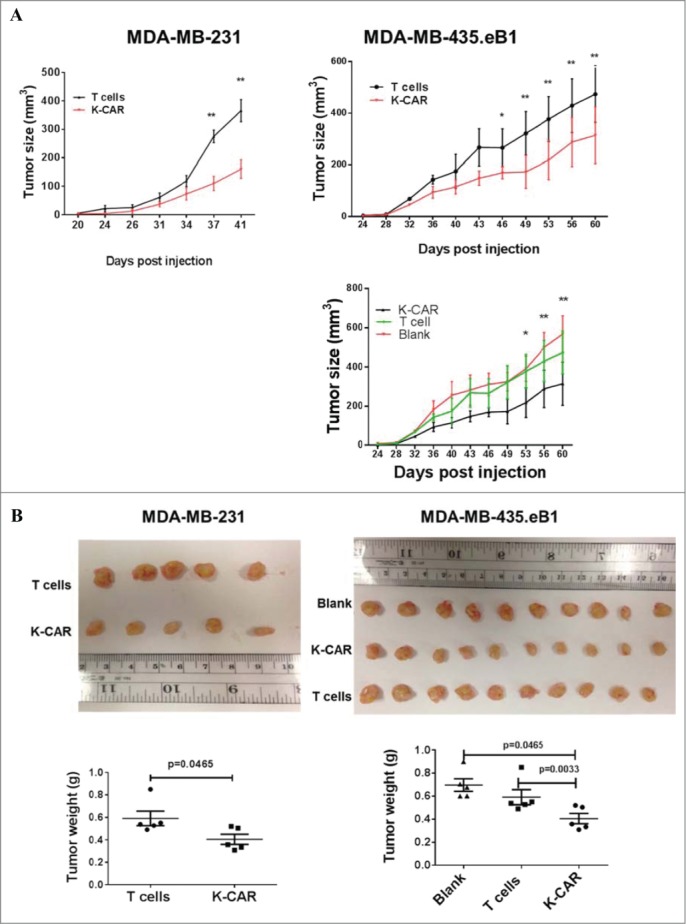
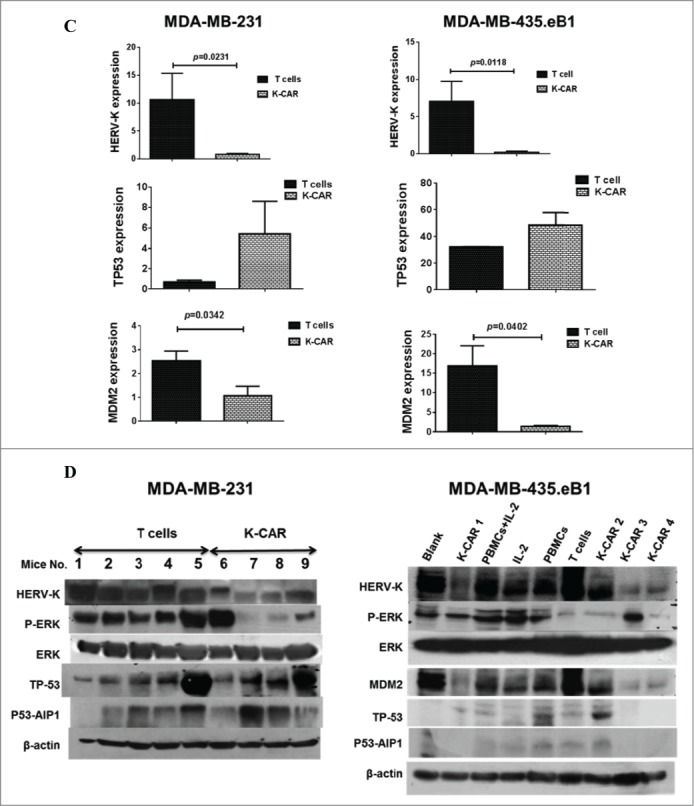
Antitumor effect in vivo and mechanism. (A) Mice were inoculated with BC cells and were untreated (black tracing), or treated with control T cells (T cells) or K-CAR T cells (K-CAR) on post-injection days 5, 13 and 21. Significantly reduced tumor growth was demonstrated in mice treated with K-CAR compared to treatment with control T cells or no treatment. (B) Tumor weights were significantly reduced in mice treated with K-CAR compared to treatment with other controls. (C) The expression of various genes in the treatment groups was detected by qRT-PCR. Significantly reduced expression of HERV-K in tumor biopsies was observed in mice treated with K-CAR in both BC cell xenograft models. K-CAR treatment downregulated expression of HERV-K, and this was accompanied by upregulation of p53 (TP53) and downregulation of MDM2 expression. (D) These profiles of gene expression were confirmed at the protein level by immunoblot assays. In addition to effects on p53 (TP-53) and MDM2expression, K-CAR T cell treatment of mice produced tumors that had decreased expression of p-ERK but not of ERK, compared with controls.
Figure 5.
(Continued)
The profiles of gene expression in tumor biopsies
In a previous study we reported that treatment of BC cells with an anti-HERV mAb impacted p53 signaling pathways, and wanted to determine whether K-CAR affected tumorigenesis by a similar mechanism. We observed significantly reduced expression of HERV-K in tumor biopsies obtained from mice treated with K-CAR compared with T cell treatment, as assessed by quantitative RT-PCR (Fig. 5C), immunoblot (Fig. 5D), or FACS (Fig. S5A–C). Of interest, p53 or P53-AIP1 was upregulated (Fig. 5C, Fig. 5D, and Fig. S5D), and oncogenes including MDM2 (Fig. 5C, Fig. 5D, and Fig. S5D) and p-ERK (Fig. 5D) were downregulated in BC cells treated with K-CAR.
Reduced tumor metastasis after K-CAR treatment
BC cells in this study were stably transfected with green fluorescent protein (GFP; pGreenPuro; System Biosciences; Fig. S4A), and imaging was used to assess metastasis of tumor cells from the primary xenograft tumor to other organs. The appearance of GFP in various organs was used for evaluating tumors and metastasis (Fig. S6A). Significantly reduced numbers of metastases to organs were found in mice treated with K-CAR compared with the other treatments (Fig. 6A). Images of various organs revealed that metastases were present in untreated mice but not in mice treated with K-CAR (Fig. 6B).
Figure 6 .
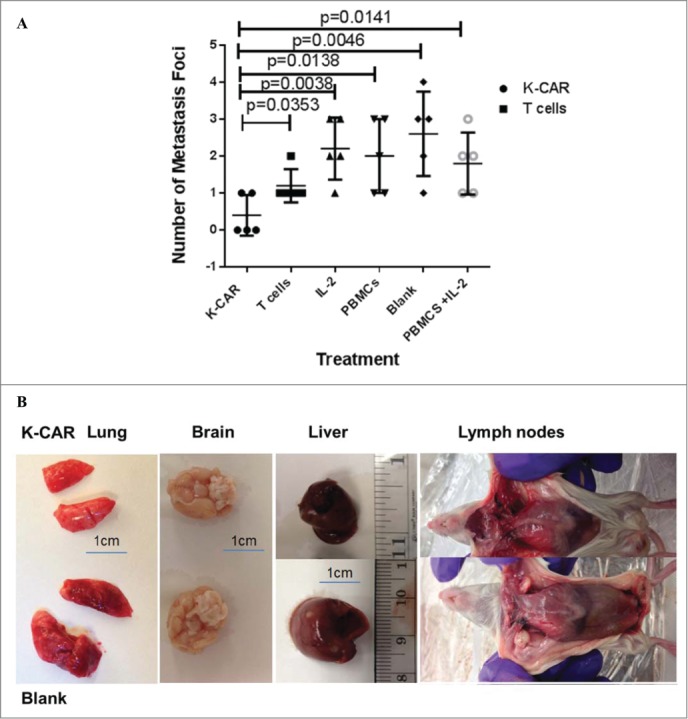
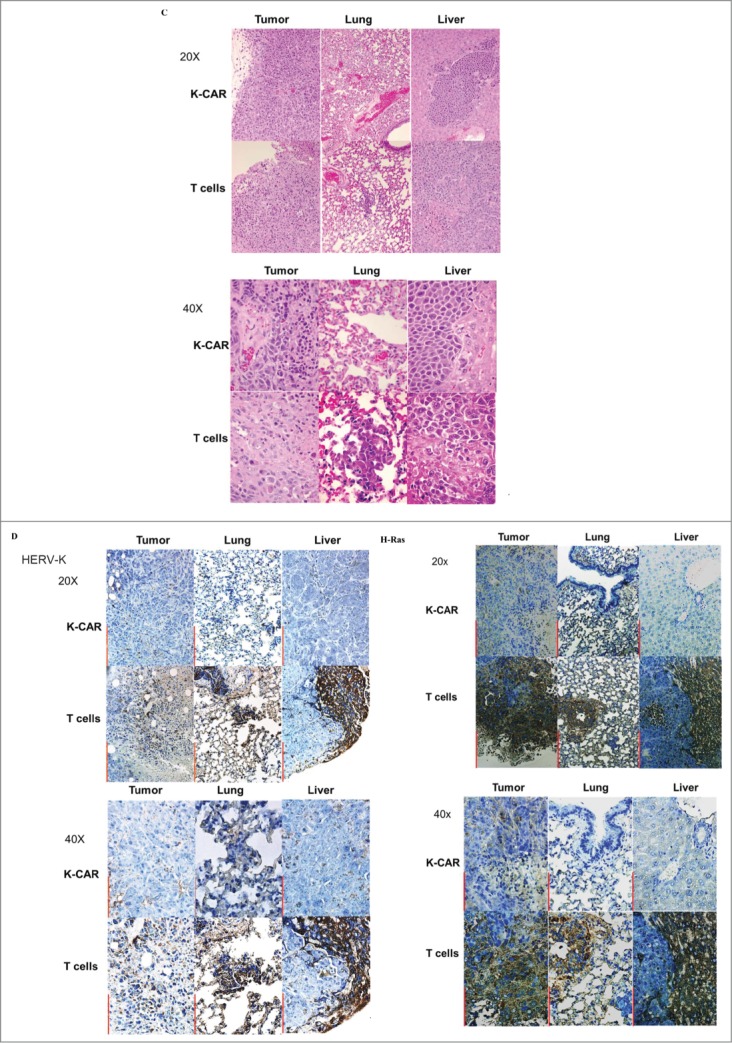
In vivo anti-metastatic effect of K-CAR treatment. (A) Mice inoculated with MDA-MB-435.eB1 cells and treated with K-CAR, vs. mice treated with control T cells, had metastases to organs that included axillary lymph node (1 metastatic focus in K-CAR T cell treatment group vs. 1 metastatic focus in the control T cell treatment group), lung (0 vs.1), liver (0 vs.1), brain (0 vs.1), and pleural lavage fluid (1 vs. 2). Thus, mice treated with K-CAR had 2 total metastatic foci to these organs, while mice treated with control T cells had 6 total foci. IL-2, PBMC, blank, and PBMCs + IL-2 treatment groups also had significantly more metastases than the K-CAR T cell treatment group. (B) Metastases that formed in other organs after K-CAR treatments or no treatment (blank) are pictured here. (C) Biopsies of tumors as well as metastases to lung and liver from K-CAR and T cell treatment groups were stained with H&E and samples are shown here in 20x and 40x. (D) The expression of HERV-K and H-Ras in biopsies from the same tissues as in (C) was determined by IHC using 6H5 mAb and H-Ras antibody. The expression of HERV-K or H-Ras was demonstrated in the tumor areas identified by H&E staining.
Figure 6.
(Continued)
Weights of body, liver, lung, and brain revealed no change of body weight in mice bearing either cell line (data not shown) and reduced lung tissue weights in both cell models treated with K-CAR compared with T cell treatment or other groups (Fig. S6B). Tissues including lung, liver, and brain were minced, and no GFP+ human cancer cells were observed in mice treated with K-CAR T cells compared with other groups (Fig. S6C, top panel). In addition, GFP+ metastatic cells harvested from pleural lavage fluid were observed in mice treated with T cells, but not with K-CAR T cells (Fig. S6C, bottom panel). Cells from minced tissues were cultured and GFP+ human BC cells were seen at two weeks post-culture only in cells from groups not treated with K-CAR (Fig. S6D). Hematoxylin and eosin (H&E) staining was further used to assess morphological features of tumor tissues and tissues from other organs (Fig. 6C). H&E staining was quantitated in Table 3 by measuring the tumor areas. K-CAR treatment resulted in smaller tumor volumes, less tumor focality and number, less infiltrative borders, and decreased mitotic activities. Also, tumor cells from the K-CAR group were more uniform with less pleomorphic nuclei and smaller nucleoli, and tumor-infiltrating lymphocytes were significantly increased in number. Metastasis of BC cells was observed in many tissues, including liver and lung tissues from mice treated with T cells (Fig. 6C) compared with K-CAR treated mice. Only one small metastatic area was observed in liver from mice treated with K-CAR compared to mice treated with T cells, which had many more metastatic foci (Fig. 6A).
Table 3.
Quantitation of H&E staining in Figure 6C by measuring tumor areas
| 20x |
40x |
||||
|---|---|---|---|---|---|
| Tumor area (mm2) | K-CAR | T cell | K-CAR | T cell | |
| H&E | Tumor | 1,500 | 2,000 | 1,790 | 2,000 |
| Lung | 0 | 77 | 0 | 1,050 | |
| Liver | 2,400 | 5,040 | 1,000 | 2,000 | |
Only one metastatic nodule was found in the entire liver of mice treated with K-CAR.
Immunohistochemistry revealed the expression of HERV-K and H-Ras in tumor and metastatic cells in several tissues obtained from mice treated with control T cells, but not in K-CAR-treated mice (Fig. 6D, Fig. S6E). HERV-K and Ras staining was quantitated in Table 4 using ImmunoRatio (http://153.1.200.58:8080/immunoratio/). K-CAR-treated tumors showed minimal IHC reactivity to HERV-K env protein or H-Ras protein, in contrast to tumors harvested from the mice treated with T cells, with the latter group showing strong immune reactivity against HERV-K mAb and H-Ras antibody. In summary, there was dramatically reduced tumor burden after treatment with K-CAR T cells, mostly due to the prevention of tumor metastasis to multiple tissues.
Table 4.
Quantitation of HERV-K+ and Ras+ staining in Figure 6D by ImmunoRatio
| 20x |
40x |
||||
|---|---|---|---|---|---|
| DAB staining(%) | K-CAR | T cell | K-CAR | T cell | |
| HERV-K | Tumor | 1.8 | 18.8 | 4.4 | 19.1 |
| Lung | 4.4 | 62.3 | 10.2 | 68.0 | |
| Liver | 1.1 | 40.3 | 2.2 | 43.2 | |
| Ras | Tumor | 1.6 | 44.6 | 1.5 | 37.7 |
| Lung | 6.2 | 35.7 | 1.9 | 39.4 | |
| Liver | 2.6 | 13.6 | 2.4 | 23.9 | |
Discussion
We have successfully used HERV-K env protein as a TAA for development of immunotherapy against BC.18,21,22,24 Here, K-CAR T cells generated from both BC patients and normal donors showed antitumor effects in vitro sand in vivo. Adoptive immunotherapy is a promising approach for cancer treatment, but solid tumors have historically shown poorer response than treatment of hematological malignancies for various reasons, including target antigen differences in the two malignancies, poor T-cell trafficking to solid tumors, and lower CAR cytotoxicity due to the immunosuppressive environment of the local tumor. A major goal of CAR-based immunotherapy is to identify tumor-specific targets and avoid off-tissue toxicity, thus emphasizing the need to select target antigens that show both efficacy and safety.
Recently, we found that serum HERV-K viral mRNA tended to be higher in BC patients who later on developed the metastatic disease.20 Our accumulated data thus provide strong evidence that HERV-K env protein may be an ideal target for CAR-based therapy against BC, especially against metastatic BC.
The SB system we employed here is frequently used for gene delivery and long-term transgene expression due to its lower cost and better safety profile.26-28 We used the SB system to transfer HERV-K-CAR into PBMCs from BC patients and NDs to propagate K-CAR T cells, and showed that these cells display CAR-dependent effector function and anti-proliferative effects. Both BC patients and normal donors can produce K-CAR T cells. The overall population of K-CAR+ T cells obtained from BC patients was significantly expanded compared to those from NDs, as analyzed 21 d after culture with aAPC and IL-2, suggesting that there may be increased memory T cells in this group. However, a slower proliferation rate was observed for K-CAR T cells generated from BC patients than from NDs. A higher percentage of CD4+ T cells and a lower percentage of CD8+ T cells were noted in BC patients compared with NDs, but there was no significant difference in the percentage of CD8+ or CD4+ T cells from K-CAR T cells obtained from BC patients or NDs. Of interest, a significantly enhanced percentage of CD8+ T cells were found in K-CAR T cells after CD4 depletion. Significantly enhanced specific lysis of target cells was observed in BC patient K-CAR T cells with CD4+ T cell depletion, compared to those without depletion. Tumor escape from immune-mediated destruction has been associated with immunosuppressive mechanisms that inhibit T cell activation,29 and suppression of CD8+ effector cells by CD4+CD25+FoxP3+ Tregs plays a key role in this immunosuppression.30 Our results indicate that K-CAR T cells from BC patients may contain a high presence of Tregs, compared with K-CAR T cells from NDs. Our data thus support the concept that Treg depletion from K-CAR T cells provides synergy for improved immune-mediated tumor control in vitro and in vivo.
K-CAR-dependent effector function and cytotoxicity against HERV-K+ BC cells was demonstrated in many of our assays, which showed inhibition of BC cell proliferation, increased specific lysis in CTL assays, and increased cell death. Antitumor effects were also greater with K-CAR T cells than T cells from the same donors. Inhibition of proliferation and enhanced cytotoxicity toward BC cells was observed to increase upon exposure to a larger number of K-CAR T cells. The specific killing occurred in an antigen-dependent manner, because no killing was observed if target cells such as MCF-10A breast cells did not express HERV-K, or if the expression of HERV-K was knocked down by shRNA in BC cells.
Enhanced release of cytokines, including IFNγ, IL-2, TNF-α, and Granzyme B, was detected in culture media of BC cells treated with K-CAR T cells vs. T cells obtained from BC patients and NDs. No significant difference in cytokine production was found in K-CAR obtained from BC patients and controls. Release of these Th1 cytokines may assist in K-CAR T cell killing of BC cells.
Antitumor effects were also demonstrated in xenograft models inoculated with two BC cell lines. Significantly reduced tumor growth of K-CAR+ T cell treatment groups was observed in both models compared with mice treated with control T cells. The expression of HERV-K was concomitantly significantly reduced in tumor biopsies treated with K-CAR in both mouse models. Interestingly, upregulation of p53 and downregulation MDM2 was detected in tumor biopsies treated with K-CAR compared with T cell treatment. These changes were observed not only at the RNA level but also at the level of protein expression. p53-inducible genes play crucial roles in cell cycle control, DNA repair, and apoptosis. The tumorigenic potential of MDM2, the principal cellular antagonist of p53, is closely linked to its repressive function of p53. In an earlier study, we showed that anti-HERV-K mAb was able to bind cell surface env signals through p53 pathway activation and induce a p53 signaling response.21
The Ras–ERK pathway is known to play a pivotal role in differentiation, proliferation and tumor progression. In an earlier study, knockdown of wild-type TP53 led to activation of p-ERK1/2,31 showing a reciprocal relationship similar to what we observed in the current study. Expression of Np9 (a spliced variant of the HERV-K env gene) is essential for maintaining ERK1/2 signaling pathway in leukemia cells,32 further supporting an important role for HERV-K in signaling via ERK1/2. The Ras–Raf–MEK–ERK signaling pathway has been proposed to play an important role in HERV-K activation.33 These observations may thus provide a mechanistic explanation of the role(s) HERV-K plays in breast tumorigenesis. Since Ras–ERK and MDM2 signaling have been demonstrated to play important roles in cell growth and tumorigenesis, our results may explain mechanistically how K-CAR treatment induces inhibition of cell proliferation in vitro and tumorigenesis in vivo.
Another important finding in this study is the anti-metastatic role of K-CAR. We observed significantly reduced or no metastasis to lung, liver, brain, and lymph nodes in mice treated with K-CAR compared with mice treated with T cells and the other treatments tested. These tumor cells present in tumor biopsies, as well as those that metastasized to other organs, were HERV-K+ BC cells. Of interest, HERV-K env protein downregulation correlated with downregulation of p-ERK and Ras proteins in most tumor biopsies and at sites of metastasis in mice treated with K-CAR T cells. Since Ras–ERK and MDM2 signaling have been demonstrated to play important roles in cell growth and tumorigenesis, our results may explain mechanistically how K-CAR treatment induces inhibition of cell proliferation in vitro and tumorigenesis in vivo.
In summary, our data provide strong evidence that K-CAR is very effective for immunotherapy against BC, and especially against metastatic BC. The mechanism of action of K-CAR is through signaling pathways involving not only p53 but also Ras, and the role of Ras signaling in mediating the pro-tumorigenic effect of HERV-K will be investigated in near future. Clinical trials are also needed to determine the efficacy of K-CARs in humans.
Materials and Methods
Study design
Patient cells and samples were analyzed for various aspects of immune response, and data were analyzed in a blinded fashion. Samples were analyzed at least in triplicate, and experiments in mice (n = 5 per group) were independently replicated at least twice. Animal studies that gave similar results were used to arrive at conclusions.
Ethics statement
PBMCs were obtained from cancer patients and NDs at MD Anderson Cancer Center according to an approved Institutional Review Board protocol (LAB04-0083) and written informed consent was provided by study participants and/or their legal guardians. Animal studies were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of MD Anderson Cancer Center. The procedures employed herein were performed according to the Declaration of Helsinki.
Plasmids
The scFv sequence from mAb clone 6H521 against HERV-K env protein was codon optimized (CoOp) (Invitrogen, Carlsbad, CA) and cloned into the SB transposon driven by the human elongation factor-1α (hEF-1α) promoter, flanked by SB inverted repeats forming CoOp6H5CD28/pSBSO (a gift from Dr. Laurence Cooper, MD Anderson Cancer Center). The HERV-K full-length env sequence obtained from BC patient 37 (diagnosis of IDC; Fig. S2B), containing the viral surface and transmembrane domains of HERV-K env, was cloned into a pLVX-DsRed-Monomer-C1 vector (Clontech Laboratories, Inc.).
Cell lines
The cell lines used in this study are listed in the Supplementary Methods.
In vitro proliferation of HERV-K-specific CAR T cells
PBMCs were isolated by Ficoll-Paque density gradient centrifugation as described previously22 and electroporated with the SB system as described previously with some minor modifications.25 Briefly, PBMCs (1 × 107 per cuvette) were resuspended in 100 µL of Amaxa Nucleofector solution (Human T-cell Kit), mixed with 8 μg of 6H5scFvCD28mZ (CoOp/pSBSO) and 5 µg of pCMV-SB11, and electroporated using the U-14 program in an Amaxa Nucleofector® (Lonza, Allendale, NJ). K562 cells stably transduced with pLVX-K env to express HERV-K env protein were used as aAPC to propagate and generate K-CAR+ T cells. After 4 h of culture, the electroporated T cells were split to maintain suitable density and co-cultured with γ-irradiated (100 Gy) aAPC at a 1:2 T cell:aAPC ratio. The aAPCs were added twice weekly. Soluble IL-21 (eBioscience) and IL-2 (Chiron) cytokines were supplemented every other day at a concentration of 30 ng/mL and 50 U/ml, respectively, to complete RPMI media in the presence of OKT3 (0.5 μg/mL), as described previously.25 CAR+ T cells were quantified weekly by FACS and were identified as those positive for both Fc and CD3+ markers, as described previously.25 The PE-conjugated F(ab′)2 fragment of goat anti-human Fcγ was used for assessing Fc positivity. Control T cells were mock transfected without added DNA or GFP, as negative controls.
FACS analysis, immunohistochemistry and immunoblot
Protocols for FACS analysis and IHC are presented in the Supplementary Methods, and were based on our previous publications.21,22
CD4+ lymphocyte depletion and cytotoxic T lymphocyte (CTL) assays
CD4+ lymphocyte depletion CTL and cytokine release assays were carried out using standard protocols described in the Supplementary Methods section.
Detection of K-CAR
K-CAR mRNA was detected by RT-PCR, as described previously,22 and in the Supplementary Methods section.
HERV-K env shRNA lentiviral packaging
shRNAs targeting the HERV-K env gene (shRNAenv; GenBank No. M14123.1) and matched scrambled shRNA sequences serving as negative controls (shRNAc) were designed using the Invitrogen RNAi Designer program, and cloned into the pGreenPuro™ vector (System Biosciences). Lentiviral particles expressing shRNA were then packaged and titered according to the manufacturer's instructions. RT-PCR, qRT-PCR, FACS, and immunoblot were subsequently performed to determine the expression of HERV-K env RNA or protein in cell lines.
Alamar blue assay of cell viability and live/dead viability/cytotoxicity tests
These fluorescence-based assays of cell viability are discussed in detail in the Supplementary Methods section.
Cancer stem cell culture
For growing tumorspheres in suspension, trypsinized cells (MDA-MB-435.eB1) and patients' tumor cells were cultured on ultra-low attachment tissue culture plates with stem cell culture medium under 5% CO2 at 37°C, as described previously.34–36
In vivo antitumor activity of HERV-K-CAR T cells
Eight-week-old female nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice were injected with 1 × 106 BC cells subcutaneously in both flanks. Another model was created by subcutaneous injection of 2 × 106 MDA-MB-435.eB1 cells in both flanks. Mice (n = 5) received 1 × 106 K-CAR cells by intravenous infusion starting on days 5 and 13 post-injection. On day 20 post-injection of BC cells, 1 × 107 K-CAR cells were infused, followed by 600 U of IL-2 (eBioscience), which was injected intraperitoneally and intravenously once a week during the treatment period. One cohort of mice (n = 5) bearing the tumor received no treatment as a blank control group. The other four cohorts were treated with IL-2, PBMC, PBMC plus IL-2, and control T cells, respectively. When tumors became measurable, tumor size was calculated and growth rates measured twice weekly. Mice were euthanized when tumor volumes exceeded 1000 mm3. Fresh lung, liver, bone, brain, pleural lavage fluid, and axillary lymph nodes were then extracted, weighed, and evaluated for metastases. RNA and protein were isolated from both groups and the expression of HERV-K env RNA was determined by qRT-PCR and immunoblot. qRT-PCR was carried out using the TaqMan® One-Step RT-PCR Master Mix Reagents Kit (Applied Biosystems, CA). The expression level of GAPDH gene was used as an internal control. The relative gene expression levels were quantified by the 2(–ΔΔCT) method as described.37
Statistical Analysis
The protocols used for statistical analysis are detailed in the Supplementary Methods section.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Perry Hackett for his permission to use the SB system in our study, Dr. Danielle Lu for pathologic analysis of mouse tumors, and Dr. Nathalie Scholler for reviewing the manuscript.
Author Contributions
LJNC and FWJ conceptualized the study. FWJ designed the overall study, provided advice, interpreted data, and participated in writing the manuscript. FZ participated in study design, performed the major experiments involving K-CAR and in vitro and in vivo experiments, and participated in writing the manuscript. JK initiated and optimized the study design of some experiments. ML designed and cloned the shRNA and the pLVX-K env plasmids, and performed experiments involving these plasmids; YW contributed to immune assays and in vivo experiments. KH provided BC patient samples and clinical information. GLJ assisted in designing experiments and interpreting data, and participated in writing the manuscript.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Funding
This work was supported in part by grant BC113114 from the United States Department of Defense, grant ES007784 from the National Institute of Environmental Health Sciences, and grants 07-2007-070 01 (GLJ) and 02-2011-104 (FWJ) from the Avon Foundation for Women.
References
- 1.Dotti G, Savoldo B, Takahashi S, Goltsova T, Brown M, Rill D, Rooney C, Brenner M. Adenovector-induced expression of human-CD40-ligand (hCD40L) by multiple myeloma cells. A model for immunotherapy. Exp Hematol 2001; 29:952-61; PMID:11495701; http://dx.doi.org/ 10.1016/S0301-472X(01)00668-3 [DOI] [PubMed] [Google Scholar]
- 2.Mami-Chouaib F, Echchakir H, Dorothee G, Vergnon I, Chouaib S. Antitumor cytotoxic T-lymphocyte response in human lung carcinoma: identification of a tumor-associated antigen. Immunol Rev 2002; 188:114-21; PMID:12445285; http://dx.doi.org/ 10.1034/j.1600-065X.2002.18810.x [DOI] [PubMed] [Google Scholar]
- 3.Davies DM, Maher J. Adoptive T-cell immunotherapy of cancer using chimeric antigen receptor-grafted T cells. Arch Immunol Ther Exp 2010; 58:165-78; PMID:20373147; http://dx.doi.org/ 10.1007/s00005-010-0074-1 [DOI] [PubMed] [Google Scholar]
- 4.Sun M, Shi H, Liu C, Liu J, Liu X, Sun Y. Construction and evaluation of a novel humanized HER2-specific chimeric receptor. Breast Cancer Res 2014; 16:R61; PMID:24919843; http://dx.doi.org/ 10.1186/bcr3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanitis E, Poussin M, Klattenhoff AW, Song D, Sandaltzopoulos R, June CH, Powell DJ Jr. Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res 2013; 1:43-53; PMID:24409448; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han EQ, Li XL, Wang CR, Li TF, Han SY. Chimeric antigen receptor-engineered T cells for cancer immunotherapy: progress and challenges. J Hematol Oncol 2013; 6:47; PMID:23829929; http://dx.doi.org/ 10.1186/1756-8722-6-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010; 18:843-51; PMID:20179677; http://dx.doi.org/ 10.1038/mt.2010.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kandalaft LE, Powell DJ Jr., Coukos G. A phase I clinical trial of adoptive transfer of folate receptor-alpha redirected autologous T cells for recurrent ovarian cancer. J Transl Med 2012; 10:157; PMID:22863016; http://dx.doi.org/ 10.1186/1479-5876-10-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlimper C, Hombach AA, Abken H, Schmidt-Wolf IG. Improved activation toward primary colorectal cancer cells by antigen-specific targeting autologous cytokine-induced killer cells. Clin Dev Immunol 2012; 2012:238924; PMID:22481963; http://dx.doi.org/ 10.1155/2012/238924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol 2013; 31:71-5; PMID:23242161; http://dx.doi.org/ 10.1038/nbt.2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, Vulto A, den Bakker M, Oosterwijk E, Debets R et al.. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther 2013; 21:904-12; PMID:23423337; http://dx.doi.org/ 10.1038/mt.2013.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazazian HH, Jr. Mobile elements: drivers of genome evolution. Science 2004; 303:1626-32; PMID:15016989; http://dx.doi.org/ 10.1126/science.1089670 [DOI] [PubMed] [Google Scholar]
- 13.Lander ES. Initial impact of the sequencing of the human genome. Nature 2011; 470:187-97; PMID:21307931; http://dx.doi.org/ 10.1038/nature09792 [DOI] [PubMed] [Google Scholar]
- 14.Ahn K, Kim HS. Structural and quantitative expression analyses of HERV gene family in human tissues. Mol Cell 2009; 28:99-103; PMID:19669627; http://dx.doi.org/ 10.1007/s10059-009-0107-y [DOI] [PubMed] [Google Scholar]
- 15.Reus K, Mayer J, Sauter M, Zischler H, Muller-Lantzsch N, Meese E. HERV-K(OLD): ancestor sequences of the human endogenous retrovirus family HERV-K(HML-2). J Virol 2001; 75:8917-26; PMID:11533155; http://dx.doi.org/ 10.1128/JVI.75.19.8917-8926.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hohn O, Hanke K, Bannert N. HERV-K(HML-2), the best preserved family of hervs: endogenization, expression, and implications in health and disease. Front Oncol 2013; 3:246; PMID:24066280; http://dx.doi.org/ 10.3389/fonc.2013.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Contreras-Galindo R, Kaplan MH, Leissner P, Verjat T, Ferlenghi I, Bagnoli F, Giusti F, Dosik MH, Hayes DF, Gitlin SD et al.. Human endogenous retrovirus K (HML-2) elements in the plasma of people with lymphoma and breast cancer. J Virol 2008; 82:9329-36; PMID:18632860; http://dx.doi.org/ 10.1128/JVI.00646-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang-Johanning F, Frost AR, Jian B, Epp L, Lu DW, Johanning GL. Quantitation of HERV-K env gene expression and splicing in human breast cancer. Oncogene 2003; 22:1528-35; PMID:12629516; http://dx.doi.org/ 10.1038/sj.onc.1206241 [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Rycaj K, Geng S, Li M, Plummer JB, Yin B, Liu H, Xu X, Zhang Y, Yan Y et al.. Expression of human endogenous retrovirus type k envelope protein is a novel candidate prognostic marker for human breast cancer. Genes Cancer 2011; 2:914-22; PMID:22593804; http://dx.doi.org/ 10.1177/1947601911431841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang-Johanning F, Li M, Esteva FJ, Hess KR, Yin B, Rycaj K, Plummer JB, Garza JG, Ambs S, Johanning GL et al.. Human endogenous retrovirus type K antibodies and mRNA as serum biomarkers of early-stage breast cancer. Int J Cancer 2014; 134:587-95; PMID:23873154; http://dx.doi.org/ 10.1002/ijc.28389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang-Johanning F, Rycaj K, Plummer JB, Li M, Yin B, Frerich K, Garza JG, Shen J, Lin K, Yan P et al.. Immunotherapeutic potential of anti-human endogenous retrovirus-K envelope protein antibodies in targeting breast tumors. J Natl Cancer Inst 2012; 104:189-210; PMID:22247020; http://dx.doi.org/ 10.1093/jnci/djr540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang-Johanning F, Radvanyi L, Rycaj K, Plummer JB, Yan P, Sastry KJ, Piyathilake CJ, Hunt KK, Johanning GL. Human endogenous retrovirus K triggers an antigen-specific immune response in breast cancer patients. Cancer Res 2008; 68:5869-77; PMID:18632641; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-6838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rycaj K, Plummer JB, Yin B, Li M, Garza J, Radvanyi L, Ramondetta LM, Lin K, Johanning GL, Tang DG, Wang-Johanning F et al.. Cytotoxicity of human endogenous retrovirus K-specific T cells toward autologous ovarian cancer cells. Clin Cancer Res 2015; 21:471-83; Epub 2014/11/06; PMID: 25370465; http://dx.doi.org/11410490 10.1158/1078-0432.ccr-14-0388 [DOI] [PubMed] [Google Scholar]
- 24.Wang-Johanning F, Frost AR, Johanning GL, Khazaeli MB, LoBuglio AF, Shaw DR, Strong TV. Expression of human endogenous retrovirus k envelope transcripts in human breast cancer. Clin Cancer Res 2001; 7:1553-60; PMID:11410490 [PubMed] [Google Scholar]
- 25.Maiti SN, Huls H, Singh H, Dawson M, Figliola M, Olivares S, Rao P, Zhao YJ, Multani A, Yang G et al.. Sleeping beauty system to redirect T-cell specificity for human applications. J Immunother 2013; 36:112-23; PMID:23377665; http://dx.doi.org/ 10.1097/CJI.0b013e3182811ce9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torikai H, Reik A, Liu PQ, Zhou Y, Zhang L, Maiti S, Huls H, Miller JC, Kebriaei P, Rabinovitch B et al.. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood 2012; 119:5697-705; PMID:22535661; http://dx.doi.org/ 10.1182/blood-2012-01-405365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kebriaei P, Huls H, Jena B, Munsell M, Jackson R, Lee DA, Hackett PB, Rondon G, Shpall E, Champlin RE et al.. Infusing CD19-directed T cells to augment disease control in patients undergoing autologous hematopoietic stem-cell transplantation for advanced B-lymphoid malignancies. Hum Gene Ther 2012; 23:444-50; PMID:22107246; http://dx.doi.org/ 10.1089/hum.2011.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh H, Manuri PR, Olivares S, Dara N, Dawson MJ, Huls H, Hackett PB, Kohn DB, Shpall EJ, Champlin RE et al.. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res 2008; 68:2961-71; PMID:18413766; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013; 5:200ra116; PMID:23986400; http://dx.doi.org/ 10.1126/scitranslmed.3006504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kline J, Brown IE, Zha YY, Blank C, Strickler J, Wouters H, Zhang L, Gajewski TF. Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin Cancer Res 2008; 14:3156-67; PMID:18483384; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-4696 [DOI] [PubMed] [Google Scholar]
- 31.Gulati AP, Yang YM, Harter D, Mukhopadhyay A, Aggarwal BB, Benzil DL, Whysner J, Albino AP, Murali R, Jhanwar-Uniyal M. Mutant human tumor suppressor p53 modulates the activation of mitogen-activated protein kinase and nuclear factor-kappaB, but not c-Jun N-terminal kinase and activated protein-1. Mol Carcinog 2006; 45:26-37; PMID:16267831; http://dx.doi.org/ 10.1002/mc.20149 [DOI] [PubMed] [Google Scholar]
- 32.Chen T, Meng Z, Gan Y, Wang X, Xu F, Gu Y, Xu X, Tang J, Zhou H, Zhang X et al.. The viral oncogene Np9 acts as a critical molecular switch for co-activating beta-catenin, ERK, Akt and Notch1 and promoting the growth of human leukemia stem/progenitor cells. Leukemia 2013; 27:1469-78; PMID:23307033; http://dx.doi.org/ 10.1038/leu.2013.8 [DOI] [PubMed] [Google Scholar]
- 33.Huang G, Li Z, Wan X, Wang Y, Dong J. Human endogenous retroviral K element encodes fusogenic activity in melanoma cells. J Carcinog 2013; 12:5; PMID:23599687; http://dx.doi.org/ 10.4103/1477-3163.109032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu F, Li J, Chen H, Fu J, Ray S, Huang S, Ai W. Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene 2011; 30:2161-72; PMID:21242971; http://dx.doi.org/ 10.1038/onc.2010.591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009; 138:645-59; PMID:19682730; http://dx.doi.org/ 10.1016/j.cell.2009.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X, Sarvestani SK, Moeinzadeh S, He X, Jabbari E. Effect of CD44 binding peptide conjugated to an engineered inert matrix on maintenance of breast cancer stem cells and tumorsphere formation. PLoS One 2013; 8:e59147; PMID:23527117; http://dx.doi.org/ 10.1371/journal.pone.0059147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402-8; PMID:11846609; http://dx.doi.org/ 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.