Abstract
Renal cell carcinoma (RCC) is an immunogenic tumor, but uses several immune-suppressive mechanisms to shift the balance from tumor immune response toward tumor growth. Although RCC has traditionally been considered to be radiation resistant, recent evidence suggests that hypofractionated radiotherapy contributes to systemic antitumor immunity. Because the efficacy of antitumor immune responses depends on the complex balance between diverse immune cells and progressing tumor cells, radiotherapy alone is unlikely to induce persistent antitumor immunity. Therefore, the combination of radiotherapy with drugs having synergistic immunomodulatory properties holds great promise with the optimal timing and sequence of modalities depending on the agent used. We highlight the immunomodulatory properties of targeted therapies, such as tyrosine kinase inhibitors, mammalian target of rapamycin (mTOR) inhibitors and vascular endothelial growth factor (VEGF) neutralizing antibodies, and will suggest a combination schedule with radiotherapy based on the available literature. We also address the combination of radiotherapy with innovative treatments in the field of immunotherapy.
Keywords: antitumor immunity, immunotherapy, radiotherapy, renal cell carcinoma, targeted therapy, treatment combination
Abbreviations
- APCs
antigen presenting cells
- APM
antigen processing machinery
- ASMase
acid sphingomyelinase
- ATP
adenosine triphosphate
- ccRCC
clear cell renal cell carcinoma
- CRT
calreticulin
- CTL
cytotoxic T lymphocyte
- CTLA-4
cytotoxic T lymphocyte associated protein 4
- DAMPs
damage-associated molecular patterns
- DCs
dendritic cells
- ER
endoplasmic reticulum
- HFRT
hypofractionated radiotherapy
- HIF-1α
hypoxia-inducible factor α
- HMGB1
high-mobility group box 1
- HSP70
heat shock protein 70
- ICAM-1
intercellular adhesion molecule 1
- ICD
immunogenic cell death
- IDO
immune regulating enzyme indoleamine-2,3-dioxygenase
- IFNγ
interferon γ
- IL-2
interleukin 2
- IL-6
Interleukin 6
- IL-10
interleukin 10
- IL-12
Interleukin 12
- M1 macrophages
pro-inflammatory macrophages
- M2 macrophages
anti-inflammatory macrophages
- MDSCs
myeloid-derived suppressor cells
- MHC
major histocompatibility complex
- MICA
MHC class I-related chain A
- mTOR
mammalian target of rapamycin
- NK cells
natural killer cells
- PDGFR
platelet-derived growth factor receptor
- PD-L1
programmed death ligand 1
- RCC
renal cell carcinoma
- ROS
reactive oxygen species
- SBRT
stereotactic body radiotherapy
- STAT3
signal transducer and activator of transcription 3
- TCR
T cell receptor
- TGF-β
transforming growth factor β
- Th1 cells
T helper 1 cells
- Th 2 cells
T helper 2 cells
- TILs
tumor infiltrating lymphocytes
- TIM-3
T cell immunoglobulin and mucin domain 3
- TKIs
tyrosine kinase inhibitors
- TNFα
tumor necrosis factor α
- Tregs
regulatory T cells
- VCAM-1
vascular cell adhesion molecule 1
- VEGF
vascular endothelial growth factor
- VHL
von Hippel-Lindau.
Introduction
RCC presents with metastatic disease in about 30% of patients, while another third of patients with localized advanced disease will ultimately develop metastases.1,2 Molecular therapies that block the VEGF or mTOR pathways are currently considered the mainstay treatment3 for metastatic RCC. Nevertheless, a durable response to targeted therapy is rare and most patients eventually develop progressive disease.4,5 We therefore have to look at new therapeutic options to improve the outcome of these patients. Since RCC is considered an immunogenic tumor,6-8 we might find the answer in the field of immunotherapy. There are some clinical cases in RCC describing responses outside the irradiated regions, following high-dose stereotactic body radiotherapy (SBRT) to metastases.9,10 These responses are termed “abscopal effects.” Both pre-clinical and clinical data11–13 suggest that these effects are immune mediated.14,15 Despite these observations, both the tumor and its microenvironment seem to be able to evade the immune system in the majority of cases. Radiotherapy alone is probably unlikely to induce persistent antitumor immunity and a combination with synergistic immunomodulatory agents might be necessary to induce long-term clinical results, as suggested by promising preclinical and clinical data.12,16-20
The current review offers insights in the specific immune escape mechanisms present in RCC with a specific focus on the potential role of radiotherapy in combination with systemic treatment to improve clinical responses by enhancing antitumor immunity.
Immune Modulation in RCC
Although the immune system tries to control the proliferation of RCC, the tumor is able to progress. By evasion of the antitumor immune response, RCC is able to shift the balance from tumor immune response toward tumor growth (Fig. 1). In the next paragraphs, these evasion mechanisms of RCC influencing both the innate21 and adaptive immune system are highlighted.22
Figure 1.

The balance between pro-immunogenic and immunosuppressive factors in the tumor microenvironment of RCC. The immune system plays a protective role in tumor control. Dendritic cells (DCs) take up apoptotic and necrotic tumor fragments and present processed tumor-derived peptides to T-helper (Th) lymphocytes as well as cross-present to cytotoxic T lymphocytes (CTLs). Tumor-activated NK cells kill tumor cells by releasing their cytotoxic granules onto the surface. On the other hand, RCC is able to evade antitumor immune responses. RCC stimulates the secretion of immunosuppressive soluble factors such as IL-10, IL-6, vascular endothelial growth factor (VEGF), arginase-I (ARG-1) and indoleamine-2,3-dioxygenase (IDO). RCC also activates transforming growth factor β (TGF-β), signal transducer and activator of transcription 3 (STAT3), promotes the accumulation of regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs) and pro-tumorigenic M2 macrophages. RCC also impairs T cell function by the decreased expression of the CD3ζ chain and the increased expression of the co-inhibitory molecules PD-L1, B7-H4 and T cell immunoglobulin and mucin domain 3 (TIM-3). Finally, RCC impairs NK cell activity by shedding soluble MHC class I-related chain A (MICA) into the circulation.
RCC is able to escape cytotoxic T lymphocyte (CTL)-mediated killing through different mechanisms (Fig. 2). T cells are initially stimulated to recognize cancer cells through cross-priming by dendritic cells (DCs). However, RCC interferes with DC activation by secreting immunosuppressive factors. Consequently, only a minority of the DCs show signs of activation23 and are able to prime naïve T cells. Moreover, deficiencies in both the proteasome and transporter associated with antigen processing, reduction of other antigen processing machinery (APM)-components, and altered expression of major histocompatibility complex (MHC)-I molecules, allows RCC to escape recognition by CTLs.24
Figure 2.
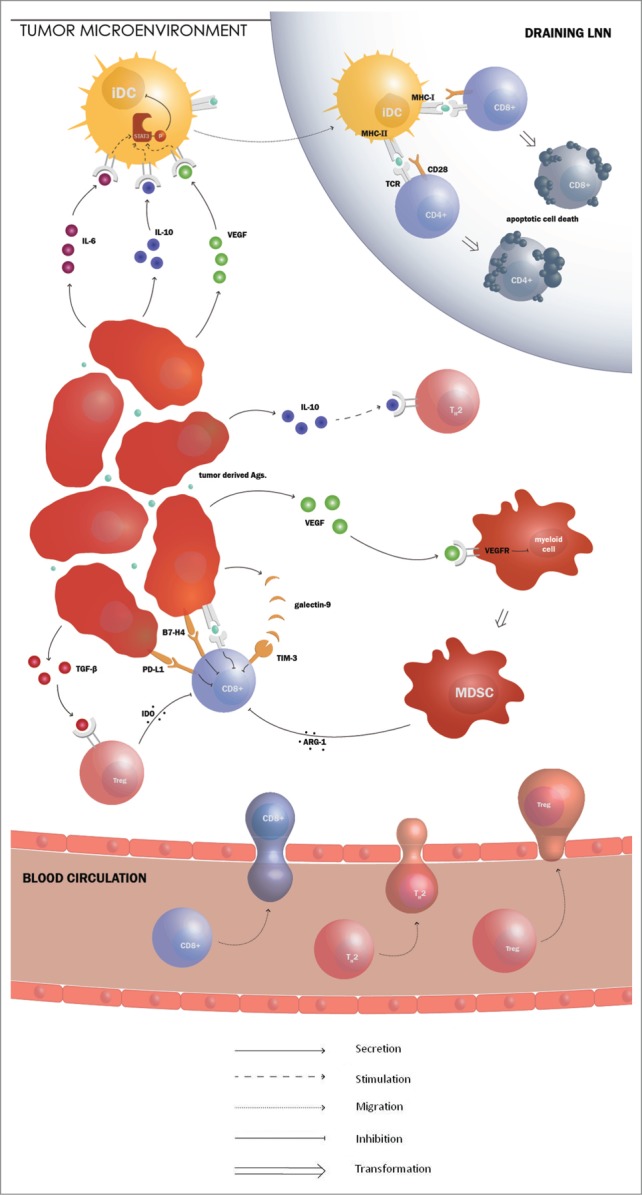
The immune evasion mechanisms of RCC hinder the adaptive immune response. Production of vascular endothelial growth factor (VEGF) by renal cell carcinoma (RCC) arrests the differentiation of myeloid cells, resulting in the accumulation of immature myeloid cells. These immature myeloid cells are called myeloid derived suppressor cells (MDSCs) and block T cell responses by producing immunosuppressive agents such as arginase I. Arginase production by MDSCs results in impaired T cell receptor (TCR) signaling, causing a disturbed lytic function of the CD8+ T cells. VEGF, along with IL-6 and IL-10, also induces the activation of signal transducer and activator of transcription 3 (STAT3). STAT3 activation is thought to be involved in the absence of functional DCs. The expression the co-inhibitory molecule programmed death ligand 1 (PD-L1) by RCC might stimulate T cell inhibition. RCC cancer cells also often express the negative co-stimulatory molecule B7-H4. Recently, a new co-inhibitory molecule, T cell immunoglobulin and mucin domain 3 (TIM-3), was described. The molecule induces T cell death by binding its ligand, galactin-9. RCC is able to counteract Th1 cell differentiation. Production of IL-10 by the tumor cells causes Th1 cell loss and Th2 cell prevalence. RCCs also produce transforming growth factor (TGF)-β, which is known to stimulate the recruitment and activation of regulatory T cells (Tregs). They downregulate the function of immune effector cells through secretion of immunosuppressive factors such as indoleamine-2,3-dioxygenase (IDO).
Most RCCs are highly vascularized because of mutations of the von Hippel–Lindau (VHL) tumor suppressor gene. pVHL is needed for the degradation of hypoxia-inducible factor α (HIF-1α). Deficient pVHL leads to accumulation of HIF-1α and stimulation of angiogenesis through HIF-induced VEGF production.25 In addition to stimulating tumor angiogenesis, VEGF also arrests the differentiation of myeloid cells, resulting in accumulation of immature myeloid cells. These immature myeloid cells are myeloid-derived suppressor cells (MDSCs) and block T cell responses by producing IL-10, transforming growth factor (TGF)-β, prostaglandin E2,26 reactive oxygen species (ROS)27 and arginase I.26,28 Compared to healthy controls, higher levels of MDSCs are found in the peripheral blood of RCC patients,24 associated with a 6–10-fold increase in arginase activity.26 Arginase production by MDSCs results in a decreased expression of the CD3ζ chain on tumor-infiltrating lymphocytes (TILs) of RCC.29 The CD3ζ chain is part of the T cell receptor (TCR) complex and normally plays a critical role in the proximal signaling events leading to T cell activation. Its reduced expression leads to impaired TCR signaling, causing a disturbed lytic function of the TILs.22 VEGF, along with IL-6 and IL-10, also induces signal transducer and activator of transcription 3 (STAT3) activation.30 STAT3 activation is thought to be involved in the accumulation of immunosuppressive cells, such as MDSCs and regulatory T cells (Tregs),31,32 and in the absence of functional DCs.31 In addition, STAT3 activation might be responsible for the reduced CTL reactivity in RCC, since STAT3 is required for the expression of HIF-1α,33 constitutively activated in the majority of RCC,34 and gene silencing of HIF-1α was seen to restore the susceptibility of tumor cells to CTL-mediated killing.30 These mechanisms might explain why both VEGF expression in tumor tissue and serum levels of VEGF are associated with poor prognosis in RCC patients.35,36
T cells are only activated when the balance between co-stimulatory and co-inhibitory signals crosses the threshold for T cell activation.37 Therefore, the expression, by both primary and metastatic RCC tumor cells, of the co-inhibitory molecule programmed death ligand 1 (PD-L1) might shift the balance toward T cell inhibition.38 The expression of the co-inhibitory molecule PD-L1 in RCC is associated with aggressive tumor behavior and poor outcome.39,40 RCC tumor cells also often express the negative co-stimulatory molecule B7-H4. Its expression is associated with adverse clinical features.38,41 Recently, a new co-inhibitory molecule, T cell immunoglobulin and mucin domain 3 (TIM-3), was described. The molecule is expressed by Th1 cells and CTLs and induces cell death by binding its ligand, galactin-9. Furthermore, the upregulated expression of TIM-3 on tumor-specific and tumor-infiltrating CD8+ T cells from patients with clear cell (cc)RCC was associated with poor prognosis.42,43
Since Th1 cells are considered to be effector cells with antitumor activity, achieving a Th1-dominated immune response against RCC cancer cells would be desirable. However, RCC is able to counteract Th1 cell differentiation. Production of IL-10 by the tumor cells causes Th1 cell loss and Th2 cell prevalence.24 Additionally, RCCs do not produce the necessary cytokines, such as IL-2 and IL-12, to foster an optimal development of tumor-specific T cells. On the contrary, they produce TGF-β, which is known to stimulate the recruitment and activation of CD4+ CD25+ FOXP3+ Tregs.22,23 Under the influence of the chemokine CCL22, Tregs accumulate at the tumor site.44 They downregulate the function of immune effector cells through secretion of IL-10, TGF-β27,45 and the immune-regulating enzyme indoleamine-2,3-dioxygenase (IDO).37,46 Tregs are detectable in the peripheral circulation. Frequencies of Tregs in the peripheral circulation of patients with RCC were elevated 3-fold compared to healthy controls24 and increased frequencies were associated with a shorter overall survival.40,47,48
RCC also influences the innate immune system (Fig. 1 Supplementary data). In patients with RCC a high frequency of natural killer (NK) cells in the lymphocytic infiltrate of the primary tumor seems to predict a better prognosis.49,50 However, in advanced RCC, NK cell frequency and activity are often decreased, correlating with poor survival.24 One possible mechanism for the impaired NK cell activity is the shedding of MHC class I-related chain A (MICA), a soluble NKG2D ligand, from the tumor cell surface into the circulation.24 This causes a down-modulation of the NK cell-activating receptor, NKG2D, resulting in decreased cytotoxicity.51 In addition, by secreting IL-10, cancer cells induce the polarization of tumor-associated macrophages from a pro-inflammatory (M1) to an anti-inflammatory (M2) phenotype.24,52 It is STAT3 signaling that plays an important role in this conversion.31 M1 macrophages are hypothesized to bear antitumor activities because they produce high levels of inflammatory cytokines, such as IL-12 and tumor necrosis factor α (TNFα). On the contrary, M2 macrophages produce anti-inflammatory cytokines, such as IL-10 and IL-6.47,53 In RCC, M2 macrophages are associated with a more advance tumor stage, while the opposite is held true for M1 macrophages.24,47,52
Immunogenic Potential of Radiotherapy in RCC
The role of radiotherapy in metastatic RCC is used to palliate symptomatic metastases3 as RCC has been traditionally considered a radiation-resistant tumor. Although RCC might be resistant to conventional fractionated radiation (daily fractions of 1.8–3.0 Gy), a recent review suggested the opposite for hypofractionated radiotherapy (HFRT), typically delivering ≥ 5 Gy per fraction, in a single or a few fractions. HFRT, results in a different tumor radiobiology compared to conventional fractionated radiotherapy. One of the effects involves increased endothelial cell apoptosis, triggered by acid sphingomyelinase (ASMase)-induced ceramide release. Others have suggested that HFRT activates de novo synthesis of ceramide. Ceramide is able to initiate an apoptotic cell death through the release of mitochondrial cytochrome c.54 Therefore, HFRT, in contrast to conventional radiotherapy, efficiently destroys tumor microvasculature and is expected to have better results in tumors that are highly dependent on angiogenesis, such as RCC. This is supported by the excellent local tumor control of HFRT.55 HFRT has already been proven to be very safe in the treatment of oligometastatic disease. A systematic review of Kothari et al. reported one year local control rates of 88% and 86% for intra- and extracranial metastases, respectively. Grade 3–4 toxicity ranged between 0 and 6%.56 A prospective phase II trial for patients with brain metastases from so-called radio-resistant primary tumors, including RCC, showed median survival rates with stereotactic radiosurgery (SRS), which were comparable to surgical series.57 A prospective phase II trial using extracranial HFRT in mRCC or inoperable primary RCC showed local control in 98% of treated lesions,58 making it an excellent alternative to metastasectomy for treatment of extracranial metastases that are technically inoperable. Future randomized trials are required to confirm the additional benefit of HFRT above conventional radiotherapy.
The encouraging results of HFRT might also be explained by the effect radiotherapy has on the immune system.59 In the next paragraphs, we provide evidence for the potential of radiotherapy in shifting the balance back toward tumor control (Fig. 3). To date, little is known about which dose/fractionation regimens optimally enhance the antitumor immune response (25), but the majority of preclinical studies has investigated the effect of HFRT (22, 25) (Table 1).
Figure 3.

The balance between pro-immunogenic and immunosuppressive effects of radiotherapy and tumor rejection. Radiation promotes the antitumor immune response. Key molecular signals that promote priming of antitumor cytotoxic T cells (CTLs) by dendritic cells (DCs) loaded with tumor antigens include exposure of calreticulin (CRT) and heat shock protein (HSP) 70 and release of ATP and high-mobility group box 1 (HMGB1). These signals are released by the tumor cells undergoing a radiation-induced immunogenic cell death. Tumor infiltration by T cells that produce interferon γ (IFNγ) and tumor necrosis factor α (TNF-α) is facilitated by upregulation of vascular cellular adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) on tumor endothelium. Radiation-induced upregulation of major histocompatibility complex class 1 (MHC-1), NKG2D ligands (NKG2DL) and the co-stimulatory molecule CD80 on surviving tumor cells improves their recognition and killing by T cells. On the other hand, radiation activates immunosuppressive transforming growth factor β (TGF-β) and Signal transducer and activator of transcription 3 (STAT3), stimulates the secretion of vascular endothelial growth factor (VEGF), and promotes accumulation of regulatory T cells (Tregs) and pro-tumorigenic M2 macrophages.
Table 1.
Immunogenic potential of radiotherapy
| A. Pro-immunogenic effects | |||
|---|---|---|---|
| Effect on the TME | Conventional RT | HFRT | References |
| Increases the surface expression of NKG2D ligand | Unknown | Yes | 81 |
| Provide tumor antigens | Unknown | Yes | 57,58 |
| DC activation | Yes | Yes | 56,61 |
| CRT exposure | Unknown | Yes | 57 |
| ATP secretion | Unknown | Yes | 57 |
| Release of HMGB1 | Unknown | Yes | 57 |
| Increase of MHC-I expression | Yes | Yes | 57,70 |
| Increase of ICAM-1 expression | Unknown | Yes | 57,70 |
| Induction of IFNγ production | Unknown | Yes | 66 |
| Induction of type 1 IFN | Unknown | Yes | 72 |
| Stimulation of CD8+ effector T cells | Unknown | Yes | 72 |
| B. Immunosuppressive effects | |||
| Effect on the TME | Conventional RT | HFRT | References |
| Induction of TGF-β | Yes | Yes | 77 |
| Secretion of VEGF | Yes | Yes | 78 |
| Induction of M2 macrophages | Unknown | Yes | 69,84 |
| Activation of STAT3 | Unknown | Yes | 14,84 |
(A) Pro-immunogenic effects: Hypofractionated radiotherapy (HFRT) (fraction sizes more than 5 Gy) is known to promote the antitumor immune response by upregulation of NKG2D ligands (NKG2DL), major histocompatibility complex class I (MHC-I) and intercellular adhesion molecule 1 (ICAM-1). HFRT activates dendritic cells (DCs) through exposure of calreticulin (CRT) and release of ATP and high-mobility group protein B1 (HMGB1). Activated DCs migrate to local lymphoid organs and stimulate CD8+ effector T cells. CD8+ effector T cells will infiltrate the tumor and produce interferon γ (IFNγ).
Conventional radiotherapy (daily fractions of 1.8–3.0 Gy) is known to promote the antitumor immune response by upregulation of MHC-I and activation of DCs.
(B) Immunosuppressive effects: HFRT is also known to activate signal transducer and activator of transcription 3 (STAT3), promote the secretion of vascular endothelial growth factor (VEGF) and the accumulation of pro-tumorigenic M2 macrophages. Conventional radiotherapy has been observed to activate the immunosuppressive transforming growth factor β (TGF-β) and VEGF.
Radiotherapy is able to hinder RCC in escaping CTL-mediated killing on different levels (Fig. 4). Firstly, irradiated dying cells provide a source of multiple tumor antigens60,61 for cross-presentation by circulating DCs.62-64 Radiotherapy stimulates DC activation by inducing immunogenic cell death (ICD), a cell death modality that is part of a ROS-dependent endoplasmic reticulum (ER) stress response.59,62 ICD stimulates an immune response against dead-cell associated antigens65 and is characterized by exposure of damage-associated molecular patterns (DAMPs), such as calreticulin (CRT)60 and heat shock protein (HSP)70 and release of high-mobility group box 1 (HMGB1)66 and adenosine triphosphate (ATP).60,67 These DAMPs are able to stimulate DC maturation,68 diversifying the TCR repertoire of tumor-specific T cells.69 Therefore, irradiated tumor cells might serve as an in situ autologous tumor vaccine.63 Radiation also induces interferon (IFN)γ production within the tumor microenvironment,70,71 which has been shown to enhance the level of APM-components and to increase the expression of MHC-I molecules on the surface of the tumor cells.14,60,62 Activation of the ceramide pathway in response to HFRT, triggers vascular endothelial cell apoptosis via the ASMase pathway. Such damage also stimulates expression of MHC molecules.72-74
Figure 4.
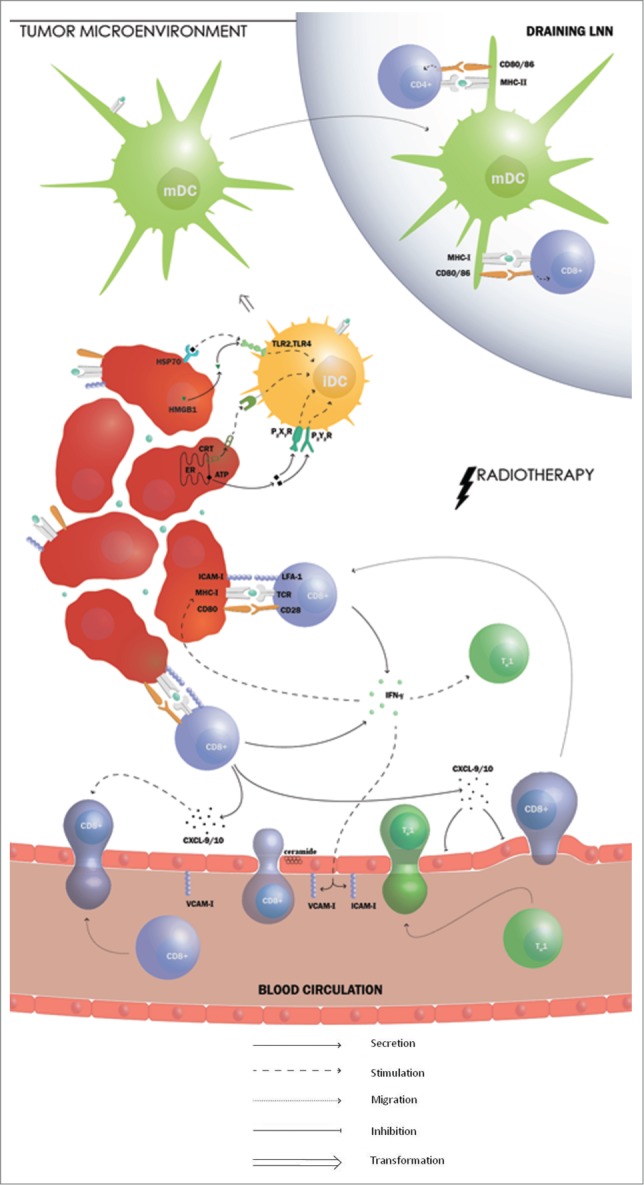
Radiotherapy stimulates the adaptive immune response in RCC. Radiotherapy is able to hinder renal cell carcinoma (RCC) in escaping cytotoxic T lymphocytes (CTL)-mediated killing on different levels. Irradiated dying cells provide a source of multiple tumor antigens for cross-presentation by circulating dendritic cells (DCs) and increases the expression of major histocompatibility complex class I (MHC-I) molecules on the surface of the tumor cells. Furthermore, radiotherapy stimulates DC activation by inducing immunogenic cell death (ICD), a cell death modality that is characterized by exposure of damage-associated molecular patterns (DAMPs), such as calreticulin (CRT) and heat shock protein (HSP)70 and release of high-mobility group box 1 (HMGB1) and adenosine triphosphate (ATP). These DAMPs are able to stimulate DC maturation. Radiation induces interferon (IFN)γ production which plays a role in the accumulation of CD8+ T cells and restores the limited recruitment of Th1-polarized lymphocytes. IFNγ also induces the expression of the adhesion molecules vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1 on tumor vasculature, facilitating T cell adhesion before transmigration. Radiotherapy stimulates the secretion of CXCL9 and CXCL10, which are known to be important T cell chemo-attractants with an antiangiogenic effect. Furthermore, activation of the ceramide pathway in response to hypofractionated radiotherapy triggers vascular endothelial cell apoptosis. Finally, the expression of the co-stimulatory molecule CD80 on DCs has also been found to be increased by radiation and could therefore shift the balance toward T cell activation.
Secondly, the upregulation of IFNγ following radiotherapy also plays a role in the trafficking of CD8+ T cells68,75,76 leading to the accumulation of CD8+ T cells in the tumor. The efficacy of high-dose radiotherapy has been proven to depend on the presence of these CD8+ T cells, since antibody-mediated depletion of CD8+ T cells completely abolished the therapeutic effect.59,71 The accumulation of CD8+ T cells is the result of different IFNγ-induced mechanisms, such as the expression of the adhesion molecules vascular cell adhesion molecule (VCAM)-170 and intercellular adhesion molecule (ICAM)-162,74 on tumor vasculature, facilitating T cell adhesion before transmigration and the secretion of CXCL9 and CXCL10, important T cell chemo-attractants with an anti-angiogenic effect.70 Besides T cells, they also attract monocytes who replenish the amount of DC.77 The expression of the co-stimulatory molecule CD80 on DCs in the tumor microenvironment has also been found to be increased by radiation78 and could therefore shift the balance toward T cell activation.
Thirdly, radiotherapy is able to restore the limited recruitment of Th1-polarized lymphocytes in the tumor microenvironment of RCC14 by shifting the balance from a tumor microenvironment dominated by TGF-β toward a tumor microenvironment enriched with IFNγ, which is responsible for the differentiation of CD4+ T cells into Th1 cells. This is important, because a Th2-dominated response was consistently observed as a poor prognostic factor for patients with RCC.79 Consequently, radiotherapy is able to induce tumor-specific Th1 cells in the non-irradiated draining lymph nodes of the irradiated tumor and favor the trafficking of effector cells into tumors.
However, radiotherapy also induces immunosuppressive mechanisms by activating TGF-β,80,81 stimulating Tregs and inducing the activation of STAT3 and VEGF.82 STAT3 and TGF-β, might hinder the response to ICD.14
Total dose, fractionation, dose distribution and timing of radiotherapy are key variables in determining the effects of radiotherapy on the immune system.83 In a murine melanoma model, a hypofractionated regimen with two fractions of 7, 5 Gy gave the best tumor control and tumor immunity while maintaining low Treg numbers.84 However, the optimal radiation regimen may not necessarily be the same for all tumor types or settings.
Radiotherapy influences components of the innate immune response as well (Fig. 1 Supplementary data). Radiation increases the surface expression of NKG2D ligand,85 which binds the NK cell-activating receptor NKG2D, increasing the susceptibility of NK cells. On the other hand, radiation might also decrease the expression of the NK cell-activating NKG2D receptor,86 through the release of TGF-β.80 In addition, radiotherapy induces the expression of MHC-I molecules. Since NK cells destroy cells that have downregulated expression of MHC class I molecules, induction of MHC-I expression might decrease recognition by NK cells.87 Therefore, it is difficult to predict the net effect of radiation therapy on NK cells. In addition, after radiotherapy a misdirected tissue repair response can promote tumor recurrence and progression. This wound healing response is orchestrated by M2 macrophages who stimulate angiogenesis and contribute to the suppression of antitumor immunity by secreting cytokines such as IL-10.73,88 In contrast, other studies show that HFRT results in the priming of MHC-I molecules and augments cytolytic activity.89,90 Radiotherapy is also able to prime macrophages for pro-inflammatory signaling in a dose-dependent manner, as shown by enhanced IFNγ-mediated NO production and increased TLR-mediated TNF-α secretion.91,92 Furthermore, conventional fractionated radiotherapy was observed to skew macrophage function to an antitumor mode in different murine carcinoma models93 and both conventional and HFRT caused a significant increase of tumor-infiltrating M1 macrophages.94 Importantly, radiotherapy was able to enhance M2 activity in C57BL/6 mice, while increasing M1 activity in CBA/CaJ mice.95 Thus, not only depending on the modulation of cytokine production, but also on the experimental model, radiotherapy has been reported to have different effect on tumor-infiltrating macrophages, therefore the net results in clinical practice is still unclear.
Repurposing of Molecular Targeted Therapies
Because the efficacy of antitumor immune responses depends on the complex balance between diverse immune cells and progressing tumor cells, radiotherapy alone is unlikely to induce persistent antitumor immunity in all treated patients. Therefore, a new role for radiotherapy in combination with synergistic immunomodulatory agents is emerging.62 Significant progress in the understanding of RCC biology has led to the development of targeted therapies such as tyrosine kinase inhibitors (TKIs), mTOR inhibitors and VEGF neutralizing antibodies. Since the pro-oncogenic pathways targeted by these therapies also drive many of the immune-evasion mechanisms of RCC, target therapies have the capacity to optimize antitumor immune responses.96 In the next paragraphs, we highlight the immunomodulatory properties of these agents and will suggest a combination schedule with radiotherapy based on the available literature. The agents and their effects are summarized in Table 2.
Table 2.
General working mechanism of approved targeted therapies and their effect on immune cells
| Drug | General working mechanism | Effect on the immune system | Refs. |
|---|---|---|---|
| Sunitinib | Blocks multiple tumor-associated tyrosine kinases, including VEGFR and PDGFR and c-kit tyrosine kinases | Immunostimulatory: Blocks STAT3 Decreases numbers and effectiveness of MDSCs and Treg cells Stimulates T cell priming by DCs Blocks VEGF signaling Reduces the expression of co-inhibitory molecules PD-1 and CTLA-4 | 85–89,92 |
| Sorafenib | Blocks multiple tumor-associated tyrosine kinases, including VEGFR and PDGFR and c-kit tyrosine kinases | Immunostimulatory: reduces Tregs, decreases NK cell inhibition, stimulates pro-inflammatory activity of macrophages Immunosuppressive: prevents upregulation of co-stimulatory molecules, reduces T cell proliferation, lowers cytokine secretion by DCs | 92,94–96 |
| Pazopanib | Blocks multiple tumor-associated tyrosine kinases, including VEGFR and PDGFR and c-kit tyrosine kinases | Unknown | 97 |
| Axitinib | Blocks multiple tumor-associated tyrosine kinases, including VEGFR and PDGFR and c-kit tyrosine kinases | Immunostimulatory: Reduces Tregs Reduces MDSCs | 98,99 |
| Temsirolimus and Everolimus = mTOR inhibitors | Blocks mTOR pathway | Immunostimulatory: enhances CD8+ T cell activation, enhance IFNγ production, enhance CD8+ T cell differentiation into memory T cells and decreases IDO expression Immunosuppressive: augments the responsiveness of Tregs to antigen | 85,102 |
| Bevacizumab | Blocks angiogenesis | Immunostimulatory: Blocks STAT3 Increases DC maturation Shifts DC differentiation toward mature DCs instead of MDSCs Increases DC priming of T cells | 85 |
Summary of the most important immunomodulatory properties of approved targeted agents in the treatment of renal cell carcinoma. The immunomodulatory properties of not all the targeted agents have been thoroughly studied already.
Abbreviations: VEGFR: vascular endothelial growth factor receptor, PDGFR platelet derived growth factor receptor, STAT3: signal transducer and activator of transcription 3, MDSCs: myeloid-derived suppressor cells, Tregs: regulatory T cells, DC: dendritic cell, VEGF: vascular endothelial growth factor, PD-1: programmed cell death protein-1, CTLA-4: cytotoxic T lymphocyte associated protein 4, NK cell: natural killer cell, mTOR: mammalian target of rapamycin, IFNγ: interferon γ, IDO: indoleamine-2,3-dioxygenase.
In RCC, TKIs not only inhibit angiogenesis and tumor growth, but also have the potential to interact with the immune system.97 TKIs approved for treatment of advanced RCC currently include sunitinib, sorafenib, pazopanib and axitinib. They all target VEGFR, PDGFR and c-kit tyrosine kinases, be it with a different affinity. The most-studied TKI in the treatment of RCC, sunitinib, has important immunostimulatory capacities. It causes downregulation of immunosuppressive Tregs and MDSCs.97,98 It reduces the level of MDSCs through three different mechanisms: the inhibition of STAT3,99 the inhibition of c-kit100 and the inhibition of VEGF receptors.96 Additionally, sunitinib stimulates T cell priming by DCs. It also reduces the expression of co-inhibitory molecules, such as PD-1 and CTL-associated protein 4 (CTLA-4).97 Importantly, sunitinib has already been observed to safely potentiate the radiation-induced antitumor response.17 To optimize the therapeutic effects of this combination, we suggest that the administration of sunitinib should be started prior to radiotherapy, since it affects T cell priming and increases radiation sensitivity by normalizing tumor vasculature.101,102 Because sunitinib also antagonizes the immunosuppressive tumor microenvironment by reducing the levels of MDSCs, Tregs and co-inhibitory molecules, it should be continued after radiotherapy as treatment consolidation.96 Unlike sunitinib, sorafenib has some immune suppressive effects on DC and CD8+ T cell function.103 Sorafenib lowers cytokine secretion by DCs, prevents upregulation of co-stimulatory molecules and reduces the capacity of APCs to stimulate T cell proliferation.104 Even though sorafenib also has some pro-immunogenic activity,105–107 we believe it might not be an ideal candidate to use before radiotherapy, since it might hinder tumor-specific T cell priming.96
Pazopanib and axitinib are more novel TKIs that also inhibit VEGFR and c-kit kinases.108,109 Not much is known about their immunomodulatory capacities. They might have similar effect as sunitinib on the level of MDSCs. Treatment with axitinib in combination with DC-based vaccination was observed to stimulate antitumor immune responses, by reducing the number of intratumoral MDSCs and Tregs and activating tumor-specific CD8+ T cells.110 We suggest that pazopanib and axitinib treatment should be combined with radiotherapy with the same sequence as proposed for sunitinib.96 The safety of the combination of pazopanib with conventional radiotherapy has already been investigated.111
mTOR inhibitors, temsirolimus and everolimus, are known to promote Tregs.112 Combining radiotherapy with a mTOR inhibitor might further boost the stimulation of immunosuppressive Tregs. Even though mTOR inhibition could also increase the quantity of memory T cells,113 it is difficult to predict to which side the balance would be shifted when radiotherapy is added to treatment with mTOR inhibitors.
Bevacizumab is a monoclonal antibody neutralizing VEGFA. Treatment with bevacizumab in combination with IFN-α is also a first-line treatment in metastatic RCC. VEGFA blockade blocks STAT3 signaling and stimulates the antigen presenting capacity of DCs which results in increased T cell proliferation.114 Therefore, combination of radiotherapy with bevacizumab might promote the formation of a radiation-induced antitumor immune response in patients with RCC. Since bevacizumab stimulates DC maturation and T cell priming and increases radiation sensitivity, we suggest that bevacizumab should be administrated prior to radiotherapy.96
Immunotherapy
The combination of radiotherapy with immunotherapies that possess synergistic immunomodulatory properties might also be promising.
Since IL-2 is known to stimulate Th1 responses and treatment with high-dose IL-2 occasionally has been observed to induce complete responses in patients with RCC,6 combining it with radiotherapy may improve clinical effects. A phase 1 study evaluating the combination of SBRT and IL-2, could not detect any dose-limiting adverse effects related to SBRT. Furthermore, response to the combination therapy was correlated to an increased frequency of proliferating early effector CD4+ memory T cells in the peripheral blood.13
Preclinical and clinical evidence suggest that inhibition of CTLA-4, a known inhibitory competitor for the co-stimulatory molecules CD80 and CD86,115 might increase the stimulation of antitumor T effector cells. Ipilimumab, an anti-CTLA-4 antibody, was able to induce tumor regression in 10% of patients with metastatic RCC in a phase II study.116 Since anti-CTLA-4 antibodies decrease co-inhibitory signaling, they might also be able to increase the strength of radiotherapy-induced T cell stimulation. In a murine carcinoma model, the combination of anti-CTLA-4 treatment and radiotherapy was observed to inhibit tumor growth through the formation of a stable interaction between TILs and tumor cells. This stable interaction was largely due to the improved formation of a NKG2D-mediated immunological synapse, complementing weak stimulatory signals from the tumor cells.117 There are already clinical cases and a phase I/II clinical trial69 describing an immune-mediated abscopal effect in melanoma patients receiving a combination of high-dose radiotherapy and ipilimumab.11,12 We suggest that anti-CTLA-4 antibodies should be administered before radiotherapy since they stimulate the removal of Tregs and continued following radiotherapy to prolong the proliferation of antitumor T effector cells.96
Since resistance to the combination of HFRT and ipilimumab in metastatic melanoma patients was correlated to an upregulation of PD-L1, addition of PD-L1 blockade might reverse T cell exhaustion and prevent resistance to the combination therapy. Importantly, preclinical evidence suggests that the combination with radiotherapy is mandatory as dual checkpoint blockade alone proved to be inferior.69 As previously described, the co-inhibitory molecule PD-L1 suppresses T cell responses in RCC, by binding PD-1, and could shift the balance toward tumor progression.29 Furthermore, the expression of PD-L1 in RCC is associated with aggressive tumor behavior and poor outcome.39,40 Blocking PD-1 pathways, therefore, has the potential to increase antitumor immunity in RCC patients. Blockade of PD-1 induced responses in 27% of patients with RCC in a phase 1b study118 and response rates were higher in patients with greater percentages of TILs and PD-L1 expression. Preclinical data confirm that HFRT in combination with anti-PD-1 and anti-PD-L1 treatment synergistically promote antitumor immunity.19,20 In RCC tumors with low inflammation, HFRT might create a more permissive tumor microenvironment, thereby increasing response rates to anti-PD-1/PD-L1 treatment in otherwise non-responding patients. As blockade of PD-1 pathways prevents T cell exhaustion, we suggest it should be administrated directly following radiotherapy. Finally, combinations of radiotherapy with inhibitors of B7-H4 and TIM-3 have not yet been investigated, but are interesting options considering their importance in the antitumor immune response as described above.
Conclusion
RCC is considered an immunogenic tumor, but uses several immune suppressive mechanisms to shift the balance from tumor immune response toward tumor growth. Radiotherapy tries to shift the balance back. However, radiotherapy alone is unlikely to induce persistent antitumor immunity. Therefore, the combination of radiotherapy with drugs having synergistic immunomodulatory properties holds great promise in preventing the immune escape in RCC and might result in superior therapeutic responses. Consequently, prospective trials examining these combinations hold great potential. It should be considered that HFRT might increase the risk of inflammatory reactions. Therefore, phase I trials, assessing the safety of these novel combinations, are essential. In addition, preclinical evidence suggests that high-dose radiation, such as typically delivered by HFRT, results in increased antitumor immunity. Preclinical data also indicate that fractionated radiotherapy might be preferable to single dose radiation. However, these findings need to be confirmed in clinical studies. Besides the optimal HFRT pattern, it is just as important to determine the optimal timing of each treatment combination. Since the optimal treatment sequence, leading to maximum immunologic and clinical benefit while maintaining tolerable toxicities, may vary depending on the specific type of agent used.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Referenences
- 1.Audenet F, Yates DR, Cancel-Tassin G, Cussenot O, Roupret M. Genetic pathways involved in carcinogenesis of clear cell renal cell carcinoma: genomics towards personalized medicine. BJU Int 2012; 109(12):1864-70; PMID:22035299; http://dx.doi.org/ 10.1111/j.1464-410X.2011.10661.x [DOI] [PubMed] [Google Scholar]
- 2.Swanson DA. Surgery for metastases of renal cell carcinoma. Scand J Surg 2004; 93(2):150-5; PMID:15285568 [DOI] [PubMed] [Google Scholar]
- 3.Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, Patard JJ, Mulders PF, Sinescu IC. European Association of Urology Guideline Group . EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol 2010; 58(3):398-406; PMID:20633979; http://dx.doi.org/ 10.1016/j.eururo.2010.06.032 [DOI] [PubMed] [Google Scholar]
- 4.Coppin C, Le L, Porzsolt F, Wilt T. Targeted therapy for advanced renal cell carcinoma. Cochrane Database Syst Rev 2008(2):CD006017; PMID:18425931; http://dx.doi.org/ 10.1002/14651858.CD006017.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iacovelli R, Alesini D, Palazzo A, Trenta P, Santoni M, De Marchis L, Cascinu S, Naso G, Cortesi E. Targeted therapies and complete responses in first line treatment of metastatic renal cell carcinoma. A meta-analysis of published trials. Cancer Treat Rev 2014; 40(2):271-5; PMID:24070900; http://dx.doi.org/ 10.1016/j.ctrv.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 6.Klapper JA, Downey SG, Smith FO, Yang JC, Hughes MS, Kammula US, Sherry RM, Royal RE, Steinberg SM, Rosenberg S. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma : a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer 2008; 113(2):293-301; PMID:18457330; http://dx.doi.org/ 10.1002/cncr.23552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elhilali MM, Gleave M, Fradet Y, Davis I, Venner P, Saad F, Klotz L, Moore R, Ernst S, Paton V. Placebo-associated remissions in a multicentre, randomized, double-blind trial of interferon gamma-1b for the treatment of metastatic renal cell carcinoma. The Canadian Urologic Oncology Group. BJU Int 2000; 86(6):613-8; PMID:11069364; http://dx.doi.org/ 10.1046/j.1464-410x.2000.00880.x [DOI] [PubMed] [Google Scholar]
- 8.Rendon RA. New surgical horizons: the role of cytoreductive nephrectomy for metastatic kidney cancer. Can Urol Assoc J 2007; 1(2 Suppl):S62-8; PMID:18542787; http://dx.doi.org/ 10.5489/cuaj.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishiyama H, Teh BS, Ren H, Chiang S, Tann A, Blanco AI, Paulino AC, Amato R. Spontaneous regression of thoracic metastases while progression of brain metastases after stereotactic radiosurgery and stereotactic body radiotherapy for metastatic renal cell carcinoma: abscopal effect prevented by the blood-brain barrier? Clin Genitourin Cancer 2012; 10(3):196-8; PMID:22409865; http://dx.doi.org/ 10.1016/j.clgc.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 10.Wersall PJ, Blomgren H, Pisa P, Lax I, Kalkner KM, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol 2006; 45(4):493-7; PMID:16760190; http://dx.doi.org/ 10.1080/02841860600604611 [DOI] [PubMed] [Google Scholar]
- 11.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys 2013; 85(2):293-5; PMID:22560555; http://dx.doi.org/ 10.1016/j.ijrobp.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366(10):925-31; PMID:22397654; http://dx.doi.org/ 10.1056/NEJMoa1112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seung SK, Curti BD, Crittenden M, Walker E, Coffey T, Siebert JC, Miller W, Payne R, Glenn L, Bageac A et al.. Phase 1 study of stereotactic body radiotherapy and interleukin-2-tumor and immunological responses. Sci Transl Med 2012; 4(137):137ra74; PMID:22674552; http://dx.doi.org/ 10.1126/scitranslmed.3003649 [DOI] [PubMed] [Google Scholar]
- 14.Durante M, Reppingen N, Held KD. Immunologically augmented cancer treatment using modern radiotherapy. Trends Mol Med 2013; 19(9):565-82; PMID:23831337; http://dx.doi.org/ 10.1016/j.molmed.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 15.Snow RM, Schellhammer PF. Spontaneous regression of metastatic renal cell carcinoma. Urology 1982; 20(2):177-81; PMID:7112827; http://dx.doi.org/ 10.1016/0090-4295(82)90356-9 [DOI] [PubMed] [Google Scholar]
- 16.Meredith RF, Raisch KP, Bonner JA, Buchsbaum DJ, Grizzle WE, Li Y, Spencer SA. Pazopanib combined with radiation: in vivo model of interaction. Cancer Biother Radiopharm 2014; 29(6):247-50; PMID:24945464; http://dx.doi.org/ 10.1089/cbr.2013.1583 [DOI] [PubMed] [Google Scholar]
- 17.Kao J, Chen CT, Tong CC, Packer SH, Schwartz M, Chen SH, Sung MW. Concurrent sunitinib and stereotactic body radiotherapy for patients with oligometastases: final report of a prospective clinical trial. Target Oncol 2014; 9(2):145-53; PMID:23660867; http://dx.doi.org/ 10.1007/s11523-013-0280-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venton G, Ducournau A, Gross E, Lechevallier E, Rochwerger A, Curvale G, Zink JV, Salas S, Deville JL. Complete pathological response after sequential therapy with sunitinib and radiotherapy for metastatic clear cell renal carcinoma. Anticancer Res 2012; 32(2):701-5; PMID:22287766 [PubMed] [Google Scholar]
- 19.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E et al.. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys 2013; 86(2):343-9; PMID:23462419; http://dx.doi.org/ 10.1016/j.ijrobp.2012.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014; 124(2):687-95; PMID:24382348; http://dx.doi.org/ 10.1172/JCI67313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer 2004; 4(1):11-22; PMID:14708024; http://dx.doi.org/ 10.1038/nrc1252 [DOI] [PubMed] [Google Scholar]
- 22.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Ann Rev Immunol 2007; 25:267-96; PMID:17134371; http://dx.doi.org/ 10.1146/annurev.immunol.25.022106.141609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teng L, Chen Y, Ding D, Dai H, Liu G, Li C. Immunosuppressive effect of renal cell carcinoma on phenotype and function of dendritic cells. Int Urol Nephrol 2013; 46(5):915-20; PMID:24202958; http://dx.doi.org/ 10.1007/s11255-013-0595-8 [DOI] [PubMed] [Google Scholar]
- 24.Frankenberger B, Noessner E, Schendel DJ. Immune suppression in renal cell carcinoma. Semin Cancer Biol 2007; 17(4):330-43; PMID:17656104; http://dx.doi.org/ 10.1016/j.semcancer.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 25.Jonasch E, Futreal PA, Davis IJ, Bailey ST, Kim WY, Brugarolas J, Giaccia AJ, Kurban G, Pause A, Frydman J et al.. State of the science: an update on renal cell carcinoma. Mol Cancer Res 2012; 10(7):859-80; PMID:22638109; http://dx.doi.org/ 10.1158/1541-7786.MCR-12-0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raber P, Ochoa AC, Rodriguez PC. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol Invest 2012; 41(6-7):614-34; PMID:23017138; http://dx.doi.org/ 10.3109/08820139.2012.680634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 2013; 138(2):105-15; PMID:23216602; http://dx.doi.org/ 10.1111/imm.12036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res 2007; 13(2 Pt 2):721s-6s; PMID:17255300; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-2197 [DOI] [PubMed] [Google Scholar]
- 29.Seliger B. Immune Escape Mechanisms of Renal Cell Carcinoma. Eur Urol Suppl 2007; 6(10):616-22; PMID: 18292536; http://dx.doi.org/19265129 10.1016/j.eursup.2007.03.009 [DOI] [Google Scholar]
- 30.Noman MZ, Buart S, Van Pelt J, Richon C, Hasmim M, Leleu N, Suchorska WM, Jalil A, Lecluse Y, El Hage F et al.. The cooperative induction of hypoxia-inducible factor-1 alpha and STAT3 during hypoxia induced an impairment of tumor susceptibility to CTL-mediated cell lysis. J Immunol 2009; 182(6):3510-21; PMID:19265129; http://dx.doi.org/ 10.4049/jimmunol.0800854 [DOI] [PubMed] [Google Scholar]
- 31.Rebe C, Vegran F, Berger H, Ghiringhelli F. STAT3 activation: A key factor in tumor immunoescape. JAKSTAT 2013; 2(1):e23010; PMID:24058791; http://dx.doi.org/ 10.4161/jkst.23010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avalle L, Pensa S, Regis G, Novelli F, Poli V. STAT1 and STAT3 in tumorigenesis: A matter of balance. JAKSTAT 2012; 1(2):65-72; PMID:24058752; http://dx.doi.org/ 10.4161/jkst.20045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, Gritsko T, Turkson J, Kay H, Semenza GL et al.. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene 2005; 24(36):5552-60; PMID:16007214; http://dx.doi.org/ 10.1038/sj.onc.1208719 [DOI] [PubMed] [Google Scholar]
- 34.Baldewijns MM, van Vlodrop IJ, Vermeulen PB, Soetekouw PM, van Engeland M, de Bruine AP. VHL and HIF signalling in renal cell carcinogenesis. J Pathol 2010; 221(2):125-38; PMID:20225241; http://dx.doi.org/ 10.1002/path.2689 [DOI] [PubMed] [Google Scholar]
- 35.Yang CC, Chu KC, Yeh WM. Expression of vascular endothelial growth factor in renal cell carcinoma is correlated with cancer advancement. J Clin Lab Anal 2003; 17(3):85-9; PMID:12696078; http://dx.doi.org/ 10.1002/jcla.10074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobsen J, Grankvist K, Rasmuson T, Ljungberg B. Prognostic importance of serum vascular endothelial growth factor in relation to platelet and leukocyte counts in human renal cell carcinoma. Eur J Cancer Prev 2002; 11(3):245-52; PMID:12131658 [DOI] [PubMed] [Google Scholar]
- 37.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol 2014; 35(2):51-60; PMID:24210163; http://dx.doi.org/ 10.1016/j.it.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seliger B, Quandt D. The expression, function, and clinical relevance of B7 family members in cancer. Cancer Immunol Immunother 2012; 61(8):1327-41; PMID:22695874; http://dx.doi.org/ 10.1007/s00262-012-1293-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clinical Cancer Res 2007; 13(6):1757-61; PMID:17363529; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-2599 [DOI] [PubMed] [Google Scholar]
- 40.Kang MJ, Kim KM, Bae JS, Park HS, Lee H, Chung MJ, Moon WS, Lee DG, Jang KY. Tumor-infiltrating PD1-Positive lymphocytes and FoxP3-positive regulatory t cells predict distant metastatic relapse and survival of clear cell renal cell carcinoma. Transl Oncol 2013; 6(3):282-9; PMID:23730407; http://dx.doi.org/ 10.1593/tlo.13256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A 2006; 103(27):10391-6; PMID:16798883; http://dx.doi.org/ 10.1073/pnas.0600937103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan J, Jiang B, Zhao H, Huang Q. Prognostic implication of TIM-3 in clear cell renal cell carcinoma. Neoplasma 2014; 61(1):35-40; PMID:24195506; http://dx.doi.org/ 10.4149/neo_2014_006 [DOI] [PubMed] [Google Scholar]
- 43.Gao J, Bernatchez C, Sharma P, Radvanyi LG, Hwu P. Advances in the development of cancer immunotherapies. Trends Immunol 2013; 34(2):90-8; PMID:23031830; http://dx.doi.org/ 10.1016/j.it.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savage PA, Malchow S, Leventhal DS. Basic principles of tumor-associated regulatory T cell biology. Trends Immunol 2013; 34(1):33-40; PMID:22999714; http://dx.doi.org/ 10.1016/j.it.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol 2006; 16(1):3-15; PMID:16153857; http://dx.doi.org/ 10.1016/j.semcancer.2005.07.008 [DOI] [PubMed] [Google Scholar]
- 46.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 2013; 34(3):137-43; PMID:23103127; http://dx.doi.org/ 10.1016/j.it.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dannenmann SR, Thielicke J, Stockli M, Matter C, von Boehmer L, Cecconi V, Hermanns T, Hefermehl L, Schraml P, Moch H et al.. Tumor-associated macrophages subvert T-cell function and correlate with reduced survival in clear cell renal cell carcinoma. Oncoimmunology 2013; 2(3):e23562; PMID:23687622; http://dx.doi.org/ 10.4161/onci.23562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liotta F, Gacci M, Frosali F, Querci V, Vittori G, Lapini A, Santarlasci V, Serni S, Cosmi L, Maggi L et al.. Frequency of regulatory T cells in peripheral blood and in tumour-infiltrating lymphocytes correlates with poor prognosis in renal cell carcinoma. BJU Int 2011; 107(9):1500-6; PMID:20735382; http://dx.doi.org/ 10.1111/j.1464-410X.2010.09555.x [DOI] [PubMed] [Google Scholar]
- 49.Noessner E, Brech D, Mendler AN, Masouris I, Schlenker R, Prinz PU. Intratumoral alterations of dendritic-cell differentiation and CD8(+) T-cell anergy are immune escape mechanisms of clear cell renal cell carcinoma. Oncoimmunology 2012; 1(8):1451-3; PMID:23243626; http://dx.doi.org/ 10.4161/onci.21356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cozar JM, Canton J, Tallada M, Concha A, Cabrera T, Garrido F, Ruiz-Cabello Osuna F. Analysis of NK cells and chemokine receptors in tumor infiltrating CD4 T lymphocytes in human renal carcinomas. Cancer Immunol Immunother 2005; 54(9):858-66; PMID:15887015; http://dx.doi.org/ 10.1007/s00262-004-0646-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chitadze G, Bhat J, Lettau M, Janssen O, Kabelitz D. Generation of soluble NKG2D ligands: proteolytic cleavage, exosome secretion and functional implications. Scand J Immunol 2013; 78(2):120-9; PMID:23679194; http://dx.doi.org/ 10.1111/sji.12072 [DOI] [PubMed] [Google Scholar]
- 52.Daurkin I, Eruslanov E, Stoffs T, Perrin GQ, Algood C, Gilbert SM, Rosser CJ, Su LM, Vieweg J, Kusmartsev S. Tumor-associated macrophages mediate immunosuppression in the renal cancer microenvironment by activating the 15-lipoxygenase-2 pathway. Cancer Res 2011; 71(20):6400-9; PMID:21900394; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-1261 [DOI] [PubMed] [Google Scholar]
- 53.Santoni M, Massari F, Amantini C, Nabissi M, Maines F, Burattini L, Berardi R, Santoni G, Montironi R, Tortora G et al.. Emerging role of tumor-associated macrophages as therapeutic targets in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother 2013; 62(12):1757-68; PMID:24132754; http://dx.doi.org/ 10.1007/s00262-013-1487-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene 2003; 22(37):5897-906; PMID:12947396; http://dx.doi.org/ 10.1038/sj.onc.1206702 [DOI] [PubMed] [Google Scholar]
- 55.De Meerleer G, Khoo V, Escudier B, Joniau S, Bossi A, Ost P, Briganti A, Fonteyne V, Van Vulpen M, Lumen N et al.. Radiotherapy for renal-cell carcinoma. Lancet Oncol 2014; 15(4):e170-7; PMID:24694640; http://dx.doi.org/ 10.1016/S1470-2045(13)70569-2 [DOI] [PubMed] [Google Scholar]
- 56.Kothari G, Foroudi F, Gill S, Corcoran NM, Siva S. Outcomes of stereotactic radiotherapy for cranial and extracranial metastatic renal cell carcinoma: a systematic review. Acta Oncol 2015; 54(2):148-57; PMID:25140860; http://dx.doi.org/ 10.3109/0284186X.2014.939298 [DOI] [PubMed] [Google Scholar]
- 57.Manon R, O'Neill A, Knisely J, Werner-Wasik M, Lazarus HM, Wagner H, Gilbert M, Mehta M; Eastern Cooperative Oncology Group . Phase II trial of radiosurgery for one to three newly diagnosed brain metastases from renal cell carcinoma, melanoma, and sarcoma: an Eastern Cooperative Oncology Group study (E 6397). J Clin Oncol 2005; 23(34):8870-6; PMID:16314647; http://dx.doi.org/ 10.1200/JCO.2005.01.8747 [DOI] [PubMed] [Google Scholar]
- 58.Svedman C, Sandstrom P, Pisa P, Blomgren H, Lax I, Kalkner KM, Nilsson S, Wersäll P. A prospective Phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol 2006; 45(7):870-5; PMID:16982552; http://dx.doi.org/ 10.1080/02841860600954875 [DOI] [PubMed] [Google Scholar]
- 59.Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, Schwendener R, Pruschy M, Knuth A, van den Broek M. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol 2012; 189(2):558-66; PMID:22685313; http://dx.doi.org/ 10.4049/jimmunol.1200563 [DOI] [PubMed] [Google Scholar]
- 60.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 2014; 5(2):403-16; PMID:24480782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res 2004; 64(21):7985-94; PMID:15520206; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-1525 [DOI] [PubMed] [Google Scholar]
- 62.Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol 2012; 2:153; PMID:23112958; http://dx.doi.org/ 10.3389/fonc.2012.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hodge JW, Guha C, Neefjes J, Gulley JL. Synergizing radiation therapy and immunotherapy for curing incurable cancers. Opportunities and challenges. Oncology 2008; 22(9):1064-70; discussion 75, 80-1, 84; PMID: 1877795624512329 [PMC free article] [PubMed] [Google Scholar]
- 64.Kulzer L, Rubner Y, Deloch L, Allgauer A, Frey B, Fietkau R, Dörrie J, Schaft N, Gaipl US. Norm- and hypo-fractionated radiotherapy is capable of activating human dendritic cells. J Immunotoxicol 2014; 11(4):328-36; PMID:24512329; http://dx.doi.org/ 10.3109/1547691X.2014.880533 [DOI] [PubMed] [Google Scholar]
- 65.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31:51-72; PMID:23157435; http://dx.doi.org/ 10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- 66.Dudek AM, Garg AD, Krysko DV, De Ruysscher D, Agostinis P. Inducers of immunogenic cancer cell death. Cytokine Growth Factor Rev 2013; 24(4):319-33; PMID:23391812; http://dx.doi.org/ 10.1016/j.cytogfr.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 67.Shiao SL, Coussens LM. The tumor-immune microenvironment and response to radiation therapy. J Mammary Gland Biol Neoplasia 2010; 15(4):411-21; PMID:21161342; http://dx.doi.org/ 10.1007/s10911-010-9194-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fuertes MB, Woo SR, Burnett B, Fu YX, Gajewski TF. Type I interferon response and innate immune sensing of cancer. Trends Immunol 2013; 34(2):67-73; PMID:23122052; http://dx.doi.org/ 10.1016/j.it.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM et al.. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015; 520(7547):373-7; PMID:25754329; http://dx.doi.org/ 10.1038/nature14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol 2008; 180(5):3132-9; PMID: 18292536; http://dx.doi.org/23583648 10.4049/jimmunol.180.5.3132 [DOI] [PubMed] [Google Scholar]
- 71.Gerber SA, Sedlacek AL, Cron KR, Murphy SP, Frelinger JG, Lord EM. IFN-gamma mediates the antitumor effects of radiation therapy in a murine colon tumor. Am J Pathol 2013; 182(6):2345-54; PMID:23583648; http://dx.doi.org/ 10.1016/j.ajpath.2013.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Finkelstein SE, Timmerman R, McBride WH, Schaue D, Hoffe SE, Mantz CA, Wilson GD. The confluence of stereotactic ablative radiotherapy and tumor immunology. Clin Devel Immunol 2011; 2011:439752; PMID:22162711; http://dx.doi.org/ 10.1155/2011/439752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gough MJ, Young K, Crittenden M. The impact of the myeloid response to radiation therapy. Clin Dev Immunol 2013; 2013:281958; PMID:23653658; http://dx.doi.org/ 10.1155/2013/281958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chiriva-Internati M, Grizzi F, Pinkston J, Morrow KJ, D'Cunha N, Frezza EE, Muzzio PC, Kast WM, Cobos E. Gamma-radiation upregulates MHC class I/II and ICAM-I molecules in multiple myeloma cell lines and primary tumors. In Vitro Cell Dev Biol Anim 2006; 42(3-4):89-95; PMID:16759154; http://dx.doi.org/ 10.1290/0508054.1 [DOI] [PubMed] [Google Scholar]
- 75.Liang H, Deng L, Burnette B, Weichselbaum RR, Fu YX. Radiation-induced tumor dormancy reflects an equilibrium between the proliferation and T lymphocyte-mediated death of malignant cells. Oncoimmunology 2013; 2(9):e25668; PMID:24319637; http://dx.doi.org/ 10.4161/onci.25668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lim JY, Gerber SA, Murphy SP, Lord EM. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8(+) T cells. Cancer Immunol Immunother 2014; 63(3):259-71; PMID:24357146; http://dx.doi.org/ 10.1007/s00262-013-1506-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, Di Pucchio T, Connolly J, Fay JW, Pascual V, et al.. Dendritic cell subsets in health and disease. Immunol Rev 2007; 219:118-42; PMID:17850486; http://dx.doi.org/ 10.1111/j.1600-065X.2007.00551.x [DOI] [PubMed] [Google Scholar]
- 78.Bernstein MB, Garnett CT, Zhang H, Velcich A, Wattenberg MM, Gameiro SR, Kalnicki S, Hodge JW, Guha C. Radiation-induced modulation of costimulatory and coinhibitory T-cell signaling molecules on human prostate carcinoma cells promotes productive antitumor immune interactions. Cancer Biother Radiopharm 2014; 29(4):153-61; PMID:24693958; http://dx.doi.org/ 10.1089/cbr.2013.1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma Y, Kepp O, Ghiringhelli F, Apetoh L, Aymeric L, Locher C, Tesniere A, Martins I, Ly A, Haynes NM et al.. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol 2010; 22(3):113-24; PMID:20403709; http://dx.doi.org/ 10.1016/j.smim.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 80.Demaria S, Formenti SC. Role of T lymphocytes in tumor response to radiotherapy. Front Oncol 2012; 2:95; PMID:22937524; http://dx.doi.org/ 10.3389/fonc.2012.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou YC, Liu JY, Li J, Zhang J, Xu YQ, Zhang HW, Qiu LB, Ding GR, Su XM, Mei-Shi. Ionizing radiation promotes migration and invasion of cancer cells through transforming growth factor-beta-mediated epithelial-mesenchymal transition. Int J Radiat Oncol Biol Phys 2011; 81(5):1530-7; PMID:22115555; http://dx.doi.org/ 10.1016/j.ijrobp.2011.06.1956 [DOI] [PubMed] [Google Scholar]
- 82.Kil WJ, Tofilon PJ, Camphausen K. Post-radiation increase in VEGF enhances glioma cell motility in vitro. Radiat Oncol 2012; 7:25; PMID:22356893; http://dx.doi.org/ 10.1186/1748-717X-7-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013; 105(4):256-65; PMID:23291374; http://dx.doi.org/ 10.1093/jnci/djs629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys 2012; 83(4):1306-10; PMID:22208977; http://dx.doi.org/ 10.1016/j.ijrobp.2011.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim JY, Son YO, Park SW, Bae JH, Chung JS, Kim HH, Chung BS, Kim SH, Kang CD. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp Mol Med 2006; 38(5):474-84; PMID:17079863; http://dx.doi.org/ 10.1038/emm.2006.56 [DOI] [PubMed] [Google Scholar]
- 86.Nausch N, Cerwenka A. NKG2D ligands in tumor immunity. Oncogene 2008; 27(45):5944-58; PMID:18836475; http://dx.doi.org/ 10.1038/onc.2008.272 [DOI] [PubMed] [Google Scholar]
- 87.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today 1990; 11(7):237-44; PMID:2201309; http://dx.doi.org/ 10.1016/0167-5699(90)90097-S [DOI] [PubMed] [Google Scholar]
- 88.Lerman OZ, Greives MR, Singh SP, Thanik VD, Chang CC, Seiser N, Brown DJ, Knobel D, Schneider RJ, Formenti SC et al.. Low-dose radiation augments vasculogenesis signaling through HIF-1-dependent and -independent SDF-1 induction. Blood 2010; 116(18):3669-76; PMID:20631377; http://dx.doi.org/ 10.1182/blood-2009-03-213629 [DOI] [PubMed] [Google Scholar]
- 89.Duerst R, Werberig K. Cells of the J774 macrophage cell line are primed for antibody-dependent cell-mediated cytotoxicity following exposure to gamma-irradiation. Cell Immunol 1991; 136(2):361-72; PMID:1831405; http://dx.doi.org/ 10.1016/0008-8749(91)90359-J [DOI] [PubMed] [Google Scholar]
- 90.Lambert LE, Paulnock DM. Modulation of macrophage function by gamma-irradiation. Acquisition of the primed cell intermediate stage of the macrophage tumoricidal activation pathway. J Immunol 1987; 139(8):2834-41; PMID:311609625481260 [PubMed] [Google Scholar]
- 91.McKinney LC, Aquilla EM, Coffin D, Wink DA, Vodovotz Y. Ionizing radiation potentiates the induction of nitric oxide synthase by interferon-gamma and/or lipopolysaccharide in murine macrophage cell lines. Role of tumor necrosis factor-alpha. Ann N Y Acad Sci 2000; 899:61-8; PMID:9766626; http://dx.doi.org/25481260 10.1111/j.1749-6632.2000.tb06176.x [DOI] [PubMed] [Google Scholar]
- 92.Schaue D, Micewicz ED, Ratikan JA, Xie MW, Cheng G, McBride WH. Radiation and inflammation. Semin Radiat Oncol 2015; 25(1):4-10; PMID:25481260; http://dx.doi.org/ 10.1016/j.semradonc.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L et al.. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013; 24(5):589-602; PMID:24209604; http://dx.doi.org/ 10.1016/j.ccr.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 94.Timke C, Winnenthal HS, Klug F, Roeder FF, Bonertz A, Reissfelder C, Rochet N, Koch M, Tjaden C, Buechler MW et al.. Randomized controlled phase I/II study to investigate immune stimulatory effects by low dose radiotherapy in primarily operable pancreatic cancer. BMC Cancer 2011; 11:134; PMID:21489291; http://dx.doi.org/ 10.1186/1471-2407-11-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coates PJ, Rundle JK, Lorimore SA, Wright EG. Indirect macrophage responses to ionizing radiation: implications for genotype-dependent bystander signaling. Cancer Res 2008; 68(2):450-6; PMID:18199539; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-3050 [DOI] [PubMed] [Google Scholar]
- 96.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012; 12(4):237-51; PMID:22437869; http://dx.doi.org/ 10.1038/nrc3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Porta C, Paglino C, Imarisio I, Ganini C, Pedrazzoli P. Immunological effects of multikinase inhibitors for kidney cancer: a clue for integration with cellular therapies? J Cancer 2011; 2:333-8; PMID:21716852; http://dx.doi.org/ 10.7150/jca.2.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res 2009; 69(6):2506-13; PMID:19244102; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kujawski M, Zhang C, Herrmann A, Reckamp K, Scuto A, Jensen M, Deng J, Forman S, Figlin R, Yu H. Targeting STAT3 in adoptively transferred T cells promotes their in vivo expansion and antitumor effects. Cancer Res 2010; 70(23):9599-610; PMID:21118964; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, Schwartz M, Divino CM, Pan PY, Chen SH. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res 2009; 69(6):2514-22; PMID:19276342; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-4709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.El Kaffas A, Giles A, Czarnota GJ. Dose-dependent response of tumor vasculature to radiation therapy in combination with Sunitinib depicted by three-dimensional high-frequency power Doppler ultrasound. Angiogenesis 2013; 16(2):443-54; PMID:23314761; http://dx.doi.org/ 10.1007/s10456-012-9329-2 [DOI] [PubMed] [Google Scholar]
- 102.Truman JP, Garcia-Barros M, Kaag M, Hambardzumyan D, Stancevic B, Chan M, Fuks Z, Kolesnick R, Haimovitz-Friedman A. Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery. PloS One 2010; 5(9); PMID:20941382; http://dx.doi.org/ 10.1371/annotation/6e222ad5-b175-4a00-9d04-4d120568a897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, Radsak MP, Weinschenk T, Singh-Jasuja H, Brossart P. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood 2008; 111(12):5610-20; PMID:18310500; http://dx.doi.org/ 10.1182/blood-2007-02-075945 [DOI] [PubMed] [Google Scholar]
- 104.Dangaj D, Lanitis E, Zhao A, Joshi S, Cheng Y, Sandaltzopoulos R, Ra HJ, Danet-Desnoyers G, Powell DJ Jr, Scholler N. Novel recombinant human b7-h4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses. Cancer Res 2013; 73(15):4820-9; PMID:23722540; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Desar IM, Jacobs JH, Hulsbergen-vandeKaa CA, Oyen WJ, Mulders PF, van der Graaf WT, Adema GJ, van Herpen CM, de Vries IJ. Sorafenib reduces the percentage of tumour infiltrating regulatory T cells in renal cell carcinoma patients. Int J Cancer 2011; 129(2):507-12; PMID:20839259; http://dx.doi.org/ 10.1002/ijc.25674 [DOI] [PubMed] [Google Scholar]
- 106.Kohga K, Takehara T, Tatsumi T, Ishida H, Miyagi T, Hosui A, Hayashi N. Sorafenib inhibits the shedding of major histocompatibility complex class I-related chain A on hepatocellular carcinoma cells by down-regulating a disintegrin and metalloproteinase 9. Hepatology 2010; 51(4):1264-73; PMID:20099300; http://dx.doi.org/ 10.1002/hep.23456 [DOI] [PubMed] [Google Scholar]
- 107.Lin JC, Liu CL, Lee JJ, Liu TP, Ko WC, Huang YC, Wu CH, Chen YJ. Sorafenib induces autophagy and suppresses activation of human macrophage. Int Immunopharmacol 2013; 15(2):333-9; PMID:23337882; http://dx.doi.org/ 10.1016/j.intimp.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gupta S, Spiess PE. The prospects of pazopanib in advanced renal cell carcinoma. Ther Adv Urol 2013; 5(5):223-32; PMID:24082917; http://dx.doi.org/ 10.1177/1756287213495099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kessler ER, Bowles DW, Flaig TW, Lam ET, Jimeno A. Axitinib, a new therapeutic option in renal cell carcinoma. Drugs Today 2012; 48(10):633-44; PMID:23110259; http://dx.doi.org/ 10.1358/dot.2012.48.10.1860768 [DOI] [PubMed] [Google Scholar]
- 110.Bose A, Lowe DB, Rao A, Storkus WJ. Combined vaccine+axitinib therapy yields superior antitumor efficacy in a murine melanoma model. Melanoma Res 2012; 22(3):236-43; PMID:22504156; http://dx.doi.org/ 10.1097/CMR.0b013e3283538293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goyal S, Shah S, Khan AJ, Danish H, Haffty BG. Evaluation of acute locoregional toxicity in patients with breast cancer treated with adjuvant radiotherapy in combination with pazopanib. ISRN Oncol 2012; 2012:896202; PMID:23304555; http://dx.doi.org/19362054 10.5402/2012/896202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weichhart T, Saemann MD. The multiple facets of mTOR in immunity. Trends Immunol 2009; 30(5):218-26; PMID:19362054; http://dx.doi.org/ 10.1016/j.it.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 113.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol 2012; 12(5):325-38; PMID:22517423; http://dx.doi.org/ 10.1038/nri3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, Hurwitz HI, Dev I, Nixon AB, Lyerly HK et al.. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother 2008; 57(8):1115-24; PMID:18193223; http://dx.doi.org/ 10.1007/s00262-007-0441-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res 2013; 19(5):997-1008; PMID:23460531; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-2214 [DOI] [PubMed] [Google Scholar]
- 116.Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis S et al.. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother 2007; 30(8):825-30; PMID:18049334; http://dx.doi.org/ 10.1097/CJI.0b013e318156e47e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ruocco MG, Pilones KA, Kawashima N, Cammer M, Huang J, Babb JS, Liu M, Formenti SC, Dustin ML, Demaria S. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clinical Invest 2012; 122(10):3718-30; PMID:22945631; http://dx.doi.org/ 10.1172/JCI61931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin in Immunol 2012; 24(2):207-12; PMID:22236695; http://dx.doi.org/ 10.1016/j.coi.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


