Abstract
The engineering of T lymphocytes to express chimeric antigen receptors (CARs) aims to establish T cell-mediated tumor immunity rapidly. In this study, we conducted a pilot clinical trial of autologous or donor- derived T cells genetically modified to express a CAR targeting the B-cell antigen CD19 harboring 4-1BB and the CD3ζ moiety. All enrolled patients had relapsed or chemotherapy-refractory B-cell lineage acute lymphocytic leukemia (B-ALL). Of the nine patients, six had definite extramedullary involvement, and the rate of overall survival at 18 weeks was 56%. One of the two patients who received conditioning chemotherapy achieved a three-month durable complete response with partial regression of extramedullary lesions. Four of seven patients who did not receive conditioning chemotherapy achieved dramatic regression or a mixed response in the haematopoietic system and extramedullary tissues for two to nine months. Grade 2–3 graft-versus-host disease (GVHD) was observed in two patients who received substantial donor-derived anti-CD19 CART (chimeric antigen receptor-modified T) cells 3–4 weeks after cell infusions. These results show for the first time that donor-derived anti-CD19 CART cells can cause GVHD and regression of extramedullary B-ALL. This study is registered at www.clinicaltrials.gov as NCT01864889.
Keywords: anti-CD19 chimeric antigen receptor (CAR) T cells, B-cell acute lymphoblastic leukemia (B-ALL), graft-versus-host disease (GVHD), refractory
Abbreviations
- Allo-HSCT
Allogeneic haematopoietic stem cell transplantation
- B-ALL
B-cell acute lymphoblastic leukemi
- CAR
chimeric antigen receptor
- CLL
chronic lymphocytic leukemia
- CNS
cerebral spinal fluid
- CRS
cytokine release syndrome
- GVHD
graft-versus-host disease
- MRD
minimal residual disease
- NHL
non-Hodgkin lymphoma
Introduction
B-ALL in adults remains a challenge for medical oncologists because of poor overall survival. For patients who had received allogeneic haematopoietic stem cell transplantation (allo-HSCT) in first remission, the overall complete remission rate to first salvage therapy was 25%.1 However, many patients never receive a potentially life-saving allo-HSCT due to failure to achieve a second CR after salvage chemotherapy. As a result, data in which all relapses are included in the analysis irrespective of whether allo-HSCT is performed show a much more dismal long-term survival for patients with relapsed B-ALL, despite intensive, highly toxic therapy.2 Furthermore, all salvage therapies currently suited to relapsed B-ALL are associated with much short-term and long-term toxicity. Moreover, leukemia infiltration into extramedullary sites may also reduce leukemia responsiveness to induction chemotherapy,3,4 whereas persisting blasts correlate with decreased overall survival and confer poor prognosis in patients with ALL.5,6 For this reason, new therapeutic regimens for this patient population are needed.
The aim of adoptive transfer of genetically engineered immune effector cells is to rapidly establish T cell–mediated tumor immunity.7,8 CART cell therapy has emerged as a promising strategy for the treatment of cancer.9-11 Expression of CD19 is restricted to B-lineage cells and possibly follicular dendritic cells, and it is found in most B-cell malignancies, including B-ALL.12 The use of the second-generation CART cells targeting CD19 (CART-19) has shown amazing clinical efficacy even in some patients with relapsed and/or refractory acute lymphoblastic leukemia (ALL),13-17 chronic lymphocytic leukemia (CLL)18-21 and non-Hodgkin lymphoma (NHL),22 providing a potential cure strategy for B-cell malignancies.18,23 With the exception of autologous CART-19 cell therapy, donor-derived cell infusion has been widely administered to patients who relapsed after allo-HSCT in several recently registered clinical trials. A few preliminary clinical reports have revealed the feasibility and safety of a strategy of donor-derived CART-19 infusions.24,25 In this report, we enrolled nine adult patients with relapsed or chemotherapy-refractory B-ALL, including three with previous allo-HSCT and six suffering from definite extramedullary involvement which were ineligible in the most other trials,16,17 and treated them with autologous or donor-derived CART-19 cells. Only two out of nine patients received conditioning regimens combined with intensive chemotherapy before CART-19 infusions. Objective clinical response including in both the haematopoietic system and extramedullary tissues was observed in 6 patients in this trial. Two patients who received donor-derived CART-19 developed treatable grade 2–3 GVHD 3–4 weeks after cell infusions. Repeated cytokine release syndrome (CRS) linked with high tumor burdens was observed in most cases. This study provides further support for CART-19 clinical trials in patients with refractory B-ALL and raises the possibility of using CART-19 in the early disease stage.
Results
Patient characteristics
Nine patients with CD19+ B-ALL were enrolled from July 2013 to March 2014 (Table 1). Nine patients with B-ALL had primary refractory disease and had never attained a minimal-residual-disease (MRD) negative remission despite many intensive chemotherapy regimens. Three had previously undergone allogeneic HSCT. Six patients had confirmed extramedullary leukemia involvement or bulky adenopathy after relapse and/or salvage therapy. Extramedullary leukemia involves sequestered sites such as the liver, kidney, bone, muscular tissues, lung, pancreas and central nervous system. Details regarding previous induction and subsequent salvage regimens following relapse are summarized in Table S1.
Table 1.
Patient Clinical Characteristics
| Patient no./ Gender/Age | Diagnosis/ Cytogenetics | Prior therapies | Best response (weeks) | Salvage therapy | Conditioning therapy | CNS status | Extramedullary involvement | Marrow blasts before therapy | Total dose of CART-19 cells | Response to CAR T cells (weeks) | Leukemia free survival / Overall survival (weeks) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/F/49 | C-ALL/Ph+ | MIVP/ HD-MTX/TKI | CR (8) | Hyper-CVAD A/HD-MTX | None | 1 | None | MRD+ | 2.2 × 108(3.0 × 106/kg) | MRD− (9)(at day 28) | 9/54+ |
| 2/M/35 | Burkitt-ALL | BFM95/HD-MTX | CR (16) | Hyper-CVAD A/ Hyper-CVAD B/MCA | C-MOAD | 1 | Lung/Pancreas | 80.8% | 3.6 × 108(5.3 × 106/kg) | Progression | 0/4 |
| 3/F/64 | C-ALL/Ph+ | IOLP/TKI/MTX/VP | CR (48), MRD+ | – | None | 1 | None | 7% | 4.0 × 108(5.9 × 106/kg) | Progression | 0/18 |
| 4/F/65* | B-ALL (lymphoma)/Complex† | R-CHOP × 6/DICE × 6/CHOPE | PR with 3–20% blasts in BM (48) | – | None | 1 | Adenopathy | 94.89% | 2.9 × 108 (6.4 × 106/kg)/5.7 × 108 (1.27 × 107/kg) | Complete response in BM and PB with partial degradation of adenopathy (20) | 0/20 |
| 5/M/23 | MyAg+ALL/Ph+ | CP/VD | PR (8) | VDCD/L-VDCP/L-MTX × 2/TKI | None | 1 | None | 86% | 7.9 × 108(1.0 × 107/kg) | Progression | 0/14 |
| 6/M/32Δ | C-ALL/Ph+ | Hyper-CVAD × 3/ TKI/Intrathecal injection/Craniocerebral radiotherapy | CR (26)CNS3 | Hyper-CVAD A /TKI/Intrathecal injection | None | 3 | CNS | MRD+ | 3.2 × 108(3.6 × 106/kg) | CNS1 (20+)(at day 28) | 20+/25+ |
| 7/M/44* | C-ALL/Ph+ | CODP × 3/TKI/Hyper-CVAD A/Allo-HSCT | CR (24) | CODP/TKI/ DLI × 5/VP/Intrathecal injection/localradiotherapy | None | 3 | CNS | MRD+ | 5.3 × 108 (7.3 × 106/kg)/8.5× 108 (1.2 × 107/kg) | MRD−, CNS1 (38+). (at day 28) Chemotherapy due to renascent subcutaneous lesions | 38+/53+ |
| 8/F/15** | Pro-ALL/E2A-PBX1 | R+Hyper-CVAD × 5/Allo-HSCT | PR | AA/MEA/FLAE/DLI × 1/ Clofarabine | None | 1 | Bone | 66.09% | 2.5 × 108(4.5 × 106/kg) | Hematological improvement and reduction of blast counts of bone marrow (23.15%) (8) | 0/8 |
| 9/F/23** | C-ALL | CODP/Hyper-CVAD A/Hyper-CVAD B/Hyper-CVAD A/Allo-HSCT | CR (40) | DLI × 4/HD-MTX | C-MOAD(Reduced doses) | 1 | Bone, liver, kidney, muscular tissues | 64.79% | 2.3 × 108(4.2 × 106/kg) | Complete response in BM and PB with partial degradation of extramedullary lesions (12) | 0/12+ |
Patients who received 2 CART-19 cell therapeutic regimens.
Patients who received donor-derived CART cell infusions.
This patient suffered from transformed B-ALL from 3 y chronic myeloid leukemia (CML).
This patient suffered from CD19+ leukemia progressed from diffuse large B cell lymphoma with complex karyotype.
F: female; M: male; C-ALL: common B-ALL; Pro-ALL: progenitor B-ALL; MyAg+ALL: myeloid antigen (MyAg) expression-ALL; Ph+: Philadelphia chromosome positive; Allo-HSCT: allo-haematopoietic stem cell transplantation; DLI: donor lymphocyte infusion; TKI: tyrosine kinase inhibitor; MRD: minimal residual disease; CNS: central nervous system; PR: partial remission; CNS3: ≥ 5/μL WBCs, cytospin positive for blasts or Traumatic spinal tap with ≥ 10/μL RBCs, cytospin positive for blasts. CNS1: no detectable leukemia in the CSF. The detailed components and doses of chemotherapy regimens for each patient are described in Supplementary.
Generation, characterization and in vitro anti-leukemia activity of CART-19 cells
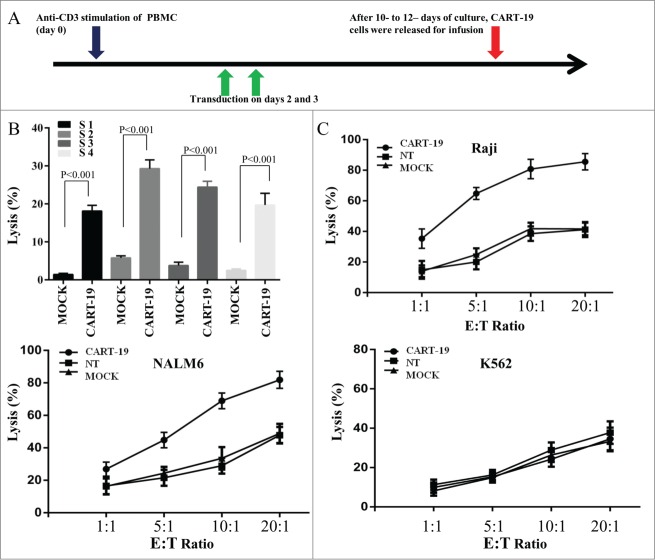
After 10–12 d of culture, cells were released for infusion (Fig. 1A). The verified transfection efficiency of the final products ranged from 13.27% to 44.06 % (Fig. S1B and Table S2). CART-19 cells were principally composed of CD8+ cells (70.87% ± 13.95%), the majority of which express CD62L (63.36% ± 16.11%), with a few of showing a central memory T cell phenotype (CD45RO+CCR7+CD62L+, 15.54% ± 9.96%) (Table S3). The final number of infused cells and the corresponding immunophenotypic data for each patient are summarized in Tables S2 and S3.
Figure 1.
CD19-specific cytotoxic activity of CART-19 cells. (A) CART-19 cells were produced by activating peripheral-blood mononuclear cells (PBMC) with anti-CD3 antibody OKT3 on day 0 and transducing T cells on days 2 and 3. After 10–12 d of culture, cells were released for infusion. (B) Cytotoxic activity of mock-transfected and CART-19 cells against primary CD19+ B-ALL blasts, evaluated in a 6 h CFSE-staining assay; results are shown at an effector:target (E:T) ratio of 20:1. (C) Cytotoxic activity of non-transfected, mock-transfected and CART-19 cells obtained from all nine patients against CD19+ Raji and NALM6 and CD19− K562 cell lines. The data are presented as the mean of triplicate values from each patients, and error bars represent SD. Arrows indicated the date of lentivirus transfection.
Compared with the nontransduced T (NT) cells and mock transduced T cells, we demonstrated that CART-19 cells possessed prominent cytolytic activity against CD19+ Raji, NALM6, cells and CD19+ primary B-ALL blast cells, but not CD19− K562 cells (Figs. 1B and 1C).
Reverse correlation between CAR molecule levels and CD19+ target cell number
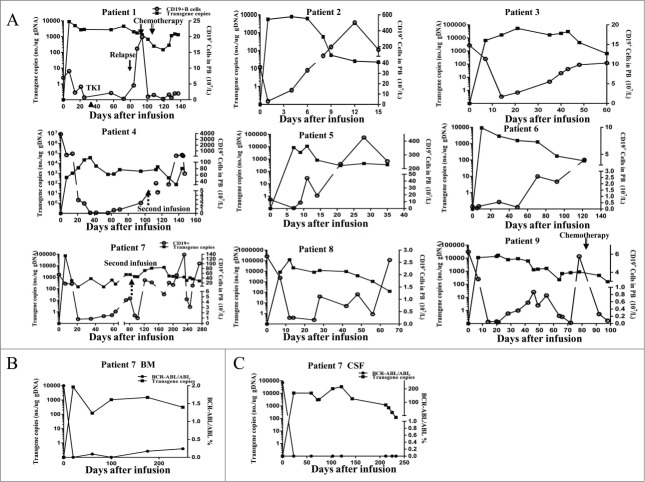
The in vivo persistence of CART-19 cells was measured by quantitative real-time Polymerase Chain Reaction (PCR) of serial peripheral blood (PB) and bone marrow (BM) aspirate samples in this cohort of patients. As shown in Fig. 2A, CAR copy numbers in PB reached their peak value 2–3 weeks after CART-19 infusions in most patients and maintained a high level (>1,000 copies/μg gDNA) for more than 6 weeks in patients 1, 3, 4, 6, 8, and 9. Similarly, high copy numbers were detected from BM samples 2–3 weeks after CART-19 infusions and maintained for at least 6–12 weeks in patients 1, 4, 6, 8 and 9 (Fig. 2B and Figs. S2A and S2B). High levels and long-term maintenance of CAR molecule expression were serially detected in the CSF of patients 6 and 7 who had definite leukemia involvement in the central nervous system (Fig. 2C and Fig. S2C). With the exception of patients 2, 5 and 6, CART-19 treatment induced a marked decrease in CD19+ cell count in PB, BM and CSF (reflected by BCR/ABL molecule marker) within 3–4 weeks, with subsequent observation showing a reverse correlation between CAR levels and CD19 count.
Figure 2.
Copy number of CAR molecules and CD19+ cells in the peripheral blood, bone marrow and Cerrebral spinal fluid. (A) Quantitative real-time PCR was performed on genomic DNA harvested from each patient's PBMCs collected before and at serial time points after CART-19 cell infusion, using primers specific for the transgene. CD19+ B cells expressed as count change from baseline in the blood after the infusion of CART-19 cells in all nine patients. Patients 2, 3, 4, 5, and 8 died before the last follow-up, the time of all patients after cell infusion range from 15 to 140 d. In all panels, ▴ indicates imatinib (TKI) therapy, ↓ indicates the time of relapse, ↑
indicates the time of relapse, ↑ indicates the time of second infusion, ↓
indicates the time of second infusion, ↓ indicates the time of chemotherapy, black squares represent the values for CAR copies by Q-PCR, and black circles indicate CD19+ B cell counts in PB. The first chemotherapy regimen: Cyclophosphamide Etoposide, Vincristine and Dexamethasone. The second chemotherapy regimen: Vincristine, Daunorubicin, Cyclophosphamide and Prednison (B, C) For patient Bone marrow and cerebralspinal fluid aspirates were obtained at serial time points after CART-19 cell infusion in patient 7. Black squares represent the values of CAR copies by Q-PCR and black circles indicate the detection of bcr/abl transcripts.
indicates the time of chemotherapy, black squares represent the values for CAR copies by Q-PCR, and black circles indicate CD19+ B cell counts in PB. The first chemotherapy regimen: Cyclophosphamide Etoposide, Vincristine and Dexamethasone. The second chemotherapy regimen: Vincristine, Daunorubicin, Cyclophosphamide and Prednison (B, C) For patient Bone marrow and cerebralspinal fluid aspirates were obtained at serial time points after CART-19 cell infusion in patient 7. Black squares represent the values of CAR copies by Q-PCR and black circles indicate the detection of bcr/abl transcripts.
Changes of serum cytokines associated with CART-19 treatment
We analyzed the fluctuation of the serum cytokine levels before and after CART-19 infusions for each patient. A transiently elevation of cytokines including IL-6, TNF-α, IFNγ, IL-8, IL-10, Granzyme B, VEGF, IL-12p40, and Granzyme A, albeit at markedly different levels, were observed in nearly all patients in the early stage after cell treatment (within 2–3 weeks), and the subsequent discontinuous fluctuation of IL-6, TNF-α, IFNγ, IL-8, and IL-10 with high levels were observed in patients 1, 2, 4, 8 and 9 (Figs. S3A and S3B). We also analyzed the rapid and extreme variations of cytokine levels from all patients treated in this trial within one month after CART cell infusion (Fig. 3 and Fig. S4A). The peak time of cytokine elevation correlated temporally with the peak levels of CART-19 cells detected in the blood for each patient, which was consisted with previous studies.18 Notably, we found that patients with CRS or GVHD treated with steroids and/or tocilizumab after 30 d exhibited a rapid drop in serum cytokines, consistent with clinical resolution of the CRS. In addition, the decline of CART-19 cells after 30 d may induce the variation of cytokines.
Figure 3.
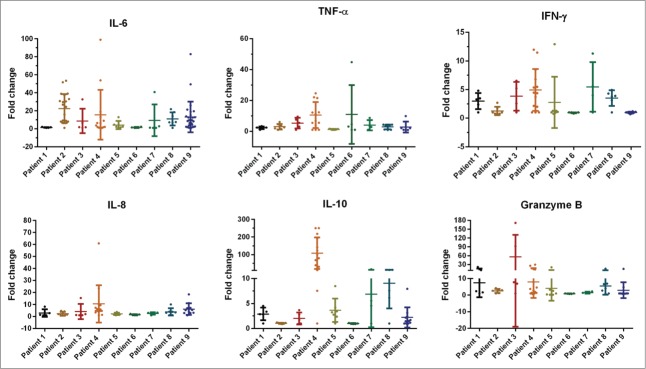
Median values for fold change of cytokines level within the first month after CART-19 cell infusion. Serum was harvested from each patient's PB, collected before and at serial time points after CART-19 cell infusion. Serum cytokines were measured by FACS.
Clinical response after CART-19 infusion
Clinical response assessment was done on day 30 (within 4 d) after CART-19 cell infusion. Patients 2 and 9 were given conditioning chemotherapy for debulking before receiving CART-19 cell infusions. Patients 3, 4 and 8 were not suitable candidates for further chemotherapy due to dyscrasia, cardiac insufficiency, or pulmonary fungal infection. Patient 5 refused any conditioning regimen before CART-19 infusions. Patients 1, 6, and 7 were not given a conditioning treatment in light of their low leukemia burden.
Prior to CART-19 infusions, three patients (patients 1, 6 and 7) had Ph+ minimal residual disease (MRD+) in BM at the molecular level and/or leukemia involvement in the cerebral spinal fluid (CNS3) that was refractory to craniocerebral radiotherapy and intrathecal chemotherapeutic drug injection (MTX/Ara-c/Dex). CART-19 infusion only led to 9 week MRD− remission in patient 1 (Fig. S2A), 38 week MRD− and CNS1 remission in patient 7 (Figs. 2A and 2B), and 20 week CNS1 remission in patient 6 (Fig. S2C). Rapid progression was confirmed morphologically 12 weeks after cell therapy in patient 1; this may have been related to imatinib (TKI) administration beginning from 5 weeks after cell infusion, given possible functional repression of T cells by TKI.26-30 Interestingly and importantly, patient 7 suffered obvious subcutaneous nodules in the limbs and chest wall with MRD− and CNS1 remission 30 weeks after the first CART-19 infusion. The nodules were confirmed to be due to leukemia involvement in subcutaneous adipose tissues by immunohistochemical examination of biopsy specimens (Fig. S5A), and they were sensitive to chemotherapy.
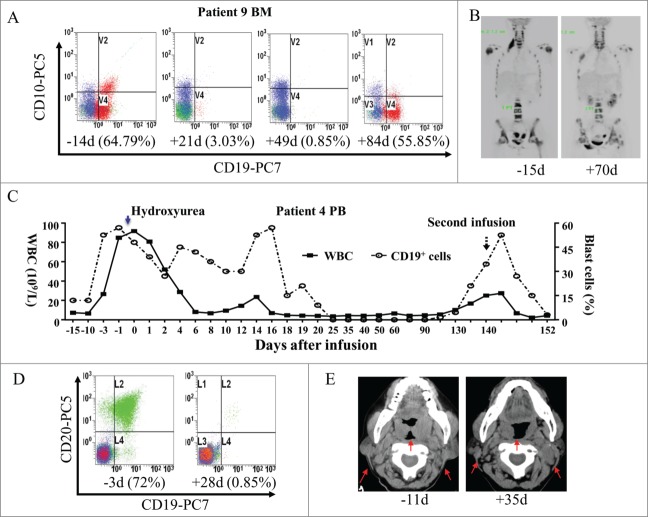
Patients 8 and 9 converted mixed to complete donor chimerism at the time of onset GVHD after donor-derived CART-19 cell infusion. Patient 8 obtained an 8-week relief of cytopenia in the blood and a moderate decrease of blasts in BM after infusion, but rapidly progressed and died shortly after receiving anti-GVHD therapy. Patient 9 gradually reached a complete hematologic remission and a partial regression of her extramedullary leukemic lesions. The bone pain of extramedullary sites could be alleviated after infusion for patient 9 (Figs. 4A and 4B). Similarly, after receiving anti-GVHD therapy consisting of steroids and cyclosporine A (CsA), approximately 50% CD19+ blasts reoccurred in BM 12 weeks after CART-19 infusion (Fig. 4A). Patients 2, 3, and 5, who showed rapid clinical progression of leukemia prior to receiving CART-19 infusion, developed aggressive disease even after CART-19 treatment and soon died (Fig. S6). During the preparation of CART-19 cells, the total white blood cell (WBC) count of patient 4 rose sharply from 1.2 × 109/L to 91.63 × 109/L with approximately 60% blasts in PB on the first day of CART-19 cell infusion. This patient gradually attained complete hematological remission and partial regression of her extensive adenopathy after CART-19 infusion. (Figs. 4C, 4D, and 4E, ). However, the disease reoccurred 20 weeks after the first CART-19 infusion, along with a decreased level of CAR molecule in PB and BM (Fig. 2A and Fig. S2B). This patient received a second CART19 treatment and succumbed from acute tumor lysis syndrome (TLS) related to her extensive and bulky adenopathy 12 d after CART-19 infusion.
Figure 4.
Clinical response to T cell infusions. (A) Flow cytometry for CD19 and CD10 expression in BM before and after treatment. Cells were gated on CD45+7AAD− cells in patient 9. (B) PET-CT scan of patient 9 before treatment and two months after treatment showed partial regression of her extramedullary leukemic lesions. (C) Samples before and after infusion of blood were obtained at the time points indicated on the x-axis and used for WBC counts and blast cell percent. Black squares represent the values of white blood cells (WBC) counts and black circles indicate CD19+ cell percent in PB. ↑ indicates the time of second infusion and ↓
indicates the time of second infusion and ↓ indicates hydroxyurea injection. (D) Flow cytometry for CD20 and CD19 expression in PB before and after treatment. Cells were gated on CD45+7AAD− cells in patient 4. (E) A CT scan shows regression of cervical lymph nodes in patient 4 after infusion of CART-19 cells. ↑
indicates hydroxyurea injection. (D) Flow cytometry for CD20 and CD19 expression in PB before and after treatment. Cells were gated on CD45+7AAD− cells in patient 4. (E) A CT scan shows regression of cervical lymph nodes in patient 4 after infusion of CART-19 cells. ↑ indicates a lymph node mass that regressed.
indicates a lymph node mass that regressed.
Adverse events associated with CART-19 treatment
Nearly all patients developed grade 1–3 chills and fever 1–2 h after CART-19 infusions, and these symptoms subsided overnight. Delayed adverse events after CART-19 therapy are summarized in Table 2. In addition, patients (patients 2 and 9) received conditioning therapy developed neutropenia and thrombocytopenia which were associated with conditioning therapy before and after CART-19 cell infusion, so we did not consider these adverse events owing to patients with CART-19 infusion in this study.
Table 2.
Adverse events after CART-19 infusions
| Patient no. | Adverse events | Grade | Time of occurrence | Description | Duration |
|---|---|---|---|---|---|
| 1 | CRS associated lung injury | 1 | 16 weeks after cell infusion | Intermittent low-grade fever and fatigue. Asymptomatic brochiectasis-like imaging features and ground-glass like change of right middle and lower lung lobes and left lower lobe. | 3 weeks |
| 2 | CRS, acute capillary leaking syndrome, lung and pancreas injuries | 4 | 10 days after infusion | High fever, acute pancreatitis, edema, oliguria, pleural effusion, ascites, dyspnea. | Continous till death after 4 weeks |
| 4 | CRS and tumor lysis syndrome | 4 | 12th day after the second infusion | High fever, rapid shrinks of adenopathy accompanied by electrolyte imbalance, oliguria, dyspnea and heart failure. | Died after 12 h |
| 7 | Oral and genital mucosa ulcers | 1 | 3 weeks after infusion | Pain, ulceration and exudation. | 12 weeks |
| Neurological symptoms | 1 | 4 weeks after the second infusion | Numbness and stiffness of lower limbs and abdominal skin. | 8 weeks | |
| 8 | Neurological symptoms | 1 | 3 weeks after infusion | Facial paralysis and headache, insomnia, irritability. | Continuous |
| GVHD (Liver injury) | 2 | 4 weeks after infusion | Jaundice associated with elevated aminotransferases. | 2 weeks | |
| 9 | Repeated CRS | 3 | 1 week after infusion | High fever and bone and muscle pain | Repeated |
| GVHD (Skin and liver) | 2 | 4 weeks after infusion | Red rash or pimples over her body, elevated aminotransferases. | 4 weeks |
CRS indicates Cytokine release syndrome; GVHD indicates graft-versus-host disease.
Consistent with previous reports, the primary toxicity was CRS,13,14,18,22,31 the severity order of which was primarily associated with tumor burdens the recurrent elevation of serum cytokines such as IL-6. Our results indicated peak levels of IL-6 after infusion of CART-19 cells in patients with severe CRS as compared with patients with CRS that was not severe (Fig. S4B). In most cases such as in patient 9 (Figs. 2A and 5A), CRS was effectively ameliorated without apparent effect on CART-19 cell expansion and persistence by the use of etanercept (anti-TNF-α) or tocilizumab (anti-IL-6R). Interestingly, patient 1 experienced 3 week intermittent low-grade fever and fatigue which may have been related to lung injury three months after CART-19 infusion. Pulmonary CT scanning showed bronchiectasis-like imaging features and ground-glass changes in the right middle and the lower lung lobes and left lower lobe (Fig. 5B). All these abnormalities completely disappeared 2 weeks after one dose of etanercept (25 mg).
Figure 5.
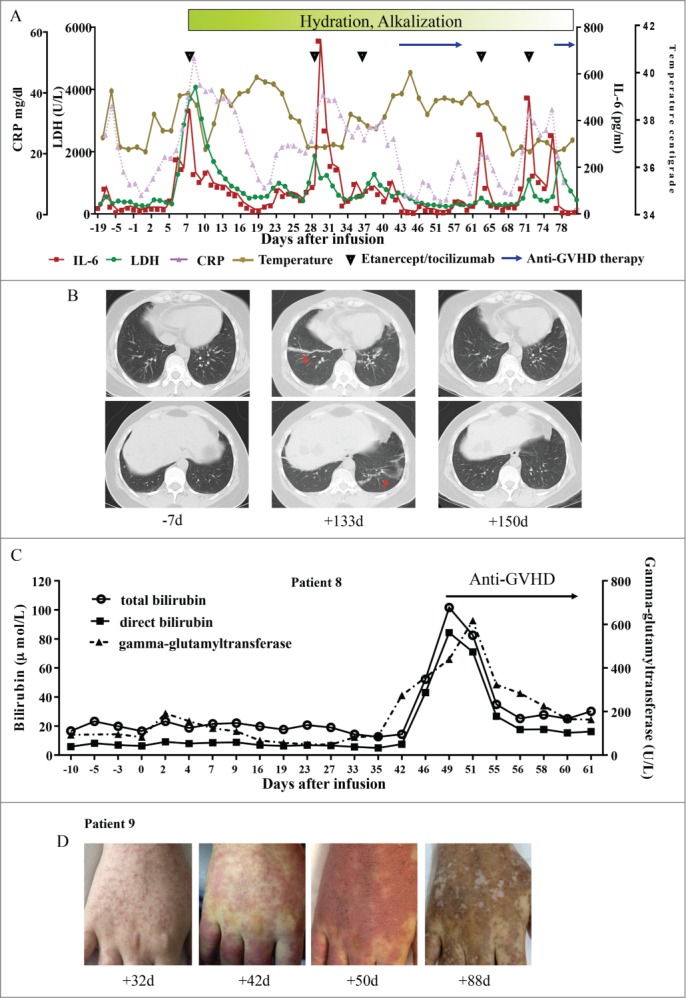
Toxic clinical response to T cell infusion. (A) This panel shows changes in serum lactate dehydrogenase (LDH) levels, IL-6, circulating C reactive protein (CRP) and body temperature before and after CART-19 infusion, with the maximum temperature per 24-h period indicated by the circles in patient 9. (B) Pulmonary CT of patient 1 before and after CART-19 infusion. ↑ indicates the bronchiectasis-like imaging features or ground-glass changes. (C) This panel shows changes in the levels of total bilirubin, direct bilirubin and indirect bilirubin during the period of in which patient 8 developed GVHD. (D) This panel shows chronically aggravated skin damage in patient 9 due to GVHD.
indicates the bronchiectasis-like imaging features or ground-glass changes. (C) This panel shows changes in the levels of total bilirubin, direct bilirubin and indirect bilirubin during the period of in which patient 8 developed GVHD. (D) This panel shows chronically aggravated skin damage in patient 9 due to GVHD.
GVHD induced by donored-derived CART19 cell infusion was observed 3–4 weeks after cell treatment in patients 8 and 9. Aggravated hyperbilirubinemia, elevated aminotransferases in patient 8 (Fig. 5C), and chronically aggravated skin damage in patient 9 (Fig. 5D), were easily controlled and reversed with short-term use of corticoid agents and/or CsA.
Discussion
CD19-targeted autologous T cell therapy has shown remarkable clinical efficacy in adult patients with relapsed and/or refractory B-ALL. This clinical trial was designed to test the clinical efficacy of autologous and donor-derived CART-19 cells in adult patients with chemotherapy-resistant/refractory disease and the related toxicities. The fact that six out of nine evaluable advanced patients experienced objective response in this trial not only demonstrates the potent in vivo antitumor activity of CART-19 cells but also suggests a promising applicability of CART-19 in CD19+ B-cell malignant diseases. Importantly, this study is the first to report that a substantial number of donor T cells specifically targeted to CD19 resulted in clinically significant GVHD in two patients.
Accumulated evidence from previously published data demonstrated that conditioning chemotherapy enhanced the engraftment of transferred T cells and improved clinical response in both hematological malignancies and solid tumors.32,33 All conditioning chemotherapy regimens, particularly the most intensive ones that combine chemotherapies and irradiation,34 appear to improve the persistence of transferred T cells as well as clinical response of malignancies. In recent reports of CRS following adoptive T-cell therapy for cancer,13,14,18,22 the incidence and severity of the syndrome also appears greater when patients have large tumor burdens. In this study, we recommended that two evaluable patients received C-MOAD conditioning regimens that were previously used before enrollment for transient debulking in an attempt to prevent life-threatening CRS. Significantly, one patient (patient 2) treated with CAR-modified T cells and conditioning regimens exhibited a pronounced, slowly progressive tumor response. Nevertheless, another patient (patient 9) exhibited complete regression in BM and PB over three months, with partial regression of extramedullary lesions after CART-19 cell treatment. Our findings suggest that conditioning regimens combined with intensive chemotherapy may prevent life-threatening CRS by reducing tumor burdens. Despite the well-documented benefits of conditioning chemotherapy in enhancing in vivo persistence and antitumor efficacy of adoptively transferred tumor-specific T cells,32,35-37 the role of the pre-infusion conditioning regimens needs to be carefully evaluated.
The persistence of CART-19 cells in ALL patients lasted approximately three months, consistent with previous reports.15,38 There are several explanations for the modest expansion and in vivo persistence of infused CART-19 cells. The first is the number of central memory T cells in CART-19.39 Phenotypic analyses from our trial showed variable levels of central memory T cells in the CART-19 cells (Table S3). Second, some studies have demonstrated that the difference in persistence of CAR-modified T cells was due primarily to the administration of IL-2.40 However, it is not clear that IL-2 injections will help the transferred cytotoxic T cells, so the patients in our trial did not receive IL-2 injections. Third, a number of studies suggest plausible mechanisms for coordinate effects of chemotherapy and CART cells41,42 in addition to the lymph-depleting effects of chemotherapy, which promote homeostatic expansion of adoptive T cells,37 including CART-19 cells. This is consistent with our finding that patients 2 and 9, who received lymphodepleting therapy, demonstrated more rapid in vivo expansion after cell infusion (Fig. 2A). Finally, there is evidence that the use of supportive treatments such as treatment for CRS and GVHD affect the survival of CART-19 cells. Administration of high lymphotoxic doses of steroids to treat GVHD in patient 9 resulted in a rapid amelioration of clinical symptoms but significantly abrogated CART-19 cell expansion and persistence (Fig. 2A). Furthermore, progression of malignancy was observed in patients 8 and 9 after anti-GVHD therapy with short-term steroid treatment, consistent with previous reports.15 Thus, factors influencing the expansion and persistence of CAR-modified cells are complicated and need to be fully elucidated.
GVHD – a severe adverse event – was observed in patients 8 and 9 who were treated with donor-derived CART-19 cells in this study. This contrasts with other recent CAR studies in which none of patients had any evidence of GVHD after infusion of donor-derived CART-19 cells.24,25 In addition, patient 7 was treated with donor-derived but autologously collected CART-19 cells and no evidence of GVHD was noted. Several groups' exhibited chimerism levels have been associated with incidence of GVHD.43,44 Some reports43 observed that achievement of complete chimerism usually preceded grade 2–4 acute GVHD. Meanwhile, other studies have also indicated that acute GVHD is a strong predictor of complete donor chimerism. These findings are also compatible with our study revealing that patients 7 and 9 had complete chimerism at the time of onset of grade 2–3 GVHD. It is speculated that this is the reason why GVHD has not been observed in those patients of the previous studies who may have been completely chimerised and tolerized before donor T cell infusion. It is also conceivable that the total number of infused cells was much higher than in previous studies24,25 and that this may also have affected the development of GVHD in this trial. It should be cautious that the enrolled patients had mixed chimerism with an enhanced risk of GVHD.
Although two patients in whom malignancy regressed after receiving donor-derived CART-19 cells experienced GVHD, we also conclude that the regression of ALL was caused by CART-19 immune responses rather than general donor-versus-host response against allogeneic antigens. Two main features of the clinical course of these patients support this conclusion. First, the regression of these two patients' leukemia cells was evident < 2 weeks after receiving CART-19 cells. Such rapid regression of leukemia cells is not consistent with the slower regression of malignancy that is typically observed after standard donor lymphocyte infusions.40,45 Another reason is that regression of malignancy after CART-19 cell infusions was associated with rapid deficiency of all B cells (Fig. 2A).
The trafficking of targeted effector T cells to tumor sites is a prerequisite for their antitumor activity.46 Indeed, recent clinical data have provided compelling evidence to support the need to evaluate T cell function at the site of disease.47,48 Data accumulated mainly in the context of adverse events demonstrate that infused T cells do, in fact, traffic throughout the body and home to sites where target antigen is expressed.49,50 Here, a quantitative PCR assay was used to assess trafficking of CART-19 cells to multiple sites. We were able to detect CART-19 cells in post-infusion BM aspirates from patients, further supporting that the hypothesis that infused CART-19 cells can migrate to the site of tumor (BM) (Fig. 2B, Figs. S2A and S2B). The highly efficient migration of CART-19 cells to the CSF in patients 6 and 7, who had CNS3 leukemia, further suggests that this treatment holds promise in preventing relapse in the CNS51 and supports the testing of chimeric antigen receptor-directed T cell therapies for CNS lymphomas and primary CNS cancers. We found that the levels of CART-19 cells to multiple sites of tumor involvement, including subcutaneous nodules, CSF, and BM (Fig. S5B), were very low nearly six months after infusion, which may have been a factor in the relapse of patient 7.
Generally, patients with CNS leukemia and isolated extramedullary leukemia are considered as having high-risk ALL with a poor prognosis. In our study, extramedullary sites such as bone and muscular tissue have obtained a partial regression might be the result of CART cell infusion. This is supported by our previous study suggesting that a predominance of CAR T cells could traffick into lung and liver tissue,52 which may lead to the tumor regression of extramedullary sites in this study. However, why other extramedullary sites, such as adipose tissues, do not obtain a regression after CAR T cell infusion remains unclear. Intriguingly, we observed that immunohistochemical examination of biopsy specimens of subcutaneous adipose tissues from patient 7 after infusion show that tumor cells were CD19+ and CD10+ and little CD3+ cells infiltrated the tumor. In addition, it has been suggested that the migration of leukemia cells into extramedullary sites during chemotherapy may leave a reservoir of viable leukemia cells in extramedullary sites that eventually may proliferate and cause relapse. There is growing evidence that leukemia cells allow for directed migration and their retention within extramedullary sites of organ infiltration.53 As such, leukemia cells reside a microenvironment that facilitate their growth and protects them from spontaneous and induced apoptosis.54 Some findings55 indicate that leukemia cells can proliferate efficiently in extramedullary sites, but cannot in the PB where tumor stroma is absent. Furthermore, it has recently been shown that the extramedullary survival of leukemia cells is resistance to death signals, and this likely contributes to the poor clinical response of ALL treated with CART-19 cells. Some studies indicated that no donor cells were detected at the site of the extramedullary tumors.56 It exhibited that immunologically active CAR T cells may not reach isolated extramedullary sites, and recruitment of CAR T cells in these tissues may not be fully operative, or may be delayed. Taken together, extramedullary leukemia present a similar set of challenges compared with solid tumors: overall lesser sensitivity to T-cell-mediated cytotoxicity, a microenvironment that presents with an array of immunosuppressive mechanisms. Hence, we need new approaches to improve therapeutic efficacy in extramedullary leukemia such as intratumoral T cell administrator while ensuring patient safety is an essential goal that requires further investigation.
In conclusion, adoptive immunotherapy with CD19-directed CAR-modified T cells is a feasible and possibly effective treatment modality for adult B-ALL patients with CNS3 leukemia. Debulking conditioning regimens combined intensive chemotherapy prior to CAR-modified T cell infusions may improve clinical response. Finally but importantly, it should be emphasized that the toxicity management of GVHD for enrolled patients with mixed chimerism treated with donor-derived CAR-modified T cells must be conducted with extreme caution.
Methods
Patient enrollment and clinical protocols
Adult patients with CD19+ B-ALL were eligible for enrollment. Relapsed or refractory disease was defined as no CR after more than two induction regimens or progression or recurrence of detectable disease after at least two cycles of salvage chemotherapy or HSCT. Patients with CNS3 (CNS3: ≥5/µL WBCs, cytospin positive for blasts or Traumatic spinal tap with ≥10/μL RBCs, cytospin positive for blasts; CNS1: no detectable leukemia in the CSF) leukemia or isolated extramedullary leukemia were eligible. The presence of CNS3 leukemia or isolated extramedullary leukemia was an exclusion criterion in most other studies. Of three patients who had previously undergone allogeneic HSCT, two patients with mixed (54% and 58% cells of donor origin) chimerism received transplant donor-derived CART-19 cells and one patient with complete chimerism was treated with donor-derived but autologously collected CART-19 cells. Patients with high tumor burden were considered for a conditioning treatment for lymphodepletion and debulking while preparing the T cell expansion only if they refused and/or were not suitable for further chemotherapy due to comorbidity. The first day of cell infusion was set as study day 0. CART-19 cells were transfused in escalating doses over a period of 3–5 consecutive days on the basis of total cell numbers not on CART-19 cells. For each patient, the total number of CART-19 cell infused was ≥3.0 × 106/kg. Adverse events during and after therapy were assessed according to the National Institutes of Health Common Terminology Criteria for Adverse Events Version 3.0 (http://ctep.cancer.gov/). All patients provided written informed consent in accordance with the Declaration of Helsinki before enrolling in the study. The protocol (ClinicalTrials.gov identifier NCT01864889) was approved by the Institutional Review Board at the Chinese PLA General Hospital. No commercial sponsor was involved in the study.
Constructs and lentivirus package
The single chain fragment variable (scFv) sequence specific for CD19 was derived from HM852952.1 (GeneBank No.). CAR.19-4-1BBζ vectors harboring anti-CD19 scFv and human 4-1BB and CD3ζ signaling domains were generated (Fig. S1A). The cassettes were cloned into a lentiviral backbone. A pseudotyped, clinical-grade lentiviral vector was produced according to current good manufacturing practices. The green fluorescence protein (GFP) harboring vector CARCD137ζ-GFP was also constructed for verification of transduction efficiency.
Generation and expansion of CAR T cells
CAR T cells were generated as previously described.52,57 CAR T cells were produced by adding the anti-CD3 monoclonal antibody OKT3 (500 ng/μL) directly to whole peripheral-blood mononuclear cells (PBMCs) suspended in culture medium containing interleukin-2 (IL-2) (500 U/mL). Lentivirus-mediated CAR transduction was performed twice, on days 2 and 3 of cell culture in 24-well plates precoated with a recombinant fibronectin fragment. After transduction, the T cell lines were expanded ex vivo in the presence of IL-2 (500 U/mL) added three times weekly, without any additional stimulation with OKT3 antibody.
Immunophenotyping
Anti-human monoclonal antibodies against CD3, CD4, CD8, CD56, CD19, CD45RO, CD62L, and CCR7, were used for immunophenotyping analysis. All these antibodies and isotype-matched monoclonal antibodies were purchased from BD Biosciences (CA, USA). Data acquisition was performed using a FACSCalibur flow cytometer (BD Biosciences).
Cytotoxicity assays
Standard 6 h carboxyfluorescein succinimidyl ester (CFSE) cytotoxicity assays were performed as previously described,52 using CD19− K562, CD19+ NALM6 and Ramos cell lines and patients' autologous primary blasts.
Quantitative PCR
We used real-time PCR to quantify the level of CAR transgenes as described previously.58 A 153-bp (base pair) fragment containing portions of the CD8a chain and adjacent 4-1BB chain was amplified. A standard curve was prepared for absolute quantitation of CAR transgene copies by making serial dilutions of the plasmid that encoded the CAR. A 7-point standard curve was generated consisting of 100 to 108 copies/μL CAR plasmid spiked into 100 ng non-transduced control genomic DNA. Amplification of β-actin was used for normalization of DNA quantities. Quantitative PCR was also used for analysis of leukemia MRD of bcr-abl fusion gene.
Cytokine measurements
Serum IL-2, IL-6, IL-10, IL-12p70, IL-12/IL23p40, IFNγ, TNF-α, VEGF, and Gramyz A levels were batch analyzed using a BD Biosciences microbead sandwich immunoassay according to the manufacturer's instruction.
Statistics
The results are shown as the mean ± standard deviation of the mean (SD) of triplicate measurements (wells). Data were plotted using GraphPad Prism version 5.0. Two-way analysis of variance (ANOVA) was used to determine the significance of the differences between means in all experiments. A P value < 0.05 was considered to be statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author Contributions
Contribution: Han-ren Dai, Wen-ying Zhang, Xiao-lei Li, and Qing-wang Han designed and performed the in vitro experiment, analyzed data, and wrote the manuscript; Ye-lei Guo, Ya-jing Zhang, and Yao Wang performed in vitro experiments; Mei-xia Chen, Kai-chao Feng, Quan-shun Wang, and Hong-li Zhu ensured compliance with regulatory requirements for the clinical trial; Chun-meng Wang, Feng-xia Shi, and Yan Zhang supervised the manufacture of cells in infusion; Su-xia Li and Wei-dong Han enrolled patients in the study, analysis data, and wrote and reviewed the manuscript.
Funding
This study was supported by the grants from the National Natural Science Foundation of China (Nos. 31270820, 81230061, and 81121004), and is also supported by the Beijing Nova Program (No. Z141107001814104) and was partially supported by a grant from the National Basic Science and Development Programme of China (Nos. 2012CB518103, 2012AA020502 and 2013BAI01B00).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Gokbuget N, Stanze D, Beck J, Diedrich H, Horst HA, Huttmann A, Kobbe G, Kreuzer KA, Leimer L, Reichle A et al.. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood 2012; 120:2032-41; PMID:22493293; http://dx.doi.org/ 10.1182/blood-2011-12-399287 [DOI] [PubMed] [Google Scholar]
- 2.Forman SJ, Rowe JM. The myth of the second remission of acute leukemia in the adult. Blood 2013; 121:1077-82; PMID:23243288; http://dx.doi.org/ 10.1182/blood-2012-08-234492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivera GK, Zhou Y, Hancock ML, Gajjar A, Rubnitz J, Ribeiro RC, Sandlund JT, Hudson M, Relling M, Evans WE et al.. Bone marrow recurrence after initial intensive treatment for childhood acute lymphoblastic leukemia. Cancer 2005; 103:368-76; PMID:15599932; http://dx.doi.org/ 10.1002/cncr.20743 [DOI] [PubMed] [Google Scholar]
- 4.Gajjar A, Harrison PL, Sandlund JT, Rivera GK, Ribeiro RC, Rubnitz JE, Razzouk B, Relling MV, Evans WE, Boyett JM et al.. Traumatic lumbar puncture at diagnosis adversely affects outcome in childhood acute lymphoblastic leukemia. Blood 2000; 96:3381-4; PMID:11071631 [PubMed] [Google Scholar]
- 5.Sandlund JT, Harrison PL, Rivera G, Behm FG, Head D, Boyett J, Rubnitz JE, Gajjar A, Raimondi S, Ribeiro R et al.. Persistence of lymphoblasts in bone marrow on day 15 and days 22 to 25 of remission induction predicts a dismal treatment outcome in children with acute lymphoblastic leukemia. Blood 2002; 100:43-7; PMID:12070006; http://dx.doi.org/ 10.1182/blood.V100.1.43 [DOI] [PubMed] [Google Scholar]
- 6.Gajjar A, Ribeiro R, Hancock ML, Rivera GK, Mahmoud H, Sandlund JT, Crist WM, Pui CH. Persistence of circulating blasts after 1 week of multiagent chemotherapy confers a poor prognosis in childhood acute lymphoblastic leukemia. Blood 1995; 86:1292-5; PMID:7632935 [PubMed] [Google Scholar]
- 7.Ruella M, Kalos M. Adoptive immunotherapy for cancer. Immunol Rev 2014; 257:14-38; PMID:24329787; http://dx.doi.org/ 10.1111/imr.12136 [DOI] [PubMed] [Google Scholar]
- 8.Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annu Rev Med 2014; 65:333-47; PMID:24274181; http://dx.doi.org/ 10.1146/annurev-med-060512-150254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maus MV, Fraietta JA, Levine BL, Kalos M, Zhao Y, June CH. Adoptive immunotherapy for cancer or viruses. Annu Rev Immunol 2014; 32:189-225; PMID:24423116; http://dx.doi.org/ 10.1146/annurev-immunol-032713-120136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turtle CJ, Hudecek M, Jensen MC, Riddell SR. Engineered T cells for anti-cancer therapy. Curr Opin Immunol 2012; 24:633-9; PMID:22818942; http://dx.doi.org/ 10.1016/j.coi.2012.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood 2010; 116:1035-44; PMID:20439624; http://dx.doi.org/ 10.1182/blood-2010-01-043737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadler LM, Anderson KC, Marti G, Bates M, Park E, Daley JF, Schlossman SF. B4, a human B lymphocyte-associated antigen expressed on normal, mitogen-activated, and malignant B lymphocytes. J Immunol 1983; 131:244-50; PMID:235150806408173 [PubMed] [Google Scholar]
- 13.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M et al.. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Translat Med 2013; 5:177ra38; PMID:23515080; http://dx.doi.org/ 10.1126/scitranslmed.3005930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF et al.. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Eng J Med 2013; 368:1509-18; PMID:23527958; http://dx.doi.org/ 10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M et al.. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Translat Med 2014; 6:224ra25; PMID:24553386; http://dx.doi.org/ 10.1126/scitranslmed.3008226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DW, Kochenderfer JN, Stetler-Stevenson MA, Cui YK, Delbrook C, Feldman SA, Fry TJ, O Rimas O, Sabatino M, Shah NN.. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015. Feb 7; 385(9967):517-28; PMID: 25319501; http://dx.doi.org/25317870 10.1016/S0140-6736(14)61403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF et al.. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Eng J Med 2014; 371:1507-17; PMID:25317870; http://dx.doi.org/ 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T Cells with Chimeric Antigen Receptors Have Potent Antitumor Effects and Can Establish Memory in Patients with Advanced Leukemia. Sci TYranslat Med 2011; 3; 95ra73; PMID:21832238; http://dx.doi.org/ 10.1126/scitranslmed.3002842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric Antigen Receptor-Modified T Cells in Chronic Lymphoid Leukemia. New Engl J Med 2011; 365:725-33; PMID:21830940; http://dx.doi.org/ 10.1056/NEJMoa1103849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholson IC, Lenton KA, Little DJ, Decorso T, Lee FT, Scott AM, Zola H, Hohmann AW. Construction and characterisation of a functional CD19 specific single chain Fv fragment for immunotherapy of B lineage leukaemia and lymphoma. Molecular immunology 1997; 34:1157-65; PMID:9566763; http://dx.doi.org/ 10.1016/S0161-5890(97)00144-2 [DOI] [PubMed] [Google Scholar]
- 21.Cooper LJ, Topp MS, Serrano LM, Gonzalez S, Chang WC, Naranjo A, Wright C, Popplewell L, Raubitschek A, Forman SJ et al.. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood 2003; 101:1637-44; PMID:12393484; http://dx.doi.org/ 10.1182/blood-2002-07-1989 [DOI] [PubMed] [Google Scholar]
- 22.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM et al.. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012; 119:2709-20; PMID:22160384; http://dx.doi.org/ 10.1182/blood-2011-10-384388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol 2013; 10:267-76; PMID:23546520; http://dx.doi.org/ 10.1038/nrclinonc.2013.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, Hakim FT, Halverson DC, Fowler DH, Hardy NM et al.. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 2013; 122:4129-39; PMID:24055823; http://dx.doi.org/ 10.1182/blood-2013-08-519413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, Diouf O, Liu E, Barrett AJ, Ito S et al.. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood 2013; 122:2965-73; PMID:24030379; http://dx.doi.org/ 10.1182/blood-2013-06-506741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohon P, Porkka K, Mustjoki S. Immunoprofiling of patients with chronic myeloid leukemia at diagnosis and during tyrosine kinase inhibitor therapy. Eur J Haematol 2010; 85:387-98; PMID:20662899; http://dx.doi.org/ 10.1111/j.1600-0609.2010.01501.x [DOI] [PubMed] [Google Scholar]
- 27.Paniagua RT, Sharpe O, Ho PP, Chan SM, Chang A, Higgins JP, Tomooka BH, Thomas FM, Song JJ, Goodman SB et al.. Selective tyrosine kinase inhibition by imatinib mesylate for the treatment of autoimmune arthritis. The Journal of clinical investigation 2006; 116:2633-42; PMID:16981009; http://dx.doi.org/ 10.1172/JCI28546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seggewiss R, Lore K, Greiner E, Magnusson MK, Price DA, Douek DC, Dunbar CE, Wiestner A. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood 2005; 105:2473-9; PMID:15572591; http://dx.doi.org/ 10.1182/blood-2004-07-2527 [DOI] [PubMed] [Google Scholar]
- 29.Cwynarski K, Laylor R, Macchiarulo E, Goldman J, Lombardi G, Melo JV, Dazzi F. Imatinib inhibits the activation and proliferation of normal T lymphocytes in vitro. Leukemia 2004; 18:1332-9; PMID:15190258; http://dx.doi.org/ 10.1038/sj.leu.2403401 [DOI] [PubMed] [Google Scholar]
- 30.Dietz AB, Souan L, Knutson GJ, Bulur PA, Litzow MR, Vuk-Pavlovic S. Imatinib mesylate inhibits T-cell proliferation in vitro and delayed-type hypersensitivity in vivo. Blood 2004; 104:1094-9; PMID:15100154; http://dx.doi.org/ 10.1182/blood-2003-12-4266 [DOI] [PubMed] [Google Scholar]
- 31.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014; 124:188-95; PMID:24876563; http://dx.doi.org/ 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF et al.. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2008; 26:5233-9; PMID:18809613; http://dx.doi.org/ 10.1200/JCO.2008.16.5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM et al.. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002; 298:850-4; PMID:12242449; http://dx.doi.org/ 10.1126/science.1076514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wrzesinski C, Paulos CM, Kaiser A, Muranski P, Palmer DC, Gattinoni L, Yu Z, Rosenberg SA, Restifo NP et al.. Increased Intensity Lymphodepletion Enhances Tumor Treatment Efficacy of Adoptively Transferred Tumor-specific T Cells. J Immunother 2010; 33:1-7; PMID:19952961; http://dx.doi.org/ 10.1097/CJI.0b013e3181b88ffc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JH, Brentjens RJ. Adoptive immunotherapy for B-cell malignancies with autologous chimeric antigen receptor modified tumor targeted T cells. Discovery medicine 2010; 9:277-88; PMID:20423671 [PMC free article] [PubMed] [Google Scholar]
- 36.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, Maric I, Raffeld M, Nathan DA, Lanier BJ et al.. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010; 116:4099-102; PMID:20668228; http://dx.doi.org/ 10.1182/blood-2010-04-281931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallen H, Thompson JA, Reilly JZ, Rodmyre RM, Cao J, Yee C. Fludarabine modulates immune response and extends in vivo survival of adoptively transferred CD8 T cells in patients with metastatic melanoma. PloS one 2009; 4:e4749; PMID:19270751; http://dx.doi.org/ 10.1371/journal.pone.0004749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brentjens RJ, Riviere I, Park JH, Davila ML, Wang XY, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O et al.. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011; 118:4817-28; PMID:21849486; http://dx.doi.org/ 10.1182/blood-2011-04-348540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. The Journal of clinical investigation 2008; 118:294-305; PMID:18060041; http://dx.doi.org/ 10.1172/JCI32103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deol A, Lum LG. Role of donor lymphocyte infusions in relapsed hematological malignancies after stem cell transplantation revisited. Cancer treatment reviews 2010; 36:528-38; PMID:20381970; http://dx.doi.org/ 10.1016/j.ctrv.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nature reviews Immunology 2008; 8:59-73; PMID:18097448; http://dx.doi.org/ 10.1038/nri2216 [DOI] [PubMed] [Google Scholar]
- 42.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, Altiok S, Celis E, Gabrilovich DI. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. The Journal of clinical investigation 2010; 120:1111-24; PMID:20234093; http://dx.doi.org/ 10.1172/JCI40269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Childs R, Clave E, Contentin N, Jayasekera D, Hensel N, Leitman S, Read EJ, Carter C, Bahceci E, Young NS et al.. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood 1999; 94:3234-41; PMID:10556212 [PubMed] [Google Scholar]
- 44.Baron F, Baker JE, Storb R, Gooley TA, Sandmaier BM, Maris MB, Maloney DG, Heimfeld S, Oparin D, Zellmer E et al.. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood 2004; 104:2254-62; PMID:15226174; http://dx.doi.org/ 10.1182/blood-2004-04-1506 [DOI] [PubMed] [Google Scholar]
- 45.Kolb HJ, Schmid C, Barrett AJ, Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood 2004; 103:767-76; PMID:12958064; http://dx.doi.org/ 10.1182/blood-2003-02-0342 [DOI] [PubMed] [Google Scholar]
- 46.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ Jr, Rosenberg SA. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. Journal of immunology 2004; 173:7125-30; PMID:15585832; http://dx.doi.org/ 10.4049/jimmunol.173.12.7125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melenhorst JJ, Scheinberg P, Chattopadhyay PK, Gostick E, Ladell K, Roederer M, Hensel NF, Douek DC, Barrett AJ, Price DA. High avidity myeloid leukemia-associated antigen-specific CD8+ T cells preferentially reside in the bone marrow. Blood 2009; 113:2238-44; PMID:18997173; http://dx.doi.org/ 10.1182/blood-2008-04-151969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN et al.. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. The New England journal of medicine 2003; 348:203-13; PMID:12529460; http://dx.doi.org/ 10.1056/NEJMoa020177 [DOI] [PubMed] [Google Scholar]
- 49.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM, Cimino PJ et al.. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013; 122:863-71; PMID:23770775; http://dx.doi.org/ 10.1182/blood-2013-03-490565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa G et al.. Identification of a Titin-Derived HLA-A1-Presented Peptide as a Cross-Reactive Target for Engineered MAGE A3-Directed T Cells. Science translational medicine 2013; 5:197ra103; PMID:23926201; http://dx.doi.org/ 10.1126/scitranslmed.3006034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pullen J, Boyett J, Shuster J, Crist W, Land V, Frankel L, Iyer R, Backstrom L, van Eys J, Harris M et al.. Extended triple intrathecal chemotherapy trial for prevention of CNS relapse in good-risk and poor-risk patients with B-progenitor acute lymphoblastic leukemia: a Pediatric Oncology Group study. J Clin Oncol 1993; 11:839-49; PMID:8487048 [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Zhang WY, Han QW, Liu Y, Dai HR, Guo YL, Bo J, Fan H, Zhang Y, Zhang YJ et al.. Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T cells. Clin Immunol 2014; 155:160-75; PMID:25444722; http://dx.doi.org/ 10.1016/j.clim.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 53.Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood 1999; 94:3658-67; PMID:10572077 [PubMed] [Google Scholar]
- 54.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell'Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood 2000; 96:2655-63; PMID:11023495 [PubMed] [Google Scholar]
- 55.Kersey JH, Wang D, Oberto M. Resistance of t(4;11) (MLL-AF4 fusion gene) leukemias to stress-induced cell death: possible mechanism for extensive extramedullary accumulation of cells and poor prognosis. Leukemia 1998; 12:1561-4; PMID:9766500; http://dx.doi.org/ 10.1038/sj.leu.2401148 [DOI] [PubMed] [Google Scholar]
- 56.Schafer H, Bader P, Kaiserling E, Klingebiel T, Handgretinger R, Kanz L, Einsele H. Extramedullary relapses at uncommon sites after allogeneic stem cell transplantation. Bone Marrow Transpl 2000; 26:1133-4; PMID:11108319; http://dx.doi.org/ 10.1038/sj.bmt.1702645 [DOI] [PubMed] [Google Scholar]
- 57.Wang QS, Wang Y, Lv HY, Han QW, Fan H, Guo B, Wang LL, Han WD. Treatment of CD33-directed Chimeric Antigen Receptor-modified T Cells in One Patient With Relapsed and Refractory Acute Myeloid Leukemia. Molecular therapy : the journal of the American Society of Gene Therapy 2015; 23:184-91; PMID:25174587; http://dx.doi.org/ 10.1038/mt.2014.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, Lindgren CG, Lin Y, Pagel JM, Budde LE et al.. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 2012; 119:3940-50; PMID:22308288; http://dx.doi.org/ 10.1182/blood-2011-10-387969 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.