Abstract
Pain and symptom management at the end of life remains suboptimal.
Pain physicians are uniquely placed to provide interventions for patients with difficult to manage pain.
Interventions such as acupuncture, radiofrequency ablation, neurolysis together with regional analgesia including neuraxial techniques and spinal cordotomy may provide the patient with improved analgesia.
Background
Currently, from discussion with colleagues around the country, few pain medicine units in the United Kingdom formally appear to contribute to the management of pain at the end of life with palliative care colleagues. There may be many reasons for this, yet there are many similarities between the two specialties, which have similar ethe. Both specialties have evolved over the past half century or so. Dame Cicely Saunders, who founded the first modern hospice - St. Christopher's Hospice in Sydenham, SE London (www.stchristophers.org.uk/), is usually seen as inspiring the beginning of specialist Palliative Care which has evolved both clinically and academically, although independently of the hospice movement, since then.1 Pain Medicine has now also evolved since the development of the scientific study of pain by Melzack and Wall in the 1950's, culminating in their famous paper on the Gate Control theory of pain.2 It is now a recognised specialty of its own, having grown as an arm of anaesthetics, now with its own faculty within the Royal College of Anaesthetists, with its own qualifications and its own Society.3
One would expect that the management of pain at the end of life in particular would now be a routine and effectively beneficial part of medicine, with the combined efforts of Palliative Care and Pain Medicine. However, despite the best intentions of clinicians, pain and symptom control at the end of life can still be suboptimal, predominantly because the entire healthcare system has been designed around cure of disease rather than palliation4 and Pain Medicine has evolved from acute pain services, being involved predominantly with non-palliative care (though some might say that they ‘palliate the living’, rather than the dying). The shadow of Harold Shipman and the ensuing reports by Inquiry Judge Dame Janet Smith5,6 have also induced a deep fear of the use of opioids in many non-specialist doctors, aggravating the situation.7,8 Many patients at the end of life may end up with sub-optimal pain and symptom control by their General Practitioners where specialists are not involved. This will change as an increasing number of GP's have a specialist interest in pain and/or palliative care, and GP's are now undergoing more training in palliative care in their training year.9 Nurses may be so afraid of hastening death that they will not administer opioid analgesia ordered by a physician.10
The pain medicine physician can collaborate with palliative care colleagues to aid in the better management of difficult pain at the end of life. Our own unit has been working closely with palliative care colleagues since 1992, when a palliative care specialist recognized how much we could offer to those patients not responding well to systemic techniques of pain management. We were able to, and continue to, offer nerve blocks, acupuncture and spinal blocks and infusions to help those presenting difficulties in pain management. This may be in the acute hospital setting, or in the hospice, and we often continue pain management at home, with the support of local community and MacMillan nurses. Somewhat surprisingly, only one reference to the collaboration of Pain Medicine and Palliative Care in the literature seems to occur over the past decade.11 This article will therefore explore ways in which increased collaboration between the two specialties may improve end of life care.
Pain medicine in end of life care
The pain physician as an expert in pain mechanisms, manifestations and treatment, is uniquely placed to offer services for end of life care that can complement those of palliative care and aid in solving difficult pain problems that cause unacceptable distress. In addition to providing interventional services, the pain expert can help in the pharmacological treatment of difficult pain, such as neuropathic pain, complex regional pain syndrome and pains not responding to large doses of opioids. For example, certain drug combinations shared with various interventions including highly invasive treatments for otherwise intractable pain, such as intrathecal infusions and spinal cordotomy, may improve pain relief in some very difficult situations. The Palliative Care physician working together with the Pain Physician can develop better models of therapy to relieve pain and suffering.
The provision of care during this challenging period has recently become a major theme of the Darzi NHS review, highlighting End of Life care.12 It is important to get it right, as an inability to deal with suffering at the end of life increases demand for euthanasia and assisted suicide.13
Pain at the end of life
Pain is defined by the International Association for the Study of Pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of that damage.”14 Pain, which can be acute or chronic, or a mixture of the two, profoundly affects quality of life and contributes significantly to suffering. Pain is sensed by the nociceptive system, but is mediated centrally by a patient's subjective perceptions and is therefore uniquely perceived by each individual, and will be affected negatively by other factors, especially at the end of life, all contributing to an overall sense of suffering. These co-factors include sleep deprivation, weakness and depression, breathlessness, gastro-intestinal symptoms such as nausea and vomiting, psycho-social factors including poor medical team co-ordination and communication, family worries and financial worries, to name a few.
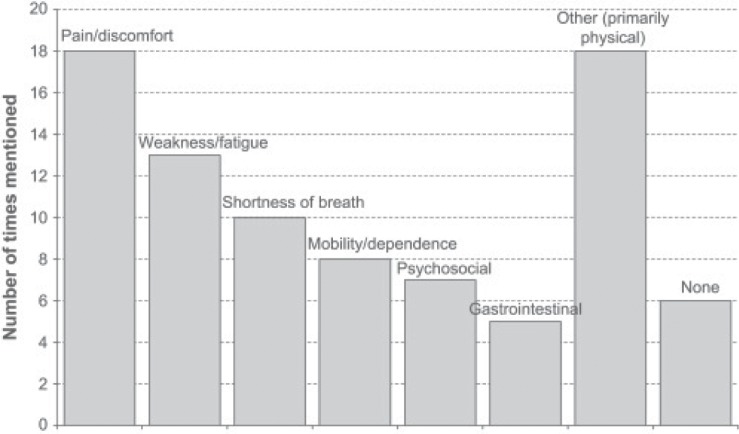
Figure 1 demonstrates the correlates of some of these co-factors15.
Figure 1.
Some co-factors occurring with pain at the end of life (see Kutner et at15).
It is easy when dealing with challenging pain management problems, to become focussed on the technicalities of drug dosing, missing other issues that may be affecting patients, their relatives and their quality of life. These important aspects are very well assessed and managed by the palliative care team. A structured assessment of all the factors affecting pain and suffering at the end of life will help to ensure optimum management of these patients. Dame Cicely Saunders evolved the concept of “total pain”, recognising that terminally ill patients will also suffer other distressing symptoms such as depression or anxiety, family stresses, social problems and spiritual and existential anxieties. Therefore it is wise to assess and manage all potential sources of additional distress. Good pain control requires the appropriate assessment of all possible areas causing distress, for which bringing a multi-disciplinary team approach will ensure greater success.16 The authors remind us (on page S32) that:
“Medical science has come to new understandings about the interplay of the physical, functional, emotional, psychological, social and spiritual aspects of wellbeing and more lately has supported the development of multidisciplinary approaches”.
Excellence in the optimization of care at the end of life is probably best delivered by a multi-professional team utilising the skills and abilities of several different specialties, including the pain physician. The modern hospice movement provides a valuable model for the delivery of carefully integrated, coordinated care, where the pain physician can be a valuable part of an integrated team. Surprisingly, there is little written on this aspect of integral care, and there appears to be no literature on the integration of Pain Medicine and Palliative Care per se.
Pain types
In those dying from cancer, some 70 – 90 % will have significant pain that requires the use of opioid medicines17. More than half of this patient group will experience breakthrough pain at least once a week18. The types of pain diagnosed in patients at the end of life will include all the classifications of pain, including acute and chronic pre-existing nociceptive pain (such as arthritis), neuropathic pain, inflammatory pain and mechanical pain.
The most challenging pains to manage effectively tend to be bone and neuropathic pain. Bone pain may occur with cancer, related to primary tumours of bone, or to metastases. It may occur in other disease states, including Paget's disease, myeloma, lupus, bone abscesses, leukaemia, traumatic fractures and arthritis. Neuropathic pain may be secondary to neuronal tumours, neuronal pressure or involvement of nerves in cancer tumours, effects of chemotherapy or radiotherapy on nerves, and effects of disease exemplified by multiple sclerosis.
Treatment of pain should aim to alleviate moderate to severe pain, using the World health Organisation (WHO) ‘Pain Ladder’ (www.who.int/cancer/palliative/painladder/en/index.html) model, but taking note also of the use of adjuvant therapies such as pregabalin and amitriptyline. Opioid requirements should be titrated according to need as suggested in the WHO model. However, non-opioid adjuvant therapies for neuropathic and somatic pain, for example anti-epileptics such as pregabalin, tricyclic anti-depressants such as amitriptyline and other agents such as the NMDA receptor antagonist ketamine are remarkably effective in many patients. Intelligent use of these medications often results in improved pain control, with fewer and less severe side effects. Opioids remain the mainstay of pain control in the terminally ill, and the WHO pain ladder continues to be the standard approach to pain management in all patients with pain, not just with cancer. When properly implemented, this program has been proved to relieve pain in more than 90 percent of patients.19
The WHO pain ladder, recommends the use of simple analgesics initially (Step 1), followed by compound products containing mild opioids such as codeine and tramadol (Step 2), and finally strong opioids equivalent to morphine (Step 3). Adjuvant therapies are considered at every level. A common error made in using the step model is not starting step 3 opioids soon enough. Earlier use of ‘strong’ opioids avoids the distressingly common problem of excessive intake of potentially toxic co-analgesics such as paracetamol and codeine, particularly in frail, elderly patients with impaired hepatic function. This was recently illustrated in an excessive death rate from co-proxamol, a combination of paracetamol and propoxyphene in those with chronic pain.20 Earlier use also facilitates appropriate use of opioids for breakthrough pain, without the complications of mixing different opioids.
Invasive techniques for pain management
Amongst the benefits that Pain Medicine can bring to management of pain at the end of life, are various invasive techniques that can aid pain relief and help to minimise side-effects from systemically administered drugs. The following is a brief description of what can be achieved.
The following are interventional techniques which pain physicians may be able to offer, working with palliative care physicians:
Acupuncture
Radiofrequency ablation of nerves and joints
Neurolysis of nerves and nerve plexuses
RF ablation of Dorsal Root ganglion
Regional analgesia, including Epidural and Intrathecal techniques
Spinal cordotomy
Acupuncture
Acupuncture can be very effective for some types of pain in patients at the end of life. It is especially useful for back pain, musculoskeletal pain and headache. The mechanism of action of acupuncture remains somewhat of a mystery, although endorphins and other neurotransmitters have been implicated (its effects are at least partially reversed by naloxone21,22. Palliative Care Patients often respond to just one session of treatment, although the majority will need more sessions. Many patients also find acupuncture very relaxing, and it is worth considering acupuncture as an adjunct to other treatments23.
Nerve, nerve root blocks and neurolysis
Specific peripheral nerve-related pain, (such as focal peripheral Neuropathies, well described by Fuller24), may be treated by neural blockade, neurolysis and pulsed radio-frequency ablation. This type of pain includes intercostal neuropathy25; neuralgia of the lateral cutaneous nerve of the thigh26; radiculopathy from nerve root irritation due to metastases, inflammation or vertebral pathology can be amenable to injections of local anaesthetic and steroid, or to pulsed radio-frequency ablation27. For occipital neuropathy contributing to headaches and / or migraine, nerve blocks may be effective. Temporary blocks using long-acting local anaesthetics (Bupivicaine or Ropivacaine), with or without steroids, can last many weeks in some patients, giving long-lasting relief, although increasingly, the use of radio-frequency ablation techniques will give long-term relief28. In conditions such as adhesive capsulitis of the shoulder (frozen shoulder), pulsed radio-frequency ablation of the suprascapular nerve is highly effective29. This technique can be used also for other nerves causing or contributing to pain, such as ilioinguinal neuropathy30.
Nerve plexus blocks are also effective for abdominal visceral pain31. Computer Tomography (CT) – guided neurolysis of the Coeliac plexus are very effective for the pain of pancreatitis, pancreatic cancer and other visceral pain including gastric, lower oesophageal and upper intestinal cancer. Once analgesia has been ascertained using local anaesthesia, permanent neurolysis may then be performed using dehydrogenated alcohol or phenol, or using pulsed or lesional (800C) radio-frequency ablation of the splanchnic nerves, through a posterior approach (although not always as successful as celiac plexus neurolysis).
Antero-lateral spinal cordotomy (tractotomy) by radiofrequency ablation
This is a highly specialised intervention, most effective for the pain from mesothelioma32, but can be used for other types of terminal pain, although not always as effective. There are four centres in the United Kingdom offering this service (see: http://www.micldtnightonmesorf.org/mesothelioma-info/what-the-specialists-say/specialist-pain-relief-service-to-help-mesothelioma-patients/).
Anterolateral cordotomy is an ablative procedure aimed at the pain-conducting tracts in the anterolateral quadrant of the spinal cord. Cordotomy provides selective loss of pain and temperature perception from multiple segments below and contralateral to the segment at which the lesion is placed32. Anterolateral cordotomy is effective for unilateral, mainly somatic pain below the midcervical dermatomes. For visceral or bilaterally occurring pain, bilateral cordotomies can be done. Most cordotomies are performed percutaneously with the patient under local anaesthesia, using fluoroscopic or CT guidance, and the lesion is created by radiofrequency. The percutaneous approach avoids risks of open operation and anaesthesia in patients in poor medical condition.
Spinal analgesia for severe pain at the end of life
There are many reasons why pain may be difficult to control at the end of life, including severe unalleviated pain, resistant to large doses of opioids and adjuvants; unacceptable side effects from excessive dosing of analgesic drugs; sensitivity to drug side effects, limiting dosage; nerve involvement by tumour, or from pressure or other damage; radiculopathy due to spinal metastases, which may be in the bone, compressing nerve roots, or actually involving the nerve roots themselves. Spinal analgesia enables better pain relief with much lower dosing and fewer side effects. Although implanted spinal infusion pumps are available, they are very expensive and do not allow for the regular changing of drug dosage that may be required in progressive disease at the end of life. If the prognosis for the patient is three months or less, external pumps carrying an infusion bag of 100 – 250 ml, infusing via a tunnelled catheter are most commonly used.
Spinal infusions of two or more drugs are usually administered (see an excellent review by Walker et al.33).
Epidural analgesia
Epidural indwelling catheters can relatively easily be used in end of life care, with appropriate service provision 34. To gain the maximum benefit, epidural catheters should be placed at the level of the pain, allowing for equal spread up and down the cord. Epidural catheters often migrate unilaterally and can cause imbalance in pain management towards either side. Gravity tends to pull the solution downwards, depending on which side of the body is lying inferior, or may result in the upper part of the body being in more pain, if the patient is sitting or standing. The presence of spinal metastases in cancer can often push the epidural catheter out, resulting in the need to replace it. Opioids administered epidurally are relatively well absorbed, especially the more lipid soluble compounds such as fentanyl and diamorphine, as discussed below. Morphine, which is more water soluble, may migrate more cranially, sometimes leading to respiratory depression as it affects the respiratory centr35. The standard dosing for epidurally administered opioid is one-tenth of the systemic dose equivalent per twenty-four hours. It can be combined with low-dose local anaesthetic such as bupivacaine, usually less than 0.1%, to minimise motor block and enable mobilisation.
Intrathecal analgesia
Intrathecally placed catheters are generally more stable than epidural catheters. Infused drug mixes with cerebro-spinal fluid, giving a better mix of drug, less dependent on gravity, and allowing even more reduced dosing of opioid. Spinal metastases tend not to push the catheter out, reducing the need to replace it.
Standard dosing of opioid for intra-thecal infusion is usually one-twentieth of the systemic equivalent, which can be started at half this rate and titrated to effect. Intrathecal local anaesthetic may be added to the solution, usually as 0.05% bupivacaine, which minimises motor block, whilst giving a synergistic improvement in pain relief.
Choice of opioid for spinal infusion
Diamorphine is the drug of choice for spinal administration in the United Kingdom. Its pharmacodynamics are ideal in this situation, although it is rapidly metabolised to morphine in-vivo (see Husaini & Russell36). Its intrathecal use in severe cancer pain, in patients already on high doses of opioids, can produce highly effective pain relief, even with those demonstrating nerve root metastases, suggesting a more highly potent action in the spine compared to systemic administration and also with other opioids. Being highly lipid soluble, it tends to remain in the spinal cord, but once metabolised to morphine, it spreads along the spine to broaden its effect. Morphine itself is more water soluble, and it is more likely to cause respiratory depression as it spreads into the brain, unlike diamorphine. Fentanyl is more lipid soluble than diamorphine, but tends to remain within the segment of spine it arrives in, with little spread, often being inadequate in advanced carcinomatosis, because of its lack of spread in the spine. (see Ummenhofer et al.37, for comparative data). Oxycodone has much lower potency when administered spinally, for reasons that are not fully elucidated38, and not as effective as systemic routes. Hydromorhone is similar to morphine and again not as effective as diamorphine39). There is less experience with sufentanil, although its pharmacokinetics are broadly similar to fentanyl and alfentanil.
Precautions with spinal infusions
Infection is minimised by tunnelling the catheter sub-cutaneously up to the shoulder, for ease of access, although it may be tunnelled a lesser distance. Increased distance from the spinal wound will minimise any spread of infection from the skin to the spine. It is especially wise to do this if chemotherapy is undergone at the same time, because of the reduced immune function when white cell counts fall.
Once stable, spinal infusions are remarkably safe. Initially, the patient must be carefully monitored for signs of opioid toxicity in a high dependency unit, as recommended by the Royal College of Anaesthetists, but after twenty four hours should be stable enough for routine observations and may be discharged to the ward. The patient can be managed at home, while being overseen by local district nurses and palliative care team including hospice/ Macmillan nurses and doctors. The infusion bag drug solution may be changed once per week, and it is recommended that the ‘recipe’ is ideally made up in sterile conditions by pharmacy. The infusion line itself should be changed regularly, at least weekly, including the filter.
Morphine infusions tend not to be used in the UK, not only because of the higher risk of late respiratory depression, but also because of the potential development of accretions at the tip of the catheter in the spine, thought to be due to granuloma formation, which seems to occur less with other opioids40.
Well-controlled pain via the spinal route is generally very successful, and gives a good quality of life, possibly even lengthening life-span by reducing opioid toxicity on immune function41. This may also improve the patients performance status to allow consideration of palliative oncology treatment such as radiotherapy and chemotherapy.
Conclusion
Pain physicians can be extraordinarily helpful, working with the palliative care team in the relief of pain. They are especially useful in the relief of difficult to manage pain which is resistant to other therapy. Indeed, it might be argued that palliative care and pain physicians are natural bedfellows and should perhaps be more closely allied departments, especially in the management of difficult pain in patients at the end of life. There is a surprising paucity of literature on the subject of using advanced pain management techniques at the end of life, perhaps something that could be addressed with closer working between Pain Medicine and Palliative Care colleagues.
References
- 1.Clark D. From margins to centre: a review of the history of palliative care in cancer. The Lancet Oncology 2007; 8(5): 430–438. [DOI] [PubMed] [Google Scholar]
- 2.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965; 150(699):971–9. [DOI] [PubMed] [Google Scholar]
- 3.Meldrum ML. A Capsule History of Pain Management. JAMA. 2003; 290:2470–2475. [DOI] [PubMed] [Google Scholar]
- 4.Max MB. Management of cancer pain. Clinical practice guideline No. 9. U.S. Public Health Service, Agency for Health Care Policy and Research; Rockville, MD, 1990. [Google Scholar]
- 5.Smith J, Shipman Inquiry Fourth Report July 2004. Crown copyright 2004.
- 6.Smith Jt, Shipman Inquiry Fifth Report December 2004. Crown copyright 2004.
- 7.Pommert K. ‘Shipman effect harms pain care’ BBC News, 7 August 2006.
- 8.McPherson A. An interesting time to die. Prospect Magazine, 2009, Issue 163, Prospect Publishing Limited. [Google Scholar]
- 9.Lloyd-Williams M. GPs' experiences of palliative care, Palliative Medicine 2003; 17; 646. [DOI] [PubMed] [Google Scholar]
- 10.Lomas C. Dying patients denied pain relief because of legal fears, Nursing Times, 18 May 2010.
- 11.Fanciullo GJ. Palliative and pain medicine: improving care for patients with serious illness, Techniques in Regional Anesthesia & Pain Management 2005; 9(3) 107–108, July 2005. [Google Scholar]
- 12.Darzi, Lord, End of Life Care Strategy: Promoting high quality care for all adults at the end of life. Department of Health, July 2008: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_086277. [Google Scholar]
- 13.Van Alphen JE, Donker GA, Marquet RL. Requests for euthanasia in general practice before and after implementation of the Dutch Euthanasia Act. B J Gen Pract, 2010; 60 (573) 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merskey H, Bogduk N., (eds). Part III: Pain Terms, A Current List with Definitions and Notes on Usage. In: Classification of Chronic Pain, Second Edition, IASP Task Force on Taxonomy, 1994; 209–214. Seattle, IASP Press. [Google Scholar]
- 15.Kutner JS, Bryant LL, Beaty BL, et al. Time Course and Characteristics of Symptom Distress and Quality of Life at the End of Life, Journal of Pain and Symptom Management 2007; 34(3) 227–236. [DOI] [PubMed] [Google Scholar]
- 16.Crawford GB, Price SD. Team working: palliative care as a model of interdisciplinary practice MJA 2003; 179: S32–S34. [DOI] [PubMed] [Google Scholar]
- 17.Hanks GW. Drug Therapy of Pain in Cancer. Der Schmerz (1992) 6: 67–91 Springer Verlag. [DOI] [PubMed] [Google Scholar]
- 18.European Pain In Cancer (EPIC) survey [http://www.paineurope.com/index.php?q=en/book_page/epic_survey].
- 19.Scottish Intercollegiate Guidelines Network. Control of pain in adults with cancer; A national clinical guideline No. 106, November 2008. Available from http://www.sign.ac.uk/pdf/SIGN106.pdf.
- 20.Simkin S, Hawton K, Sutton L, et al. Co-proxamol and suicide: preventing the continuing toll of overdose deaths. Quart J Med 2005; 98:159–70. [DOI] [PubMed] [Google Scholar]
- 21.Hongchien H, Tan EC, Fukunaga H, et al. Naloxone reversal of acupuncture analgesia in the monkey. Experimental Neurology 1981; 73(1) 298–303. [DOI] [PubMed] [Google Scholar]
- 22.Goldman N, Chen M, Fujita T, et al. Adenosine Al receptors mediate local anti-nociceptive effects of acupuncture. Nature Neuroscience. 30 May 2010. EPub. [DOI] [PMC free article] [PubMed]
- 23.Roccia L, Rogora GA. Acupuncture and relaxation, Minerva Med. 1976; 67(29):1918–20. [PubMed] [Google Scholar]
- 24.Fuller G. Focal peripheral neuropathies. J Neurol Neurosurg Psychiatry 2003;74: Suppl. 2, S20–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong FC, Lee TW, Yuen KK, et al. Intercostal nerve blockade for cancer pain; effectiveness and selection of patients. Hong Kong Med J 2007;13(4) 266–70. [PubMed] [Google Scholar]
- 26.Myers KG, George RJ. Painful neuropathy of the lateral cutaneous nerve of the thigh in patients with AIDS: successful treatment by injection with bupivacaine and triamcinolone AIDS. 1996;10(ll):1302–3. [DOI] [PubMed] [Google Scholar]
- 27.Cohen SP, Sireci A, Wu CL, et al. Pulsed Radiofrequency of the Dorsal Root Ganglia is Superior to Pharmacotherapy or Pulsed Radiofrequency of the Intercostal Nerves in the Treatment of Chronic Postsurgical Thoracic Pain. Pain Physician. 2006; 9(3) 227–236. [PubMed] [Google Scholar]
- 28.Loeser JD. Cranial neuralgias. In: Loeser JD. (ed) Bonica's Management of Pain. 3rd ed. Philadelphia: Lippincott, Williams & Wilkins, 2001:855–66. [Google Scholar]
- 29.Shah RV, Racz GB. Pulsed Mode Radiofrequency Lesioning of the Suprascapular Nerve for the Treatment of Chronic Shoulder Pain. Pain Physician. 2003; 6(4)503–506. [PubMed] [Google Scholar]
- 30.Cohen SP, Foster A. Pulsed radiofrequency as a treatment for groin pain. Urology and orchialgia 2003. Mar; 61(3):645. [DOI] [PubMed] [Google Scholar]
- 31.Garcea G, Thomasset S, Berry DP, et al. Percutaneous splanchnic nerve radiofrequency ablation for chronic abdominal pain. ANZ J Surg. 2005; 75(8): 640–4. [DOI] [PubMed] [Google Scholar]
- 32.Jackson MB, Pounder D, Price C, et al. Percutaneous cervical cordotomy for the control of pain in patients with pleural mesothelioma. Thorax. 1999; 54(3): 238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker SM, Goudas LC, Cousins MJ, et al. Combination Spinal Analgesic Chemotherapy: A Systematic Review. Anesth Analg 2002; 95(3):674–715. [DOI] [PubMed] [Google Scholar]
- 34.Lee MA, Leng ME, Tiernan EJ, et al. A simple method of using epidural analgesia in palliative medicine. Pall Med 2001; 15(4) 347–9. [DOI] [PubMed] [Google Scholar]
- 35.Sands Robert P, Jr, Yarussi Anthony T, de Leon-Casasola Oscar A. Complications and side effects associated with epidural bupivacaine/morphine analgesia. Acute Pain 1998; 1(2) 43–50. [Google Scholar]
- 36.Husaini SW, Russell IF. Intrathecal Diamorphine Compared to Morphine for Postoperative Analgesia after Caesarian Section under Spinal Anaesthesia. BJA 1998; 81(2)135–139. [DOI] [PubMed] [Google Scholar]
- 37.Ummenhofer WC, Arends RH, Shen DD, et al. Comparative Spinal Distribution and Clearance Kinetics of Intrathecally Administered Morphine, Fentanyl, Alfentanil, and Sufentanil. Anesthesiology 2000; 92(3) 739–753. [DOI] [PubMed] [Google Scholar]
- 38.Lemberg KK, Kontinen VK, Siiskonen AO, et al. Antinociception by spinal and systemic oxycodone: why does the route make a difference?: In vitro and in vivo studies in rats. Anesthesiology 2006; 105(4) 801–812. [DOI] [PubMed] [Google Scholar]
- 39.Brose WG, Tanelian DL, Brodsky JB, et al. CSF and blood pharmacokinetics of hydromorphone and morphine following lumbar epidural administration. Pain 1991; 45(1) 11–15. [DOI] [PubMed] [Google Scholar]
- 40.Phillips JA, Escott EJ, Moossy JJ, Kellermier HC. Imaging Appearance of Intrathecal Catheter Tip Granulomas: Report of Three Cases and Review of the Literature. American Journal of Roentgenology, 2007; 1896, W375–W381. [Google Scholar]
- 41.Roy S, Wang J, Kelschenbach J, Koodie L, Martin J. Modulation of Immune Function by Morphine: Implications for Susceptibility to Infection. J Neuroimmune Pharmacol. 2006; Mar 1(1): 77–89. [DOI] [PubMed] [Google Scholar]