Abstract
Chronic post-surgical pain (CPSP) is a recognised adverse consequence of surgery; surgery is common, therefore the population at risk is considerable.
Putative risk factors for CPSP include genetic predisposition, demographic, clinical (pain history, type of surgery, anaesthesia, acute pain severity), and psychological factors (vulnerability vs resilience).
Evidence of prevention is limited: long-term benefit from pre-emptive/perioperative analgesia has not been demonstrated consistently.
Large scale prospective studies with detailed pre, intra and postoperative multifactorial assessments are required to refine understanding of the aetiology and prognosis of CPSP.
Keywords: pain postoperative, pain intractable, epidemiology
Introduction
Acute pain following surgery is a predictable, physiological response to tissue damage. Patients are prepared for some degree of pain or discomfort but expect that it will pass. However, up to one third of patients undergoing common surgical procedures report persistent or intermittent pain of varying severity at one year postoperatively (1). Chronic pain is difficult and costly to treat, with wider costs associated with increased health service use as well as reduced quality of life and economic productivity. The burden of disease from chronic post-surgical pain (CPSP) is potentially enormous if we consider the volume of surgical procedures performed annually.
Defining chronic pain after surgery
As with all clinical conditions, prevalence estimates vary depending upon the case definition used, the sampling method and the timing of the postoperative assessment. There is no standardised definition for chronic pain after surgery; most epidemiological studies use the International Association for the Study of Pain (IASP) definition for chronic pain. Macrae and Davies (2) were the first to propose that specific criteria should be satisfied in order for chronic pain to be defined as post-surgical. These were:
The pain must develop after a surgical procedure.
The pain is of at least two months duration.
Other causes for the pain have been excluded.
The possibility that the pain is from a pre-existing condition has been excluded.
The first two criteria are straightforward, although pain duration of two months is shorter than the IASP recommends. These definitions assume a constant pain experience, whereby acute postoperative pain continues to eventually become chronic. However, empirical studies show that a proportion of patients are pain-free or have mild pain in the acute postoperative period, with new onset pain or altered sensations developing weeks or months after the initial surgical insult (3,4). Excluding pre-existing conditions and disentangling indeterminate pains can be difficult, particularly if pain was present preoperatively e.g. prolapsed painful hernia or angina.
Prevalence
Numerous primary studies and secondary reviews have reported on CPSP prevalence. Estimates from epidemiological studies vary widely but overall, between 10 and 30% of surgical patients will report some degree of persistent pain at one year postoperatively, with higher rates (>40%) observed after major thoracic surgery (5–7). A smaller proportion, up to 5% of all surgical patients, report severe, disabling pain at one year (8,9). In general, estimates of CPSP prevalence are derived from postoperative cross-sectional studies, prospective surgical cohorts and clinical trials. One UK study considered frequency and cause of chronic pain from the secondary care perspective: a survey of 5000 patients attending outpatient pain clinics revealed that 22% of patients attributed surgery as the main or major contributory cause of their chronic pain (10).
Most studies of CPSP have focused on common elective surgical procedures, including inguinal herniorrhaphy, breast surgery, orthopaedic and cardiothoracic surgeries. Systematic reviews have summarised the frequency of CPSP after inguinal herniorrhaphy: the first published review included data from 40 epidemiological studies and RCTs, concluding that moderate to severe chronic pain occurred in 10% of patients at one year (4). Updated reviews suggest that 12% of patients will suffer CPSP and 6% persistent testicular pain after inguinal herniorrhaphy (11). In addition to persistent groin pain, a national registry-based Danish study found that 7% of males suffered from moderate to severe pain during sexual activity after hernia repair (12). Chronic pain has now been described as the most common and serious long term problem after repair of an inguinal hernia (13).
Most studies of chronic pain after breast surgery focus on patients undergoing mastectomy or breast conservation surgery with axillary sampling/biopsy. One systematic review reported prevalence estimates ranging from 13 to 69%, although not all of the included primary studies assessed patients beyond six months postoperatively (14). Kudel and colleagues (15) found that women with multiple pains after breast surgery (phantom breast pain, scar pain or ‘other pain as a result of mastectomy’) were more likely to have a greater degree of disability and distress, suggesting an additive negative impact from concurrent postoperative pain syndromes.
The term ‘post-mastectomy pain syndrome’ encompasses a range of pain and altered sensations in the chest, arm, axilla and shoulder region (15–17). Common descriptors reported by women include stabbing pain, tenderness, tightness, pulling/dragging sensations and numbness (3,16,17). In an early study, Wallace et al (18) assessed women one to five years after mastectomy with or without breast reconstruction and also women having cosmetic breast augmentation. Rates of CPSP were higher after mastectomy with reconstruction [49%] and breast augmentation [38%] compared to women having mastectomy [31%]. Despite changes in surgical practice whereby radical mastectomy has mostly been replaced with less invasive breast conservation surgery with axillary surgery and adjuvant treatment, rates of CPSP and other postoperative symptoms remain remarkably high. However, chemotherapy and radiotherapy can also induce neuropathic pain (19), which may explain the high incidence of chronic pain in this patient group. A Scottish longitudinal study of women with post-mastectomy pain at three years postoperatively found that half continued to suffer from persistent or intermittent pain and arm morbidity up to 12 years after surgery (17). A Danish study of breast cancer survivors found a high incidence of pain (29%), paraesthesia (47%) and phantom sensations (19%) five to ten years after surgery, with adjuvant radiotherapy being a significant contributory factor (20). In a review of possible risk factors for CPSP after breast cancer treatment, Andersen and Kehlet acknowledged the increased risk associated with radiotherapy and nerve damage, but expressed frustration that study heterogeneity precluded robust conclusions (21).
Recent questionnaire studies of patients who underwent thoracic surgery found rates of post-thoracotomy pain syndrome (PTPS) of 33% following thoracotomy and 25% after video-assisted thoracic surgery (VATS) at 12 to 36 months post-operatively (22). Of those describing pain, up to 12% rated it as severe on movement. Interestingly, a similar survey investigating patients after lung transplantation found a far lower incidence of pain, between 5 and 10%, which may suggest a protective role of immunosuppression in the development of long term pain (23).
Risk factors
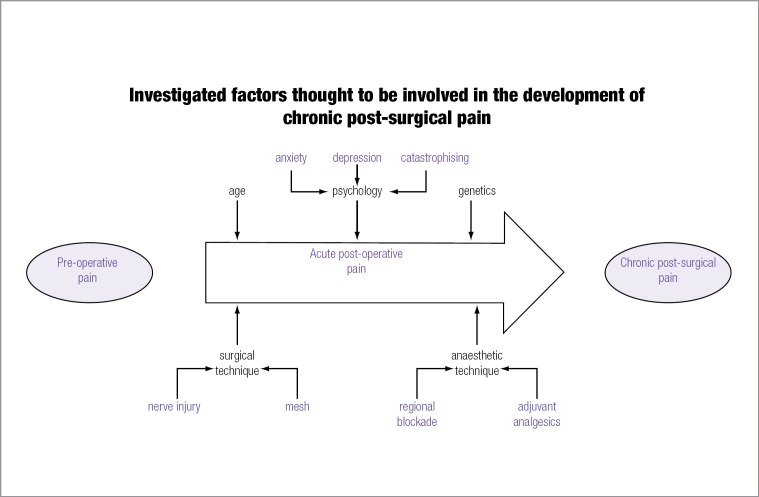
The application of epidemiological methods has been important for identifying risk factors thought to contribute to the development of CPSP. Putative factors include: the presence of preoperative pain, age, psychological factors, intraoperative nerve handling, surgical technique/approach, acute postoperative pain, genetic predisposition, type of anaesthesia, and postoperative management. A brief description of selected factors is given here.
Preoperative pain
In a sample of patients having elective abdominal surgery, higher levels of pain immediately before surgery (OR 2.96; 95% CI 1.32, 6.60) and the presence of chronic pain before surgery (lasting >6 months) (OR 1.75; 95% CI 1.03, 2.98) independently predicted moderate to intense pain in the acute postoperative period (24). Preoperative pain at or near the operative site has been found to predict chronic pain after hernia and amputation surgery (25–27). Patients with chronic post-herniorrhaphy pain were more likely to have presented with a painful hernia and to have another chronic pain syndrome: headache, backache, irritable bowel syndrome (26). Cross-sectional studies report a two- to three-fold increased risk of CPSP among patients with preoperative pain although these estimates are often based on patient recall (9). Methodologically, it is important to measure pain status preoperatively, as retrospective recall of preoperative pain may be biased by presence and intensity of current pain (28).
Age
Younger patients are more likely to develop CPSP (3,9,21,22,29,30) but this finding is inconsistent across studies (31). In a prospective study of women undergoing breast cancer surgery, the probability of CPSP incidence decreased by 5% with each one-year increase in age [OR 0.95, 95% CI 0.91–0.99] (32). These data correlate with a Scottish study of CPSP after hernia surgery, where a 5% reduction in odds of developing pain was found per one-year increase in age (9). Mechanisms explaining the increased incidence of chronic pain in younger patients are unknown, but may relate to reduction in peripheral nociceptive function with increased age.
Psychological factors
Early studies investigating risk factors for CPSP mostly focused on demographic, somatic and perioperative biomedical factors, however there has been recent interest in the role of psychological factors, particularly vulnerability and resilience, in predicting late surgical outcome. Psychological vulnerability has been defined as ‘a reaction readiness defined by a low threshold for being influenced and a risk of inexpedient reactions in social interactions and health-related behaviour’ (33). Psychological distress predicts acute postoperative pain, but less is known about the interrelationship between psychological distress, acute pain and the processes involved in the transition from acute to chronic pain after surgery. Individuals with high trait or dispositional anxiety are thought to be more hypersensitive and psychologically more reactive to threatening stimuli (24,34). State-trait anxiety and depression are predictive of acute postoperative pain; preoperative anxiety is correlated with high postoperative anxiety, postoperative pain intensity, analgesic requirements and longer length of hospital stay (24).
Research has widened from measurement of affect to include other constructs of distress and health related beliefs, including pain catastrophising. Catastrophising, argued to be a stable, trait-like characteristic, has mostly been assessed in non-surgical chronic pain populations or within the acute postoperative period (35,36). Recent studies have explored the role of health-related beliefs, including catastrophising, in predicting late surgical outcome. Preoperative catastrophising and comorbidities independently predicted pain ratings two years after knee arthroplasty (37). A similar cohort study, with larger sample size, found that pain catastrophising was the strongest independent predictor of poor pain outcome after total knee replacement (38).
Dispositional optimism, psychological ‘robustness’, expectation of pain control and expectations about functional recovery before surgery are associated with acute postoperative pain and, more recently, CPSP. Emotional distress [anxiety, depression, illness behaviour, somato-sensory amplification] were significant risk factors for clinically meaningful acute pain at one month after breast cancer surgery, but made no independent contribution to persistent pain at three months postoperatively (32,39). A large prospective surgical cohort of 625 patients undergoing minor, intermediate and major operative procedures, found that fear of the long-term consequences of surgery (OR 1.9; 95% CI 1.9, 1.1, 3.3) predicted increased pain at six months postoperatively, independent of the type of surgical procedure and other somatic factors (40). Fear related to surgery was the most consistent psychological predictor of unfavourable outcome, whereas dispositional optimism was related to better long-term functional recovery after surgery.
Intraoperative nerve injury
During surgery, nerves are at risk of injury from partial or complete division, stretching, contusion, crushing, electrical damage from diathermy, entrapment or compression (e.g. from rib retraction) (21,22,41,42). Additional risks occur during prosthetic implantation, where damage or kinking may occur, for example, when stapling or suturing mesh into position during hernia repair. Chronic inflammation can occur to non-absorbable material or implants (e.g. staples, sternal bone wires) or neuroma formation following entrapment of fibres in scar tissue. Relatively few studies have assessed whether intraoperative nerve handling, or elective preservation or division of major sensory nerves, contributes to the development of chronic pain or numbness.
The largest study to investigate intraoperative nerve handling and CPSP is a multicentre prospective study of 955 patients undergoing open mesh herniorrhaphy (8). Nerve handling was carefully recorded and patients with moderate to severe pain were examined by clinicians after one year. Non-identification of nerves was significantly associated with CPSP, as was nerve division; prognostic significance of not identifying one or more inguinal nerves was maintained after adjustment for age and sex (8). This is biologically plausible given that nerves may be inadvertently sectioned, entrapped or secured to other structures.
It is entirely conceivable that duration of surgery, associated with persistent surgical nociception and sustained peripheral injury, could trigger changes in the central nervous system. Yet there is relatively little data to support the theory that the more severe the surgical insult or tissue damage, the greater the risk of persistent pain (24). Rates of severe acute postoperative pain and chronic pain are higher after thoracic surgery but can also occur after minimally invasive surgery, including laparoscopic procedures and vasectomy (7,43,44). However, in a heterogeneous sample of patients having minor, intermediate and major surgery, Peters and colleagues (40) found that surgery lasting longer than three hours predicted increased pain, poor functional outcome and poor global recovery at six months postoperatively. Severe acute postoperative pain (OR 3.2, 95% CI 1.6, 6.3) also predicted increased pain at six months.
Acute post-operative pain
Higher levels of pain in the acute postoperative period have consistently been found to predict pain weeks and months after surgery; this association has been replicated in many surgical cohorts, including studies of hernia, breast, thoracic and laparoscopic cholecystectomy surgery (see reviews 1,2,6). Similarly, severe pain immediately before or after lower limb amputation predicts chronic pain in the residual or phantom limb (45). Whilst these data on severe acute pain predicting chronic pain are relatively consistent, the precise nature of the mechanisms involved and whether these relationships are associative or causal has yet to be determined (46).
Genetics
Patients undergoing elective surgery are broadly exposed to similar levels of tissue injury, nerve damage and nociceptive barrage, yet only a proportion of these develop a chronic pain state. It is then reasonable to assume that, as with many other diseases, there are both genetic and environmental component factors in every causal mechanism. A study of patients undergoing median sternotomy and venous saphenectomy found a higher than expected proportion of patients with CPSP at both operative sites, suggesting a constitutional predisposition for chronic pain (30,47). Pain sensitivity varies widely; increased susceptibility has been demonstrated in animals and in humans (48). Pain susceptibility is known to be influenced by several genes and human genetic studies have mostly focused on polymorphisms in candidate genes. Further research on the genetics of CPSP is currently being undertaken and is likely to be illuminating (46).
Future directions
The increase in awareness of CPSP has led to huge body of research investigating, for example, pre-emptive analgesia and other preventive strategies. Evidence of prevention is currently limited as trials of pre-emptive (e.g. gabapentinoids) and perioperative analgesia and are either contradictory or inconclusive, possibly due to poor design and small sample sizes which lack statistical power (6, 46). Many studies investigating efficacy of perioperative anaesthetic or analgesic agents show an effect in the acute postoperative period, but benefit is often not demonstrated in the long-term [for reviews see 46,49]. However, treating all patients with perioperative drugs in the hope of preventing conversion to chronic pain is both impractical and costly. These drugs are not without side-effects so, as with all preventative approaches, a balance must be struck between inconveniencing all and providing an unknown degree of benefit to a few (50). The challenge for researchers is to accurately predict and identify susceptible patients who are at greatest risk of CPSP and to enable targeted prevention.
As the development of CPSP is likely to be multifactorial, involving the interplay of psychology, genetics, pre-existing pain, and the inflammatory response to tissue damage, as well as surgical, anaesthetic and analgesic techniques, its full investigation can only be achieved with multicentre collaboration of detailed prospective studies (51). Agreement about core domains to be included within future studies e.g. pain, pain-related physical and emotional functioning, psychological factors and surgical/intraoperative factors, would provide a framework for researchers to collate minimum datasets across multiple patient cohorts and healthcare settings. This would allow data aggregation for systematic reviews, and would also provide larger samples sizes for the development and refinement of prognostic models. Further exploration of the complex relationship between biophysical, surgical and psychological processes along the transitional pathway will aid our understanding both of the aetiology and trajectory of chronic pain after surgery.
Although there is still much to learn about mechanisms, the relationship between acute postoperative pain and subsequent CPSP has been well demonstrated empirically, and the immediate goal for all extended surgical teams should, therefore, be the control of pain during the acute postoperative period.
Acknowledgements – funding
JQ's research into chronic post-surgical pain is funded by the Oxford Biomedical Research Centre. JB has research funding from IASP (Collaborative Research Grant 2011), Cancer Research UK and Chief Scientist Office.
Contributor Information
Julie Bruce, Principal Research Fellow Warwick Clinical Trials Unit, University of Warwick, Coventry CV4 7AL.
Jane Quinlan, Consultant in Anaesthesia and Acute Pain Management Nuffield Division of Anaesthetics, John Radcliffe Hospital, Oxford OX3 9DU.
References
- 1.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367(9522):1618–25. [DOI] [PubMed] [Google Scholar]
- 2.Macrae WA, Davies HTO. Chronic postsurgical pain. In: Crombie IK. ed. Epidemiology of pain. Seattle: IASP Press; 1999:125–42. [Google Scholar]
- 3.Smith WCS, Bourne D, Squair J, Phillips DO, Chambers WA. A retrospective cohort study of post mastectomy pain syndrome. Pain 1999;83:91–95. [DOI] [PubMed] [Google Scholar]
- 4.Poobalan AS, Bruce J, Smith WCS, King PM, Krukowski ZH, et al. A review of chronic pain after inguinal herniorrhaphy Clin J Pain 2003;19(1):48–54. [DOI] [PubMed] [Google Scholar]
- 5.Steegers MAH, Snik DM, Verhagen AF, van der Drift MA, Wilder-Smith OHG. Only half of the chronic pain after thoracic surgery shows a neuropathic component J Pain 2008;9(10):955–61. [DOI] [PubMed] [Google Scholar]
- 6.Macrae W, Bruce J. Chronic pain after surgery. In: Wilson PR, Watson PJ, Haythornthwaite JA, Jensen TS. (eds) Clinical pain management: chronic pain. London: Hodder Arnold, 2008:405–14. [Google Scholar]
- 7.Macrae WA. Chronic post-surgical pain: 10 years on Br J Anaesth 2008;101(1):77–86. [DOI] [PubMed] [Google Scholar]
- 8.Alfieri S, Rotondi F, Di Giorgio A, Fumagalli U, Salzano A, et al. (Groin Pain Trial Group). Influence of preservation versus division of ilioinguinal, iliohypogastric, and genital nerves during open mesh herniorrhaphy: prospective multicentric study of chronic pain. Ann Surg 2006;243(4):553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poobalan AS, Bruce J, King PM, Chambers WA, Krukowski ZH, et al. Chronic pain and quality of life following open inguinal hernia repair. Brit J Surg 2001;88(8):1122–6. [DOI] [PubMed] [Google Scholar]
- 10.Crombie IK, Davies HTO, Macrae WA. Cut and thrust: antecedent surgery and trauma among patients attending a chronic pain clinic. Pain 1998;76(1–2):167–71. [PubMed] [Google Scholar]
- 11.Aasvang E, Kehlet H. Chronic postoperative pain: the case of inguinal herniorrhaphy. Br J Anaesth 2005;95(1):69–76. [DOI] [PubMed] [Google Scholar]
- 12.Aasvang EK, Mohl B, Bay-Nielsen M, Kehlet H. Pain related sexual dysfunction after inguinal herniorrhaphy. Pain 2006;122(3):258–63. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins JT, O'Dwyer PJ. Inguinal hernias. BMJ 2008;336:269–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain 2003;104(102):1–13. [DOI] [PubMed] [Google Scholar]
- 15.Kudel I, Edwards RR, Kozachik S, Block BM, Agarwal S, et al. Predictors and consequences of multiple persistent postmastectomy pains. J Pain Symptom Manage 2007;34(6):619–27. [DOI] [PubMed] [Google Scholar]
- 16.Baron RH, Fey JV, Borgen PI, Stempel MM, Hardick KR, et al. Eighteen sensations after breast cancer surgery: a 5-year comparison of sentinel lymph node biopsy and axillary lymph node dissection. Ann Surg Oncol 2007;14(5):1653–61. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald L, Bruce J, Scott NW, Smith WCS, Chambers WA. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Brit J Cancer 2005;92(2):225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace MS, Wallace AM, Lee J, Dobke MK. Pain after breast surgery: a survey of 282 women. Pain 1996;66(2–3):195–205. [DOI] [PubMed] [Google Scholar]
- 19.Jung BF, Herrmann D, Griggs J, Oaklander AL, Dworkin RH. Neuropathic pain associated with non-surgical treatment of breast cancer. Pain 2005;118(1):10–14. [DOI] [PubMed] [Google Scholar]
- 20.Peuckmann V, Ekholm O, Rasmussen NK, Groenvold M, Christiansen P, et al. Chronic pain and other sequelae in long-term breast cancer survivors: Nationwide survey in Denmark. Eur J Pain 2009;13:478–85. [DOI] [PubMed] [Google Scholar]
- 21.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain 2011;12(7):725–46. [DOI] [PubMed] [Google Scholar]
- 22.Wildgaard K, Ravn J, Nikolajsen L, Jakobsen E, Jensen TS, et al. Consequences of persistent pain after lung cancer surgery: a nationwide questionnaire study. Acta Anaesthesiol Scand 2011;55:60–8. [DOI] [PubMed] [Google Scholar]
- 23.Wildgaard K, Iversen M, Kehlet H. Chronic pain after lung transplantation: a nationwide study. Clin J Pain 2010;26(3):217–22. [DOI] [PubMed] [Google Scholar]
- 24.Caumo W, Schmidt AP, Schneider CN, Bergmann J, Iwamoto CW, et al. Preoperative predictors of moderate to intense acute postoperative pain in patients undergoing abdominal surgery. Acta Anaesthesiol Scand 2002;46(10):1265–71. [DOI] [PubMed] [Google Scholar]
- 25.Liem MS, van Duyn EB, van der Graaf Y, van Vroonhoven TJ (Coala Trial Group). Recurrences after conventional anterior and laparoscopic inguinal hernia repair: a randomized comparison. Ann Surg 2003;237(1):136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright D, Paterson C, Scott N, Hair A, O'Dwyer PJ. Five-year follow-up of patients undergoing laparoscopic or open groin hernia repair. Ann Surg 2002;235(3):333–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikolajsen L, Ilkjær S, Krøner K, Christensen JH, Jensen TS. The influence of preamputation pain on postamputation stump and phantom pain. Pain 1997;72(3):393–405. [DOI] [PubMed] [Google Scholar]
- 28.Kalso E. Memory for pain. Acta Anaesthesiol Scand 1997;4l5(110):129–30. [DOI] [PubMed] [Google Scholar]
- 29.Gjeilo KH, Klepstad P, Wahba A, Lydersen S, Stenseth R. Chronic pain after cardiac surgery: a prospective study. Acta Anaesthesiol Scand 2010;54:70–8. [DOI] [PubMed] [Google Scholar]
- 30.Bruce J, Drury N, Poobalan AS, Jeffrey RR, Smith WC, et al. The prevalence of chronic chest and leg pain following cardiac surgery: a historical cohort study. Pain 2003;104(1–2):265–73. [DOI] [PubMed] [Google Scholar]
- 31.Page B, Paterson C, Young D, O'Dwyer PJ. Pain from primary inguinal hernia and the effect of repair on pain. Br J Surg 2002;89(10):1315–8. [DOI] [PubMed] [Google Scholar]
- 32.Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain 2006;7(9):626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinrichs-Rocker A, Schulz K, Jarvinen I, Lefering R, Simanski C, et al. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP) – A systematic review. Eur J Pain 10.1016/j.ejpain.2008.07.015. [DOI] [PubMed]
- 34.Johnston M, Vöegle C. Benefits of psychological preparation for surgery: a meta-analysis. Ann Behav Med 1993;15(4):245–56. [Google Scholar]
- 35.Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7(4):524–32. [Google Scholar]
- 36.Granot M, Ferber SG. The roles of catastrophising and anxiety in the prediction of postoperative pain intensity: a prospective study. Clin J Pain 2005;21:439–45. [DOI] [PubMed] [Google Scholar]
- 37.Forsythe ME, Dunbar MJ, Hennigar AW, Sullivan MJ, Gross M. Prospective relation between catastrophising and residual pain following knee arthoplasty: two-year follow-up. Pain Res Manage 2008;13(4):335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riddle DL, Wade JB, Jiranek WA, Kong X. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clin Orthop Relat Res 2010;468(3):798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katz J, Poleshuck EL, Andrus CH, Hogan LA, Jung BF, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain 2005;119(1–3):16–25. [DOI] [PubMed] [Google Scholar]
- 40.Peters ML, Sommer M, de Rijke JM, Kessels F, Heineman E, et al. Somatic and psychologic predictors of long-term unfavourable outcome after surgical intervention. Ann Surg 2007;245(3):487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wantz GE. Testicular atrophy and chronic residual neuralgia as risks of inguinal hernioplasty. Surg Clin North Am 1993;73(3):571–82. [DOI] [PubMed] [Google Scholar]
- 42.Ravichandran D, Kalambe BG, Pain JA. Pilot randomized controlled study of preservation or division of ilioinguinal nerve in open mesh repair of inguinal hernia. Br J Surg 2000;87(9):1166–7. [DOI] [PubMed] [Google Scholar]
- 43.Grant AM, Scott NW, O'Dwyer PJ. (MRC Laparoscopic Groin Hernia Trial Group). Five-year follow-up of a randomized trial to assess pain and numbness after laparoscopic or open repair of groin hernia. Br J Surg 2004;91(12):1570–4. [DOI] [PubMed] [Google Scholar]
- 44.Manikandan R, Srirangam SJ, Pearson E, Collins GN. Early and late morbidity after vasectomy: a comparison of chronic scrotal pain at 1 and 10 years. BJU Int 2004;93(4):571–4. [DOI] [PubMed] [Google Scholar]
- 45.Hanley MA, Jenson MP, Smith DG, Ehde DM, Edwards WT, et al. Preamputation pain and acute pain predict chronic pain after lower extremity amputation. J Pain 2007;8(2):102–9. [DOI] [PubMed] [Google Scholar]
- 46.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother 2009;9(5):723–44. [DOI] [PubMed] [Google Scholar]
- 47.Devor M. Evidence for heritability of pain in patients with traumatic neuropathy. Pain 2004;108(1–2):200–1. [DOI] [PubMed] [Google Scholar]
- 48.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet 2005;14(1):135–43. [DOI] [PubMed] [Google Scholar]
- 49.Joshi GP, Bonnet F, Shah R. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg 2008;107(3):1026–40. [DOI] [PubMed] [Google Scholar]
- 50.Dworkin RH, McDermott MP, Raja SN. Preventing chronic postsurgical pain: how much of a difference makes a difference? Anesthesiol 2010;112(3):516–8. [DOI] [PubMed] [Google Scholar]
- 51.Kehlet H, Rathmell JP. Persistent postsurgical pain: the path forward through better design of clinical studies. Anesthesiol 2010;112(3):514–5. [DOI] [PubMed] [Google Scholar]