Summary points
1. Addiction can occur with the repeated exposure of a biogenetically predisposed person to an addictive substance or behaviour.
2. In the patient with pain on opioid therapy, use the ‘4 Cs’ to diagnose addiction.
3. Screening and risk stratification of all patients considered for opioid therapy is a key element of ‘universal precautions’ in pain management.
4. There are a number of established and new screening tools including the CAGE, Opioid Risk Tool and Screener and Opioid Assessment for Patients with Pain, which can be utilized in the office setting.
5. There are a number of potential ambiguous drug-related behaviours that should trigger a re-evaluation by the clinician.
6. Treating the higher-risk patient with opioids requires more assessment, more structure and more monitoring. Written opioid prescribing agreements and urine drug testing can be helpful strategies.
7. Essential documentation includes the ‘6As’: Analgesia, Activity, Adverse effects, Ambiguous drug behaviours, Affect and Adequate prescription information.
Keywords: Opioid-related disorders/prevention and control, pain, intractable/complications, pain, intractable/drug therapy
Introduction
There has been a rapid rise in North America in the misuse of prescription opioids and a parallel rise in the related consequences of addiction and deaths from overdose.1 Clinicians and policy-makers are struggling to find solutions that reduce opioid misuse without negatively impacting pain management. The highest risk for adverse events is in patients with a previous or current substance abuse or addiction disorder.
Current knowledge about addiction
Addiction is best described as a chronic disease of brain reward centres, which evolved to ensure survival of the organism and species. Reward centres have evolved to grab our attention, dominate motivation and compel behaviour towards survival even in the presence of danger. Eating, sex, social interaction and unexpected novel stimuli activate these reward circuits under normal circumstances. All of the usual drugs of abuse and certain behaviours have an ability to turn on reward circuits to a much greater extent for a longer period of time than other stimuli. Addictive drugs hijack brain circuits that take over behaviour, leading to progressive loss of control over drug intake in spite of medical, emotional, interpersonal, occupational and legal consequences.3–6
Basic science and clinical research are increasingly identifying the altered neurochemistry of biogenetically vulnerable individuals as having a primary role in the development of addictive disorders.7 In these susceptible individuals, who usually have a positive family history of addictive disorders, polydrug abuse often begins in the early teenage years and progresses over time. Although we do not yet have any reliable, inexpensive and easily measurable genetic markers for addiction, research is ongoing.
Certain psychological traits (i.e. pathological shyness) and psychiatric conditions are associated with an increased risk of comorbid addictive disorders. Mood disorders, bipolar disorder, obsessive–compulsive disorder, attention-deficit hyperactivity disorder and borderline, antisocial and psychopathic personality disorders are all over-represented in addicted populations compared with the general population.8
To summarize, the development of substance abuse/addiction requires repeated exposure to a potentially addictive substance, taken in a manner that optimizes euphoric effect, in an individual with a particular biopsychogenetic vulnerability living in a particular social milieu (see Figure 1). Without the presence of risk factors, it is unlikely that an individual will develop an addiction disorder as a result of taking appropriately prescribed and monitored opioids for pain. However, physicians can certainly enable the ‘rekindling’ of a previous addictive disorder by failing to screen patients for risk factors or by ignoring the early symptoms and signs of a relapsing addiction.
Figure 1.
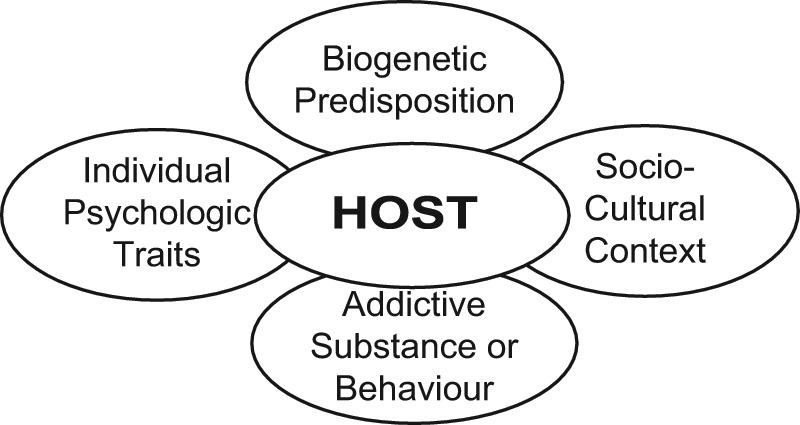
The biopsychosocial model of addiction.2
Defining opioid addiction in the patient with pain
Under the current classification system for psychiatric illness, DSM-IV-TR, the term ‘substance dependence’ continues to be used instead of the term ‘addiction’. This terminology was partly a well-meaning attempt to decrease the stigma attached to the term ‘addict’. However, it has resulted in a less precise definition, especially when referring to substances with a therapeutic use such as opioids. Under DSM-IV, one can make the diagnosis of ‘opioid dependence’ based solely on symptoms related to tolerance, physical dependence and withdrawal.9 These criteria may be quite appropriate for the diagnosis of alcohol or heroin addiction, but are not appropriate when the drug in question is prescribed for a therapeutic purpose. This confusion around definitions is due to be corrected in DSM-V.
To help clarify, the Liaison Committee on Pain and Addiction (LCPA) of the American Academy of Pain Medicine (AAPM), the American Pain Society (APS) and the American Society of Addiction Medicine (ASAM) endorsed and published a consensus document in 2001, which includes a set of appropriate and clinically useful definitions for assessing the use of opioids in the context of pain treatment:
[Addiction]is a primary, chronic, neurobiological disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviours that include one or more of the following: [also known as the ‘4 Cs’]
impaired Control over drug use;
Compulsive use;
continued use despite harm (Consequences);
Craving.
It should be emphasized that no single event is diagnostic of addictive disorder. Rather, the diagnosis is made in response to a pattern of behaviour that usually becomes obvious over time.10
Physicians have an ethical obligation to try to relieve pain and suffering without doing harm. Therefore, when considering long-term opioid therapy, it is important to screen and risk-stratify all patients and to structure treatment and monitoring strategies based on risk level. This is the basis for the concept of the ‘universal precautions’ in pain management, published by Gourlay and Heit.11 This paper is highly recommended reading for all healthcare professionals who are prescribing opioids for chronic non-cancer pain (CNCP). Most of these recommendations have been incorporated into the Canadian Guideline for Safe and Effective Use of Opioids in Chronic Non-cancer Pain, 2010.12
Screening for misuse/addiction risk
There is no foolproof way to predict which patient will manifest a substance abuse or addictive disorder when prescribed long-term opioid therapy. The best that can be expected of the conscientious clinician is to screen for key indicators in the patient’s history, family history and psychological make-up that statistically put the patient at increased risk of developing an addiction to any psychoactive substance, including opioids.13
A patient with a history of problematic use of one substance is at higher risk for misusing other psychoactive substances. The purpose of screening is not necessarily to deny patients opioids for pain, but to identify those at higher risk so that they may receive more detailed assessment with more careful prescribing and monitoring.
One simple and straightforward screening question to use in a primary care setting is to ask: ‘How many times in the past year have you used an illegal drug or used a prescription medication for non-medical reasons?’An answer of ‘once’ or more has been shown to have a sensitivity of 100% and a specificity of 73.5% when compared with a more detailed interview by an addiction professional.14
The CAGE questions have been used for over 25 years to screen for alcohol dependence and have been validated in numerous populations.15 A derivation of the CAGE, called the CAGE-AID, includes screening questions for both alcohol and drugs:
In the past, have you ever:
tried to Cut down or Change your pattern of drinking or drug use?
been Annoyed by others’ concerns about your drinking or drug use?
felt Guilty about the consequences of your drinking or drug use?
had a drink or used a drug in the morning (Eye-opener) to decrease hangover or withdrawal symptoms?
One positive response to any of the CAGE-AID questions would suggest caution. Two or more positive responses require further assessment for a serious alcohol or drug problem. The predictive value is highly dependent on the population screened. Therefore, it is also important to ask about frequency and amount of alcohol consumed. The recently published Canadian Low-Risk Alcohol Drinking Guidelines suggest that a woman drinking more than two standard drinks on a daily basis, more than three drinks on a single drinking occasion or more than 10 drinks regularly per week is at increased risk. Men who drink more than three standard drinks per day regularly, more than four drinks on a single drinking occasion or more than a total of 15 drinks per week are at increased risk.16 A standard drink is considered to be 341 mL (12 oz) of 5% alcohol beer, cider or cooler; 142 mL (5 oz) of 12% alcohol wine; or 43 mL (1.5 oz) of 40% distilled alcohol.
An initial interview with a spouse or significant other can provide valuable collateral information regarding his or her observations of the patient and allow the voicing of any concerns he or she may have regarding the prescribing of opioid therapy. This is also an opportunity to educate and to encourage future contact if concerns do develop.
It is important to ask about the home environment when assessing the risks of a given patient for opioid therapy. A low-risk patient who lives in a high-risk environment (e.g. with others who misuse or divert drugs) is at increased risk.
Finally, a review of previous medical records or communication with previous treating clinicians can also provide useful information for risk assessment.
Depending on skill level and time available, the clinician may require the assistance of other addiction or mental health professionals in evaluating the above factors in a patient with chronic pain.
Office-based screening tools
In 2005, the Opioid Risk Tool (ORT) was published and prospectively validated.17 It is a simple, office-based tool that requires the clinician to ask five questions and provides a score that stratifies patients into low-, moderate- and high-risk groups. As the ORT depends on honest reporting by the patient, it may be more susceptible to deception. This screening tool, and information regarding the derivation and clinical use of the tool, can be downloaded from www.emergingsolutionsinpain.com.
The Screener and Opioid Assessment for Patients with Pain – Revised (SOAPP-R) requires more time to complete and score but may be a preferred screening tool if one is looking after a higher-risk population.18,19 It is available from www.pain.edu.
Other opioid risk screening tools continue to be developed and are being validated clinically.
In spite of one’s best efforts at screening, it is possible that some patients with a primary underlying addictive disorder will be missed. In other patients who are picked up on screening, the relative importance of addiction versus pain in a new patient can be very difficult to assess, even for the addiction specialist, and may only become clearer after a careful trial of therapy with ‘tight’ prescribing boundaries, agreed-upon goals and close monitoring.
In those patients with recognized risk factors for addiction, the clinician and the properly informed patient may jointly choose to undertake a cautious trial of opioid therapy in spite of the risk. The concurrent use of physical rehabilitative and psychological strategies is important in a biopsychosocial approach to overall management and helps the clinician to better understand the patient’s motivation. Liaison or co-management with an addiction specialist would be helpful in such cases.
Evaluating ambiguous drug-related behaviours
When prescribing long-term opioids for pain, patients found to be at increased risk for misuse or addictions require closer attention than those at lower risk. In addition, patients who demonstrate repeated, ambiguous drug-related behaviours may need re-evaluation, closer monitoring and tighter control over prescribing. Examples of ambiguous drug behaviours include:
selling, stealing or forging prescriptions for opioid analgesics;
injecting oral opioid formulations;
obtaining prescription drugs from other doctors or non-medical sources;
concurrent abuse of alcohol or illicit drugs;
repeated dose escalation or other non-compliance despite multiple warnings;
use of additional opioids other than those prescribed;
repeated visits to other physicians or emergency rooms without advising the primary prescriber;
admitting to seeking euphoria or relief of anxiety from opioids;
drug-related deterioration in function at work, in the family or socially;
repeated resistance to change in therapy despite evidence of adverse drug effects;
missed appointments with follow-up phone calls for renewals;
repeated ‘lost’ prescriptions with implausible excuses;
non-compliance with other suggested non-drug treatments;
repeated failure to abide by a written prescribing agreement; and
evidence of intoxication or withdrawal in the office or pharmacy.20
The differential diagnosis for such ambiguous drug behaviours includes:
a previously hidden or developing addictive disorder;
pseudoaddiction due to inadequately treated pain;
psychiatric illness that has developed since opioid therapy began;
encephalopathy/confusion due to concomitant illness or drug side-effects;
family/social stresses related to relationships, losses, finances or employment;
lack of understanding by patients regarding scheduled dosing, titration, etc.; and
criminal intent to sell prescription opioids for profit.
Physicians have an ethical duty to refer a patient who appears to have an active addictive disorder to an addiction treatment professional for further assessment and treatment. This may require that the treating clinician taper the patient off opioids or refer the patient to an opioid agonist treatment (OAT) programme in which methadone or buprenorphine can be given in a structured, supervised setting. The clinician treating pain can then focus on other non-opioid treatments. Any future use of opioid therapy to treat pain in such patients should be done with concurrent follow-up by an addiction professional and strict precautions should be followed, as outlined in the next section.
Treating the higher-risk patient with opioids
How can the clinician manage a patient with a high-risk history who has legitimate severe pain that may benefit from a trial of opioid therapy? Some ‘experience-based’ suggestions include:
1. More assessment, which may include:
a more thorough biopsychosocial assessment prior to starting treatment;
a detailed assessment of baseline functional status;
collateral information from significant others (pharmacists, previous physicians, partner, etc.);
consideration of a tapering off of all opioids for 4– 6 weeks to evaluate the patient’s ‘basal pain state’ and assess for opioid hyperalgesia;
an assessment by an addiction specialist regarding the current need for an addiction treatment programme or to assess the quality of the patient’s recovery programme; or
an evaluation by a psychiatrist or psychologist as required to assess for concurrent mental health disorders.
2. More structure, which may include:
more use of addiction counselling, self-help and recovery groups;
more use of concurrent treatment modalities, including physical methods, cognitive–behavioural methods and adjuvant analgesics;
one prescriber for all psychoactive medications (including psychiatric medications);
one pharmacist for all prescriptions, and open communication with him or her;
shorter dispensing intervals (i.e. weekly or even daily) initially, until the patient demonstrates consistent responsible medication use;
little or no use of immediate-release/short-acting opioids for breakthrough or incident pain;
no replacement of ‘lost’ medications and no early refills; and
no phone repeats for any psychoactive medications.
In very high-risk patients, consider minimizing the amount of extra opioid the patient has in his or her possession by using a once-daily opioid formulation dispensed daily or with signed buprenorphine patches provided every 7 days or signed fentanyl patches every 3 days provided one dose at a time by the pharmacist.Used patches can still contain a significant amount of leftover medication and should be returned to the pharmacist before the next dose is dispensed.
Opioid prescribing agreements
Most opioid guidelines recommend the use of a written prescribing agreement signed by both clinician and patient – especially the patient who is at elevated risk for opioid misuse or is not well known to the clinician. Such an agreement is a boundary-setting tool, which can help to document informed consent. Not all authors endorse the use of written agreements because of the potential adverse impact on the doctor–patient relationship.21 The language level of a prescribing agreement should be appropriate for the comprehension level of the patient. (See the downloadable supplement for two examples of written prescribing agreements.)
3. More monitoring may include:
more frequent follow-up assessment;
random body fluid screening (urine drug testing (UDT) for the prescribed opioid and for the presence of illicit drugs) (see the downloadable file for further information about UDT);
more attention to medication side-effects and patient response;
more focus on evidence of improved function, rather than simply pain relief, as a goal of therapy;
collateral information from the pharmacist, partner and other healthcare professionals;
asking the patient to bring in all medications in original pill bottles on each visit so the clinician can examine the labels and amounts left over;
random pill or patch counts;
concurrent care by an addiction treatment professional; and
routinely consulting prescription monitoring databases where available and accessible.
Can high-risk patients benefit from opioid therapy?
In a randomized controlled trial, Jamison et al.22 demonstrated that high-risk patients with pain who were provided opioid therapy in a structured programme involving compliance checklists, monthly urine drug test (UDT) and both individual and group motivational counselling were able to manage opioid therapy in a similar way to a low-risk control group.Such a programme, of course, would require extra resources and cooperation between pain and addiction professionals.
Patients already on methadone or buprenorphine therapy (OAT) for opioid addiction who develop acute or chronic pain are often undertreated for their pain.Special assessment and treatment considerations apply (see the downloadable handout for more information).
With careful prescribing and ‘adherence’ monitoring, including regular follow-ups, periodic UDT, collateral information, random pill counts and essential documentation, one published prospective study of 500 patients demonstrated a 50% reduction in the rate of misuse behaviours compared with a historical control group.23
A systematic review of the impact of written agreements and UDT concluded that there is weak evidence for effectiveness in reducing opioid misuse.24
Essential follow-up documentation
Optimal follow-up documentation can provide early clues to the clinician regarding the development of problematic opioid use and helps to demonstrate quality of care in patients on long-term opioids. It can include the ‘6 As’ (derived from the original ‘4 As’ published by Passik25):
Analgesia (pain relief) using a numerical rating scale with or without percentage pain relief.
Activities of daily living (physical functioning), focusing on specific examples of activities the patient is able to perform since being on opioids.
Affect – the current status of symptoms of anxiety or depression.
Adverse effects, and suggested remedies – especially any evidence of cognitive impairment.
Ambiguous drug-taking behaviours and response by the clinician.
Accurate medication documentation according to local laws.
Even though a skilled drug seeker with a good scanner and a computer can forge and alter almost any prescription, try to make prescriptions as tamper-proof as possible. Some suggestions for high-risk patients include:
routinely using coloured rather than black ink;
using tamper-evident prescription pads with a background pattern that demonstrates any attempts at alteration;
writing some type of government-issued identification number, such as the patient’s health number, on the prescription so that the pharmacist can verify before dispensing (now the law in many jurisdictions);
asking the patient to use one pharmacist for all prescriptions –write the name of the pharmacy on the prescription;
when writing medication amounts, use both numbers and words (i.e. ‘100’ and ‘one hundred only’);
filling in any remaining white space on the prescription to prevent additions; and
sending prescriptions electronically or by fax to the pharmacist where allowed by law.
If, in spite of all precautions, the patient continues to demonstrate ambiguous, drug-related behaviours, then consider tapering off him or her from opioids using a humane opioid tapering protocol (available in the downloadable handout), offer to explore other treatment options and offer to refer the patient to an addiction medicine or opioid substitution programme for assessment and appropriate treatment. This course of treatment can be communicated in a non-judgemental way using a patient-centred framework for care.26
As chronic pain and addiction are each common conditions in our society, there is potential for a patient to suffer from both problems. This makes it very important for the clinician to routinely screen and risk stratify all patients presenting with chronic pain. Treating a higher-risk patient with opioids requires more assessment, more structure and more monitoring to reduce harms to the individual and society from the misuse of prescription opioids. The use of ‘universal precautions’ for pain management, which includes the use of validated screening tools, written prescribing agreements, careful prescribing and appropriate monitoring, can be helpful strategies. Important outcome documentation includes analgesia, activity, adverse effects, ambiguous drug behaviours, affect and adequate prescription records. All of these strategies can be provided in a respectful, patient-centred framework of care.
Footnotes
This research received no specific grant from any funding agency in the commercial, public or not-for-profit sectors.
The author received no financial support to write this paper however he has consulted for and is on the speakers’ bureau for a number of pharma companies who make opioid analgesics, such as: Janssen, Mundipharm, Palladin, PurduePharma and Sanofi-Aventis.
Further reading
Jovey RD. Pain and addiction: prevalence, neurobiology, definitions. In: Mogil JS (ed.) Pain 2010: an updated review (refresher course syllabus). 13th World Congress on Pain. Seattle: IASP Press, 2010.
Smith HS and Passik SD (eds) Pain and chemical dependency. New York: Oxford University Press, 2008.
Multiple-choice questions
- One of the most important risk factors for the development of addiction to any substance is:
- the potency of the substance
- a person’s biogenetic predisposition
- repeated exposure to an addictive substance
- injection drug use
- Which of the following is not considered a criterion for addiction in a patient on prescribed opioids?
- loss of control
- withdrawal when the opioid is stopped
- compulsive use
- craving
- consequences related to substance use
- The purpose of screening for substance misuse risk in a patient for whom you are considering opioid therapy is:
- to avoid the use of opioids in a high-risk patient
- to refer all patients at elevated risk to an addiction programme
- to exercise more care in prescribing and monitoring opioids
- to advise all high-risk patients that you cannot provide pain management services
- Recommended follow-up documentation for a patient on long-term opioid therapy includes which of the following outcomes:
- pain relief
- functional improvements
- side-effects and management
- ambiguous drug-related behaviours
- changes in affect
- (a) and (b) above
- all of the above
Answers
1b; 2b; 3c; 4g.
References
- 1. Centers for Disease Control. Morbidity and mortality weekly report, Vol. 60, 1 November 2011. Available at: www.cdc.gov (accessed 23 January 2012). [Google Scholar]
- 2. American Society of Addiction Medicine. Principles of addiction medicine, 2nd ed. Chevy Chase (MD): American Society of Addiction Medicine Inc., 1998. [Google Scholar]
- 3. Robinson TE, Berridge KC. Mechanisms of action of addictive stimuli. Incentive-sensitization and addiction. Addiction 2001;96:103–114. [DOI] [PubMed] [Google Scholar]
- 4. Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci 2005;8(11):1458–1463. [DOI] [PubMed] [Google Scholar]
- 5. Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci 2006; 8:1445–1449. [DOI] [PubMed] [Google Scholar]
- 6. Koob GF. The neurobiology of addiction: a neuro adaptational review relevant for diagnosis. Addiction 2006; 101(Suppl 1): 23–30. [DOI] [PubMed] [Google Scholar]
- 7. Nestler EJ. Genes and addiction. Nat Gene 2000; 26:277–281. [DOI] [PubMed] [Google Scholar]
- 8. Regier DA, Narrow WE, Rae DS, Manderscheid RW, Locke BZ, Goodwin FK. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry 1993;50(2):85–94. [DOI] [PubMed] [Google Scholar]
- 9. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
- 10. American Academy of Pain Medicine, American Pain Society, American Society of Addiction Medicine. Definitions related to the use of opioids for the treatment of pain. Glenview, IL: American Academy of Pain Medicine, 2001. [Google Scholar]
- 11. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med 2005;6: 107–112. [DOI] [PubMed] [Google Scholar]
- 12. National Opioid Use Guideline Group 2010. Canadian Guideline for Safe and Effective Use of Opioids for Chronic Non-Cancer Pain, www.nationalpaincentre.mcmasterca/opioid/ (accessed 14 November 2011).
- 13. Savage SR. Assessment for addiction in pain-treatment settings. Clin J Pain 2002;18:S28–S38. [DOI] [PubMed] [Google Scholar]
- 14. Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. A single-question screening test for drug use in primary care. Arch Intern Med 2010;170(13):1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ewing JA. Detecting alcoholism: the CAGE questionnaire. JAMA 1984;252:1905–1970. [DOI] [PubMed] [Google Scholar]
- 16. Butt P, Beirness D, Cesa F, Gliksman L, Paradis C, Stockwell T. Alcohol and health in Canada: a summary of evidence and guidelines for low-risk drinking. Ottawa, ON: Canadian Centre on Substance Abuse, 2011. Available at: http://www.ccsa.ca/eng/priorities/alcohol/canada-low-risk-alcoholdrinking-guidelines/Pages/default.aspx (accessed 1 December 2011). [Google Scholar]
- 17. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med 2005;6:432–442. [DOI] [PubMed] [Google Scholar]
- 18. Butler SF, Fernandez K, Benoit C, et al. Validation of the Revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R) J Pain 2008; 9(4):360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Passik SD, Kirsh KL, Casper D. Addiction-related assessment tools and pain management: instruments for screening, treatment planning and monitoring compliance. Pain Med 2008; 9(Suppl 2): S145–S166. [Google Scholar]
- 20. Passik SD. Issues in long-term opioid therapy: unmet needs, risks, and solutions [review]. Mayo Clin Proc 2009;84(7):593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arnold RM, Han PK, Seltzer D. Opioid contracts in chronic nonmalignant pain management: objectives and uncertainties [review]. Am J Med 2006;119(4):292–296. [DOI] [PubMed] [Google Scholar]
- 22. Jamison RN, Ross EL, Michna E, et al. Substance misuse treatment for high-risk chronic pain patients on opioid therapy: a randomized trial. Pain 2010;150(3):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manchikanti L, Cash KA, Damron KS, et al. Controlled substance abuse and illicit drug use in chronic pain patients: An evaluation of multiple variables. Pain Physician 2006;9:215–225. [PubMed] [Google Scholar]
- 24. Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med 2010;152(11):712–720. [DOI] [PubMed] [Google Scholar]
- 25. Passik SD, Kirsh KL, Whitcomb L, et al. Monitoring outcomes during long-term opioid therapy for noncancer pain: results with the Pain Assessment and ocumentation Tool. J Opioid Manag 2005;1(5):257–266. [DOI] [PubMed] [Google Scholar]
- 26. Nicolaidis C. Police officer, deal-maker, or health care provider? Moving to a patient-centered framework for chronic opioid management. Pain Med 2011;12(6):890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]


