Abstract
The endocrine effects of opioids used for the management of persistent pain are poorly understood by clinicians and patients, and hormone levels are rarely measured. It is recognized that opioids exert this effect via the hypothalamic-pituitary-gonadal axis. Additional effects on adrenal hormones, weight, blood pressure and bone density may also occur. Symptoms and signs of sex hormone deficiency occur in both men and women but are under-reported and are often clinically unrecognized.
The potential effects of long term opioid therapy on the endocrine system should be explained to patients before opioid therapy is commenced. Monitoring of sex hormones is recommended; if there are deficiencies opioids should be tapered and withdrawn, if this is clinically acceptable.
If opioid therapy has to continue, hormone replacement therapy should be initiated and monitored by an endocrinologist.
Keywords: Opioids, endocrine, sex hormones, hypogonadism, testosterone, hormone replacement
Opioids have been used as the principal method of relieving severe pain, especially acute pain and pain from advanced cancer, for centuries. Regular opioids around the clock continue to be the mainstay in the management of pain associated with end-of- life conditions and they are enshrined in the World Health Organization’s analgesic ladder. Five thousand years ago Papaver somniferum was cultivated by the Sumerians. Since then it has been intertwined with the human experience of many subsequent cultures, acting as a blessing but also as a curse, as both analgesic and anxiolytic, and stupefacient and muse. Wars have been fought over it; some might say that this continues to be the case. In 1804 Friedrich Sertürner isolated morphine and in 1843 Alexander Wood used the first injectable form. Opioid analgesics became recognisable as we know them now.
The analgesic properties of opioids have relieved the suffering of countless numbers of people, and their place in the management of acute pain and cancer pain is well established. However, approximately 50% of recipients with non-cancer pain suffer adverse effects and in 20% this leads to discontinuation of therapy.1 Adverse effects, well known to both health professionals and lay people, include somnolence, constipation, nausea, itching and respiratory depression. Less well known adverse effects include cardiovascular instability, headache and muscle spasm of both smooth and striated muscle, leading to urine retention, slowing of gut motility and myoclonic jerks.2 Fewer still will be aware of the potential for impairment of immune function, which is known to be opioid specific.3 Pain clinicians will probably know of opioid-induced hyperalgesia, although they rarely diagnose it.
The least well recognised long-term effects are suppression of endocrine function and effect on cognitive function.3 With an ageing population, increasing survival rates from cancer and increasing use of opioids for persistent non-cancer pain, the number of prescriptions of opioids in the community has increased from 6 million to 15 million in the UK between 1999 and 2008 (NHS Information Centre). The USA has 5% of the world’s population but consumes 80% of global opioid supply. The number of accidental fatal overdoses related to prescription opioids in the USA is now more than that for the recreational use of heroin and cocaine combined.4
It is imperative that all those managing and prescribing opioids for persistent pain are aware of the long-term effects of opioids on endocrine function and are able to diagnose deficiencies, monitor hormone levels, understand and explain to the patient the possible consequences of these deficiencies, strive to taper and withdraw opioids when appropriate and liaise with other clinicians to replace hormone deficiencies.
The endocrine effects of opioids
Hypogonadism
In 1895 Reverend RH Graves5 noted how ‘opium ate out the virility of the individual’ and in 1925 Surgeon General HS Cumming6 stated ‘opium makes a man effeminate’. Katz7 has previously quoted Charles Bruce, who, in 1839, referred to the opium addicts in Assam as ‘more effeminate than women’.8
It has been reported on many occasions that opioids, given by any route, suppress the hypothalamic–pituitary–gonadal axis and have a measurable impact on gonadal function.7
Physiology of opioid–endocrine interactions
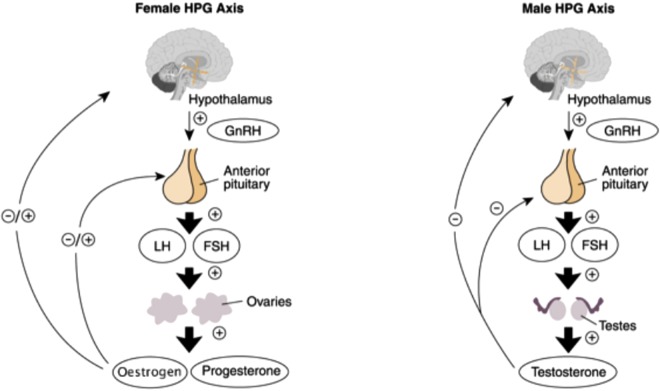
The hypothalamus is central to the regulation of sex hormones. It exerts its control via the secretion of gonadotrophin-releasing hormone (GnRH) from the preoptic area into the hypophyseal portal circulation at the median eminence. GnRH stimulates the release of follicle-stimulating hormone (FSH) and luteinising hormone (LH) from the anterior pituitary by activating its own GnRH receptor, which, via increased levels of calcium and protein kinase C, leads to the formation and secretion of FSH and LH (Figure 1).
Figure 1.
Representation of the normal female and male hypothalamic–pituitary–gonadal axis9. (Reproduced with permission from NIAAA).
GnRH is normally released in bursts in all studied vertebrates. These pulses produce circadian peaks which are essential for the correct functioning of the reproductive system by determining when each hormone is released. Non-pulsatile secretion of GnRH results in downregulation of the pituitary and leads to failed LH secretion.7
FSH is responsible for the early growth of ovarian follicles in females and maintenance of the spermatogenic epithelium in males. LH is responsible for the final maturation of follicles and their oestrogen secretion, as well as ovulation and the initial formation of the corpus luteum in the female. In the male it stimulates Leydig cells to secrete testosterone.10 These hormones are also required for the appropriate development of the human – physiologically, physically and socially.
The hypothalamic–pituitary axis is constantly under the effect of multiple substances including neurotransmitters, steroid hormones and endogenous opioids. Exogenous opioids exert an effect on the same receptors as endogenous opioids and have been shown to interfere with the release (including its pulsatile nature) of GnRH.7,10,11 Naloxone showed increased GnRH levels, and thereby increased LH concentration and pulse frequency, which suggested a basal level of opioid-based inhibition of LH secretion. It has been suggested that morphine inhibits the biosynthesis of GnRH.11 Opioids also decrease the negative feedback of sex steroids on the anterior pituitary, as well as its response to GnRH.7 In contrast, FSH secretion is not, or only minimally, affected.
With the reduction in LH levels, testosterone and oestradiol are commensurately lowered. Li Shizhen wrote of opium in his Compendium of Materia Medica (1578) that ‘lay people use it for the art of sex, particularly to “arrest seminal emission” ’11. Animal studies have added credence to Shizhen’s observation, demonstrating inhibited sexual receptivity in both sexes of rats with opioids and the converse with naloxone.12 This appears to be due to decreased arousal rather than impotence. Alarmingly, related studies showed that prepubertal opioid exposure inhibited sexual maturation.13 In humans, menstrual irregularities, including amenorrhoea, occurred following treatment with opioids via the oral and intrathecal routes. Daniell14 noted that there was also a decrease in adrenal androgens, and thereby explaining the decreased libido and sexual performance so often encountered in those exposed to long-term opioids. It appears that decreased sexual behaviour may also be due to the direct action of opioids on µ and ∂ receptors in the hypothalamus.15,16
The term ‘sex hormones’ encompasses the sex steroids (testosterone and oestradiol) and the non-steroid hormones (GnRH, FSH and LH).
Testosterone is the principal hormone of the testes, synthesised from cholesterol in Leydig cells and from androstenedione in the adrenal cortex. Its secretion is controlled by LH at 4–9 mg/day. Small amounts are secreted by the ovaries and possibly the adrenal cortex in women. It is 98% protein bound (gonadal steroid-binding globulin and albumin) in the plasma. Only the free and weakly albumin-bound testosterone is available to act on androgen receptors. Some target cells convert testosterone to dihydrotestosterone, which forms more stable hormone–receptor complexes. In males testosterone has a circadian rhythm, with the highest levels in the morning. Maximal variation may be in the order of 35%.
Testosterone imparts a negative feedback on LH release from the pituitary. It develops and maintains the male secondary sex characteristics and encourages male sexual behaviours. It is anabolic, increases rate of growth and, along with FSH, promotes spermatogenesis. It does all of this by binding to intracellular receptors and forming complexes that bind to DNA, and thus facilitating certain gene expression.
Oestrogens (17ß-oestradiol, oestrene and oestriol) are the primary female sex steroids and are biosynthesised from testosterone and androstenedione. They are secreted by the granulosa cells in ovarian follicles. Ninety-eight per cent of oestrogens are protein bound. They are metabolised by the liver and excreted in the urine. Oestrogens facilitate the development of ovarian follicles, aid in the regulation of the menstrual cycle and the requisite changes in female internal anatomy, and have anabolic effects on the uterus and fallopian tubes. They decrease FSH levels and alter LH levels. They are responsible for female sexual behaviours and libido and are largely responsible for breast development. These effects, combined with the absence of androgens, lead to female secondary sexual characteristics. Oestrogen, being a steroid hormone, exerts its effects via two principal intracellular receptors.
In summary, opioids lead to decreased secretion of GnRH, which in turn leads to reduced levels of LH. This results in decreased testosterone and oestradiol secretion, which leads to the signs and symptoms listed in Table 2. It is important to note that these changes develop over weeks to years.
Table 2.
Diagnosis in the male: causes of primary hypogonadism.
| Primary hypogonadism | Examples |
|---|---|
| Testicular disorders (primary gonadal failure) | Undescended testes |
| Bilateral torsion | |
| Orchitis | |
| Klinefelter syndrome | |
| Noonan syndrome | |
| Haemochromatosis | |
| Androgen-resistant states | |
| External testicular insults | Trauma |
| Radiotherapy | |
| Chemotherapy | |
| Autoimmune syndromes | Anti-Leydig cell antibody associated disorders |
Diagnosis of hypogonadism in the male
The diagnosis of hypogonadism is dependent on history and examination in conjunction with laboratory testing. In the postpubertal male the potential causes of primary hypogonadism mentioned in Table 3 can cause diagnostic difficulty, especially with regard to their gradual onset. If hypogonadism is suspected, it is therefore important to differentiate primary gonadal failure from that of the hypothalamic–pituitary axis.
Table 3.
Diagnosis in the male: causes of secondary hypogonadism.
| Secondary hypogonadism | Examples |
|---|---|
| Gonadotrophin-releasing hormone deficiency | Opioids |
| Hypothalamic lesions | |
| Prader–Willi syndrome | |
| Hyperprolactinaemia | Prolactinoma |
| Haemochromatosis | |
| Myotonic dystrophy | |
| Pituitary disorders | Tumour |
| Irradiation |
According to the World Health Organization’s Second International Consultation on Erectile Dysfunction, which considered the circadian rhythm and pulsatile nature of testosterone secretion, two blood samples should be taken between 8.00 a.m. and 11.00 a.m. (when testosterone levels are presumed to be at their highest, although the circadian rhythm can diminish with increasing age). Samples should be sent for measurement of serum testosterone, sex hormone-binding globulin (SHBG), prolactin, FSH and LH levels.
Normal to high FSH/LH levels might indicate primary hypogonadism (see Table 3). It is important to measure the level of FSH as it has a longer half-life and demonstrates less variability than LH. Secondary hypogonadism (Table 4) is indicated by low testosterone and normal to low FSH/LH levels (Box 1).
Table 4.
Low threshold levels for total and free testosterone levels.18
| Age (years) | Threshold for abnormally low total testosterone (2.5th percentile) | Threshold for abnormally low free testosterone (2.5th percentile) |
|---|---|---|
| 40–49 | 251 ng/dL | 5.3 ng/dL |
| 50–59 | 216 ng/dL | 4.2 ng/dL |
| 60–69 | 196 ng/dL | 3.7 ng/dL |
| 70–79 | 156 ng/dL | 2.2 ng/dL |
Box 1: According to the Endocrine Society of Australia Consensus Guidelines17.
Primary hypogonadism = Serum Testosterone <231ng/dl with LH> 1.5 times the upper limit of normal (1.5 x ULN)
Secondary hypogonadism = Serum testosterone <231ng/dl without elevation of LH
With ageing comes diminished diurnal fluctuation in serum testosterone. The level falls markedly throughout the day, reinforcing the importance of early morning sampling. In hypogonadism in this age group total testosterone levels may be normal if sex hormone-binding globulin (SHBG) levels are raised. SHBG levels increase with age, and thereby decrease the bioavailibility of testosterone.
Recent data from the Massachusetts Male Ageing Study (MMAS) provide perspectives on normal androgen ranges, as shown in Table 1.
Table 1.
Clinical effects of reduced sex hormone secretion.
| Male | Female | Males and females |
|---|---|---|
| Reduced testicular volume | Menstrual irregularities | Infertility |
| Erectile dysfunction | Reduced breast size | Reduced libido |
| Loss of muscle mass | Hot flushes | Depression and anxiety |
| Glucose intolerance | ||
| Hypercholesteraemia | ||
| Gynaecomastia | ||
| Polyuria | ||
| Diarrhoea | ||
| Night sweats | ||
| Fatigue | ||
| Osteoporosis | ||
| Anosmia | ||
| Myalgia | ||
| Galactorrhoea |
Diagnosis of hypogonadism in the female
Hypogonadism in females can similarly be due to a hypothalamic–pituitary axis or primary gonadal defect. The signs and symptoms are closely linked to the menstrual cycle in the postmenarche and premenopausal years. Common signs include oligomenorrhoea, amenorrhoea and failure to conceive. More subtle symptoms such as hot flushes and anxiety may occur, rarely with changes in pubic hair distribution and breast size21. Although the clinical effects can be as severe for women as for men, the outward or visible changes are not so obvious and have not been studied in any detail, especially in postmenopausal women.
Although androgen deficiency has been shown to be symptomatic in females using intrathecal opioid therapy and those on maintenance methadone, testosterone levels have not been routinely measured. Dihydroepiandrosterone (DHEA) is thought to be lowered and it is known that LH levels are markedly reduced. DHEA is a marker of adrenal androgen production, and approximately 50% of androgens produced in the female are adrenal in origin. There is a paucity of data with regards to hormone levels in females, and further studies are needed to quantify the clinical significance of this potentially very interesting aspect of opioid-induced androgen deficiency (OPIAD).
Effect of opioids on adrenal hormones
Acute and chronic pain, as physiological responses, result in increased secretion of adrenocorticotrophic hormone (ACTH) and cortisol. However, the chronic use of exogenous opioids has been found in several studies to decrease ACTH and cortisol levels and cortisol responses to adrenocorticotropin challenges.19 Opioids also affect the circadian rhythms of cortisol secretion, resulting in persistently raised levels of ACTH and cortisol and eventually blunting the stress response.20 Levels of dehydroepiandrosterone sulphate (DHEAS), a precursor of adrenal androgens, have also been markedly reduced in both male and female chronic opioid users.14,18 Occasionally, opioid administration may cause frank adrenal insufficiency, but the risk factors for this are unknown at present.15
Prolactin
Acute administration of opioids stimulates prolactin release from the anterior pituitary through an effect at the hypothalamus.15 This effect can be blocked by metoclopramide, suggesting that it is mediated through dopaminergic mechanisms. The effect of long-term administration of opioids on prolactin is less clear. There is an occasional increase in prolactin release, possibly dependent on the type of opioid. The clinical significance of this is unknown, but it can cause galactorrhoea.
Thyroid hormones
In general opioids do not appear to alter thyroid function in any meaningful way,19 though opioids can stimulate thyroid-stimulating hormone (TSH) via the hypothalamus.15 This is unimportant in individuals with normal free thyroxine, but individuals with hypothyroidism may have prolonged and exaggerated responses to opioids.22
Growth hormone
Acute administration of opioids leads to increased growth hormone (GH) secretion, through mechanisms involving opioid receptors, feedback levels and gene transcription. The minimum dose required is approximately 15 mg morphine.15 Abs et al.23 found GH deficiency in approximately 15% of patients receiving long-term intrathecal opioids, but not in all patients. Naloxone has been shown to inhibit GH in healthy subjects, but to increase it in obese women. The effect of chronic opioid administration on GH is complex and currently poorly understood, but it appears to be related to sex hormones, body composition and degree of insulin resistance.15
Vasopressin
Tramadol has been found to cause hyponatraemia through serotonin-induced release of vasopressin.24 The effect of opioids on the posterior pituitary is unclear: both increased and decreased levels of vasopressin have been found, depending on hydration status.22
Oxytocin
Studies on pregnant women have shown that morphine inhibits oxytocin production in the early stages of labour and during breastfeeding post partum.25
Obesity and diabetes
An increasing number of data suggests that opioids play a role in regulating food intake and food choice, and perhaps the reward associated with good-tasting foods, via central mechanisms.15 Chronic opioid use is associated with weight gain, hyperglycaemia and worsening diabetes. This may be a central action via the sympathetic nervous system and impaired insulin secretion.26 Hypogonadism is associated with increase insulin resistance and risk for diabetes mellitus,15 a risk that is improved by testosterone replacement.27
Catecholamine metabolism
Opioid therapy increases catecholamine secretion via the hypothalamus and brain stem. Patients on long-term opioids should be screened for hypertension.22
Bone metabolism
There are many risk factors for decreased bone mineral density and osteoporosis in patients treated with opioids, including possible poor nutritional status, hypogonadism, inhibition of osteoblasts, decreased osteocalcin synthesis, abnormal calcium and parathyroid hormone and increased bone resorption, mediated by interleukin 1. There is an increased risk of bone fracture in patients using opioids.3
Route of administration
Opioids for chronic use are administered orally or intrathecally. Other routes, for example repeated injection, may occasionally be used, though this practice is not recommended.28
Hormonal changes occurring after intrathecal administration were reported by several authors.23,29,30
It is estimated that 90% of patients receiving intrathecal opioids will develop hypogonadism. Oral opioids have the same effect, though the onset of action may be slower.
Type and dose of opioid
Classes of opioids differ in their effect on gonadal suppression. Tramadol and buprenorphine do not significantly alter testosterone levels in animals and humans, and buprenorphine does not suppress serum cortisol.
The incidence of hypogonadism was higher in cancer survivors receiving a dose equivalent to or more than 200 mg morphine per day for at least 1 year ompared with age-matched survivors not on opioid therapy, suggesting that the effects are dose related.31 Duration of opioid therapy also seems to increase the chance of hormonal suppression, though this needs to be studied further.
There is a possible gender difference in endocrine effects, biased towards more symptomatology in women, but further studies are needed.22
When and what to measure
Before commencing chronic opioid therapy it is recommended that the following are measured:
blood pressure;
electrolytes (especially if tramadol is used);
fasting glucose levels;
thyroid function (to exclude hypothyroidism);
testosterone, sex-binding globulin, LH/FSH and oestradiol levels; and
bone density (in an ‘at-risk’ group).
Monitoring
There are no accepted standards, but it seems reasonable to repeat the above tests every 6 months.
Consider morning fasting blood cortisol, DHEA, ACTH and GH levels. (Remember that an abnormally high fasting blood cortisol level can represent loss of diurnal variation, and advice should be sought.)
Repeat bone density yearly in ‘at-risk’ group.
Measure prolactin levels if there is galactorrhoea.
Replacement therapy
There are no accepted standards for the management of opioid-induced endocrine dysfunction.
The best option is to taper and withdraw opioids and monitor the response over a period of a few months if this is appropriate. It is not known if switching opioids is of any benefit. Buprenorphine seems to have less effect on adrenal hormones but has a greater effect on TSH than morphine. Response to different opioids is largely unknown at the time of writing.
If opioid withdrawal cannot be achieved, and the patient has definite symptoms of endocrine dysfunction, hormone replacement is recommended, with monitoring by an endocrinologist.
Testosterone can be replaced, in both men and women, as a transdermal patch or gel or by injection. Careful monitoring is required as side-effects include site reactions, polycythaemia and increased risk of prostate cancer in men and menstrual irregularities and hirsutism in women.
Oestrogen replacement therapy is best monitored by a gynaecologist.
Conclusion
The use of opioids for the management of pain continues to increase and therapy that involves these drugs has significant adverse effects in both the short and long term. Both patients and prescribing physicians should be aware, or made aware, of them. Careful risk–benefit analysis should be applied and all possible opioid-sparing manoeuvres considered.
The effects of opioids on the endocrine system are becoming increasingly apparent, although more research is required to further elucidate the mechanisms by which these happen. It affects both sexes, but the clinical signs are more obvious in males. Direct action of opioids on the hypothalamus reduces the release of GnRH, and thereby adversely affects LH levels, and subsequently testosterone synthesis and secretion.
Symptoms include infertility, decreased sexual function, loss of muscle mass and anxiety and/or depression. Osteoporosis may result from long-term opioid therapy, leading to fractures and their associated morbidity and cost.
More research is required to elucidate the aetiology of opioid-induced endocrine dysfunction. Differences between methods of opioid delivery, the effects of OPIAD in both sexes and the potential value of opioid rotation need further investigation.
The potential long-term effects of opioids on the endocrine system should be explained to the patient before therapy is commenced and regular monitoring should be perfomed.
The use of opioid therapy should be minimised by implementing a biopsychosocial approach to pain management.
The benefits of opioid therapy should be carefully assessed; if therapeutic goals have not been achieved, opioids should be tapered and withdrawn.
If it is necessary to continue opioids, hormone replacement therapy should be initiated and monitored by an endocrinologist.
Footnotes
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
The authors declare that they do not have any conflict of interest.
Further reading
Elliott JA, Horton E and Fibuch E. The endocrine effects of long-term oral opioid therapy: a case report and review of the literature. J Opioid Manag 2011; 7(2): 145–154.
Multiple choice questions (more than one answer may be correct for each question)
-
Which serum levels of the following hormones may be reduced by taking oral opioids for more than one year?
adrenocorticotropic hormone
testosterone
growth hormone
luteinising hormone
thyroid-stimulating hormone
-
Which of the following possible side-effects of taking opioids for persistent pain should be explained to women of childbearing age?
greater risk of pregnancy
weight gain
osteoporosis
amenorrhoea
fatigue
-
High fasting blood cortisol level may indicate which of the following:
loss of diurnal variation
opioid overuse
adrenal tumour
stress
overexercising
-
Testosterone replacement therapy:
can cause secondary polycaethaemia
should be offered to women who have low levels of free testosterone
leads to an increase in muscle mass
increases the risk of prostate cancer
can be safely prescribed by any doctor
-
Long-term use of opioids for non-cancer pain:
is usually effective in relieving pain
carries an increased risk of accidental overdose
requires no monitoring
may cause hypertension
is known to impair cognitive function
Answers
1. a) True; b) True; c) False; d) True; e) False.
2. a) False; b) True; c) True; d) True; e) True.
3. a) True; b) True; c) True; d) False; e) False.
4. a) True; b) True; c) False; d) True; e) False.
5. a) False; b) True; c) False; d) True; e) True.
References
- 1. Moore AR, McQuay HJ. Prevalence of opioid adverse events in chronic nonmalignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther 2005; 7: 1046–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Furlan AD, Sandoval JA, Mailis-Gagnon A, et al. Opioids for chronic non-cancer pain: a meta-analysis of effectiveness and side effects. Can Med Assoc J 2006; 174: 1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raghavan S, Harvey AD, Humble SR. New opioid side effects and implications for long-term therapy. Trends Anaesth Crit Care 2011; 1: 18–21. [Google Scholar]
- 4. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med 2010; 363(21): 1981–1983. [DOI] [PubMed] [Google Scholar]
- 5. Graves RH. Forty Years in China, Baltimore: Woodward, 1895. [Google Scholar]
- 6. Cumming HS, Control of DrugAddiction Mainly a Police Problem, The American City Magazine (November 1925, file 126, box 3, sub-series 1, series 3.
- 7. Katz N, Mazer NA. The impact of opioids on the endocrine system. Clin J Pain 2009; 25(2): 170–175. [DOI] [PubMed] [Google Scholar]
- 8. Bruce CA. Report on the manufacture of tea and on the extent and produce of the tea plantations in Assam. Calcutta: : Bishop’s College Press, 1839. [Google Scholar]
- 9. Hiller-Sturmhofel S, Bartke A. The endocrine system: An overview. Alcohol Health Res World 1998; 22(3): 153–164. [PMC free article] [PubMed] [Google Scholar]
- 10. Ganong WF. Review of medical physiology. USA: Lange/McGraw-Hill, 2005. [Google Scholar]
- 11. Luo Xiwen tr. Bencao Gangmu: Compendium of Materia Medica. Foreign Languages Press, 2003. First published 1578
- 12. Van Vugt DA, Baby N, Stewart M, Reid RL. The paradoxical stimulatory effect of morphine on LH secretion is dose-dependent and naloxone-reversible. Neuroendocrinology 1989; 50: 109–116. [DOI] [PubMed] [Google Scholar]
- 13. De la Rosa RE, Hennessey JV. Hypogonadism and methadone: hypothalamic hypogonadism after long-term use of high-dose methadone. Endocr Pract 1996; 2:4–7. [DOI] [PubMed] [Google Scholar]
- 14. Daniell HW. DHEAS deficiency during consumption of sustained-action prescribed opioids: evidence for opioid-induced inhibition of adrenal androgen production. J Pain 2006; 7(12): 901–907. [DOI] [PubMed] [Google Scholar]
- 15. Vuong C, Van Uum SHM, O’Dell L, et al. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev 2010; 31(1): 98–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Furth WR, van Emst MG, van Ree JM. Opioids and sexual behaviour of male rats: involvement of the medial preoptic area. Behav Neurosci 1995; 109; 123–134. [DOI] [PubMed] [Google Scholar]
- 17. Daniell Harry W. The Endocrine Society’s clinical guidelines on testosterone therapy in adult men with androgen deficiency syndromes. J Clin Endocrinol Metab 2010; 95(6): 2536–2559. [DOI] [PubMed] [Google Scholar]
- 18. O’Donne AB, Araujo AB, McKinlay JB. The health of normally aging men: The Massachusetts Male Aging Study (1987–2004). Exp Gerontol 2004; (7): 975–984. [DOI] [PubMed] [Google Scholar]
- 19. Katz N. The impact of opioids on the endocrine system. Pain Management Rounds 2005; 1(9). www.painmanagementrounds.org (accessed 5 February 2012) [Google Scholar]
- 20. Faccinetti F, Grasso A, Petraglia F, et al. Impaired circadian rhythmicity of β-lipotrophin, β-endorphin and ACTH in heroin addicts. Acta Endocrinol 1984; 105(2): 149–155. [DOI] [PubMed] [Google Scholar]
- 21. Daniell HW. Opioid endocrinopathy in women consuming prescribed sustained-action opioids for control of non-malignant pain. J Pain 2008; 9(1): 28–36. [DOI] [PubMed] [Google Scholar]
- 22. Thosani S, Jiminez C. Opioid-induced biochemical alterations of the neuroendocrine axis. Expert Rev Endocrinol Metab 2011; 6(5): 705–713. [DOI] [PubMed] [Google Scholar]
- 23. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endo Metab 2000; 85(6): 2215–2222. [DOI] [PubMed] [Google Scholar]
- 24. Sarret D, Le Berre JP, Zemraoui N. Tramadol-induced hyponatraemia. Am J Kidney Dis 2008; 52(5): 1026. [DOI] [PubMed] [Google Scholar]
- 25. Lindow SW, Hendricks MS, Nugent FA, et al. Morphine suppresses the oxytocin response in breast-feeding women. Gynecol Obstet Invest 1999; 48(1): 33–37. [DOI] [PubMed] [Google Scholar]
- 26. Giugliano D. Morphine, opioid peptides and pancreatic islet function. Diabetes Care 1984; 7: 92–98. [DOI] [PubMed] [Google Scholar]
- 27. Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006; 154: 899–906. [DOI] [PubMed] [Google Scholar]
- 28. Opioids for persistent pain; good practice. 2010. The British Pain Society, Faculty of Pain Medicine of the Royal College of Anaesthetists, Royal College of General Practitioners and the Faculty of Addiction Medicines of the Royal College of Psychiatrists. Available at: http://www.britishpainsociety.org/book_opioid_main.pdf (accessed 5 February 2012)
- 29. Roberts LJ, Finch PM, Pullan PT, et al. Sex hormone suppression by intrathecal opioids: a prospective study. Clin J Pain 2002; 18: 144–148. [DOI] [PubMed] [Google Scholar]
- 30. Thimineur MA, Kravitz E, Vodapally MS. Intrathecal opioid treatment for chronic non-malignant pain: a 3-year prospective study. Pain 2004; 109: 242–249. [DOI] [PubMed] [Google Scholar]
- 31. Rajagopal A, Vassilopoulou-Sellin R, Palmer JL, et al. Symptomatic hypogonadism in male survivors of cancer with chronic exposure to opioids. Cancer 2004; 100(4): 851–858. [DOI] [PubMed] [Google Scholar]