Abstract
Failed back surgery syndrome (FBSS) is a generalised disorder that is characterised by chronic pain in the lower back and/or legs that persists or recurs following anatomically successful spinal surgery. This paper aims to (1) assess the burden of failed back surgery in terms of its epidemiology, impact on health outcomes and costs and (2) summarise the evidence base for the cost-effectiveness of interventions for the management of FBSS. A narrative review based on a search of MEDLINE (PubMed) up to August 2012 was undertaken. Despite advances in technology and surgical techniques and increasing rates of spine surgery, a proportion of individuals continue to suffer from FBSS. Estimates from randomised controlled trials indicate that 5–50% of patients may have an unsuccessful outcome following lumbar spinal surgery. The understanding of the epidemiology and burden of FBSS remains poor and further research is needed in this area. The impact of FBSS on an individual’s health-related quality of life and its economic cost to society are considerable and more disabling than other common chronic pain and chronic medical conditions, such as heart failure and motor neuron disease. There is a substantive body of evidence in FBSS patients showing spinal cord stimulation (SCS) to be cost-effective (<£10,000 per quality-adjusted life year). In 2008, the National Institute for Health and Clinical Excellence recommended SCS as a treatment option for FBSS, either as an alternative to further lumbar surgery or as an adjunct to conservative medical management. The clinical and cost-effectiveness of SCS in the subgroup of those with FBSS receiving workers’ compensation remains less clear. Intrathecal morphine pumps may also be a potentially cost-effective strategy for FBSS. The findings of this review emphasise the importance of identifying strategies to prevent the development of FBSS and effective guidelines for the management of established FBSS. The continued development and application of new neuromodulation therapies and technological innovations in the field of FBSS need to be accompanied by the collection of clinical and economic data in order to demonstrate to healthcare policy makers and payers that such innovations provide benefit to the patient at good value for money.
Keywords: Costs, cost-effectiveness, epidemiology, quality of life, failed back surgery syndrome, chronic low back pain
Failed back surgery syndrome (FBSS) is a disorder that is characterised by chronic pain in the lower back and/or legs that persists or recurs following anatomically successful spinal surgery.1,2 FBSS results when the outcome of lumbar spinal surgery does not meet the pre-surgical expectation of the patient and surgeon.3
The economist, Paul Samuelson, defines economics as:4
The study of how men and society end up choosing, with or without the use of money, to employ scarce productive resources that could have alternative uses, to produce various commodities and distribute them for consumption, now or in the future, among various people and groups in society. It analyses the costs and benefits of improving patterns of resource allocation.
Thus, in considering the economic impact of FBSS, it is important to address not only the cost or financial consequences of FBSS, but also the impact of FBSS in terms of health outcomes (or ‘loss of health’).
This paper is organised into three sections. First, we consider ‘the burden of FBSS’, that is the epidemiology, impact on health outcomes and cost of illness of FBSS. Second, we summarise the evidence base for the cost-effectiveness (or ‘value for money’) of interventions aimed at managing FBSS. Finally, we draw this information together to make recommendations for clinicians and health policy makers regarding the management and funding of services for FBSS, and also identify gaps for future research.
A search of MEDLINE (PubMed) up to August 2012 was undertaken. The following search terms were used: (FBSS OR failed back surgery syndrome OR chronic low back pain) AND (costs OR cost benefit OR cost effectiveness OR economic). Studies addressing the above-mentioned issues are discussed in detail below.
Burden of FBSS
Epidemiology
Low back pain is common. It has a reported point prevalence in the general adult population that ranges from 12% to 40% and affects 60–80% of the population at some point in life.5,6 This wide range in prevalence estimates reflects both the lack of consensus regarding the definition of low back pain and also variation in the methodologies applied to estimating rates of low back pain in the population. Chronic low back pain (CLBP) is identified by the length of time a patient suffers from low back pain, the location of the pain and the aetiology of the symptoms. Approximately 5–10% of patients with low back pain develop CLBP that lasts longer than 3 months.7
Unfortunately there is little or no good literature on the epidemiology of FBSS – CLBP that persists or recurs following anatomically successful spinal surgery.1,2 In 1997, there were 317,000 lumbar surgeries performed in the United States.8 In 2002, 5 years later, there were more than 1 million spinal procedures performed.9 Despite no clear indication and an absence of demonstrated efficacy for spinal fusion, between 1990 and 2000, there was a 220% increase in spinal fusion surgery.9,10 International data show that the rate of back surgery in the United States is at least 40% higher than in any other country, and more than five times higher than in the United Kingdom.11 Given that a proportion of spinal surgeries are unsuccessful, concomitant with these increasing rates of spinal surgery, it is likely that the number of patients with FBSS has risen.
One approach to estimating the incidence of FBSS is to consider the outcomes of clinical trials of lumbar spinal surgery, that is identify the proportion of surgical patients who report an unsuccessful outcome following operation. Table 1 summarises the outcomes of a sample of randomised controlled trials of lumbar spinal surgery. Results show that the incidence of FBSS ranges across studies from 4% to 50%.12–15 This range reflects the heterogeneity in study populations, criteria to assess surgical success, surgical procedures, duration of follow-up and recency (and therefore technological innovation). It is, therefore, inappropriate to pool these studies in order to derive a single estimate of FBSS incidence. Instead, further research is needed to explore and better understand the heterogeneity in FBSS incidence across clinical trials using contemporary methods such as individual patient data meta-analysis and -regression.16 High-quality spinal surgery registries such as SWESPINE may also be useful in obtaining data on FBSS epidemiology.17
Table 1.
Rates of unsuccessful outcomes in selected randomised controlled trials of lumbar spinal surgery in chronic low back pain
| First Author (year) | Patient aetiology | Comparison | Follow-up (years) | Outcome | Proportion of surgical patients with an unsuccessful outcome |
|---|---|---|---|---|---|
| Webber (1983)12 | Radiculopathy secondary to lumbar disc herniation | Discectomy vs conservative medical management | 1 4 10 |
‘Good’ outcome: patient completely satisfied | 39/60 (65%) 40/60 (67%) 35/60 (58%) |
| Brox (1996)13 | Chronic back pain after previous surgery for disc herniation | Instrumented fusion vs cognitive intervention + exercises | 1 | ‘Success’: best grades for Prolo scale and global back question | 14/28 (50%) |
| Fritzell (2003)14 | Chronic low back pain | Non-instrumented posterolateral fusion vs instrumented posterolateral fusion vs interbody fusion with solid autogenous bone grafts (360). | 2 | The patient assessment: ‘much better’ or ‘better’ | PLF: 43/71 (60%) VSP: 46/68 (68%) 360 group: 43/72 (60%) |
| Peul (1997)15 | Severe sciatica for 6 to 12 weeks | Microdiscectomy vs conservative medical management | 1 2 |
Satisfactory outcome: global perceived recovery ‘complete’ or ‘nearly complete’ | 135/141 (96%) 225/281 (81%)a |
PLF: non-instrumented posterolateral fusion; VSP: instrumented posterolateral fusion.
Includes both surgery and control groups as there was no difference in outcome between the two groups.
Based on the figures in Table 1, the frequency of FBSS cases in the general population could range from a lower estimate of 0.02% to a figure as high as 2% (see Table 2).
Table 2.
Frequency of FBSS in the general population
| Lower estimate | Upper estimate | |
|---|---|---|
| Low back pain5,6 | 12% | 40% |
| CLBP (5–10%)7 | 0.6% | 4% |
| FBSS (4–50%)12–15 | 0.02% | 2% |
| 20/100,000 | 2000/100,000 |
CLBP: chronic low back pain; FBSS, failed back surgery syndrome.
Health outcomes
There is limited evidence on the health burden associated with CLBP and FBSS. A recent retrospective analysis of a US insurance claims database compared the co-morbidities of 101,294 patients with CLBP with a cohort of age- and sex-matched controls.18 Patients suffering from CLBP had a greater co-morbidity burden including a significantly higher (p < 0.0001) frequency of musculoskeletal and neuropathic pain conditions and common sequelae of pain such as depression (13.0% vs 6.1%), anxiety (8.0% vs 3.4%) and sleep disorders (10.0% vs 3.4%).
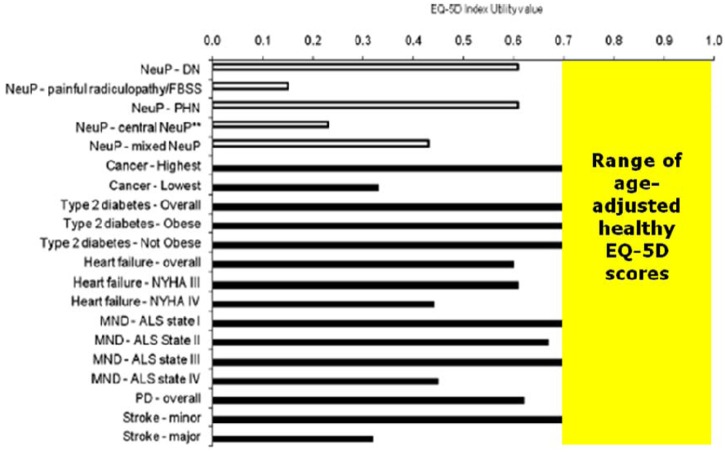
Compared with other chronic pain models (e.g. rheumatoid arthritis), FBSS patients experience greater levels of pain, a lower health-related quality of life (as measured by the EQ-5D and Short Form-36), greater disability (as measured by the Oswestry Disability Index), and a higher rate of unemployment (see Table 3).19 Similarly, a recent systematic review and meta-analysis confirmed that FBSS patients experience very low utilities and poorer levels of health-related quality of life compared with other chronic diseases (e.g. heart failure, type 2 diabetes) as well as other neuropathic pain conditions (e.g. diabetic polyneuropathy; see Figure 1).20 We hypothesise that poorer quality of life levels may reflect not only more severe pain but also additional co-morbidities.
Table 3.
Comparison of health burden of FBSS and rheumatoid arthritis
| FBSS | Rheumatoid arthritis | |
|---|---|---|
| Work disability rate | 78% | 50% |
| Disability | ||
| Oswestry Disability Scalea (mean) | 56.4 | 27 |
| Health-related quality of life | ||
| EQ-5D indexb (mean) | 0.16 | 0.42 to 0.752 |
| Short-Form 36 domainsa (mean) | ||
| Physical functioning | 23.4 | 62.3 |
| Role – physical | 5.1 | 49.0 |
| Bodily pain | 16.3 | 58.0 |
| General health | 45.7 | 52.1 |
| Vitality | 31.2 | 52.2 |
| Social functioning | 35.2 | 70.3 |
| Role – emotional | 36.4 | 72.3 |
| Mental health | 52.7 | 69.2 |
Note: table adapted from Riley RD, Lambert PC and Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. Brit Med J 2010; 340: c221 with permission from BMJ Publishing Group Ltd.
Higher score indicates lower level of disability or health-related quality of life; brange of means presented.
Figure 1.
Comparison of EQ-5D index values for FBSS with other neuropathic pain and common chronic medical diseases. This figure is modified from Doth AH, Hansson PT, Jensen MP, et al. The burden of neuropathic pain: a systematic review and meta-analysis of health utilities. Pain 2010; 149: 338–344 with permission from Elsevier.
Cost of illness
Cost of illness studies measure the economic burden of a disease and estimate the maximum amount that could potentially be saved, or gained, if a disease were to be eradicated.21,22 The total cost of illness – or ‘economic burden’ – has the following three components: (i) direct (medical and non-medical) costs, (ii) indirect costs and (iii) intangible costs. Direct medical costs include the costs of treating the disease and the costs of physician services, medical devices, medications, etc. Direct medical costs are typically the easiest to estimate because records are kept of such transactions by clinicians, third-party payers, employees or patients. Non-medical direct costs are those related to goods and services consumed directly because of the illness, but which are not considered to be health related (e.g. transportation costs to health care providers, costs of making adaptations to the home and costs of social care). Non-medical direct costs are often overlooked when considering the economic burden of a disease, but can constitute an important source of related costs. Indirect costs are those reflecting the economic value of consequences for which there is no direct monetary transfer. These include morbidity costs due to lost productivity through presenteeism or absenteeism. Indirect costs are often more difficult to assess than direct costs. The most common method of assessing indirect costs is the human capital approach, which assumes that the economic value of an employee’s productivity is equal to the cost of their salary and benefits. Intangible costs reflect the value of decreased enjoyment of life because of illness and are rarely included when estimating the economic burden of illness, largely because of general societal discomfort with placing a monetary value on these aspects of a disease. Cost of illness studies are usually conducted from one of two perspectives, either from a healthcare system perspective (that considers only direct medical costs) or from a societal perspective (considering both direct and indirect costs).
A systematic review identified 21 international cost of illness studies of low back pain published in the period from 1997 to 2007.23 Although methodologies were heterogeneous, studies consistently found the costs of low back pain to be a substantial burden on society.24 The fact that costs are heavily skewed by the most expensive claims with the longest duration suggests that most costs for low back pain are for CLBP. Indirect costs resulting from lost productivity represented a majority of the overall costs. The one UK study included estimated that, in 1998, direct costs of low back pain were £1.6 billion (£209 per capita) and that the overall costs varied between £6.6 billion and £12.3 billion depending on the costing method used.24 The largest proportion of direct medical costs were spent on chiropractic and physiotherapy (each 15%), outpatient care (17%) and primary care (13%).
No cost of illness studies were identified that have been specifically undertaken in an FBSS population. However, there is evidence to suggest that the economic burden of FBSS may be higher than CLBP. A recent study compared the healthcare service use and costs for CLBP patients with and without neuropathic components in the US population.25 The mean annual cost of care/patient was ~160% higher for CLBP patients with a neuropathic component than those without, i.e. US$2577 versus US$1007 (at 2008 prices). Except for physiotherapy, CLBP patients with neuropathic pain made significantly greater use of all resources during the follow-up period than patients without including medication, hospital visits, diagnostic assessment and interventional procedures. Between 2004 and 2006, a cohort study of 158 FBSS patients receiving Washington State workers’ compensation was undertaken to compare the cost-effectiveness of three treatment strategies (namely spinal cord stimulation (SCS), pain clinic and usual care).26 In addition to prospectively collecting the costs of each of the strategies, the authors assessed patient costs in the year prior to study entry. Mean medical and productivity loss costs were similar across three groups at baseline and ranged from US$18,195 to US$21,402, and US$22,403 to US$26,170 per FBSS patient, respectively. These medical costs are similar to those reported in an analysis of the cost-effectiveness of intrathecal morphine therapy for FBSS. The annual cost for medical therapy, excluding further surgery or implantation of a spinal cord stimulator or intrathecal pump, was estimated to be US$18,883 per patient (at 1990 prices).27 However, the costs from both of these US studies appear to be considerably higher than those reported in a clinical trial (PROCESS trial) of SCS in FBSS undertaken in European, Canadian and Australian centres. The mean annual cost of medical treatment in this trial was €5188 per patient (at 2005–6 prices).28 However, it is important to note that variance in medical costs was high (e.g. standard deviation of €5878 per patient in the PROCESS trial) indicating that these costs are likely to skewed by the small number of high-cost FBSS patients, and therefore considerable caution should be applied in comparing mean costs across studies.
Cost-effectiveness of treatments for the management of FBSS
Economic evaluations serve a different purpose to cost illnesses by focusing on the costs (and outcomes) of interventions, rather than estimating the cost of a particular disease.29 Full economic evaluations are therefore defined as studies that measure both costs and outcomes of two or more competing interventions. Economic evaluations allow healthcare policy makers to determine the appropriate distribution of resources towards competing interventions – so-called ‘value for money’ decisions. The incremental cost-effectiveness ratio (ICER) is used to summarise the difference in costs between two interventions relative to the difference in their outcomes.
For intervention B to be regarded as ‘good value for money’ (compared with intervention A), we would expect it to have a small additional cost and a high gain in outcomes, in other words a low ICER. Similarly, if, relative to intervention A, intervention B is expensive and provides little (or no) gain in outcomes (i.e. has a high ICER), we would regard it as ‘poor value for money’.
As with cost of illness, costs in economic evaluations may encompass both direct costs of treatment as well as indirect costs including time away from work or family role, loss of productivity and cost of carers.29 However, probably the greatest challenge for economic evaluations is the definition and method of outcome quantification. In this respect, there are three types of full economic evaluation. In a cost-effectiveness analysis (CEA), outcome is measured in disease specific natural units, e.g. cost per pain-free day. In a cost–utility analysis (CUA), outcome is measured using the generic measure of the quality-adjusted life year (QALY). In CUA, health outcomes are assessed using ‘utility’ scores (a score that typically ranges from 0 (‘worst quality of life’) to 1 (‘best quality of life’) and can be assessed using outcomes such as EuroQOL (EQ-5D).30 The QALY is calculated by combining utility with overall survival. Although a number of economic evaluations in healthcare continue to undertake CEA, the CUA is often regarded as the gold standard, allowing policy makers to directly compare the value for money of interventions across diseases. In the UK, the National Institute for Health and Clinical Excellence (NICE) considers the CUA as a ‘reference method’, and has set the willingness to pay threshold at £30,000 (~US$50,000) per QALY, i.e. interventions costing more than £30,000 per QALY are normally regarded as not cost-effective.31 In a cost–benefit analysis (CBA), outcomes are measured in monetary units, although, given the difficulties in valuing health outcomes in this way, CBA is infrequently used in healthcare?.29
Four economic evaluations have assessed the cost-effectiveness of SCS for FBSS26,32–34 and one study has examined the cost-effectiveness of implantable morphine pumps.27 The characteristics and results (ICERs) of these FBSS studies are summarised in Tables 4 and 5, respectively.
Table 4.
Full economic evaluations of treatments for the management of FBSS – summary of study characteristics
| First author (Year) | Country | Year of costs | Perspective | Study design | Comparisons | Time horizon (years) |
|---|---|---|---|---|---|---|
| SCS | ||||||
| North (2007)32 | United States | 1991–5 | Health services | Randomised controlled trial | SCS vs reoperation | 3 |
| Simpson (2009)33 | United Kingdom | 2007 | Health services | Decision analytic model | SCS vs reoperation; SCS+CMM vs CMM | 15 |
| Taylor (2010)34 | United Kingdom | 2009 | Health services | Decision analytic model | SCS vs reoperation; SCS+CMM vs CMM | 15 |
| Hollingworth (2012)26 | United States | 2007 | Societal | Prospective cohort study | SCS vs pain; clinic vs CMM | 2 |
| IMT implantable pump | ||||||
| deLissovoy (1997)27 | United States | 1993–4 | Health services | Decision analytic model | IMT vs CMM | 5 |
CMM: conventional medical management; IMT: intrathecal morphine therapy; SCS: spinal cord stimulation.
Table 5.
Full economic evaluations of treatments for the management of FBSS – summary of study results
| Author (year) | Intervention and result | Comparator and result | Difference | ICER | |
|---|---|---|---|---|---|
| SCS | |||||
| North (2007)32 | SCS | Reoperation | |||
| Costs/patient (US$) | 31,350 | 38,160 | –6629 | ||
| Number of successes | 7/19 (37%) | 7/21 (33%) | +4% | SCS dominant | |
| QALYs | 2.14 | 2.10 | +0.04 | ||
| Simpson (2009)33 | SCS | SCS + CMM | |||
| Costs/patient (£) | 88,443 | 78,408 | +10,035 | ||
| QALYs | 5.30 | 4.05 | +1.26 | £7996/QALY | |
| SCS | Reoperation | ||||
| Costs/patient (£) | 87,674 | 78,244 | +9430 | ||
| QALYs | 6.94 | 5.60 | +1.34 | £7043/QALY | |
| Taylor (2010)34 | SCS alone | SCS+CMM | |||
| Costs/patient (£) | 89,013 | 81,896 | +7027 | ||
| QALYS | 5.31 | 4.06 | +1.25 | £5624/QALY | |
| SCS | Reoperation | ||||
| Costs/patient (£) | 88,970 | 82,713 | +6257 | ||
| QALYs | 5.13 | 4.15 | +0.98 | £6392/QALY | |
| Hollingworth (2010)26 | SCS | Pain clinic | |||
| Costs/patient (US$) | NR | NR | +20,074a | ||
| % success | 10% | 3% | +7%a | US$131,146/success | |
| SCS | CMM | ||||
| Costs/patient (US$) | NR | NR | +29,358a | ||
| QALYs | 10% | 10% | 0%a | US$334,704/success | |
| IMT implantable pump | |||||
| De Lissovoy (1997)27 | IMT | CMM | |||
| Costs/patient (US$) | 125,103 | 85,186 | +39,916b | ||
| Months of pain relief | US$12,276/year of pain relief | ||||
CMM: conventional medical management; ICER: incremental cost-effectiveness ratio; IMT: intrathecal morphine therapy; QALY: quality-adjusted life year; SCS: spinal cord stimulation; NR: not reported.
Adjusted for baseline covariates; bworst case scenario.
Three of the four studies support the cost- effectiveness of SCS for FBSS. The analysis by North et al.32 of a randomised controlled trial at 3-year follow-up found SCS to be a ‘dominant’ economic option compared with reoperation, i.e. SCS was less costly and more effective.32 The CUAs of Simpson et al33 and Taylor et al34 both report an ICER for SCS of under £10,000 per QALY, in comparison with both reoperation and conventional medical management (CMM), when modelled over a 15-year time horizon. Based on this economic evidence, in 2008, NICE concluded ‘SCS is cost effective both as an adjunct to CMM and as alternative to reoperation’, and therefore recommended the use of SCS for the treatment of chronic neuropathic pain, including FBSS.35
However, a recently published analysis by Hollingworth and colleagues26 is less positive, concluding that ‘we found no evidence of that SCS is cost-effective intervention’. It is important to note that this more recent economic analysis was based on a study undertaken in a subgroup of FBSS patients, i.e. workers’ compensation recipients, and therefore its findings may not be applicable to the wider FBSS population. As this CEA study reported a cost per patient success outcome, it is not possible to interpret its findings in terms of a willingness to pay threshold of £30,000 per QALY (or ~US$50,000 per QALY). Furthermore, since its publication, the design and conduct of this workers’ compensation study has received considerable criticism, it being argued that its findings are prone to bias.36,37
The final economic evaluation identified was a decision analytical model, a CEA, of the use of intrathecal morphine pump in the pain management of simulated cohort of FBSS patients.27 The authors reported that, over the 5-year time horizon of the study, the ICER for intrathecal morphine, compared with CMM, ranged from US$7212 (best case) to $21,276 (worst case) per year of pain relief. As this was not a CUA, again it is not possible to conclude whether this intervention is below an acceptable threshold of cost-effectiveness.
Implications and conclusions
As presented above, CLBP is common and presents significant social and economic burden. However, our understanding of the epidemiology of FBSS remains poor and is therefore an important area for future research. The impact of FBSS on an individual’s health-related quality of life and its economic cost to society are considerable, and more disabling both when compared with other common chronic pain and also other chronic medical conditions (e.g. heart failure and motor neuron disease). These findings emphasise the need both to identify strategies to prevent FBSS and to develop evidenced-based guidelines for the management of established FBSS.
A number of full economic evaluations for the use of SCS in FBSS have been undertaken. These include evaluation by NICE, which recommends SCS as a treatment option for FBSS either as an alternative to further lumbar surgery or as an adjunct to conservative medical management. Furthermore, there is evidence to support the use of intrathecal morphine pumps as a cost-effective strategy for FBSS.
With the continued development and application of new and innovative neuromodulation therapies in the field of FBSS, it is critical that these developments are accompanied by the collection of clinical and economic data, in order to demonstrate their value for money to healthcare payers and policy makers.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: RST and RJT have received financial reimbursement as consultants for Medtronic International.
References
- 1. Wilkinson HA. The Failed Back Syndrome: Etiology and Therapy. 2nd ed. Philadelphia: Harper & Row, 1991. [Google Scholar]
- 2. North RB, Campbell J, James CS, et al. Failed back surgery syndrome: 5-year follow-up in 102 patients undergoing repeated operation. Neurosurgery 1991; 28: 685–691. [PubMed] [Google Scholar]
- 3. Waguespack A, Schofferman J, Slosar P, Reynold J. Etiology of long-term failures of lumbar spine surgery. Pain Med 2002; 3: 18–22. [DOI] [PubMed] [Google Scholar]
- 4. Samuelson P. Economics. Columbus, OH: McGraw-Hill, 1948. [Google Scholar]
- 5. Hoy D, Brooks P, Blyth F, Buchbinder R. The Epidemiology of low back pain. Best Pract Res Clin Rheumatol 2010; 24: 769–781. [DOI] [PubMed] [Google Scholar]
- 6. Juniper M, Le TK, Mladsi D. The epidemiology, economic burden, and pharmacological treatment of chronic low back pain in France, Germany, Italy, Spain and the UK: a literature-based review. Expert Opin Pharmacother 2009; 10: 2581–2592. [DOI] [PubMed] [Google Scholar]
- 7. Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber-Moffett J, Kovacs F, et al. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J 2006; 15: S192–S300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics. National Hospital Discharge Survey. Series 13, No.144. Washington, DC: US Department of Health and Human Services, Center for Disease Control, 1997. [Google Scholar]
- 9. Deyo RA, Mirza SK. Trends and variations in the use of spine surgery. Clin Orthop Relat Res 2006; 443: 139–146. [DOI] [PubMed] [Google Scholar]
- 10. Deyo RA, Mirza SK. The case for restraint in spinal surgery: Does quality management have a role to play? Eur Spine J 2009; 18(S3): S331–S337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cherkin DC, Deyo RA, Loeser JD, Bush T, Waddell G. An international comparison of back surgery rates. Spine 1994; 19: 1201–1206. [DOI] [PubMed] [Google Scholar]
- 12. Weber H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine 1983; 8: 131–140. [PubMed] [Google Scholar]
- 13. Brox JA, Reikera A, Nygaard O, et al. Lumbar instrumented fusion compared with cognitive intervention and exercises in patients with chronic back pain after previous surgery for disc herniation: A prospective randomized controlled study. Pain 2006; 122: 145–155. [DOI] [PubMed] [Google Scholar]
- 14. Fritzell P, Hagg O, Nordwall A, the Swedish Lumbar Spine Group. Complications in lumbar fusion surgery for chronic low back pain: comparison of three surgical techniques used in a prospective randomized study. A report from the Swedish Lumbar Spine Study Group. Eur Spine J 2003; 12(2): 178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peul WC, van Houwelingen HC, van den Hout WB, et al. ; Leiden-The Hague Spine Intervention Prognostic Study Group. Surgery versus prolonged conservative treatment for sciatica. N Engl J Med 2007; 356: 2245–2256. [DOI] [PubMed] [Google Scholar]
- 16. Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. Brit Med J 2010; 340: c221. [DOI] [PubMed] [Google Scholar]
- 17.SWESPINE, the Swedish Spine Register. Available at: http://www.4s.nu/patientsida_eng/index.html (accessed 11 October 2012).
- 18. Gore M, Sadosky A, Stacey BR, Tai KS, Leslie D. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine (Phila Pa 1976) 2012; 37: E668–E677. [DOI] [PubMed] [Google Scholar]
- 19. Thomson S, Jacques L. Demographic characteristics of patients with severe neuropathic pain secondary to failed back surgery syndrome. Pain Pract 2009; 9: 206–215. [DOI] [PubMed] [Google Scholar]
- 20. Doth AH, Hansson PT, Jensen MP, et al. The burden of neuropathic pain: a systematic review and meta-analysis of health utilities. Pain 2010; 149: 338–344. [DOI] [PubMed] [Google Scholar]
- 21. Byford S, Torgerson DJ, Raftery R. Cost of illness studies. BMJ 2000; 320:1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shiell A, Gerard K, Donaldson C. Cost of illness studies: an aid decision-making? Health Policy 1987; 8: 317–323. [Google Scholar]
- 23. Dagenais S, Roffey DM, Wai EK, Haldeman S, Caro J. Can cost utility evaluations inform decision making about interventions for low back pain? Spine J 2009; 9: 944–957. [DOI] [PubMed] [Google Scholar]
- 24. Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain 2000; 84: 95–103. [DOI] [PubMed] [Google Scholar]
- 25. Mehra M, Hill K, Nicholl D, Schadrack J. The burden of chronic low back pain with and without a neuropathic component: a healthcare resource use and cost analysis. J Med Econ 2012; 15: 1–8. [DOI] [PubMed] [Google Scholar]
- 26. Hollingworth W, Turner JA, Welton NJ, Comstock BA, Deyo RA. Costs and cost-effectiveness of spinal cord stimulation (SCS) for failed back surgery syndrome: an observational study in a workers’ compensation population. Spine (Phila Pa 1976) 2011; 36(24): 2076–2083. [DOI] [PubMed] [Google Scholar]
- 27. de Lissovoy G, Brown RE, Halpern M, Hassenbusch SJ, Ross E. Cost-effectiveness of long-term intrathecal morphine therapy for pain associated with failed back surgery syndrome. Clin Ther 1997; 19: 96–112. [DOI] [PubMed] [Google Scholar]
- 28. Manca A, Kumar K, Taylor RS, et al. Quality of life, resource consumption and costs of spinal cord simulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). Eur J Pain 2008; 12: 1047–1058. [DOI] [PubMed] [Google Scholar]
- 29. Drummond M, O’Brien B, Stoddart G, Torrance GW, eds. Methods for the Economic Evaluation of Health Care Programs. 2nd ed. Oxford: Oxford University Press, 1997. [Google Scholar]
- 30. Kind P. The EuroQoL instrument: an index of health-related quality of life. In: Spilker B. (ed.) Quality of Life and Pharmacoeconomics in Clinical Trials. 2nd ed. Philadelphia: Lippincott-Raven, 1996, pp. 191–201. [Google Scholar]
- 31. Rawlins MD, Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ 2004; 329: 224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. North RB, Shipley J, Taylor RS. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a cost effectiveness and cost utility analysis based on a randomized, controlled trial. Neurosurg 2007; 61: 361–368. [DOI] [PubMed] [Google Scholar]
- 33. Simpson EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J. Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation. Health Technol Assess 2009; 13(17): iii, ix,–x, 1–154. [DOI] [PubMed] [Google Scholar]
- 34. Taylor RS, Ryan J, O’Donnell R, Eldabe S, Kumar K, North RB. The cost-effectiveness of spinal cord stimulation in the treatment of failed back surgery syndrome. Clin J Pain 2010; 26: 463–469. [DOI] [PubMed] [Google Scholar]
- 35.National Institute for Health and Clinical Excellence. Spinal Cord Stimulation for Chronic Pain of Neuropathic or Ischaemic Origin. London: National Institute for Health and Clinical Excellence, 2008. Available at: http://www.nice.org.uk/nicemedia/pdf/TA159Guidance.pdf (accessed 11 October 2012). [Google Scholar]
- 36. Wasan AD. Spinal cord stimulation in a workers’ compensation population: how difficult it can be to interpret a clinical trial. Pain 2010; 148: 3–4. [DOI] [PubMed] [Google Scholar]
- 37. North RB, Shipley J, Taylor RS, Eldabe S. Questions about Turner et al. Spinal cord stimulation for failed back surgery syndrome: outcomes in a worker’s compensation setting. Pain 2010; 151: 550–551. [DOI] [PubMed] [Google Scholar]